Improving the Detectability of Microplastics in River Waters by Single Particle Inductively Coupled Plasma Mass Spectrometry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Instrumentation
2.1.1. Inductively Coupled Plasma Mass Spectrometry (ICP-MS)
2.1.2. Raman Microscopy
2.1.3. Field Emission Scanning Electron Microscopy (FESEM)
2.2. Standards
2.3. River Water Samples
2.4. Procedures
2.4.1. SP-ICP-MS Analysis
2.4.2. Acidic Pre-Treatment of River Water Samples for SP-ICP-MS Analysis
2.4.3. Preparation of Ag-Labelled Bacterial Suspension
2.4.4. FESEM Analysis
2.4.5. Raman Microscopy Analysis
3. Results
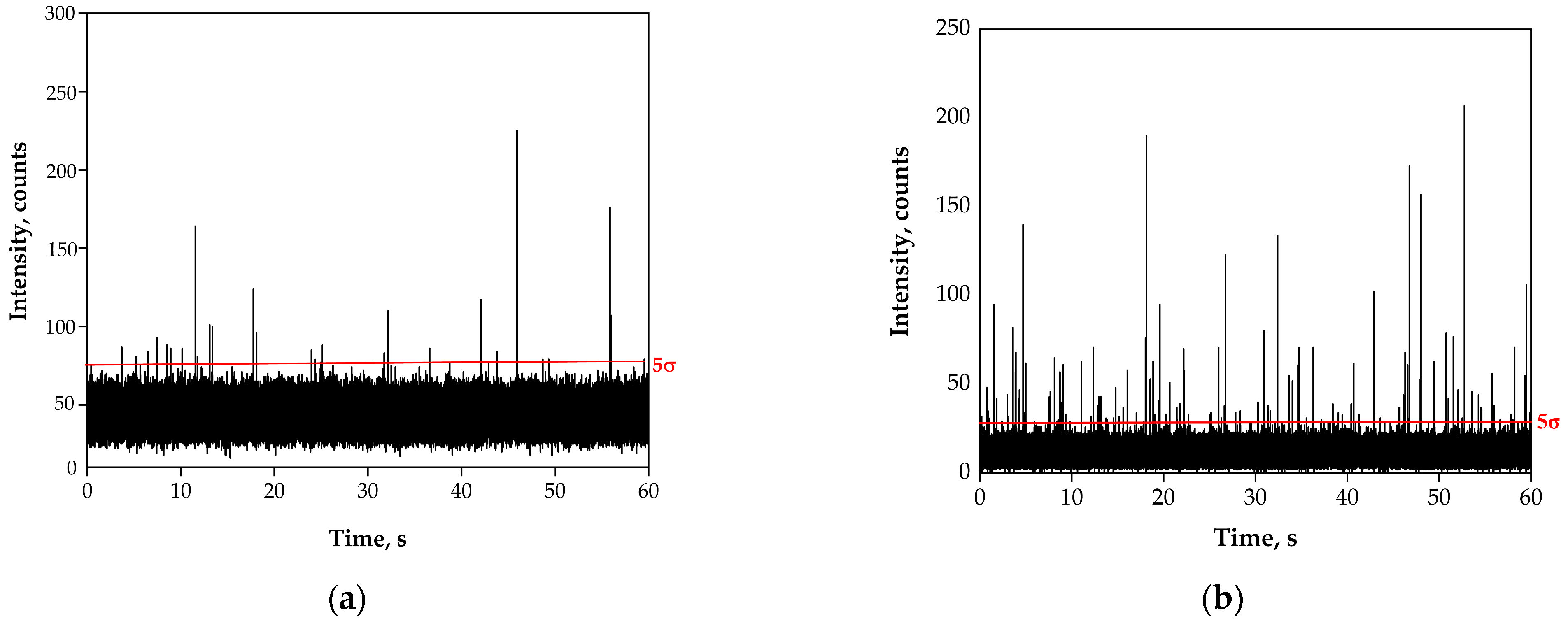
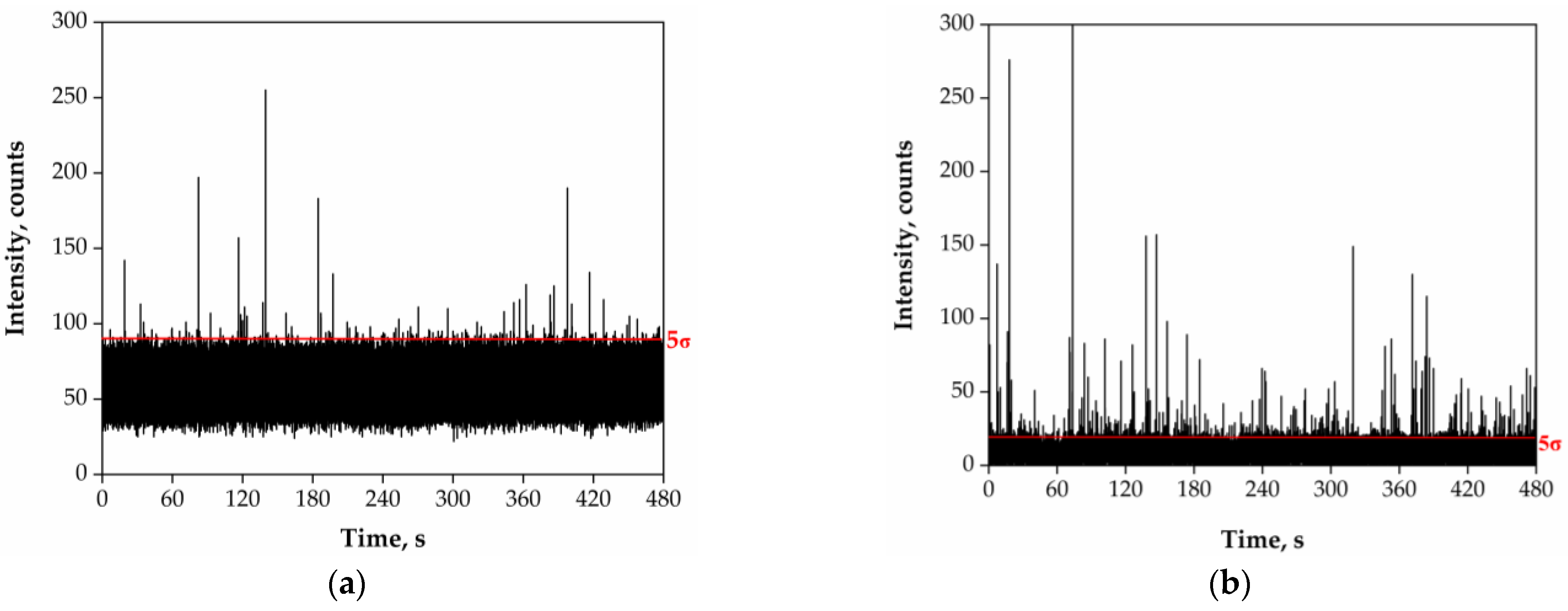
3.1. Preliminary Analysis of River Water Samples by SP-ICP-MS
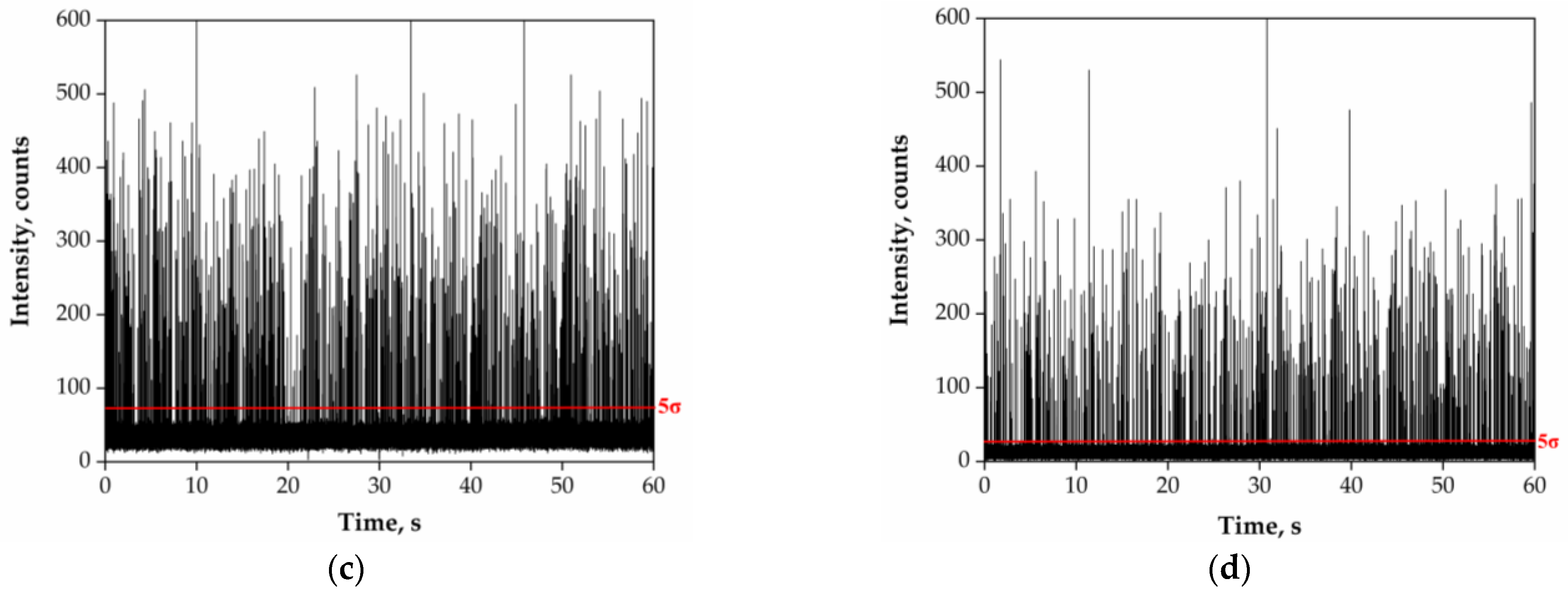
3.2. Acidic Pre-Treatment of River Water Samples
3.3. Analysis of River Water Samples by Scanning Electron and Raman Microscopies
4. Discussion
4.1. Acidic Pre-Treatment of River Water Samples
4.2. Analysis of River Water Samples by SP-ICP-MS
4.3. Analysis of River Water Samples by Electron and Raman Microscopy
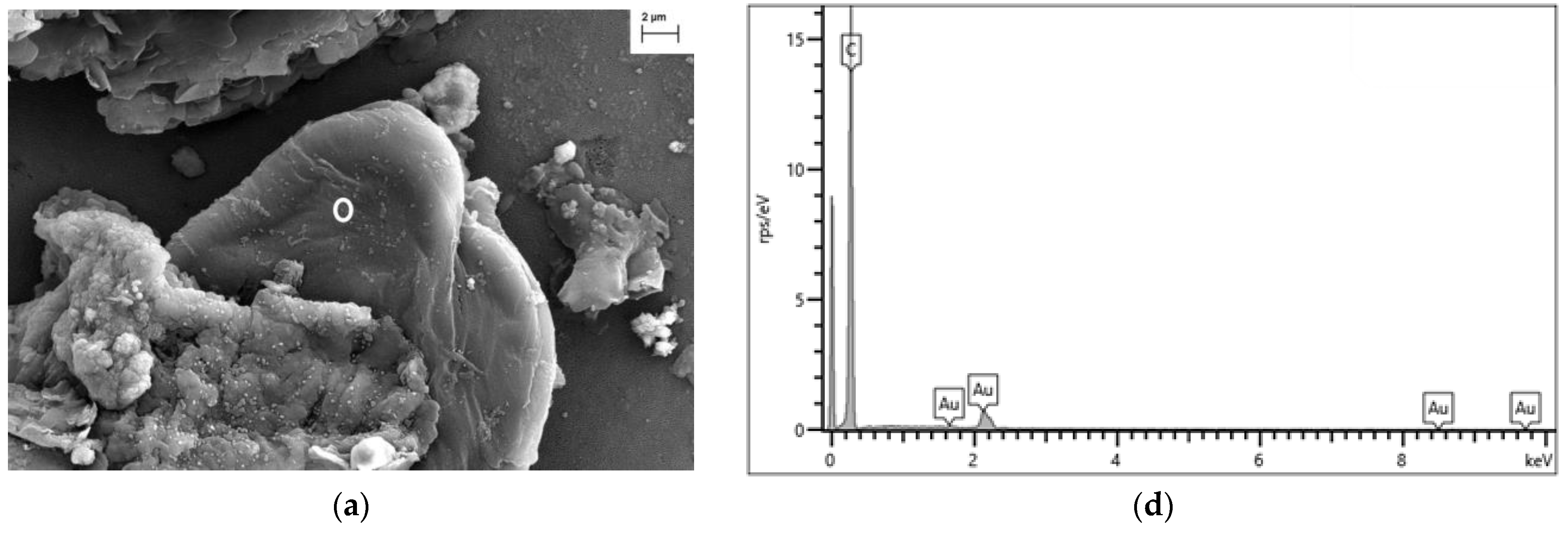
4.3.1. FESEM-EDX Analysis
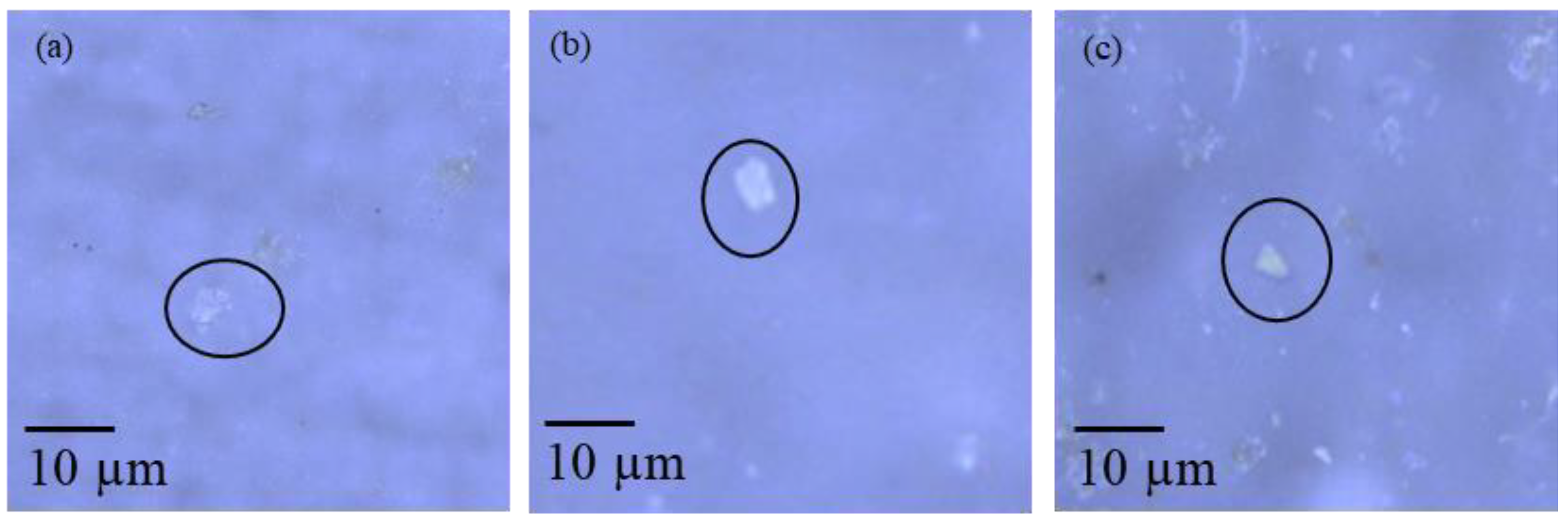
4.3.2. Raman Microscopy
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Alexy, P.; Anklam, E.; Emans, T.; Furfari, A.; Galgani, F.; Hanke, G.; Koelmans, A.; Pant, R.; Saveyn, H.; Sokull Kluettgen, B. Managing the Analytical Challenges Related to Micro- and Nanoplastics in the Environment and Food: Filling the Knowledge Gaps. Food Addit. Contam. Part A 2020, 37, 1–10. [Google Scholar] [CrossRef]
- Hartmann, N.B.; Hüffer, T.; Thompson, R.C.; Hassellöv, M.; Verschoor, A.; Daugaard, A.E.; Rist, S.; Karlsson, T.; Brennholt, N.; Cole, M.; et al. Are We Speaking the Same Language? Recommendations for a Definition and Categorization Framework for Plastic Debris. Environ. Sci. Technol. 2019, 53, 1039–1047. [Google Scholar] [CrossRef]
- Frias, J.P.G.L.; Nash, R. Microplastics: Finding a Consensus on the Definition. Mar. Pollut. Bull. 2019, 138, 145–147. [Google Scholar] [CrossRef]
- Gigault, J.; ter Halle, A.; Baudrimont, M.; Pascal, P.Y.; Gauffre, F.; Phi, T.L.; El Hadri, H.; Grassl, B.; Reynaud, S. Current Opinion: What is a Nanoplastic? Environ. Pollut. 2018, 235, 1030–1034. [Google Scholar] [CrossRef] [PubMed]
- Bianco, A.; Passananti, M. Atmospheric Micro and Nanoplastics: An Enormous Microscopic Problem. Sustainability 2020, 12, 7327. [Google Scholar] [CrossRef]
- Murphy, F.; Ewins, C.; Carbonnier, F.; Quinn, B. Wastewater Treatment Works (WwTW) as a Source of Microplastics in the Aquatic Environment. Environ. Sci. Technol. 2016, 50, 5800–5808. [Google Scholar] [CrossRef]
- Magnusson, K.; Norén, F. Screening of Microplastic Particles in and Down-Stream a Wastewater Treatment Plant; IVL Swedish Environmental Research Institute: Stockholm, Sweden, 2014. [Google Scholar]
- Enfrin, M.; Dumée, L.F.; Lee, J. Nano/Microplastics in Water and Wastewater Treatment Processes—Origin, Impact and Potential Solutions. Water Res. 2019, 161, 621–638. [Google Scholar] [CrossRef] [PubMed]
- Tagg, A.S.; Sapp, M.; Harrison, J.P.; Sinclair, C.J.; Bradley, E.; Ju-Nam, Y.; Ojeda, J.J. Microplastic Monitoring at Different Stages in a Wastewater Treatment Plant Using Reflectance Micro-FTIR Imaging. Front. Environ. Sci. 2020, 8, 145. [Google Scholar] [CrossRef]
- D’Avignon, G.; Gregory-Eaves, I.; Ricciardi, A. Microplastics in Lakes and Rivers: An Issue of Emerging Significance to Limnology. Environ. Rev. 2022, 30, 228–244. [Google Scholar] [CrossRef]
- Cordova, M.R.; Nurhati, I.S.; Shiomoto, A.; Hatanaka, K.; Saville, R.; Riani, E. Spatiotemporal Macro Debris and Microplastic Variations Linked to Domestic Waste and Textile Industry in the Supercritical Citarum River, Indonesia. Mar. Pollut. Bull. 2022, 175, 113338. [Google Scholar] [CrossRef] [PubMed]
- Winkler, A.; Antonioli, D.; Masseroni, A.; Chiarcos, R.; Laus, M.; Tremolada, P. Following the Fate of Microplastic in Four Abiotic and Biotic Matrices along the Ticino River (North Italy). Sci. Total Environ. 2022, 823, 153638. [Google Scholar] [CrossRef] [PubMed]
- Pol, W.; Żmijewska, A.; Stasińska, E.; Zieliński, P. Spatial-Temporal Distribution of Microplastics in Lowland Rivers Flowing through Two Cities (Ne-Poland). Water Air Soil Pollut. 2022, 233, 140. [Google Scholar] [CrossRef]
- Yuan, W.; Christie-Oleza, J.A.; Xu, E.G.; Li, J.; Zhang, H.; Wang, W.; Lin, L.; Zhang, W.; Yang, Y. Environmental Fate of Microplastics in the World’s Third-Largest River: Basin-Wide Investigation and Microplastic Community Analysis. Water Res. 2022, 210, 118002. [Google Scholar] [CrossRef]
- Devereux, R.; Westhead, E.K.; Jayaratne, R.; Newport, D. Microplastic Abundance in the Thames River during the New Year Period. Mar. Pollut. Bull. 2022, 177, 113534. [Google Scholar] [CrossRef]
- Zhdanov, I.; Lokhov, A.; Belesov, A.; Kozhevnikov, A.; Pakhomova, S.; Berezina, A.; Frolova, N.; Kotova, E.; Leshchev, A.; Wang, X.; et al. Assessment of Seasonal Variability of Input of Microplastics from the Northern Dvina River to the Arctic Ocean. Mar. Pollut. Bull. 2022, 175, 113370. [Google Scholar] [CrossRef]
- Treilles, R.; Gasperi, J.; Tramoy, R.; Dris, R.; Gallard, A.; Partibane, C.; Tassin, B. Microplastic and Microfiber Fluxes in the Seine River: Flood Events versus Dry Periods. Sci. Total Environ. 2022, 805, 150123. [Google Scholar] [CrossRef]
- Sá, B.; Pais, J.; Antunes, J.; Pequeno, J.; Pires, A.; Sobral, P. Seasonal Abundance and Distribution Patterns of Microplastics in the Lis River, Portugal. Sustainability 2022, 14, 2255. [Google Scholar] [CrossRef]
- Laermanns, H.; Reifferscheid, G.; Kruse, J.; Földi, C.; Dierkes, G.; Schaefer, D.; Scherer, C.; Bogner, C.; Stock, F. Microplastic in Water and Sediments at the Confluence of the Elbe and Mulde Rivers in Germany. Front. Environ. Sci. 2021, 9, 590. [Google Scholar] [CrossRef]
- de Carvalho, A.R.; Garcia, F.; Riem-Galliano, L.; Tudesque, L.; Albignac, M.; ter Halle, A.; Cucherousset, J. Urbanization and Hydrological Conditions Drive the Spatial and Temporal Variability of Microplastic Pollution in the Garonne River. Sci. Total Environ. 2021, 769, 144479. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.; Zhong, X.; Duan, Z.; Yi, X.; Cheng, F.; Xu, W.; Yang, X. Micro- and Nanoplastics Released from Biodegradable and Conventional Plastics during Degradation: Formation, Aging Factors, and Toxicity. Sci. Total Environ. 2022, 833, 155275. [Google Scholar] [CrossRef]
- Laborda, F.; Trujillo, C.; Lobinski, R. Analysis of Microplastics in Consumer Products by Single Particle-Inductively Coupled Plasma Mass Spectrometry Using the Carbon-13 Isotope. Talanta 2021, 221, 121486. [Google Scholar] [CrossRef] [PubMed]
- Bolea-Fernandez, E.; Rua-Ibarz, A.; Velimirovic, M.; Tirez, K.; Vanhaecke, F. Detection of Microplastics Using Inductively Coupled Plasma-Mass Spectrometry (ICP-MS) Operated in Single-Event Mode. J. Anal. At. Spectrom. 2020, 35, 455–460. [Google Scholar] [CrossRef]
- Liu, Z.; Zhu, Y.; Lv, S.; Shi, Y.; Dong, S.; Yan, D.; Zhu, X.; Peng, R.; Keller, A.A.; Huang, Y. Quantifying the Dynamics of Polystyrene Microplastics UV-Aging Process. Environ. Sci. Technol. Lett. 2022, 9, 50–56. [Google Scholar] [CrossRef]
- Li, F.; Armstrong, D.W.; Houk, R. Behavior of Bacteria in the Inductively Coupled Plasma: Atomization and Production of Atomic Ions for Mass Spectrometry. Anal. Chem. 2005, 77, 1407–1413. [Google Scholar] [CrossRef]
- Riisom, M.; Gammelgaard, B.; Lambert, I.H.; Stürup, S. Development and Validation of an ICP-MS Method for Quantification of Total Carbon and Platinum in Cell Samples and Comparison of Open-Vessel and Microwave-Assisted Acid Digestion Methods. J. Pharm. Biomed. Anal. 2018, 158, 144–150. [Google Scholar] [CrossRef]
- Abril, G.; Etcheber, H.; Delille, B.; Frankignoulle, M. Carbonate Dissolution in the Turbid and Eutrophic Loire Estuary. Mar. Ecol. Prog. Ser. 2003, 259, 129–138. [Google Scholar] [CrossRef]
- Laborda, F.; Gimenez-Ingalaturre, A.C.; Bolea, E.; Castillo, J.R. Single Particle Inductively Coupled Plasma Mass Spectrometry as Screening Tool for Detection of Particles. Spectrochim. Acta Part B At. Spectrosc. 2019, 159, 105654. [Google Scholar] [CrossRef]
- Currie, L.A. Limits for Qualitative Detection and Quantitative Determination Application to Radiochemistry. Anal. Chem. 1968, 40, 586–593. [Google Scholar] [CrossRef]
- Pace, H.E.; Rogers, N.J.; Jarolimek, C.; Coleman, V.A.; Higgins, C.P.; Ranville, J.F. Determining Transport Efficiency for the Purpose of Counting and Sizing Nanoparticles via Single Particle Inductively Coupled Plasma Mass Spectrometry. Anal. Chem. 2011, 83, 9361–9369. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.S.; Elsamahy, T.; Koutra, E.; Kornaros, M.; El-Sheekh, M.; Abdelkarim, E.A.; Zhu, D.; Sun, J. Degradation of Conventional Plastic Wastes in the Environment: A Review on Current Status of Knowledge and Future Perspectives of Disposal. Sci. Total Environ. 2021, 771, 144719. [Google Scholar] [CrossRef]
- Li, P.; Lai, Y.; Li, Q.; Dong, L.; Tan, Z.; Yu, S.; Chen, Y.; Sharma, V.K.; Liu, J.; Jiang, G. Total Organic Carbon as a Quantitative Index of Micro- and Nano-Plastic Pollution. Anal. Chem. 2022, 94, 740–747. [Google Scholar] [CrossRef]
- López, A.D.F.; Fabiani, M.; Lassalle, V.L.; Spetter, C.V.; Severini, M.D.F. Critical Review of the Characteristics, Interactions, and Toxicity of Micro/Nanomaterials Pollutants in Aquatic Environments. Mar. Pollut. Bull. 2022, 174, 113276. [Google Scholar] [CrossRef]
- Yu, Y.; Mo, W.Y.; Luukkonen, T. Adsorption Behaviour and Interaction of Organic Micropollutants with Nano and Microplastics—A Review. Sci. Total Environ. 2021, 797, 149140. [Google Scholar] [CrossRef] [PubMed]
- Confederación Hidrográfica del Ebro. Plan Hidrológico Del Río Gállego; CHE: Zaragoza, Spain, 2007. [Google Scholar]
- Instituto Geológico y Minero de España. Geochemical Database. Available online: https://info.igme.es/Geoquimica/ (accessed on 29 March 2023).
- Geochemical Atlas of Europe. Available online: http://weppi.gtk.fi/publ/foregsatlas/index.php (accessed on 29 March 2023).
- Gonzalez de Vega, R.; Goyen, S.; Lockwood, T.E.; Doble, P.A.; Camp, E.F.; Clases, D. Characterisation of Microplastics and Unicellular Algae in Seawater by Targeting Carbon via Single Particle and Single Cell ICP-MS. Anal. Chim. Acta 2021, 1174, 338737. [Google Scholar] [CrossRef]
- Laborda, F.; Gimenez-Ingalaturre, A.C.; Bolea, E.; Castillo, J.R. About Detectability and Limits of Detection in Single Particle Inductively Coupled Plasma Mass Spectrometry. Spectrochim. Acta Part B At. Spectrosc. 2020, 169, 105883. [Google Scholar] [CrossRef]
- Lampman, S. Characterization and Failure Analysis of Plastics; ASM International: Almer, The Netherlands, 2003. [Google Scholar]
- Azevedo, A.G.; Barros, C.; Miranda, S.; Machado, A.V.; Castro, O.; Silva, B.; Saraiva, M.; Silva, A.S.; Pastrana, L.; Carneiro, O.S.; et al. Active Flexible Films for Food Packaging: A Review. Polymers 2022, 14, 2442. [Google Scholar] [CrossRef] [PubMed]
- Ranakoti, L.; Gangil, B.; Mishra, S.K.; Singh, T.; Sharma, S.; Ilyas, R.A.; El-Khatib, S. Critical Review on Polylactic Acid: Properties, Structure, Processing, Biocomposites, and Nanocomposites. Materials 2022, 15, 4312. [Google Scholar] [CrossRef]
- Wanner, P. Plastic in Agricultural Soils—A Global Risk for Groundwater Systems and Drinking Water Supplies?—A Review. Chemosphere 2021, 264, 128453. [Google Scholar] [CrossRef]

| River | Country | Technique | Type of Plastic | Size | Plastic Concentration | Ref. |
|---|---|---|---|---|---|---|
| Citarum | Indonesia | Optical microscopy FTIR Raman microscopy | PET PS Cellophane Nylon PP PE | ˂300 µm - >1000 µm | 3.35 ± 0.54 m−3 | [11] |
| Ticino | Italy | Optical microscopy FTIR microscopy | LDPE PET PP | >20 µm | 33 ± 21 m−3 | [12] |
| Biala Czarna Hancza | Poland | Optical microscopy | - | >0.04–4 µm | 10.83 ± 3.96 L−1 10.29 ± 3.90 L−1 | [13] |
| Yangtze | China | Optical microscopy Raman microscopy | PP PE PA PS PVC PET PC | >0.11 µm | 1.27 ± 0.83 L−1 | [14] |
| Thames | United Kingdom | Optical microscopy FTIR | Rubber PVC PE | >0.5 mm - 5 mm | 51 ± 10 L−1 | [15] |
| Northern Dvina | Russia | FTIR | PE PP EEA | >0.5 mm | 0.6–1.4 × 104 km−2 | [16] |
| Seine | France | Optical microscopy FTIR microscopy | PP PE PES | 32–2528 µm | 15.5 ± 4.9 L−1 | [17] |
| Lis | Portugal | Optical microscopy FTIR FTIR microscopy | PP PVC PC Nylon | 14–4726 µm | 234 ± 398 m−3 | [18] |
| Elbe Mulde | Germany | Optical microscopy Pyr-GC-MS | PE PP PS | >50 µm | 15 ± 2 m−3 0.33–1.19 mg m−3 | [19] |
| Garone | France | Optical microscopy ATR-FTIR | PE PP PS | 700 µm - 5 mm | 0.15 ± 0.46 m−3 | [20] |
| Sample | XCsize (μm) | Number of Particle Events Detected | Particle Concentration (×104 L−1) |
|---|---|---|---|
| UP water | 1.25 | 3 ± 2 | - |
| RW01 | 1.44 | 38 ± 10 | 295 ± 32 |
| RW02 | 1.44 | 40 ± 12 | 310 ± 25 |
| RW03 | 1.53 | 28 ± 8 | 217 ± 21 |
| RW04 | 1.48 | 10 ± 3 | 109 ± 12 |
| RW05 | 1.49 | 28 ± 7 | 217 ± 15 |
| RW06 | 1.55 | 22 ± 5 | 171 ± 20 |
| RW07 | 1.63 | 17 ± 6 | 132 ± 19 |
| RW08 | 1.54 | 15 ± 4 | 116 ± 26 |
| RW09 | 1.41 | 16 ± 4 | 124 ± 15 |
| RW10 | 1.47 | 19 ± 8 | 147 ± 10 |
| RW11 | 1.39 | 13 ± 3 | 101 ± 9 |
| RW12 | 1.45 | 17 ± 4 | 132 ± 9 |
| RW13 | 1.51 | 10 ± 5 | 78 ± 7 |
| RW14 | 1.47 | 27 ± 6 | 209 ± 19 |
| RW15 | 1.46 | 24 ± 4 | 186 ± 13 |
| RW16 | 1.52 | 6 ± 2 | 47 ± 5 |
| RW17 | 1.46 | 20 ± 4 | 155 ± 20 |
| RW18 | 1.48 | 19 ± 3 | 147 ± 27 |
| RW19 | 1.47 | 16 ± 2 | 124 ± 30 |
| RW20 | 1.49 | 12 ± 2 | 93 ± 20 |
| RW21 | 1.35 | 20 ± 3 | 155 ± 32 |
| RW22 | 1.31 | 18 ± 4 | 140 ± 16 |
| RW23 | 1.37 | 42 ± 11 | 326 ± 42 |
| RW24 | 1.37 | 27 ± 10 | 209 ± 21 |
| RW25 | 1.31 | 43 ± 9 | 334 ± 44 |
| RW26 | 1.50 | 11 ± 3 | 85 ± 6 |
| RW27 | 1.35 | 29 ± 6 | 225 ± 30 |
| RW28 | 1.32 | 16 ± 5 | 124 ± 10 |
| Sample | HNO3 (% v/v) | Mean Diameter (μm) | Particle Concentration (×106 L−1) | Particle Recovery (%) |
|---|---|---|---|---|
| 2 µm | - | 2.12 ± 0.02 | 313 ± 10 | - |
| RW07 + 2 µm | - | 2.54 ± 0.03 | 227 ± 11 | 73 |
| RW07 + 2 µm | 10 | 2.23 ± 0.01 | 261 ± 5 | 83 |
| 3 µm | - | 3.41 ± 0.03 | 220 ± 21 | - |
| RW07 + 3 µm | - | 3.41 ± 0.02 | 169 ± 19 | 77 |
| RW07 + 3 µm | 10 | 3.15 ± 0.03 | 130 ± 2 | 60 |
| Sample | Baseline Intensity (Counts) | BEC (mg L−1) | XCsize (μm) | Number of Particle Events Detected | Particle Concentration (×104 L−1) |
|---|---|---|---|---|---|
| direct analysis | |||||
| UP water | 6 ± 2 | 27 | 1.26 | 3 ± 2 | - |
| RW01 | 45 ± 1 | 150 | 1.72 | 88 ± 10 | 188 ± 21 |
| RW02 | 41 ± 2 | 137 | 1.70 | 84 ± 11 | 180 ± 24 |
| RW03 | 57 ± 2 | 190 | 1.84 | 45 ± 15 | 96 ± 31 |
| RW05 | 50 ± 3 | 167 | 1.82 | 41 ± 16 | 88 ± 34 |
| RW14 | 46 ± 2 | 153 | 1.75 | 230 ± 21 | 490 ± 45 |
| RW23 | 30 ± 1 | 100 | 1.62 | 110 ± 4 | 249 ± 9 |
| RW24 | 18 ± 1 | 60 | 1.48 | 119 ± 10 | 253 ± 21 |
| RW25 | 27 ± 1 | 90 | 1.58 | 95 ± 8 | 182 ± 17 |
| RW27 | 20 ± 2 | 67 | 1.50 | 91 ± 18 | 194 ± 39 |
| acidic pre-treament (10% HNO3 24 h) | |||||
| Proc. blank | 7 ± 1 | 23 | 1.25 | 2 ± 2 | - |
| RW01 | 7 ± 1 | 23 | 1.28 | 50 ± 8 | 106 ± 18 |
| RW02 | 7 ± 1 | 23 | 1.26 | 46 ± 5 | 97 ± 11 |
| RW03 | 9 ± 1 | 30 | 1.33 | 256 ± 37 | 554 ± 78 |
| RW05 | 9 ± 1 | 30 | 1.31 | 214 ± 49 | 470 ± 70 |
| RW14 | 8 ± 1 | 26 | 1.32 | 164 ± 40 | 349 ± 84 |
| RW23 | 7 ± 2 | 23 | 1.25 | 107 ± 11 | 227 ± 15 |
| RW24 | 7 ± 1 | 23 | 1.27 | 104 ± 11 | 221 ± 23 |
| RW25 | 6 ± 1 | 20 | 1.24 | 120 ± 19 | 277 ± 37 |
| RW27 | 7 ± 1 | 23 | 1.27 | 93 ± 10 | 197 ± 22 |
| KnowitAllTM | ||
|---|---|---|
| Sample | Composition | HQI |
| RW01 | Polylactic acid | 76.2 |
| PMMA | 73.9 | |
| Polylactic acid | 64.7 | |
| Polylactic acid | 65.3 | |
| PMMA | 65.1 | |
| RW02 | HDPE | 90.6 |
| Polylactic acid | 66.4 | |
| HDPE | 91.4 | |
| RW03 | PVA | 73.1 |
| Polylactic acid | 78.4 | |
| PE | 90.9 | |
| RW23 | PVA | 66.8 |
| PE | 85.2 | |
| PP | 89.5 | |
| PP | 87.7 | |
| RW25 | PMMA | 87.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trujillo, C.; Pérez-Arantegui, J.; Lobinski, R.; Laborda, F. Improving the Detectability of Microplastics in River Waters by Single Particle Inductively Coupled Plasma Mass Spectrometry. Nanomaterials 2023, 13, 1582. https://doi.org/10.3390/nano13101582
Trujillo C, Pérez-Arantegui J, Lobinski R, Laborda F. Improving the Detectability of Microplastics in River Waters by Single Particle Inductively Coupled Plasma Mass Spectrometry. Nanomaterials. 2023; 13(10):1582. https://doi.org/10.3390/nano13101582
Chicago/Turabian StyleTrujillo, Celia, Josefina Pérez-Arantegui, Ryszard Lobinski, and Francisco Laborda. 2023. "Improving the Detectability of Microplastics in River Waters by Single Particle Inductively Coupled Plasma Mass Spectrometry" Nanomaterials 13, no. 10: 1582. https://doi.org/10.3390/nano13101582
APA StyleTrujillo, C., Pérez-Arantegui, J., Lobinski, R., & Laborda, F. (2023). Improving the Detectability of Microplastics in River Waters by Single Particle Inductively Coupled Plasma Mass Spectrometry. Nanomaterials, 13(10), 1582. https://doi.org/10.3390/nano13101582