Adsorptive Elimination of Heavy Metals from Aqueous Solution Using Magnetic Chitosan/Cellulose-Fe(III) Composite as a Bio-Sorbent
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Preparation
2.2. Synthesis M-Ch/CNF-Fe(III) Bio-Composite
2.3. Characterization of M-Ch/CNF-Fe(III) Bio-Composite
2.4. Adsorption of Cr(VI), Cu(II), and Pb(II)
2.5. Adsorption Isotherm
2.6. Adsorption Kinetics
2.7. Desorption Studies
3. Results
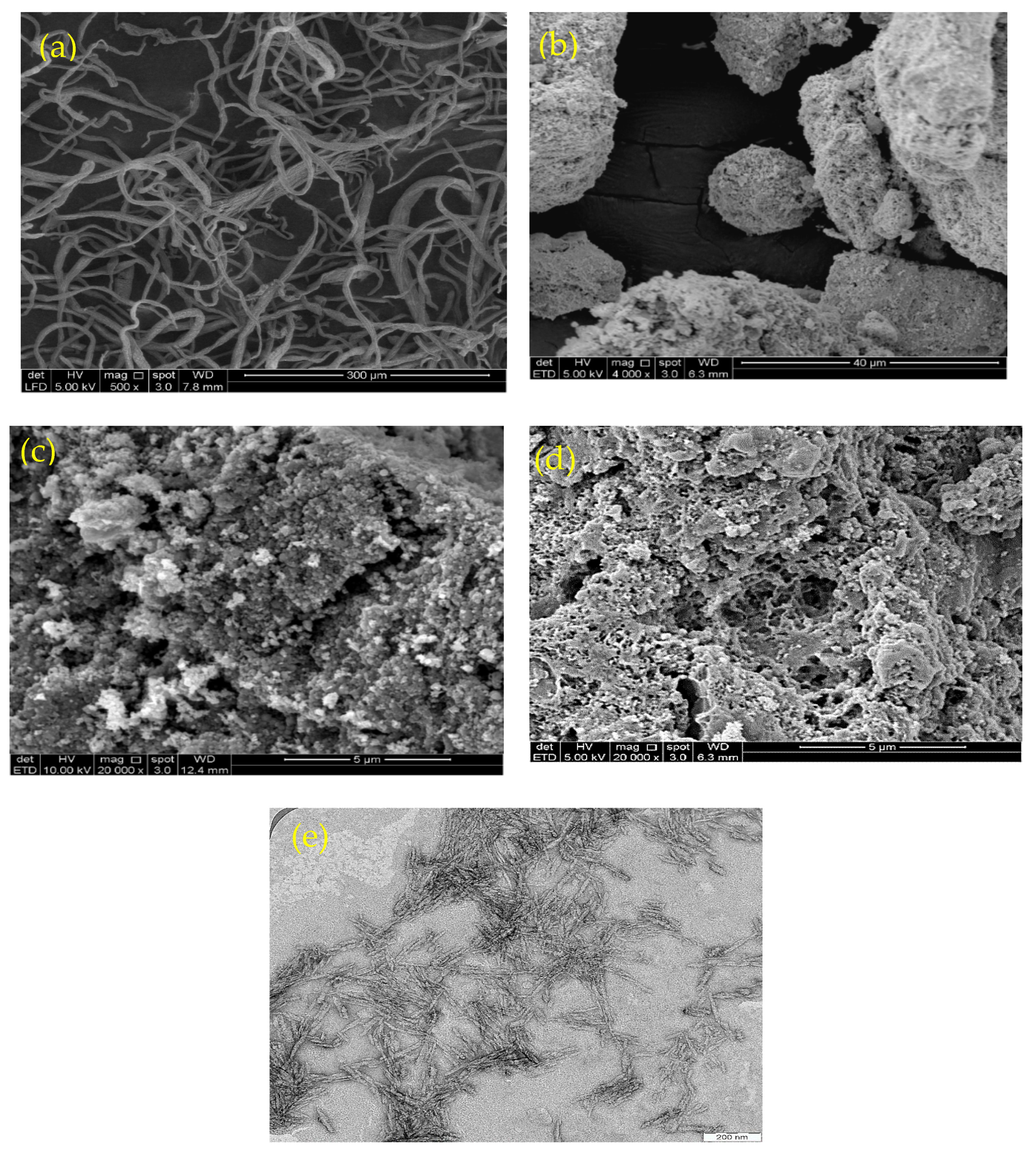
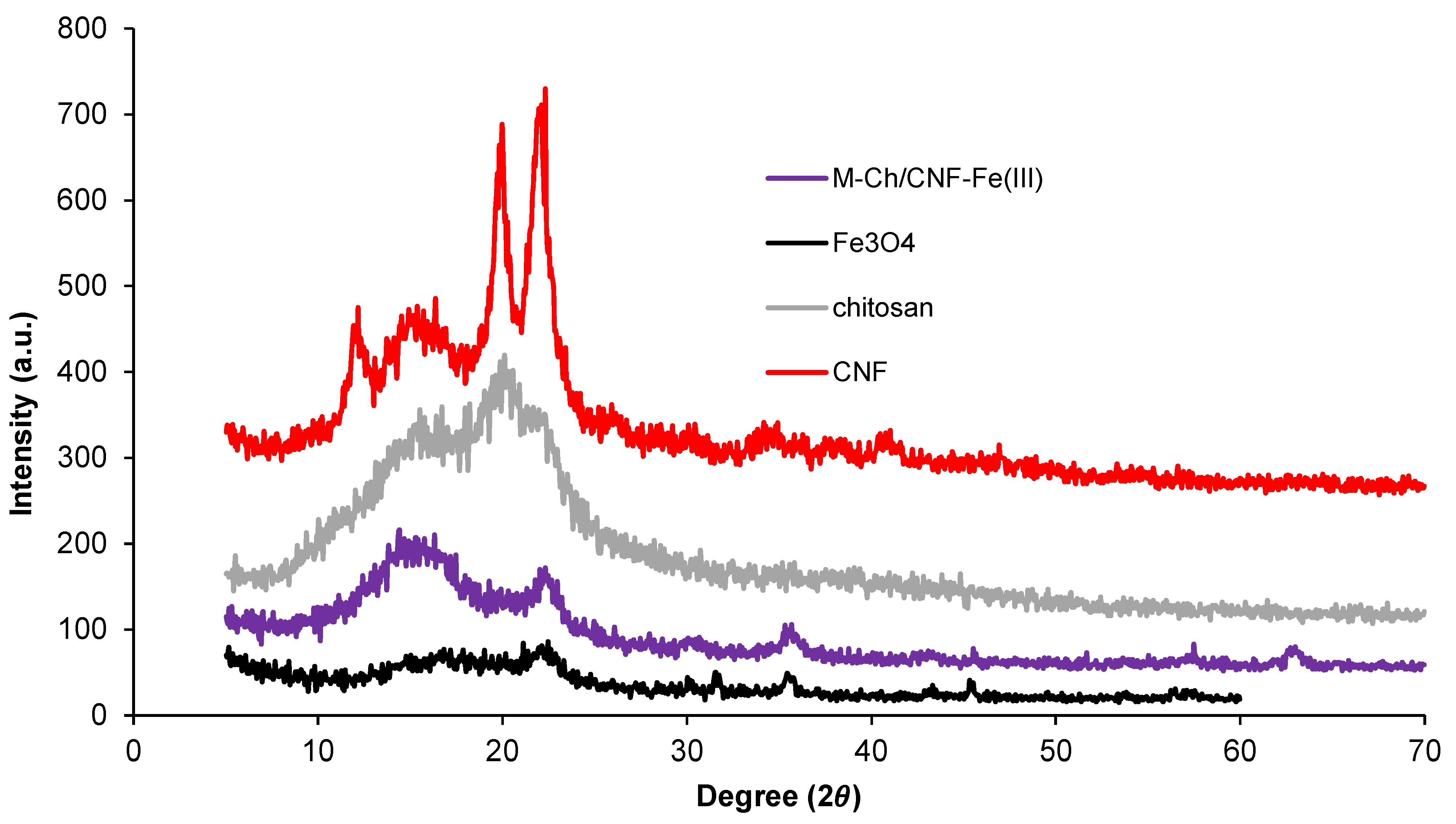
3.1. Characterization of M-Ch/CNF-Fe(III) Composite
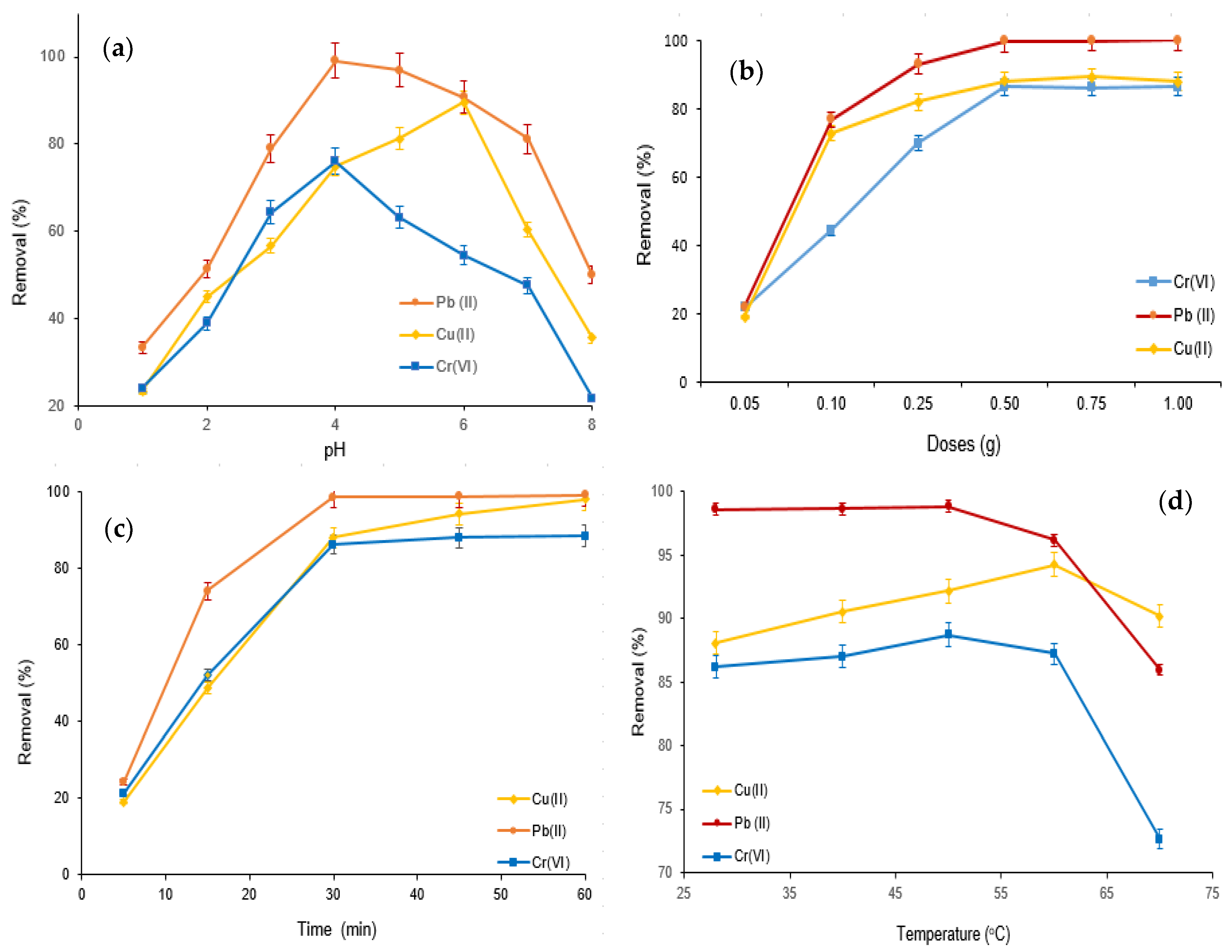
3.2. Adsorption of Cr(VI), Cu(II), and Pb(II) Using M-Ch/CNF-Fe(III) Composite
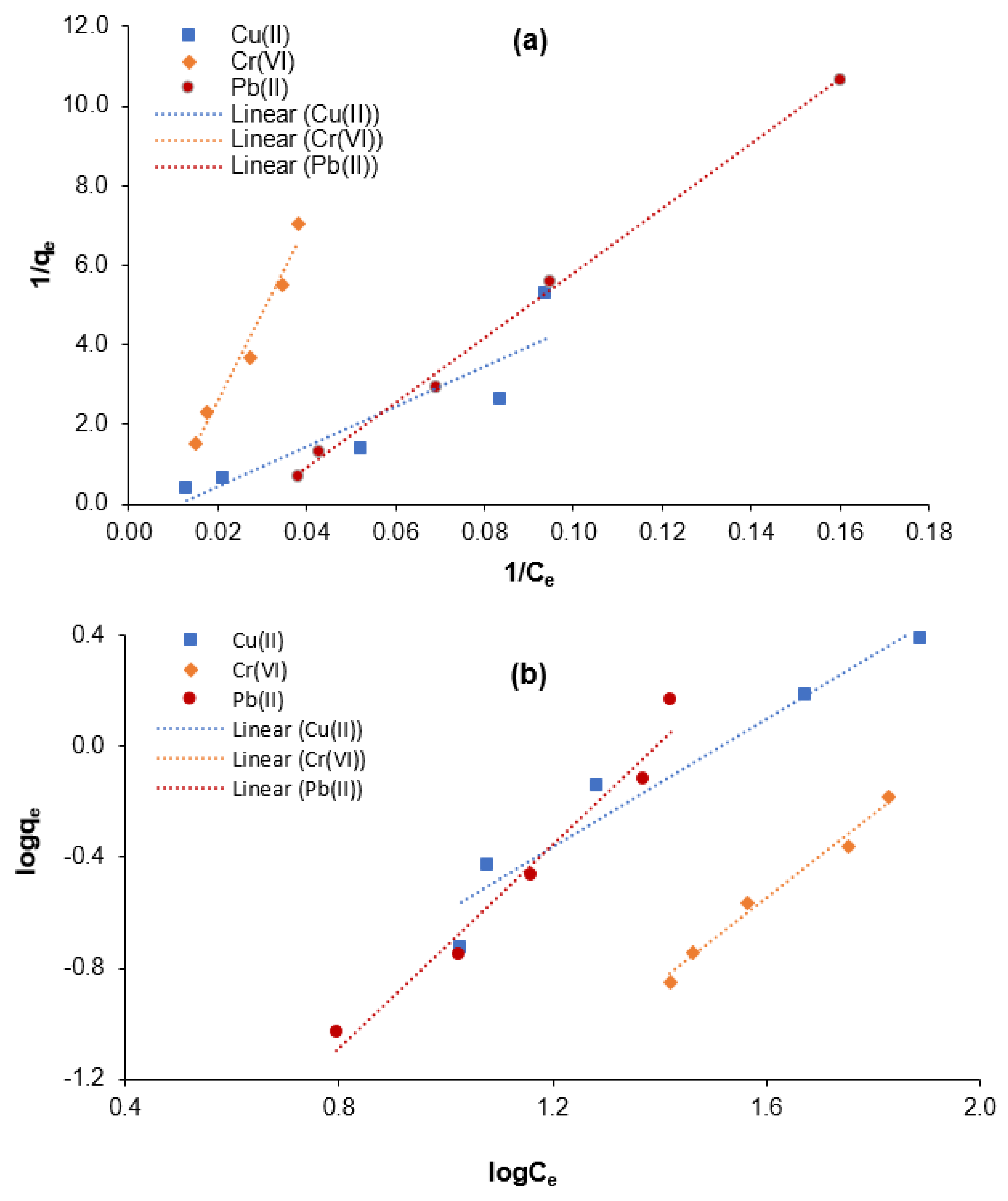
3.3. Adsorption Equilibrium Isotherm for Cr(VI), Cu(II), and Pb(II) Elimination
3.4. Adsorption Kinetics for Cr(VI), Cu(II), and Pb(II) Elimination
3.5. Reusability of M-Ch/CNF-Fe(III) Composite
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rathi, B.S.; Kumar, P.S.; Vo, D.-V.N. Critical review on hazardous pollutants in water environment: Occurrence, monitoring, fate, elimination technologies and risk assessment. Sci. Total Environ. 2021, 797, 149134. [Google Scholar] [CrossRef] [PubMed]
- Khalid, N.; Aqeel, M.; Noman, A.; Khan, S.M.; Akhter, N. Interactions and effects of microplastics with heavy metals in aquatic and terrestrial environments. Environ. Pollut. 2021, 290, 118104. [Google Scholar] [PubMed]
- Hossain, M.S.; Rashdi, S.A.; Hamed, Y.; Al-Gheethi, A.; Omar, F.M.; Zulkifli, M.; Ahmad Yahaya, A.N. Implementation of FeSO4·H2O as an Eco-Friendly Coagulant for the Elimination of Organic Pollutants from Tertiary Palm Oil Mill Effluent: Process Optimization, Kinetics, and Thermodynamics Studies. Water 2022, 14, 3602. [Google Scholar] [CrossRef]
- Khalid, A.M.; Hossain, M.S.; Ismail, N.; Khalil, N.A.; Balakrishnan, V.; Zulkifli, M.; Yahaya, A.N.A. Isolation and Characterization of Magnetic Oil Palm Empty Fruits Bunch Cellulose Nanofiber Composite as a Bio-Sorbent for Cu(II) and Cr(VI) Removal. Polymers 2021, 13, 112. [Google Scholar] [CrossRef]
- Tumolo, M.; Ancona, V.; De Paola, D.; Losacco, D.; Campanale, C.; Massarelli, C.; Uricchio, V.F. Chromium Pollution in European Water, Sources, Health Risk, and Remediation Strategies: An Overview. Int. J. Environ. Res. Public Health 2020, 17, 5438. [Google Scholar]
- Karimi-Maleh, H.; Ayati, A.; Ghanbari, S.; Orooji, Y.; Tanhaei, B.; Karimi, F.; Alizadeh, M.; Rouhi, J.; Fu, L.; Sillanpää, M. Recent advances in removal techniques of Cr(VI) toxic ion from aqueous solution: A comprehensive review. J. Mol. Liq. 2021, 329, 115062. [Google Scholar] [CrossRef]
- Shabbir, Z.; Sardar, A.; Shabbir, A.; Abbas, G.; Shamshad, S.; Khalid, S.; Natasha; Murtaza, G.; Dumat, C.; Shahid, M. Copper uptake, essentiality, toxicity, detoxification and risk assessment in soil-plant environment. Chemosphere 2020, 259, 127436. [Google Scholar] [CrossRef] [PubMed]
- Razak, M.R.; Yusof, N.A.; Aris, A.Z.; Nasir, H.M.; Haron, M.J.; Ibrahim, N.A.; Johari, I.S.; Kamaruzaman, S. Phosphoric acid modified kenaf fiber (K-PA) as green adsorbent for the removal of copper (II) ions towards industrial waste water effluents. React. Funct. Polym. 2020, 147, 104466. [Google Scholar] [CrossRef]
- Lian, Z.; Li, Y.; Xian, H.; Ouyang, X.-k.; Lu, Y.; Peng, X.; Hu, D. EDTA-functionalized magnetic chitosan oligosaccharide and carboxymethyl cellulose nanocomposite: Synthesis, characterization, and Pb(II) adsorption performance. Int. J. Biol. Macromol. 2020, 165, 591–600. [Google Scholar]
- Department of Environment (DoE). Environmental Quality (Industrial Effluent) Regulation; Department of Environment (DoE): Kuala Lumpur, Malaysia, 2009.
- Shadi, A.M.H.; Kamaruddin, M.A.; Niza, N.M.; Emmanuel, M.I.; Hossain, M.S.; Ismail, N. Electroflotation treatment of stabilized landfill leachate using titanium-based electrode. Int. J. Environ. Sci. Technol. 2021, 18, 2425–2440. [Google Scholar]
- Ali, I.; Tatiana, K.k.; Zaw Htay, T.; Thu Aung, H.; Ekaterina, M.; Nahid Siddiqui, M.; Almalki, A.S.A.; Alhadhrami, A.; Alsubaie, A.; Hameed, A.M.; et al. Economic and fast electro-flotation extraction of heavy metals from wastewater. Environ. Technol. 2022, 43, 4019–4028. [Google Scholar] [CrossRef] [PubMed]
- Bashir, A.; Malik, L.A.; Ahad, S.; Manzoor, T.; Bhat, M.A.; Dar, G.N.; Pandith, A.H. Removal of heavy metal ions from aqueous system by ion-exchange and biosorption methods. Environ. Chem. Lett. 2019, 17, 729–754. [Google Scholar] [CrossRef]
- Jain, A.; Rai, S.; Srinivas, R.; Al-Raoush, R.I. Bioinspired modeling and biogeography-based optimization of electrocoagulation parameters for enhanced heavy metal removal. J. Clean. Prod. 2022, 338, 130622. [Google Scholar] [CrossRef]
- Zhang, Q.; Ye, X.; Li, H.; Chen, D.; Xiao, W.; Zhao, S.; Xiong, R.; Li, J. Cumulative effects of pyrolysis temperature and process on properties, chemical speciation, and environmental risks of heavy metals in magnetic biochar derived from coagulation-flocculation sludge of swine wastewater. J. Environ. Chem. Eng. 2020, 8, 104472. [Google Scholar] [CrossRef]
- Balladares, E.; Jerez, O.; Parada, F.; Baltierra, L.; Hernández, C.; Araneda, E.; Parra, V. Neutralization and co-precipitation of heavy metals by lime addition to effluent from acid plant in a copper smelter. Miner. Eng. 2018, 122, 122–129. [Google Scholar] [CrossRef]
- Almasian, A.; Giahi, M.; Chizari Fard, G.; Dehdast, S.A.; Maleknia, L. Removal of heavy metal ions by modified PAN/PANI-nylon core-shell nanofibers membrane: Filtration performance, antifouling and regeneration behavior. Chem. Eng. J. 2018, 351, 1166–1178. [Google Scholar] [CrossRef]
- Bari, M.F.; Hossain, M.S.; Mujtaba, I.M.; Jamaluddin, S.B.; Hussin, K. Simultaneous extraction and separation of Cu(II), Zn(II), Fe(III) and Ni(II) by polystyrene microcapsules coated with Cyanex 272. Hydrometallurgy 2009, 95, 308–315. [Google Scholar] [CrossRef]
- Mohamed, S.H.; Hossain, M.S.; Kassim, M.H.M.; Balakrishnan, V.; Habila, M.A.; Zulkharnain, A.; Zulkifli, M.; Yahaya, A.N.A. Biosorption of Cr(VI) Using Cellulose Nanocrystals Isolated from the Waterless Pulping of Waste Cotton Cloths with Supercritical CO2: Isothermal, Kinetics, and Thermodynamics Studies. Polymers 2022, 14, 887. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Choi, H.-J. Persimmon leaf bio-waste for adsorptive removal of heavy metals from aqueous solution. J. Environ. Manag. 2018, 209, 382–392. [Google Scholar] [CrossRef]
- Nguyen, T.C.; Loganathan, P.; Nguyen, T.V.; Kandasamy, J.; Naidu, R.; Vigneswaran, S. Adsorptive removal of five heavy metals from water using blast furnace slag and fly ash. Environ. Sci. Pollut. Res. 2018, 25, 20430–20438. [Google Scholar] [CrossRef]
- Karimi, M.H.; Mahdavinia, G.R.; Massoumi, B.; Baghban, A.; Saraei, M. Ionically crosslinked magnetic chitosan/κ-carrageenan bioadsorbents for removal of anionic eriochrome black-T. Int. J. Biol. Macromol. 2018, 113, 361–375. [Google Scholar] [CrossRef] [PubMed]
- Ge, H.; Hua, T. Synthesis and characterization of poly(maleic acid)-grafted crosslinked chitosan nanomaterial with high uptake and selectivity for Hg(II) sorption. Carbohydr. Polym. 2016, 153, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Kuang, S.-P.; Wang, Z.-Z.; Liu, J.; Wu, Z.-C. Preparation of triethylene-tetramine grafted magnetic chitosan for adsorption of Pb(II) ion from aqueous solutions. J. Hazard. Mater. 2013, 260, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Rahmi; Ishmaturrahmi; Mustafa, I. Methylene blue removal from water using H2SO4 crosslinked magnetic chitosan nanocomposite beads. Microchem. J. 2019, 144, 397–402. [Google Scholar] [CrossRef]
- Afifah Khalil, N.; Syafiqah Abdul Rahman, A.; Mubin Abu Huraira, A.; Nur Dalillah Fattin Janurin, S.; Noor Syimir Fizal, A.; Ahmad, N.; Zulkifli, M.; Sohrab Hossain, M.; Naim Ahmad Yahaya, A. Magnetic chitosan hydrogel beads as adsorbent for copper removal from aqueous solution. Materials Today: Proceedings 2023, 74, 499–503. [Google Scholar] [CrossRef]
- Oyewo, O.A.; Elemike, E.E.; Onwudiwe, D.C.; Onyango, M.S. Metal oxide-cellulose nanocomposites for the removal of toxic metals and dyes from wastewater. Int. J. Biol. Macromol. 2020, 164, 2477–2496. [Google Scholar] [CrossRef]
- Fatah, I.Y.A.; Khalil, H.P.S.A.; Hossain, M.S.; Aziz, A.A.; Davoudpour, Y.; Dungani, R.; Bhat, A. Exploration of a Chemo-Mechanical Technique for the Isolation of Nanofibrillated Cellulosic Fiber from Oil Palm Empty Fruit Bunch as a Reinforcing Agent in Composites Materials. Polymers 2014, 6, 2611–2624. [Google Scholar] [CrossRef]
- Hu, D.; Huang, H.; Jiang, R.; Wang, N.; Xu, H.; Wang, Y.-G.; Ouyang, X.-k. Adsorption of diclofenac sodium on bilayer amino-functionalized cellulose nanocrystals/chitosan composite. J. Hazard. Mater. 2019, 369, 483–493. [Google Scholar] [CrossRef]
- Mondol, M.M.H.; Jhung, S.H. Adsorptive removal of pesticides from water with metal–organic framework-based materials. Chem. Eng. J. 2021, 421, 129688. [Google Scholar] [CrossRef]
- Wu, M.; Deng, W.; Zhang, Y.; Chen, C.; Liu, Z.; Fatehi, P.; Li, B. Facile Fabrication of Cellulose Nanofibrils/Chitosan Beads as the Potential pH-Sensitive Drug Carriers. Polymers 2022, 14, 2286. [Google Scholar] [CrossRef]
- Bassyouni, M.; Zoromba, M.S.; Abdel-Aziz, M.H.; Mosly, I. Extraction of Nanocellulose for Eco-Friendly Biocomposite Adsorbent for Wastewater Treatment. Polymers 2022, 14, 1852. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, M.; Gao, H.; Yin, Z.; Chen, C.; Yang, H.; Fang, L.; Jagadeesha Angadi, V.; Yi, Z.; Li, D. Construction of CeO2/YMnO3 and CeO2/MgAl2O4/YMnO3 photocatalysts and adsorption of dyes and photocatalytic oxidation of antibiotics: Performance prediction, degradation pathway and mechanism insight. Appl. Surf. Sci. 2023, 608, 154977. [Google Scholar] [CrossRef]
- Zhu, H.Y.; Fu, Y.Q.; Jiang, R.; Jiang, J.H.; Xiao, L.; Zeng, G.M.; Zhao, S.L.; Wang, Y. Adsorption removal of congo red onto magnetic cellulose/Fe3O4/activated carbon composite: Equilibrium, kinetic and thermodynamic studies. Chem. Eng. J. 2011, 173, 494–502. [Google Scholar] [CrossRef]
- Mohamed, S.H.; Hossain, M.S.; Mohamad Kassim, M.H.; Ahmad, M.I.; Omar, F.M.; Balakrishnan, V.; Zulkifli, M.; Yahaya, A.N.A. Recycling Waste Cotton Cloths for the Isolation of Cellulose Nanocrystals: A Sustainable Approach. Polymers 2021, 13, 626. [Google Scholar] [CrossRef]
- Gupta, K.; Joshi, P.; Gusain, R.; Khatri, O.P. Recent advances in adsorptive removal of heavy metal and metalloid ions by metal oxide-based nanomaterials. Coord. Chem. Rev. 2021, 445, 214100. [Google Scholar] [CrossRef]
- Dong, J.; Du, Y.; Duyu, R.; Shang, Y.; Zhang, S.; Han, R. Adsorption of copper ion from solution by polyethylenimine modified wheat straw. Bioresour. Technol. Rep. 2019, 6, 96–102. [Google Scholar] [CrossRef]
- Einollahi Peer, F.; Bahramifar, N.; Younesi, H. Removal of Cd (II), Pb (II) and Cu (II) ions from aqueous solution by polyamidoamine dendrimer grafted magnetic graphene oxide nanosheets. J. Taiwan Inst. Chem. Eng. 2018, 87, 225–240. [Google Scholar] [CrossRef]
- Vishnu, D.; Dhandapani, B. The symbiotic effect of integrated Muraya koenigii extract and surface-modified magnetic microspheres–a green biosorbent for the removal of Cu(II) and Cr(VI) ions from aqueous solutions. Chem. Eng. Commun. 2021, 208, 851–862. [Google Scholar] [CrossRef]
- Mat Yasin, N.M.F.; Hossain, M.S.; Abdul Khalil, H.P.S.; Zulkifli, M.; Al-Gheethi, A.; Asis, A.J.; Yahaya, A.N.A. Treatment of Palm Oil Refinery Effluent Using Tannin as a Polymeric Coagulant: Isotherm, Kinetics, and Thermodynamics Analyses. Polymers 2020, 12, 2353. [Google Scholar] [CrossRef]
- Fan, T.; Liu, Y.; Feng, B.; Zeng, G.; Yang, C.; Zhou, M.; Zhou, H.; Tan, Z.; Wang, X. Biosorption of cadmium(II), zinc(II) and lead(II) by Penicillium simplicissimum: Isotherms, kinetics and thermodynamics. J. Hazard. Mater. 2008, 160, 655–661. [Google Scholar] [CrossRef]
- Sun, X.; Yang, L.; Li, Q.; Zhao, J.; Li, X.; Wang, X.; Liu, H. Amino-functionalized magnetic cellulose nanocomposite as adsorbent for removal of Cr(VI): Synthesis and adsorption studies. Chem. Eng. J. 2014, 241, 175–183. [Google Scholar] [CrossRef]
- Liu, B.; Chen, C.; Li, W.; Liu, H.; Liu, L.; Deng, S.; Li, Y. Effective removal of Cr(VI) from aqueous solution through adsorption and reduction by magnetic S-doped Fe-Cu-La trimetallic oxides. J. Environ. Chem. Eng. 2022, 10, 107433. [Google Scholar] [CrossRef]
- Daneshfozoun, S.; Abdullah, M.A.; Abdullah, B. Preparation and characterization of magnetic biosorbent based on oil palm empty fruit bunch fibers, cellulose and Ceiba pentandra for heavy metal ions removal. Ind. Crops Prod. 2017, 105, 93–103. [Google Scholar] [CrossRef]
- Anush, S.M.; Vishalakshi, B. Modified chitosan gel incorporated with magnetic nanoparticle for removal of Cu(II) and Cr(VI) from aqueous solution. Int. J. Biol. Macromol. 2019, 133, 1051–1062. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, Z.; Chen, H.; Kai, C.; Jiang, M.; Wang, Q.; Zhou, Z. Polyethylenimine functionalized Fe3O4/steam-exploded rice straw composite as an efficient adsorbent for Cr(VI) removal. Appl. Surf. Sci. 2018, 440, 1277–1285. [Google Scholar] [CrossRef]
- Touihri, M.; Guesmi, F.; Hannachi, C.; Hamrouni, B.; Sellaoui, L.; Badawi, M.; Poch, J.; Fiol, N. Single and simultaneous adsorption of Cr(VI) and Cu (II) on a novel Fe3O4/pine cones gel beads nanocomposite: Experiments, characterization and isotherms modeling. Chem. Eng. J. 2021, 416, 129101. [Google Scholar] [CrossRef]
- Jain, M.; Yadav, M.; Kohout, T.; Lahtinen, M.; Garg, V.K.; Sillanpää, M. Development of iron oxide/activated carbon nanoparticle composite for the removal of Cr(VI), Cu(II) and Cd(II) ions from aqueous solution. Water Resour. Ind. 2018, 20, 54–74. [Google Scholar] [CrossRef]
- Sasidharan, R.; Kumar, A. Response surface methodology for optimization of heavy metal removal by magnetic biosorbent made from anaerobic sludge. J. Indian Chem. Soc. 2022, 99, 100638. [Google Scholar] [CrossRef]
- Ragheb, E.; Shamsipur, M.; Jalali, F.; Mousavi, F. Modified magnetic-metal organic framework as a green and efficient adsorbent for removal of heavy metals. J. Environ. Chem. Eng. 2022, 10, 107297. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Y.; Zheng, H.; Zhang, B.; Zuo, Q.; Fan, K. Functionalized dual modification of covalent organic framework for efficient and rapid trace heavy metals removal from drinking water. Chemosphere 2022, 290, 133215. [Google Scholar] [CrossRef]

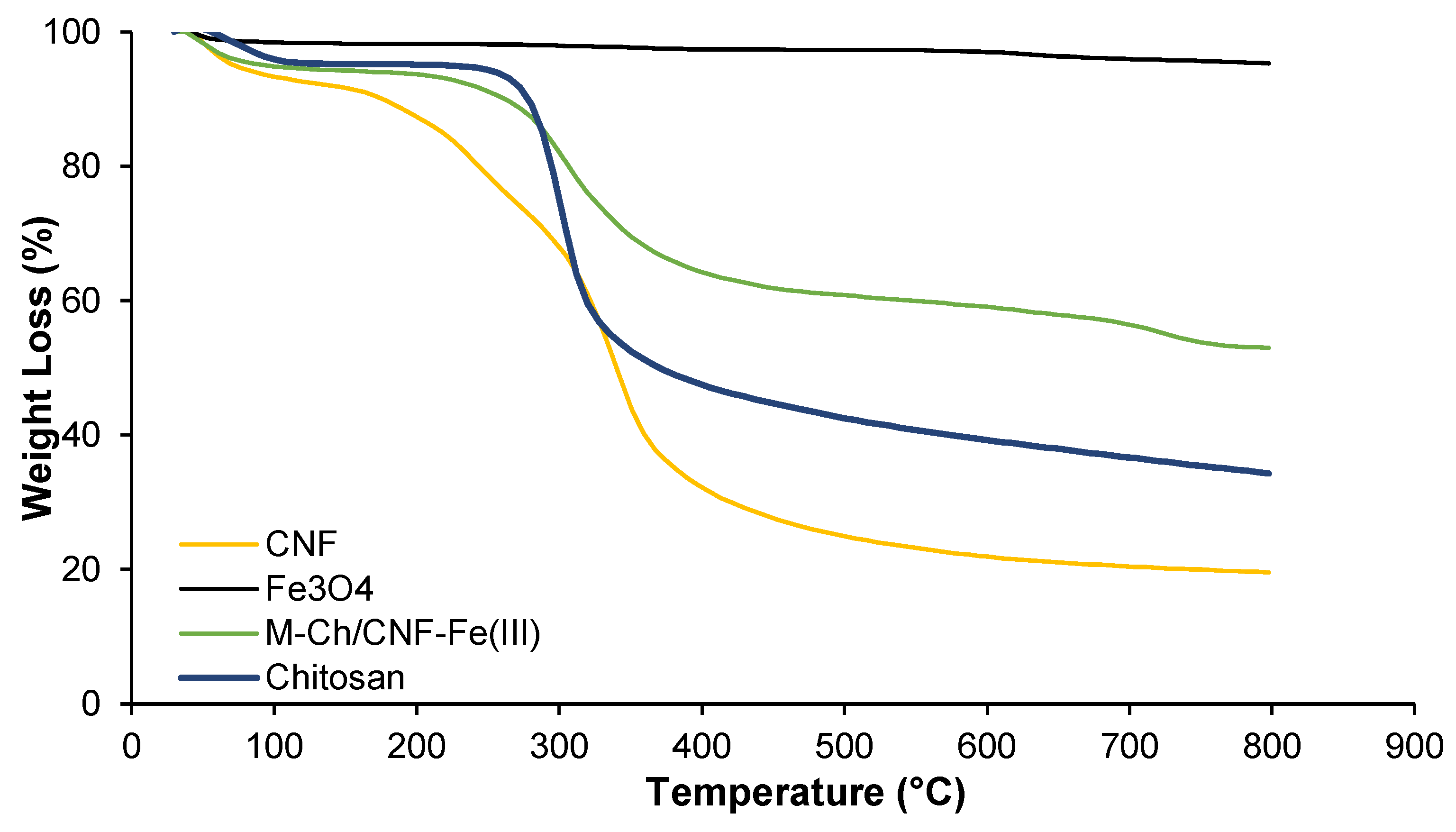
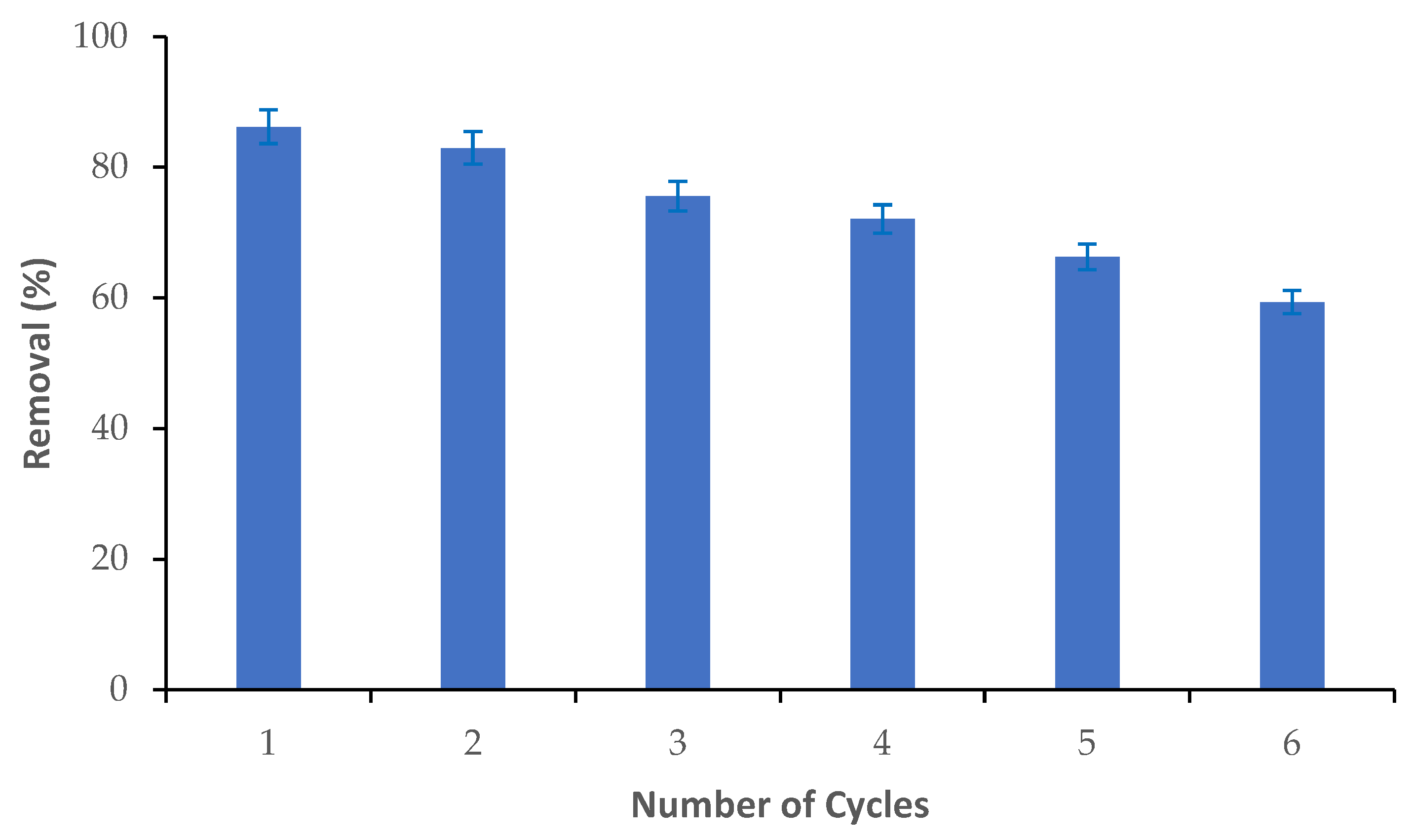
| Materials | Tonset (°C) | Tmax (°C) | Weight Loss (%) |
|---|---|---|---|
| Fe3O4 | 700 | 750 | 5 |
| Chitosan | 257 | 362 | 66 |
| CNF | 173 | 408 | 81 |
| M-Ch/CNF-Fe(III) | 239 | 328 | 50 |
| Langmuir Isotherm | Freundlich Isotherm | |||||
|---|---|---|---|---|---|---|
| R2 | a (L/mg) | b (mg/mg) | R2 | kf (L/mg) | n | |
| Cu(II) | 0.8228 | 0.0113 | 1.7655 | 0.9378 | 0.0179 | 0.8670 |
| Cr(VI) | 0.9677 | 0.0084 | 0.5412 | 0.9825 | 0.0011 | 0.6628 |
| Pb(II) | 0.9967 | 0.0001 | 0.4232 | 0.9668 | 0.0028 | 0.5453 |
| Metal Ions | Temperature (°C) | Pseudo-1st-Order | Pseudo-2nd-Order | |||||
|---|---|---|---|---|---|---|---|---|
| qe (exp) (mg/mg) | qe (mg/mg) | K1 (1/min) | R2 | qe (mg/mg) | K2 (mg/mg.min) | R2 | ||
| Cu(II) | 28 | 2.669 | 3.782 | 0.056 | 0.9482 | 2.869 | 0.073 | 0.9985 |
| 40 | 2.715 | 3.749 | 0.061 | 0.9896 | 2.914 | 0.098 | 0.9999 | |
| 50 | 2.734 | 3.685 | 0.072 | 0.9971 | 2.989 | 0.198 | 0.9998 | |
| 60 | 2.784 | 3.402 | 0.079 | 0.9982 | 3.029 | 0.207 | 0.9999 | |
| 70 | 2.815 | 3.309 | 0.076 | 0.9865 | 3.196 | 0.218 | 0.9999 | |
| Cr(VI) | 28 | 4.496 | 5.435 | 0.024 | 0.9726 | 4.997 | 0.092 | 0.9996 |
| 40 | 4.498 | 5.102 | 0.014 | 0.9896 | 5.089 | 0.106 | 0.9901 | |
| 50 | 5.084 | 4.253 | 0.042 | 0.9069 | 5.172 | 0.133 | 0.9864 | |
| 60 | 5.106 | 4.256 | 0.048 | 0.9648 | 5.281 | 0.147 | 0.9984 | |
| 70 | 5.109 | 3.831 | 0.049 | 0.9907 | 5.328 | 0.149 | 0.9995 | |
| Pb(II) | 28 | 5.590 | 6.424 | 0.042 | 0.9242 | 5.475 | 0.016 | 0.9996 |
| 40 | 5.596 | 5.557 | 0.043 | 0.9891 | 5.573 | 0.075 | 0.9979 | |
| 50 | 5.604 | 5.342 | 0.058 | 0.9556 | 5.585 | 0.132 | 0.9993 | |
| 60 | 5.619 | 5.046 | 0.068 | 0.9979 | 5.593 | 0.295 | 0.9998 | |
| 70 | 5.627 | 4.443 | 0.096 | 0.9663 | 5.594 | 0.306 | 0.9999 | |
| Magnetic Adsorbent | Metals Ion | Removal Capacity (mg/g) | References |
|---|---|---|---|
| Silica-coated amino-functionalized magnetic Muraya koenigii extracts | Cr(VI) Cu(II) | 71.12 73.71 | [39] |
| Magnetic nanoparticles incorporated with chitosan gel | Cr(VI) Cu(II) | 83.33 90.90 | [45] |
| Magnetic pinecone gel beads | Cr(VI) Cu(II) | 212.22 68.64 | [47] |
| Magnetically activated carbon nanoparticles | Cr(VI) Cu(II) Pb(II) | 2.38 0.74 0.25 | [48] |
| Polyethylenimine-functionalized Fe3O4/steam-exploded rice straw composite | Cr(VI) | 280.11 | [46] |
| Magnetically modified alkali-conditioned anaerobically digested sludge | Cu(II) Cd(II) Pb(II) | 29.72 28.55 28.60 | [49] |
| Modified magnetic metal–organic framework | Hg(II) Cd(II) Pb(II) | 431 393 397 | [50] |
| Functionalized double-modified covalent organic framework | Pb(II) | 14.22 | [51] |
| M-Ch/CNF-Fe(III) composite | Cu(II) Cr(VI) Pb(II) | 391.21 185.22 99.86 | Present study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalid, A.M.; Hossain, M.S.; Khalil, N.A.; Zulkifli, M.; Arafath, M.A.; Shaharun, M.S.; Ayub, R.; Ahmad Yahaya, A.N.; Ismail, N. Adsorptive Elimination of Heavy Metals from Aqueous Solution Using Magnetic Chitosan/Cellulose-Fe(III) Composite as a Bio-Sorbent. Nanomaterials 2023, 13, 1595. https://doi.org/10.3390/nano13101595
Khalid AM, Hossain MS, Khalil NA, Zulkifli M, Arafath MA, Shaharun MS, Ayub R, Ahmad Yahaya AN, Ismail N. Adsorptive Elimination of Heavy Metals from Aqueous Solution Using Magnetic Chitosan/Cellulose-Fe(III) Composite as a Bio-Sorbent. Nanomaterials. 2023; 13(10):1595. https://doi.org/10.3390/nano13101595
Chicago/Turabian StyleKhalid, Aina Mardhia, Md. Sohrab Hossain, Nor Afifah Khalil, Muzafar Zulkifli, Md. Azharul Arafath, Maizatul Shima Shaharun, Rashid Ayub, Ahmad Naim Ahmad Yahaya, and Norli Ismail. 2023. "Adsorptive Elimination of Heavy Metals from Aqueous Solution Using Magnetic Chitosan/Cellulose-Fe(III) Composite as a Bio-Sorbent" Nanomaterials 13, no. 10: 1595. https://doi.org/10.3390/nano13101595








