Influence of Natural Mordenite Activation Mode on Its Efficiency as Support of Nickel Catalysts for Biodiesel Upgrading to Renewable Diesel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Acid and Acid–Base Treatment of Natural Mordenite
2.3. Nickel Catalysts Preparation
2.4. Catalysts’ Characterization
2.5. Catalysts’ Evaluation
3. Results and Discussion
3.1. Catalysts’ Characterization
3.1.1. Textural Properties
3.1.2. Morphology of the Materials
3.1.3. Structural Properties of the Materials
3.1.4. Reducibility and Acidity Characteristics
3.2. Catalysts’ Evaluation
3.3. Spent Catalysts’ Characteristics
4. Conclusions
- Natural mordenite is a promising support for Ni catalysts used for the SDO of biodiesel to green diesel;
- Double activation of natural mordenite using acid (HCl) solution followed by alkaline (NaOH) solution optimized its supporting characteristics, finally resulting in a supported nickel catalyst with (i) enhanced specific surface area and mean pore diameter facilitating mass transfer; (ii) easier nickel phase reduction (iii) enhanced Ni0 dispersion and thus high active surface; (iv) balanced population of moderate and strong acid sites; (v) resistance to sintering; and (vi) low coke formation.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, J.; Wang, J.; Huang, Z.; Liu, T.; Li, H. Photothermal technique-enabled ambient production of microalgae biodiesel: Mechanism and life cycle assessment. Bioresour. Technol. 2023, 369, 128390. [Google Scholar] [CrossRef] [PubMed]
- Hassan, T.; Rahman, M.M.; Rahman, M.A.; Nabi, M.N. Opportunities and challenges for the application of biodiesel as automotive fuel in the 21st century. Biofuels Bioprod. Bioref. 2022, 16, 1353–1387. [Google Scholar] [CrossRef]
- Zhang, Z.; Cheng, J.; Qiu, Y.; Zhang, X.; Zhou, J.; Cen, K. Competitive conversion pathways of methyl palmitate to produce jet biofuel over Ni/desilicated meso-Y zeolite catalyst. Fuel 2019, 244, 472–478. [Google Scholar] [CrossRef]
- Dhillon, G.; Vasudevan, P.T. Deoxygenation of Methyl Oleate and Commercial Biodiesel Over W and Ni-W Catalysts. Waste Biomass Valor. 2021, 12, 2357–2364. [Google Scholar] [CrossRef]
- Fani, Κ.; Lycourghiotis, S.; Bourikas, K.; Kordouli, E. Biodiesel Upgrading to Renewable Diesel over Nickel Supported on Natural Mordenite Catalysts. Ind. Eng. Chem. Res. 2021, 60, 18695–18706. [Google Scholar] [CrossRef]
- Hachemi, I.; Jeništová, K.; Mäki-Arvela, P.; Kumar, N.; Eränen, K.; Hemming, J.; Murzin, D.Y. Comparative study of sulfur-free nickel and palladium catalysts in hydrodeoxygenation of different fatty acid feedstocks for production of biofuels. Catal. Sci. Technol. 2016, 6, 1476–1487. [Google Scholar] [CrossRef]
- Hachemi, I.; Murzin, D.Y. Kinetic modeling of fatty acid methyl esters and triglycerides hydrodeoxygenation over nickel and palladium catalysts. Chem. Eng. J. 2018, 334, 2201–2207. [Google Scholar] [CrossRef]
- Marinescu, M.; Popovici, D.R.; Bombos, D.; Vasilievici, G.; Rosca, P.; Oprescu, E.E.; Bolocan, I. Hydrodeoxygenation and hydrocracking of oxygenated compounds over CuPd/γ-Al2O3–ZSM-5 catalyst. Reac. Kinet. Mech. Cat. 2021, 133, 1013–1026. [Google Scholar] [CrossRef]
- Sun, P.; Liu, S.; Zhou, Y.; Zhang, S.; Yao, Z. Production of Renewable Light Olefins from Fatty Acid Methyl Esters by Hydroprocessing and Sequential Steam Cracking. ACS Sustain. Chem. Eng. 2018, 6, 13579–13587. [Google Scholar] [CrossRef]
- Şenol, O.İ.; Viljava, T.-R.; Krause, A.O.I. Hydrodeoxygenation of methyl esters on sulphided NiMo/γ-Al2O3 and CoMo/γ-Al2O3 catalysts. Catal. Today 2005, 100, 331–335. [Google Scholar] [CrossRef]
- Roy, P.; Jahromi, H.; Rahman, T.; Adhikari, S.; Feyzbar-Khalkhali-Nejad, F.; Hassan, E.B.; Oh, T.-S. Understanding the effects of feedstock blending and catalyst support on hydrotreatment of algae HTL biocrude with non-edible vegetable oil. Energy Convers. Manag. 2022, 268, 115998. [Google Scholar] [CrossRef]
- Nikolopoulos, I.; Kogkos, G.; Andriopoulou, C.; Kordouli, E.; Dracopoulos, V.; Bourikas, K.; Kordulis, C.; Lycourghiotis, A. Cobalt−Alumina Coprecipitated Catalysts for Green Diesel Production. Ind. Eng. Chem. Res. 2021, 60, 18672–18683. [Google Scholar] [CrossRef]
- Ranga, C.; Alexiadis, V.I.; Lauwaert, J.; Lødeng, R.; Thybaut, J.W. Effect of Co incorporation and support selection on deoxygenation selectivity and stability of (Co)Mo catalysts in anisole HDO. Appl. Catal. A Gen. 2019, 571, 61–70. [Google Scholar] [CrossRef]
- Li, H.; Fang, Z.; Smith, R.L.; Yang, S. Efficient valorization of biomass to biofuels with bifunctional solid catalytic materials. Prog. Energy Combust. Sci. 2016, 55, 98–194. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, W.; Li, Y.; Li, F.; Liu, J.; Fan, L.; Fu, J.; Liu, X.; Lyu, Y. Quantitative synergy between metal and acid centers over the Ni/Beta bifunctional catalyst for methyl laurate hydrodeoxygenation to bio-jet fuel. Fuel Proc. Technol. 2023, 241, 107602. [Google Scholar] [CrossRef]
- Makarouni, D.; Lycourghiotis, S.; Kordouli, E.; Bourikas, K.; Kordulis, C.; Dourtoglou, V. Transformation of limonene into p-cymene over acid activated natural mordenite utilizing atmospheric oxygen as a green oxidant: A novel mechanism. Appl. Catal. B Environ. 2018, 224, 740–750. [Google Scholar] [CrossRef]
- Lycourghiotis, S.; Makarouni, D.; Kordouli, E.; Bourikas, K.; Kordulis, C.; Dourtoglou, V. Activation of natural mordenite by various acids: Characterization and evaluation in the transformation of limonene into p-cymene. Mol. Catal. 2018, 450, 95–103. [Google Scholar] [CrossRef]
- Makarouni, D.; Kordulis, C.; Dourtoglou, V. Solvent-Driven Selectivity on the One-Step Catalytic Synthesis of Manoyl Oxide Based on a Novel and Sustainable “Zeolite Catalyst–Solvent” System. Catal. Lett. 2022, 152, 1298–1307. [Google Scholar] [CrossRef]
- Nugrahaningtyas, K.D.; Rahmawati, N.; Rahmawati, F.; Hidayat, Y. Synthesis and Characterization of CoMo/Mordenite Catalyst for Hydrotreatment of Lignin Compound Models. Open Chem. J. 2019, 17, 1061–1070. [Google Scholar] [CrossRef]
- Trisunaryanti, W.; Triyono, T.; Denti, F.A. Preparation of Ni-Mo/Mordenite catalysts under the variation of Mo/Ni ratio and their characterizations for stearic acid conversion. Indones. J. Chem. 2003, 3, 80–90. [Google Scholar] [CrossRef]
- Lycourghiotis, S.; Makarouni, D.; Kordouli, E.; Bourikas, K.; Kordulis, C.; Dourtoglou, V. The Influence of Calcination on the Physicochemical Properties of Acidactivated Natural Mordenite. Curr. Catal. 2020, 9, 138–147. [Google Scholar] [CrossRef]
- Trisunaryanti, W.; Triyono, T.; Armunanto, R.; Hastuti, L.P.; Ristiana, D.D.; Ginting, R.V. Hydrocracking of α-cellulose using Co, Ni, and Pd supported on mordenite catalysts. Indones. J. Chem. 2018, 18, 166. [Google Scholar] [CrossRef]
- Liu, Q.; Zuo, H.; Wang, T.; Ma, L.; Zhang, Q. One-step hydrodeoxygenation of palm oil to isomerized hydrocarbon fuels over Ni supported on nano-sized SAPO-11 catalysts. Appl. Catal. A: Gen. 2013, 468, 68–74. [Google Scholar] [CrossRef]
- Lycourghiotis, S.; Kordouli, E.; Bourikas, K.; Kordulis, C.; Lycourghiotis, A. The role of promoters in metallic nickel catalysts used for green diesel production: A critical review. Fuel Process. Technol. 2023, 244, 107690. [Google Scholar] [CrossRef]
- Chung, K.-H. Dealumination of mordenites with acetic acid and their catalytic activity in the alkylation of cumene. Microporous Mesoporous Mater. 2008, 111, 544–550. [Google Scholar] [CrossRef]
- Van laak, A.N.C.; Gosselink, R.W.; Sagala, S.L.; Meeldijk, J.D.; de Jongh, P.E.; de Jong, K.P. Alkaline treatment on commercially available aluminum rich mordenite. Appl. Catal. A Gen. 2010, 382, 65–72. [Google Scholar] [CrossRef]
- Paixão, V.; Monteiro, R.; Andrade, M.; Fernandes, A.; Rocha, J.; Carvalho, A.P.; Martins, A. Desilication of MOR zeolite: Conventional versus microwave assisted heating. Appl. Catal. A Gen. 2011, 402, 59–68. [Google Scholar] [CrossRef]
- Zafeiropoulos, G.; Nikolopoulos, N.; Kordouli, E.; Sygellou, L.; Bourikas, K.; Kordulis, C.; Lycourghiotis, A. Developing Nickel–Zirconia Co-Precipitated Catalysts for Production of Green Diesel. Catalysts 2019, 9, 210. [Google Scholar] [CrossRef]
- ALOthman, Z.A. A Review: Fundamental Aspects of Silicate Mesoporous Materials. Materials 2012, 5, 2874–2902. [Google Scholar] [CrossRef]
- Yusniyanti, F.; Trisunaryanti, W.; Triyono, T. Acid-Alkaline Treatment of Mordenite and Its Catalytic Activity in the Hydrotreatment of Bio-Oil. Indones. J. Chem. 2021, 21, 37–45. [Google Scholar] [CrossRef]
- Cuevas-García, R.; Téllez-Romero, J.G.; Ramírez, J.; Sarabia-Bañuelos, P.; Puente-Lee, I.; Salcedo-Luna, C.; Hernández-González, S.; Nolasco-Arizmendi, V.A. Effect of the preparation method on particle size and reaction selectivity on naphthalene hydrogenation over Ni/H-MOR catalysts. Catal. Today 2021, 360, 63–71. [Google Scholar] [CrossRef]
- Yusuf, M.; Leeke, G.; Wood, J. Anisole Hydrodeoxygenation over Nickel-Based Catalysts: Influences of Solvent and Support Properties. Energy Fuels 2023, 37, 1225–1237. [Google Scholar] [CrossRef]
- Nikolopoulos, I.; Kogkos, G.; Tsavatopoulou, V.D.; Kordouli, E.; Bourikas, K.; Kordulis, C.; Lycourghiotis, A. Nickel—Alumina Catalysts for the Transformation of Vegetable Oils into Green Diesel: The Role of Preparation Method, Activation Temperature, and Reaction Conditions. Nanomaterials 2023, 13, 616. [Google Scholar] [CrossRef]
- Kordulis, C.; Bourikas, K.; Gousi, M.; Kordouli, E.; Lycourghiotis, A. Development of nickel based catalysts for the transformation of natural triglycerides and related compounds into green diesel: A critical review. Appl. Catal. B Environ. 2016, 181, 156–196. [Google Scholar] [CrossRef]
- Arora, P.; Grennfelt, E.L.; Olsson, L.; Creaser, D. Kinetic study of hydrodeoxygenation of stearic acid as model compound for renewable oils. Chem. Eng. J. 2019, 364, 376–389. [Google Scholar] [CrossRef]
- Pedroza, M.A.P.; Segtovich, I.S.V.; de Morais Sermoud, V.; da Silva, M.A.P. Hydrodeoxygenation of stearic acid to produce diesel–like hydrocarbons: Kinetic modeling, parameter estimation and simulation. Chem. Eng. Sci. 2022, 254, 117576. [Google Scholar] [CrossRef]
- Kaluža, L.; Kubička, D. The comparison of Co, Ni, Mo, CoMo and NiMo sulfided catalysts in rapeseed oil hydrodeoxygenation. Reac. Kinet. Mech. Cat. 2017, 122, 333–341. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, L.; Li, G.; Hu, C. Insights into the Influence of ZrO2 Crystal Structures on Methyl Laurate Hydrogenation over Co/ZrO2 Catalysts. ACS Catal. 2021, 11, 7099–7113. [Google Scholar] [CrossRef]
- Kaluža, L.; Soukup, K.; Koštejn, M.; Čejka, J.; Karban, J.; Palcheva, R.; Laube, M.; Gulková, D. On Stability of High-Surface-Area Al2O3, TiO2, SiO2-Al2O3, and Activated Carbon Supports during Preparation of NiMo Sulfide Catalysts for Parallel Deoxygenation of Octanoic Acid and Hydrodesulfurization of 1-Benzothiophene. Catalysts 2022, 12, 1559. [Google Scholar] [CrossRef]
- Smirnov, A.A.; Alekseeva, M.V.; Bulavchenko, O.A.; Yakovlev, V.A. Studying the Effect of the Process Temperature on the Degree of Bio-Oil Hydrotreatment at Low Hydrogen Contents over NiCu–SiO2 Catalyst with a High Metal Loading. Catal. Ind. 2019, 11, 65–73. [Google Scholar] [CrossRef]
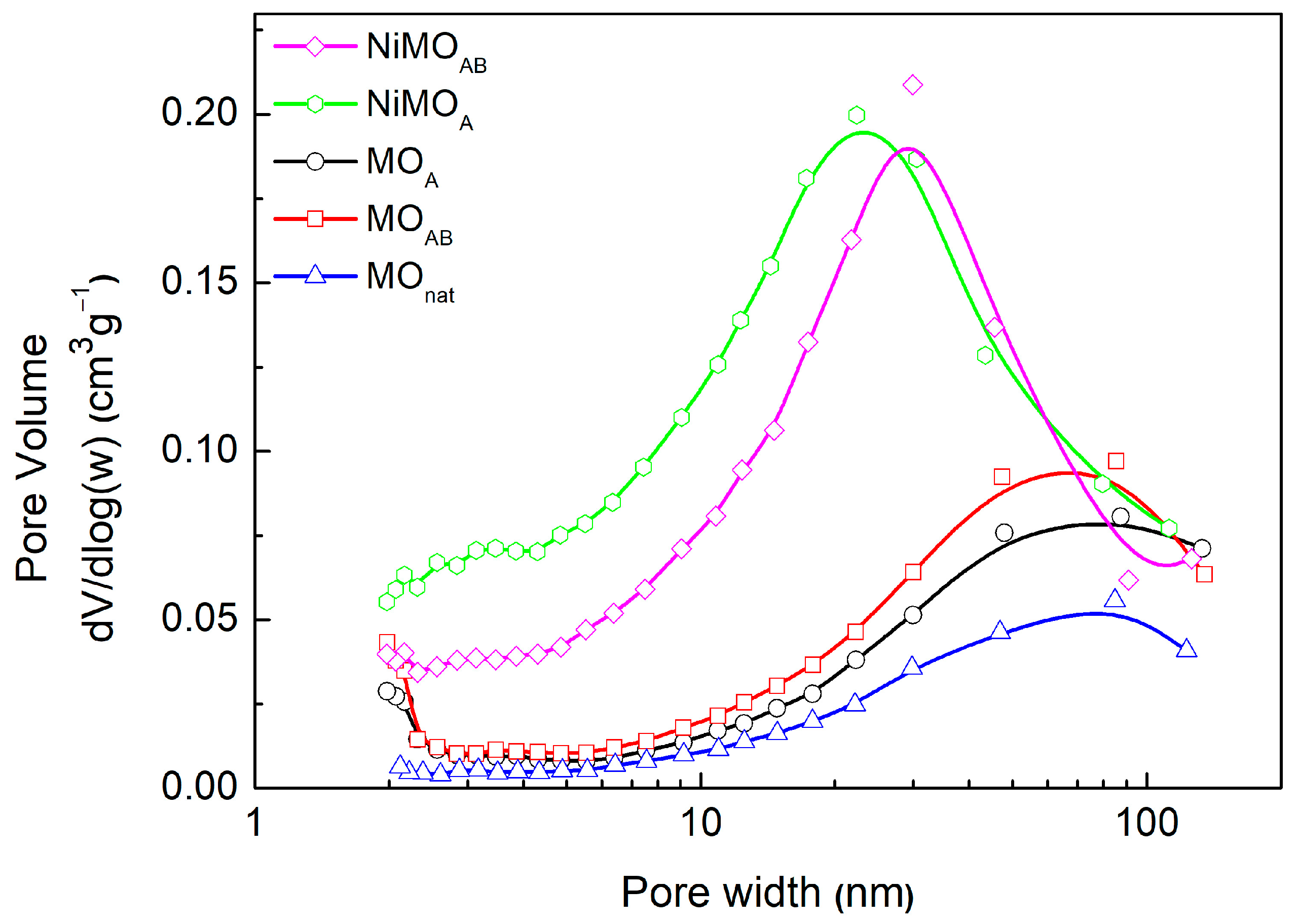
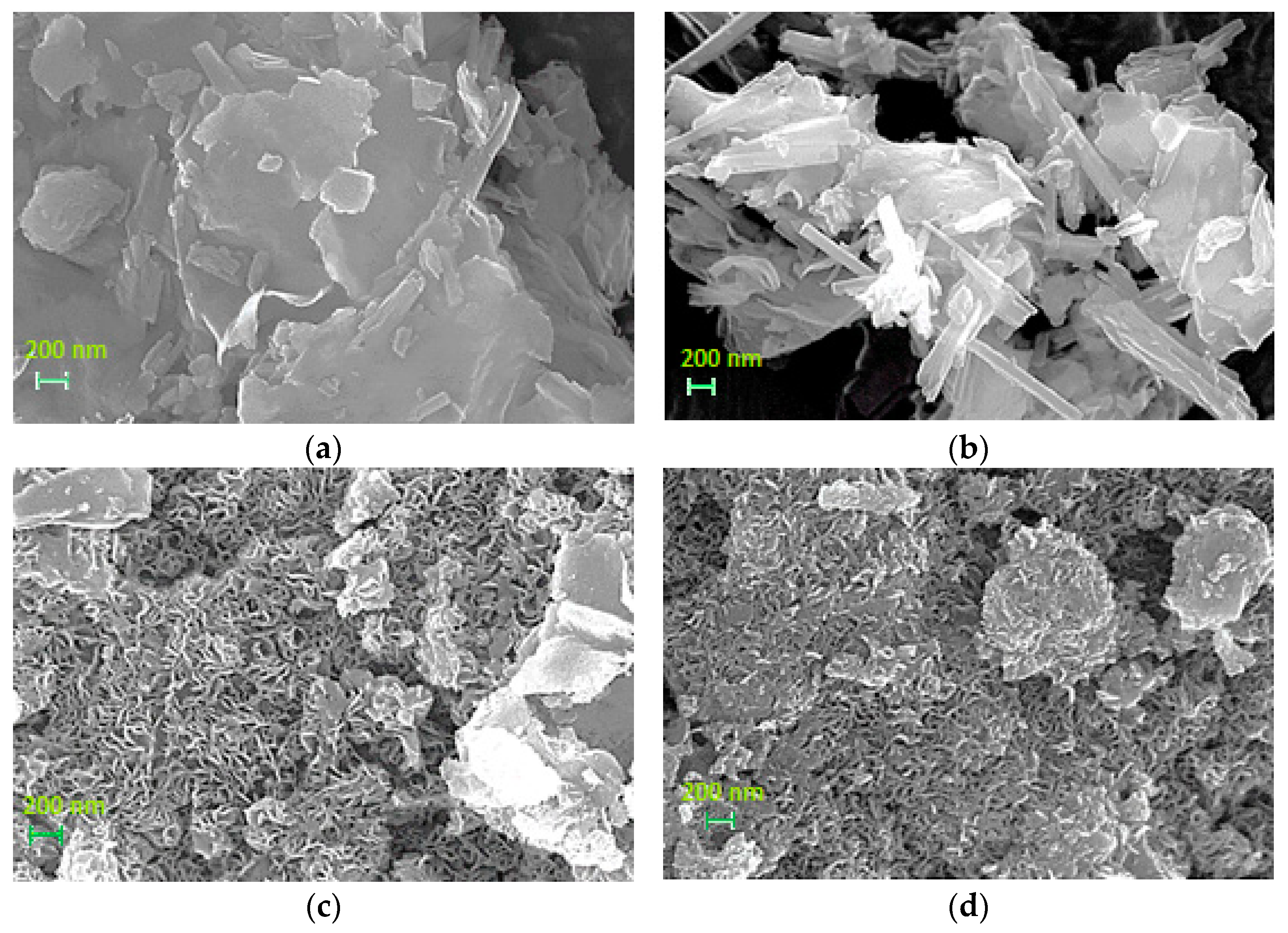
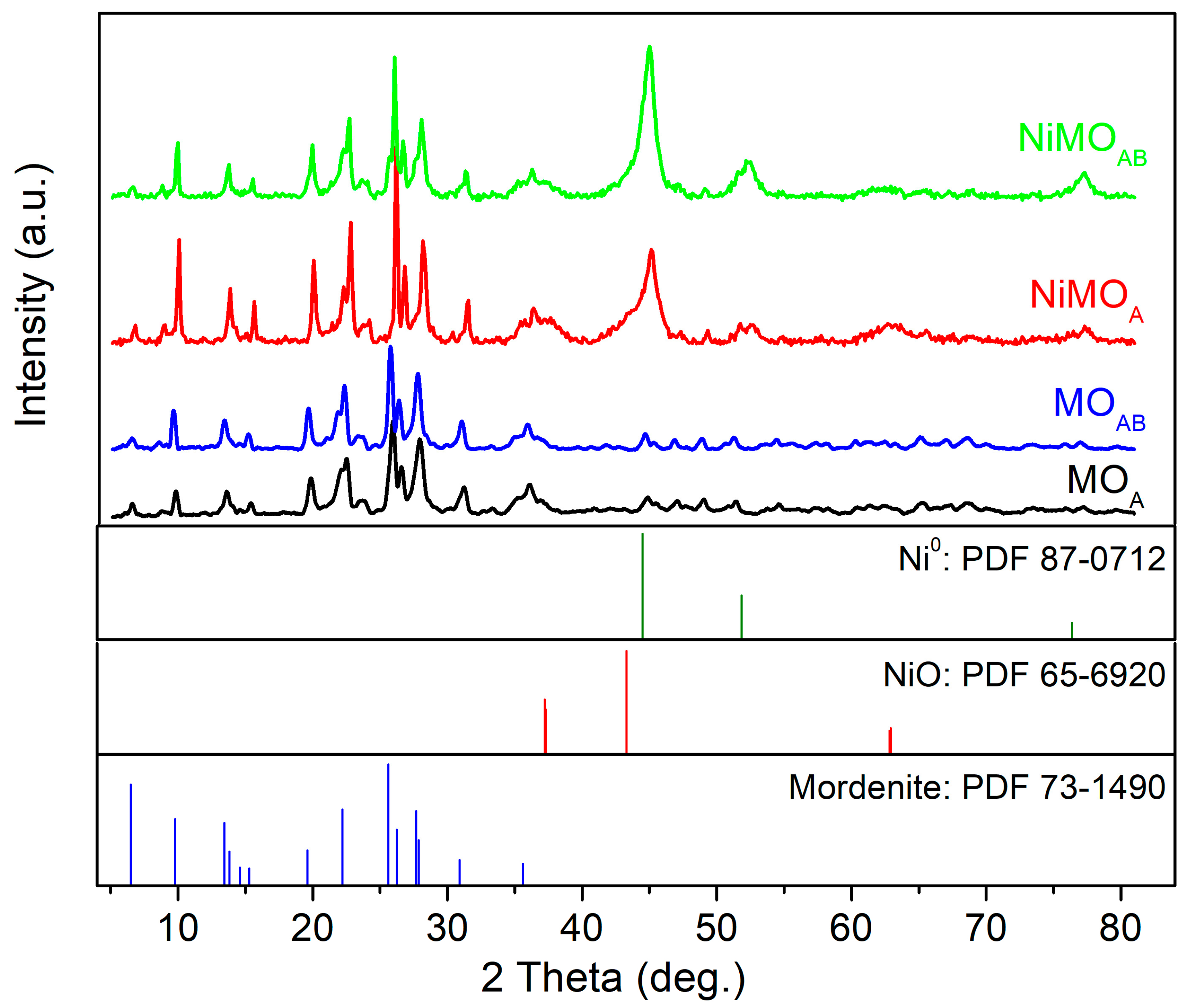

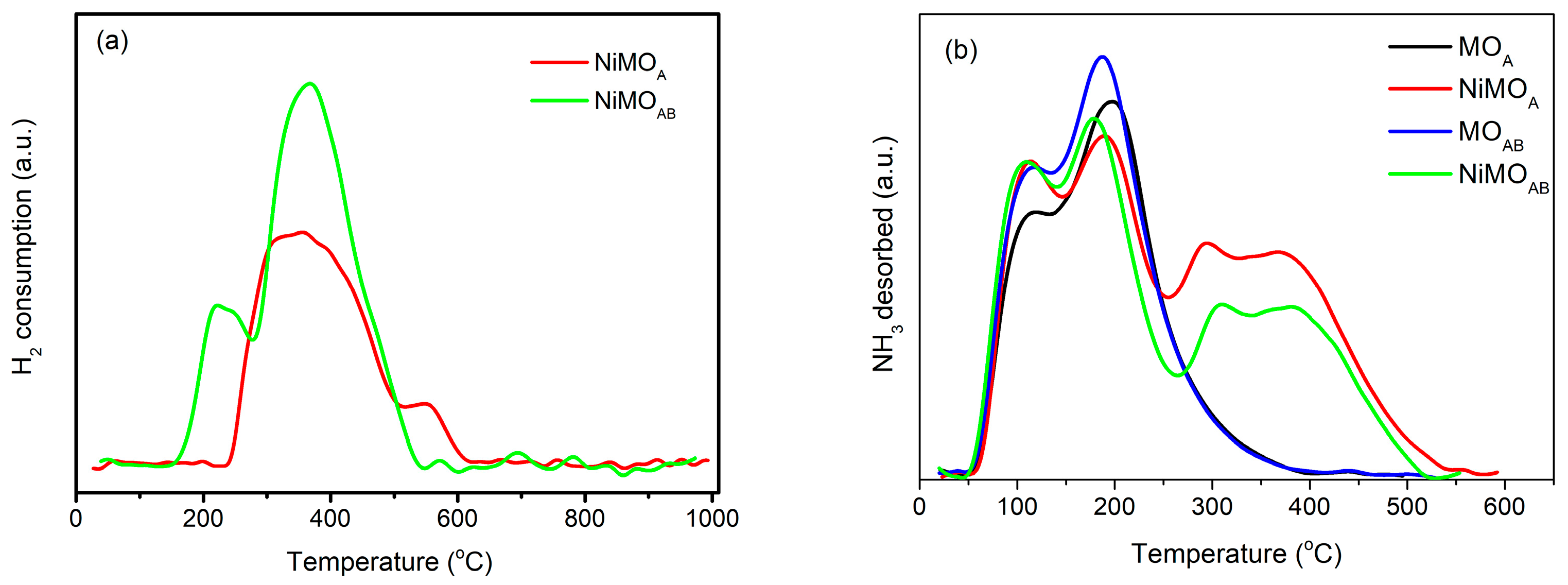
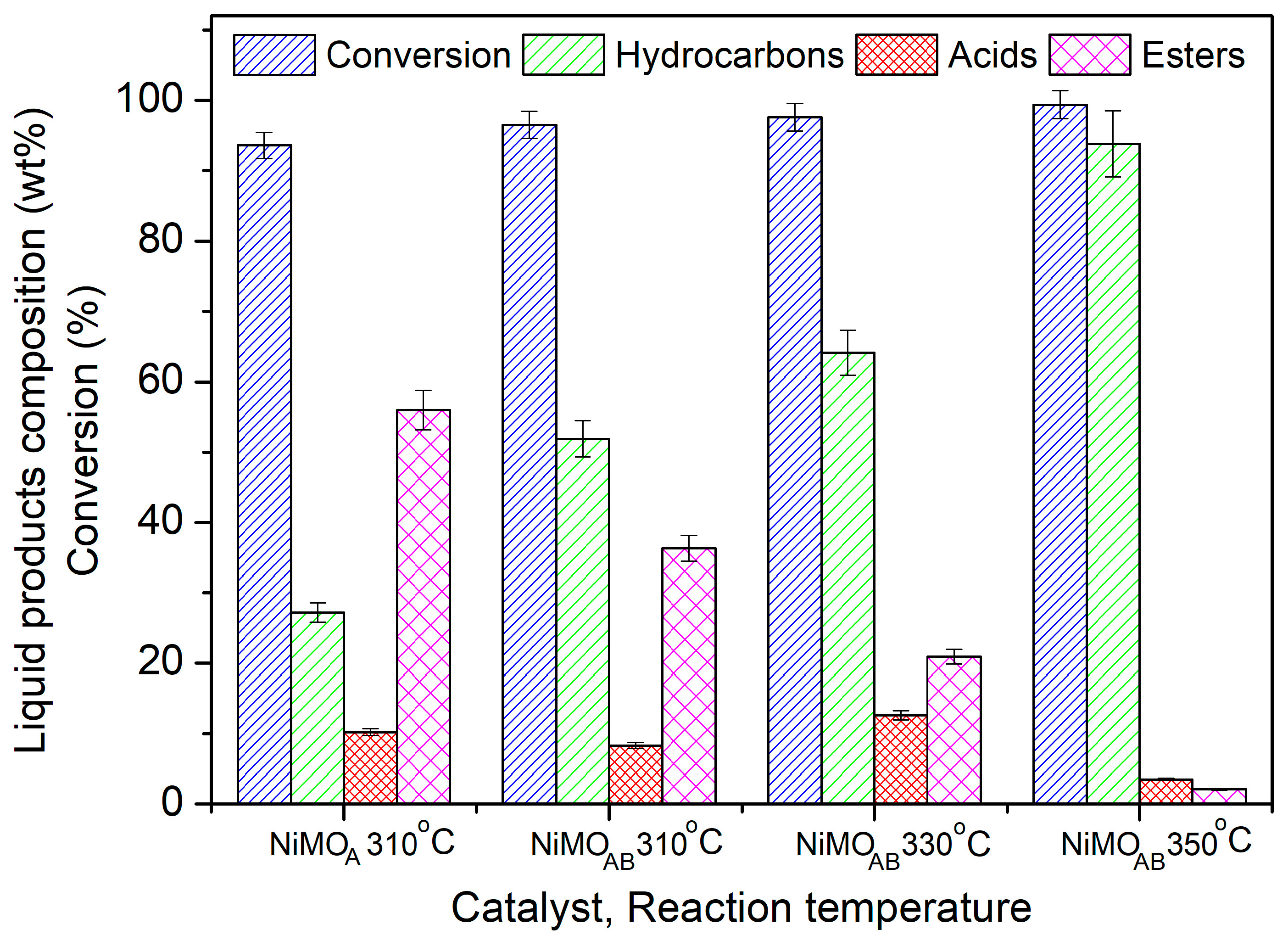
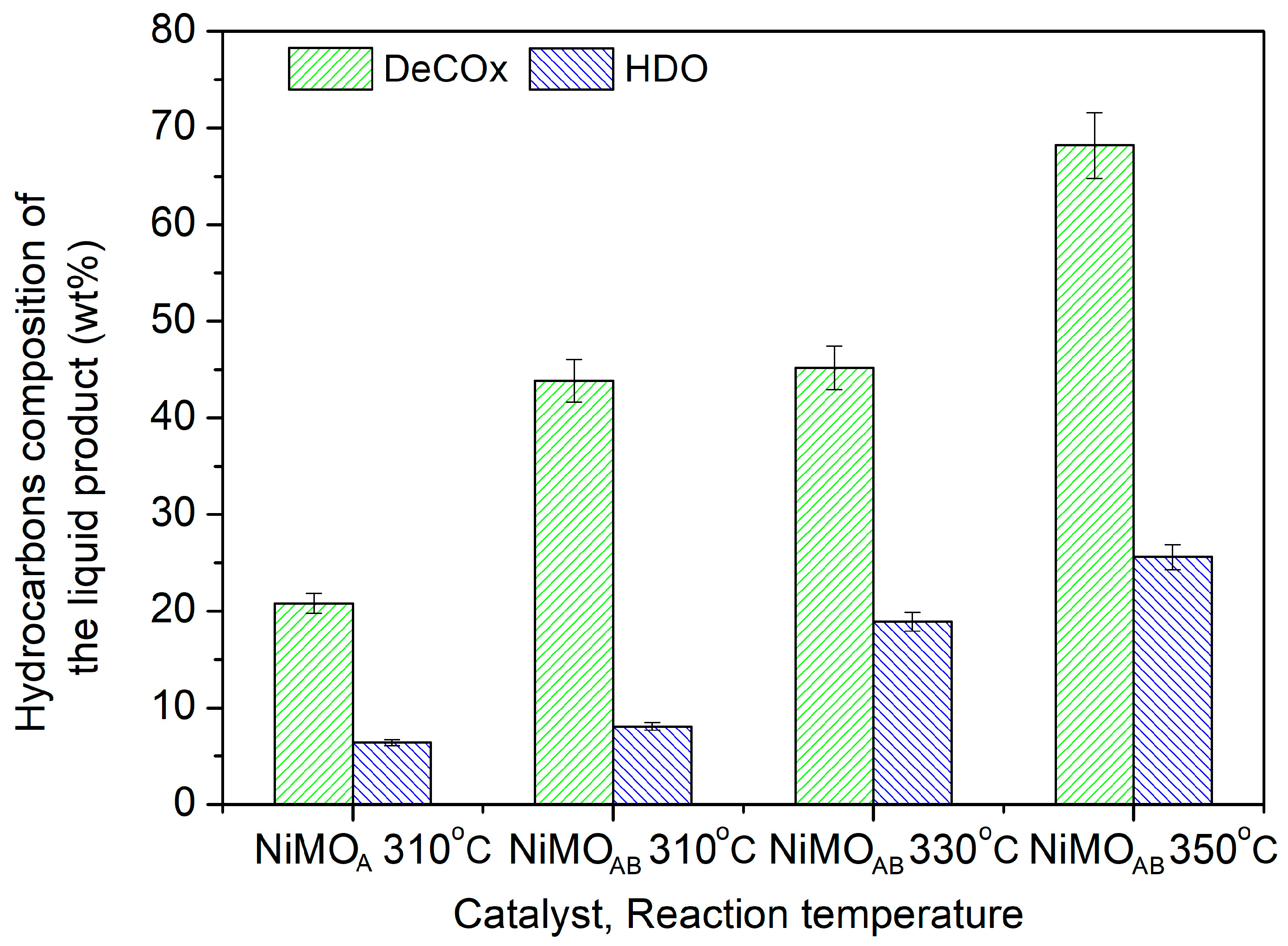
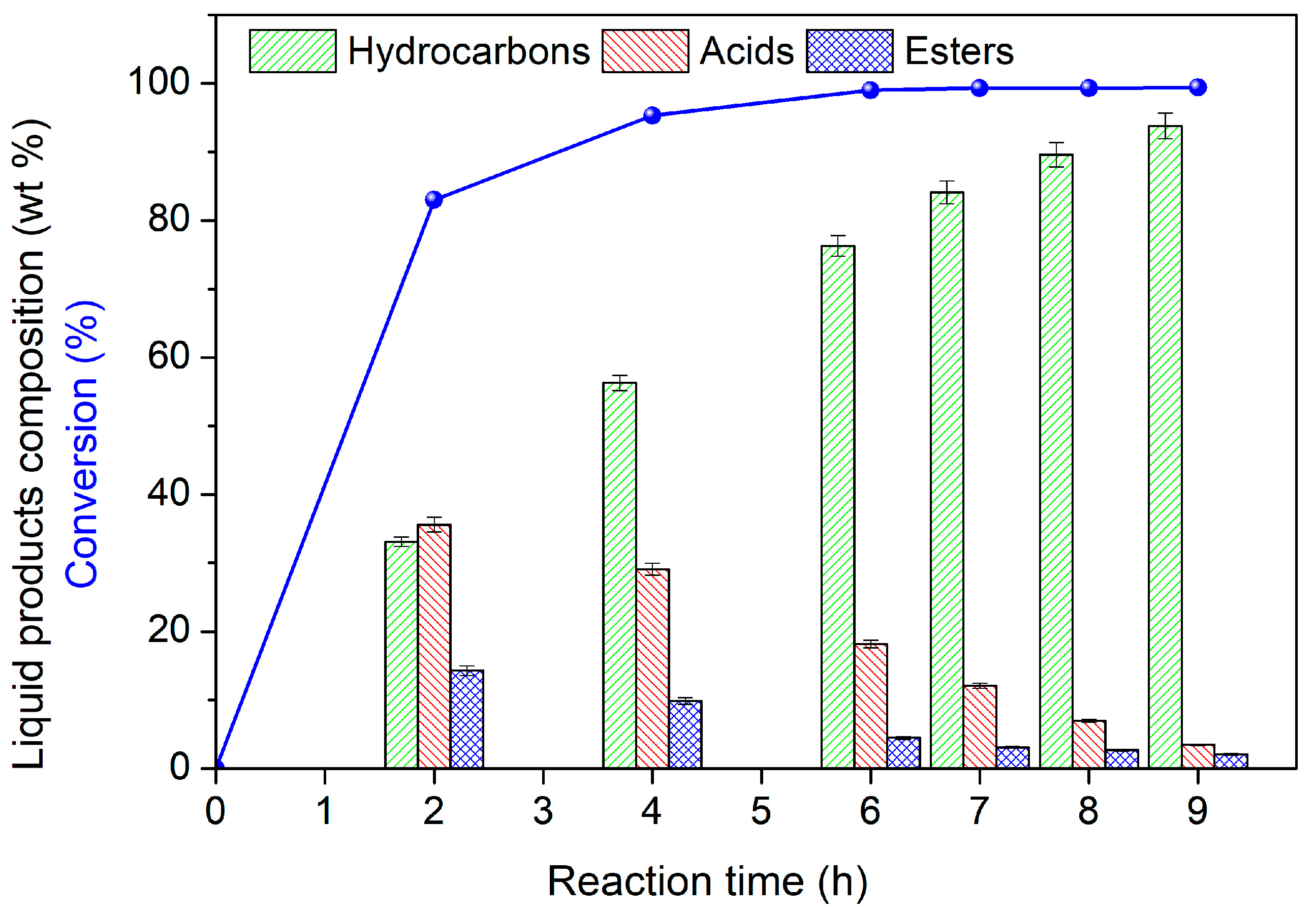

| Sample | Si/Al Ratio | SBET (m2‧g−1) | Smicro (m2‧g−1) | 1 SNi0 (m2‧g−1) | VBJH (cm3‧g−1) | dBJH (nm) | MCSNi0 (nm) |
|---|---|---|---|---|---|---|---|
| MOnat. | 4.59 | 16 | 8 | - | 0.06 | 19.5 | - |
| MOA | 5.85 | 156 | 131 | - | 0.09 | 20.4 | - |
| MOAB | 3.51 | 250 | 217 | - | 0.11 | 21.1 | - |
| NiMOA | - | 96 | 21 | 18.37 | 0.25 | 10.1 | 11 |
| NiMOAB | - | 124 | 73 | 20.21 | 0.20 | 14 | 10 |
| Sample | Total Acidity (a.u.) | Acid Site Concentration (%) | |||
|---|---|---|---|---|---|
| Physisorbed (104–108 °C) | Weak (183–196 °C) | Medium (292–309 °C) | Strong (367–437 °C) | ||
| MOA | 385,090 | - | 94.2 | 5 | 0.8 |
| (498,440) | (22.7) | (72.8) | (3.9) | (0.6) | |
| MOAB | 414,151 | - | 92.5 | 7.3 | 0.2 |
| (527,211) | (21.4) | (72.7) | (5.7) | (0.2) | |
| NiMOA | 689,360 | - | 47.8 | 5.4 | 46.8 |
| (791,768) | (12.9) | (41.6) | (4.7) | (40.8) | |
| NiMOAB | 429,509 | - | 58.8 | 27.5 | 13.7 |
| (602,812) | (28.7) | (41.9) | (19.6) | (9.8) | |
| Sample | Reaction Temperature (°C) | SBET (m2‧g−1) | SNi0 (m2‧g−1) | 1 C (wt.%) | 2 dNi0 (nm) |
|---|---|---|---|---|---|
| spNiMOA | 310 | 29 | 11 | 9 | 19 |
| spNiMOAB | 310 | 22 | 14 | 7.8 | 15 |
| spNiMOAB | 330 | 17 | 8.5 | 6.6 | 24 |
| spNiMOAB | 350 | 16 | 8 | 4.6 | 26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fani, K.; Lycourghiotis, S.; Bourikas, K.; Kordouli, E. Influence of Natural Mordenite Activation Mode on Its Efficiency as Support of Nickel Catalysts for Biodiesel Upgrading to Renewable Diesel. Nanomaterials 2023, 13, 1603. https://doi.org/10.3390/nano13101603
Fani K, Lycourghiotis S, Bourikas K, Kordouli E. Influence of Natural Mordenite Activation Mode on Its Efficiency as Support of Nickel Catalysts for Biodiesel Upgrading to Renewable Diesel. Nanomaterials. 2023; 13(10):1603. https://doi.org/10.3390/nano13101603
Chicago/Turabian StyleFani, Konstantina, Sotiris Lycourghiotis, Kyriakos Bourikas, and Eleana Kordouli. 2023. "Influence of Natural Mordenite Activation Mode on Its Efficiency as Support of Nickel Catalysts for Biodiesel Upgrading to Renewable Diesel" Nanomaterials 13, no. 10: 1603. https://doi.org/10.3390/nano13101603






