Tunning the Zeolitic Imidazole Framework (ZIF8) through the Wet Chemical Route for the Hydrogen Evolution Reaction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
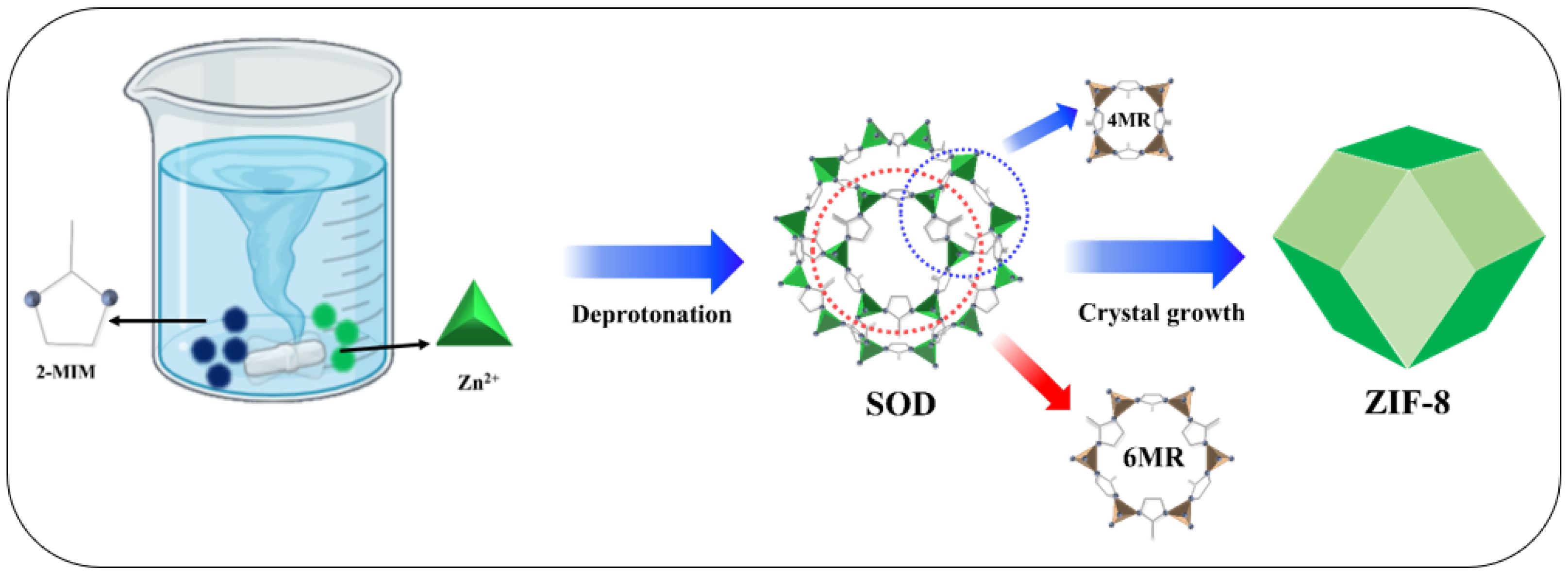
2.2. Synthesis of ZIF8 Crystals
2.3. ZIF8 Characterization
2.4. Electrochemical Measurement
3. Results
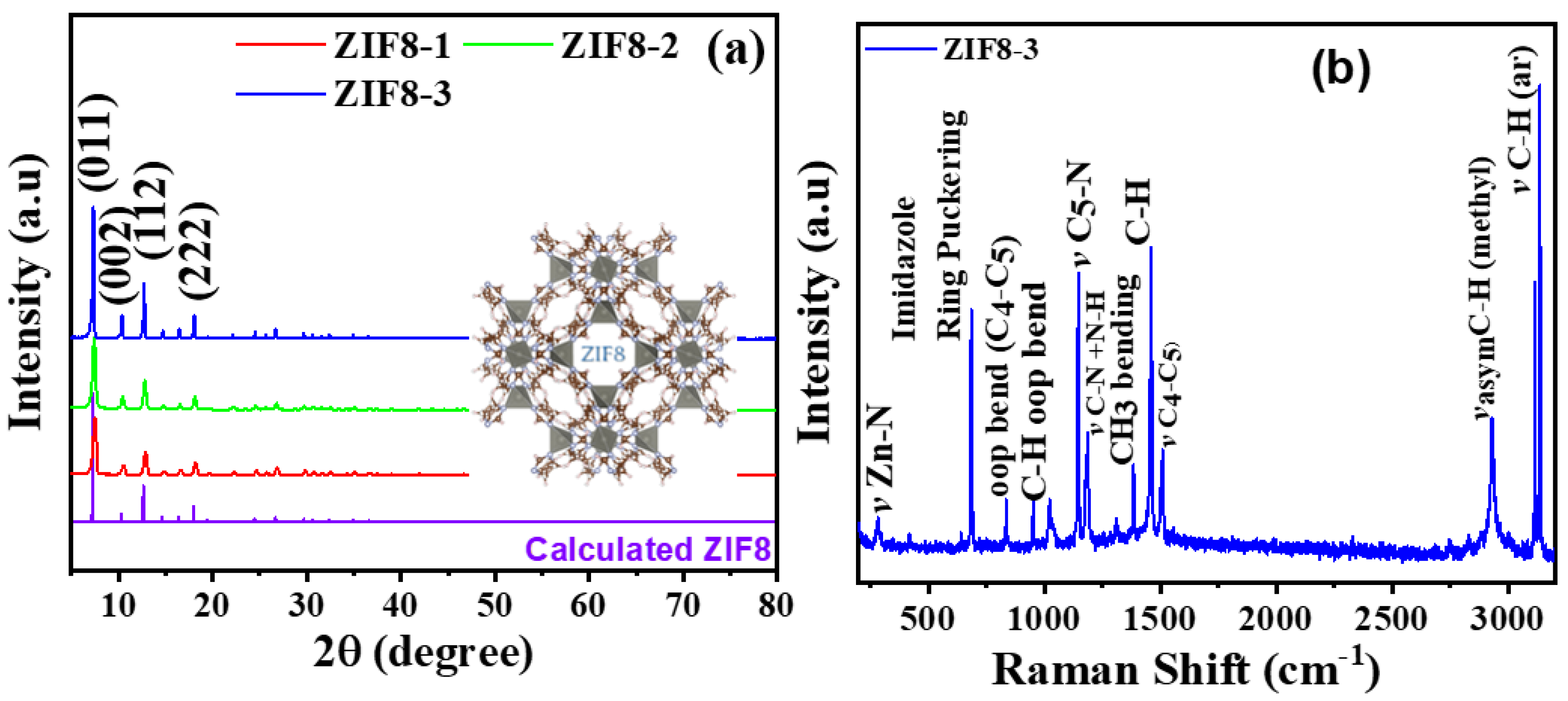
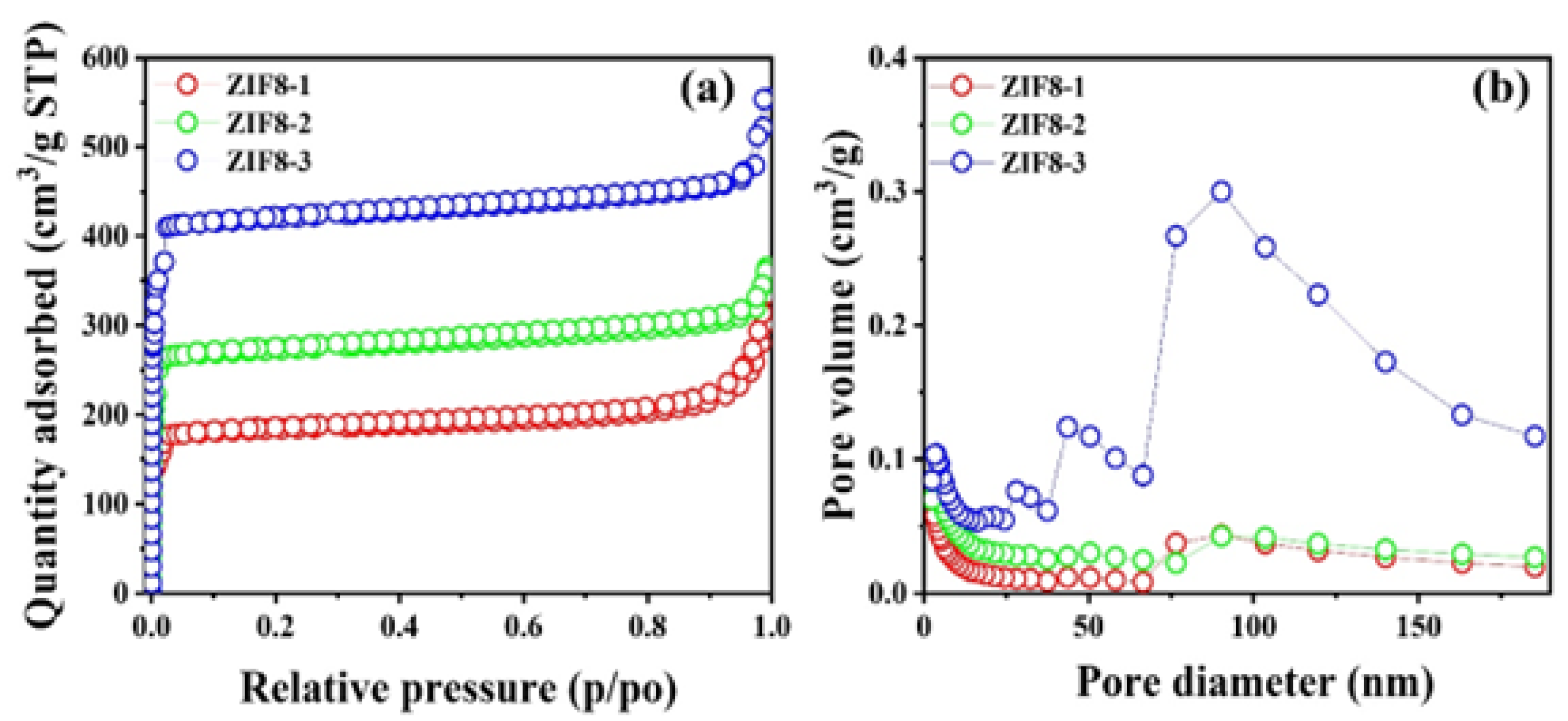
3.1. Morphology and Structural Analysis
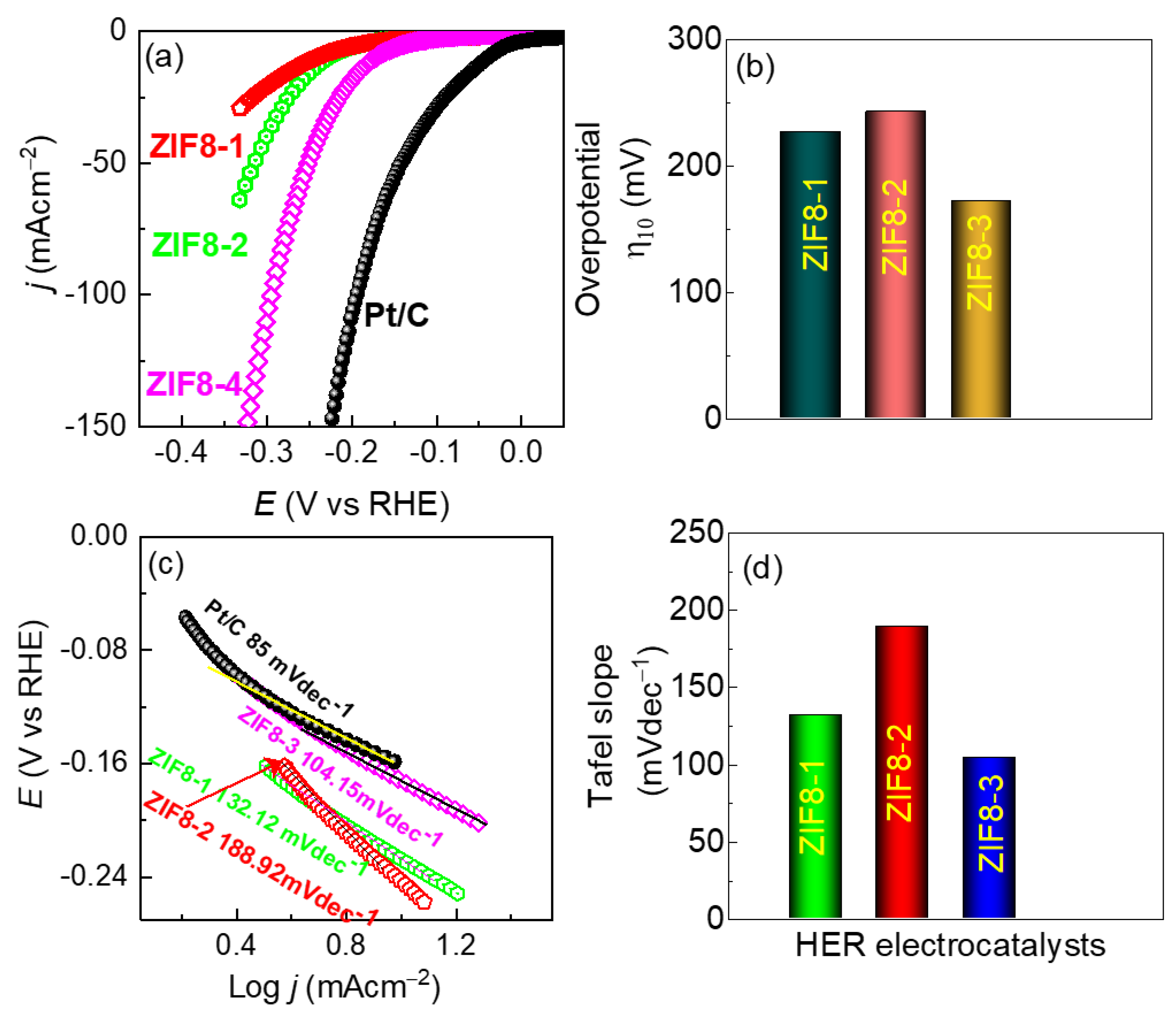
3.2. HER Performance of the As-Synthesized ZIF8
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yun, S.; Zhang, Y.; Xu, Q.; Liu, J.; Qin, Y. Recent advance in new-generation integrated devices for energy harvesting and storage. Nano Energy 2019, 60, 600–619. [Google Scholar] [CrossRef]
- Shao, Y.; Jiang, Z.; Zhang, Q.; Guan, J. Progress in Nonmetal-Doped Graphene Electrocatalysts for the Oxygen Reduction Reaction. ChemSusChem 2019, 12, 2133–2146. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Raza, R.; Shakir, M.I.; Iftikhar, A.; Alvi, F.; Ullah, M.K.; Hamid, A.; Kim, J.-S. Promising electrochemical study of titanate based anodes in direct carbon fuel cell using walnut and almond shells biochar fuel. J. Power Sources 2019, 434, 126679. [Google Scholar] [CrossRef]
- Arshad, A.; Yun, S.; Si, Y.; Han, F.; Zhang, Y.; Zhang, Y.; Wang, Z.; Wang, C. Aloe vera-peel derived porous carbon integrated Co/Mn-oxide based nano-hybrids: An efficient electrocatalyst in advanced photovoltaics. J. Power Sources 2020, 451, 227731. [Google Scholar] [CrossRef]
- Zhang, H.; Gao, Y.; Xu, H.; Guan, D.; Hu, Z.; Jing, C.; Sha, Y.; Gu, Y.; Huang, Y.; Chang, Y.; et al. Combined Corner-Sharing and Edge-Sharing Networks in Hybrid Nanocomposite with Unusual Lattice-Oxygen Activation for Efficient Water Oxidation. Adv. Funct. Mater. 2022, 32, 2207618. [Google Scholar] [CrossRef]
- Han, F.; Yun, S.; Zhang, C.; Xu, H.; Wang, Z. Steel slag as accelerant in anaerobic digestion for nonhazardous treatment and digestate fertilizer utilization. Bioresour. Technol. 2019, 282, 331–338. [Google Scholar] [CrossRef]
- Jia, B.; Yun, S.; Shi, J.; Han, F.; Wang, Z.; Chen, J.; Abbas, Y.; Xu, H.; Wang, K.; Xing, T. Enhanced anaerobic mono- and co-digestion under mesophilic condition: Focusing on the magnetic field and Ti-sphere core–shell structured additives. Bioresour. Technol. 2020, 310, 123450. [Google Scholar] [CrossRef]
- Sarwar, S.; Ali, A.; Liu, Z.; Li, J.; Uprety, S.; Lee, H.; Wang, R.; Park, M.; Bozack, M.J.; Adamczyk, A.J.; et al. Towards thermoneutral hydrogen evolution reaction using noble metal free molybdenum ditelluride/graphene nanocomposites. J. Colloid Interface Sci. 2020, 581, 847–859. [Google Scholar] [CrossRef]
- Su, D.; Jin, H.; Gong, Y.; Li, M.; Pang, Z.; Wang, Y. From “waste to gold”: A one-pot method to synthesize ultrafinely dispersed Fe2O3-based nanoparticles on N-doped carbon for synergistic and efficient water splitting. J. Mater. Chem. A 2015, 3, 11756–11761. [Google Scholar] [CrossRef]
- Xu, G.-R.; Bai, J.; Yao, L.; Xue, Q.; Jiang, J.-X.; Zeng, J.-H.; Chen, Y.; Lee, J.-M. Polyallylamine-Functionalized Platinum Tripods: Enhancement of Hydrogen Evolution Reaction by Proton Carriers. ACS Catal. 2016, 7, 452–458. [Google Scholar] [CrossRef]
- Shrestha, N.K.; Patil, S.A.; Han, J.; Cho, S.; Inamdar, A.I.; Kim, H.; Im, H. Chemical etching induced microporous nickel backbones decorated with metallic Fe@hydroxide nanocatalysts: An efficient and sustainable OER anode toward industrial alkaline water-splitting. J. Mater. Chem. A 2022, 10, 8989–9000. [Google Scholar] [CrossRef]
- Song, Y.; Chen, Y.; Wang, Z.; Zhao, W.; Qin, C.; Yu, H.; Wang, X.; Bakenov, Z.; Zhang, Y. Defective ZnOx@ porous carbon nanofiber network inducing dendrite-free zinc plating as zinc metal anode for high-performance aqueous rechargeable Zn/Na4Mn9O18 battery based on hybrid electrolyte. J. Power Sources 2022, 518, 230761. [Google Scholar] [CrossRef]
- Zhang, T.; Wu, M.-Y.; Yan, D.-Y.; Mao, J.; Liu, H.; Hu, W.-B.; Du, X.-W.; Ling, T.; Qiao, S.-Z. Engineering oxygen vacancy on NiO nanorod arrays for alkaline hydrogen evolution. Nano Energy 2018, 43, 103–109. [Google Scholar] [CrossRef]
- Patil, S.A.; Cho, S.; Jo, Y.; Shrestha, N.K.; Kim, H.; Im, H. Bimetallic Ni-Co@hexacyano nano-frameworks anchored on carbon nanotubes for highly efficient overall water splitting and urea decontamination. Chem. Eng. J. 2021, 426, 130773. [Google Scholar] [CrossRef]
- Li, Y.; Sun, Y.; Qin, Y.; Zhang, W.; Wang, L.; Luo, M.; Yang, H.; Guo, S. Recent advances on water-splitting electrocatalysis mediated by noble-metal-based nanostructured materials. Adv. Energy Mater. 2020, 10, 1903120. [Google Scholar] [CrossRef]
- Sazali, N. Emerging technologies by hydrogen: A review. Int. J. Hydrogen Energy 2020, 45, 18753–18771. [Google Scholar] [CrossRef]
- Zhang, G.; Hu, J.; Nie, Y.; Zhao, Y.; Wang, L.; Li, Y.; Liu, H.; Tang, L.; Zhang, X.; Li, D.; et al. Integrating Flexible Ultralight 3D Ni Micromesh Current Collector with NiCo Bimetallic Hydroxide for Smart Hybrid Supercapacitors. Adv. Funct. Mater. 2021, 31, 2100290. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, X.; Liu, H.; Li, J.; Chen, Y.; Duan, H. 3D-printed multi-channel metal lattices enabling localized electric-field redistribution for dendrite-free aqueous Zn ion batteries. Adv. Energy Mater. 2021, 11, 2003927. [Google Scholar] [CrossRef]
- Liang, X.; Wu, C.-M.L. Metal-free two-dimensional phosphorus carbide as an efficient electrocatalyst for hydrogen evolution reaction comparable to platinum. Nano Energy 2020, 71, 104603. [Google Scholar] [CrossRef]
- Shiraz, H.G.; Crispin, X.; Berggren, M. Transition metal sulfides for electrochemical hydrogen evolution. Int. J. Hydrogen Energy 2021, 46, 24060–24077. [Google Scholar] [CrossRef]
- Wang, H.; Li, J.; Li, K.; Lin, Y.; Chen, J.; Gao, L.; Nicolosi, V.; Xiao, X.; Lee, J.-M. Transition metal nitrides for electrochemical energy applications. Chem. Soc. Rev. 2020, 50, 1354–1390. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Wang, B.; Cai, H.; Hao, H.; Li, J.; Yang, T.; Yang, S. Understanding the doping effect on hydrogen evolution activity of transition-metal phosphides: Modeled with Ni2P. Appl. Catal. B Environ. 2021, 295, 120283. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, X.; Wei, Y.; Liu, J.; Cheng, M.; Wang, L.; Li, Y.; Wu, J.; Wei, T.; Hu, J.; et al. Core-shell and hollow meso@microporous carbon derived from ZIF-8@CMK-3 composites for Li-S batteries. J. Alloys Compd. 2023, 934, 167861. [Google Scholar] [CrossRef]
- Zhao, X.; Zhu, M.; Tang, C.; Quan, K.; Tong, Q.; Cao, H.; Jiang, J.; Yang, H.; Zhang, J. ZIF-8@MXene-reinforced flame-retardant and highly conductive polymer composite electrolyte for dendrite-free lithium metal batteries. J. Colloid Interface Sci. 2022, 620, 478–485. [Google Scholar] [CrossRef]
- Rabani, I.; Tahir, M.S.; Afzal, F.; Truong, H.B.; Kim, M.; Seo, Y.-S. High-efficient mineralization performance of photocatalysis activity towards organic pollutants over ruthenium nanoparticles stabilized by metal organic framework. J. Environ. Chem. Eng. 2023, 11, 109235. [Google Scholar] [CrossRef]
- Rabani, I.; Lee, J.-W.; Choi, S.R.; Park, J.-Y.; Patil, S.A.; Turpu, G.; Kim, M.; Soo-Seo, Y. Structural engineering of ruthenium decorated zeolitic imidazole framework nanocomposite for hydrogen evolution reactions and supercapacitors. J. Energy Storage 2023, 62, 106885. [Google Scholar] [CrossRef]
- Sonawane, A.V.; Murthy, Z.V.P. Synthesis and characterization of ZIF-8-based PVDF mixed matrix membranes and application to treat pulp and paper industry wastewater using a membrane bioreactor. Environ. Sci. Water Res. Technol. 2022, 8, 881–896. [Google Scholar] [CrossRef]
- Qin, Y.; Xie, J.; Liu, S.; Bai, Y. Selective methanol-sensing of SnS-supported ultrathin ZIF-8 nanocomposite with core-shell heterostructure. Sens. Actuators B Chem. 2022, 368, 132230. [Google Scholar] [CrossRef]
- Chen, X.; An, X.; Tang, L.; Chen, T.; Zhang, G. Confining platinum clusters in ZIF-8-derived porous N-doped carbon arrays for high-performance hydrogen evolution reaction. Chem. Eng. J. 2021, 429, 132259. [Google Scholar] [CrossRef]
- Sun, C.; Duan, X.; Song, J.; Zhang, M.; Jin, Y.; Zhang, M.; Song, L.; Cao, H. Rh particles in N-doped porous carbon materials derived from ZIF-8 as an efficient bifunctional electrocatalyst for the ORR and HER. RSC Adv. 2021, 11, 13906–13911. [Google Scholar] [CrossRef]
- Kida, K.; Okita, M.; Fujita, K.; Tanaka, S.; Miyake, Y. Formation of high crystalline ZIF-8 in an aqueous solution. Crystengcomm 2012, 15, 1794–1801. [Google Scholar] [CrossRef]
- Zheng, G.; Chen, Z.; Sentosun, K.; Pérez-Juste, I.; Bals, S.; Liz-Marzán, L.M.; Pastoriza-Santos, I.; Pérez-Juste, J.; Hong, M. Shape control in ZIF-8 nanocrystals and metal nanoparticles@ZIF-8 heterostructures. Nanoscale 2017, 9, 16645–16651. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Peng, Y.; Wang, H.; Gu, Z.; Duan, C. Synthesis of Au@ZIF-8 single- or multi-core–shell structures for photocatalysis. Chem. Commun. 2014, 50, 8651–8654. [Google Scholar] [CrossRef] [PubMed]
- Schejn, A.; Aboulaich, A.; Balan, L.; Falk, V.; Lalevée, J.; Medjahdi, G.; Aranda, L.; Mozet, K.; Schneider, R. Cu2+-doped zeolitic imidazolate frameworks (ZIF-8): Efficient and stable catalysts for cycloadditions and condensation reactions. Catal. Sci. Technol. 2014, 5, 1829–1839. [Google Scholar] [CrossRef]
- Li, J.; Chang, H.; Li, Y.; Li, Q.; Shen, K.; Yi, H.; Zhang, J. Synthesis and adsorption performance of La@ZIF-8 composite metal–organic frameworks. RSC Adv. 2020, 10, 3380–3390. [Google Scholar] [CrossRef] [PubMed]
- Thanh, M.T.; Thien, T.V.; Du, P.D.; Hung, N.P.; Khieu, D.Q. Iron doped zeolitic imidazolate framework (Fe-ZIF-8): Synthesis and photocatalytic degradation of RDB dye in Fe-ZIF-8. J. Porous Mater. 2017, 25, 857–869. [Google Scholar] [CrossRef]
- Zhou, K.; Mousavi, B.; Luo, Z.; Phatanasri, S.; Chaemchuen, S.; Verpoort, F. Characterization and properties of Zn/Co zeolitic imidazolate frameworks vs. ZIF-8 and ZIF-67. J. Mater. Chem. A 2017, 5, 952–957. [Google Scholar] [CrossRef]
- Karagiaridi, O.; Lalonde, M.B.; Bury, W.; Sarjeant, A.A.; Farha, O.K.; Hupp, J.T. Opening ZIF-8: A Catalytically Active Zeolitic Imidazolate Framework of Sodalite Topology with Unsubstituted Linkers. J. Am. Chem. Soc. 2012, 134, 18790–18796. [Google Scholar] [CrossRef]
- Guan, D.; Shi, C.; Xu, H.; Gu, Y.; Zhong, J.; Sha, Y.; Hu, Z.; Ni, M.; Shao, Z. Simultaneously mastering operando strain and reconstruction effects via phase-segregation strategy for enhanced oxygen-evolving electrocatalysis. J. Energy Chem. 2023, in press. [Google Scholar] [CrossRef]
- Kumari, G.; Jayaramulu, K.; Maji, T.K.; Narayana, C. Temperature Induced Structural Transformations and Gas Adsorption in the Zeolitic Imidazolate Framework ZIF-8: A Raman Study. J. Phys. Chem. A 2013, 117, 11006–11012. [Google Scholar] [CrossRef]
- Markham, L.M.; Mayne, L.C.; Hudson, B.S.; Zgierski, M.Z. Resonance Raman studies of imidazole, imidazolium, and their derivatives: The effect of deuterium substitution. J. Phys. Chem. 1993, 97, 10319–10325. [Google Scholar] [CrossRef]
- Radhakrishnan, D.; Narayana, C. Guest dependent Brillouin and Raman scattering studies of zeolitic imidazolate framework-8 (ZIF-8) under external pressure. J. Chem. Phys. 2016, 144, 134704. [Google Scholar] [CrossRef] [PubMed]
- Rabani, I.; Park, Y.-J.; Lee, J.-W.; Tahir, M.S.; Kumar, A.; Seo, Y.-S. Ultra-thin flexible paper of BNNT–CNF/ZnO ternary nanostructure for enhanced solid-state supercapacitor and piezoelectric response. J. Mater. Chem. A 2022, 10, 15580–15594. [Google Scholar] [CrossRef]
- Rabani, I.; Hussain, S.; Vikraman, D.; Seo, Y.-S.; Jung, J.; Jana, A.; Shrestha, N.K.; Jalalah, M.; Noh, Y.-Y.; Patil, S.A. 1D-CoSe2 nanoarray: A designed structure for efficient hydrogen evolution and symmetric supercapacitor characteristics. Dalton Trans. 2020, 49, 14191–14200. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cheng, H.; Cheng, M.; Liu, Z.; Huang, D.; Zhang, G.; Shao, B.; Liang, Q.; Luo, S.; Wu, T.; et al. The application of Zeolitic imidazolate frameworks (ZIFs) and their derivatives based materials for photocatalytic hydrogen evolution and pollutants treatment. Chem. Eng. J. 2020, 417, 127914. [Google Scholar] [CrossRef]
- Patil, S.A.; Rabani, I.; Vikraman, D.; Bathula, C.; Shrestha, N.K.; Kim, H.; Hussain, S.; Im, H. Template-free synthesis of one-dimensional cobalt sulfide nanorod array as an attractive architecture for overall water splitting. Int. J. Energy Res. 2020, 45, 2785–2796. [Google Scholar] [CrossRef]
- McCrory, C.C.L.; Jung, S.; Peters, J.C.; Jaramillo, T.F. Benchmarking Heterogeneous Electrocatalysts for the Oxygen Evolution Reaction. J. Am. Chem. Soc. 2013, 135, 16977–16987. [Google Scholar] [CrossRef] [PubMed]
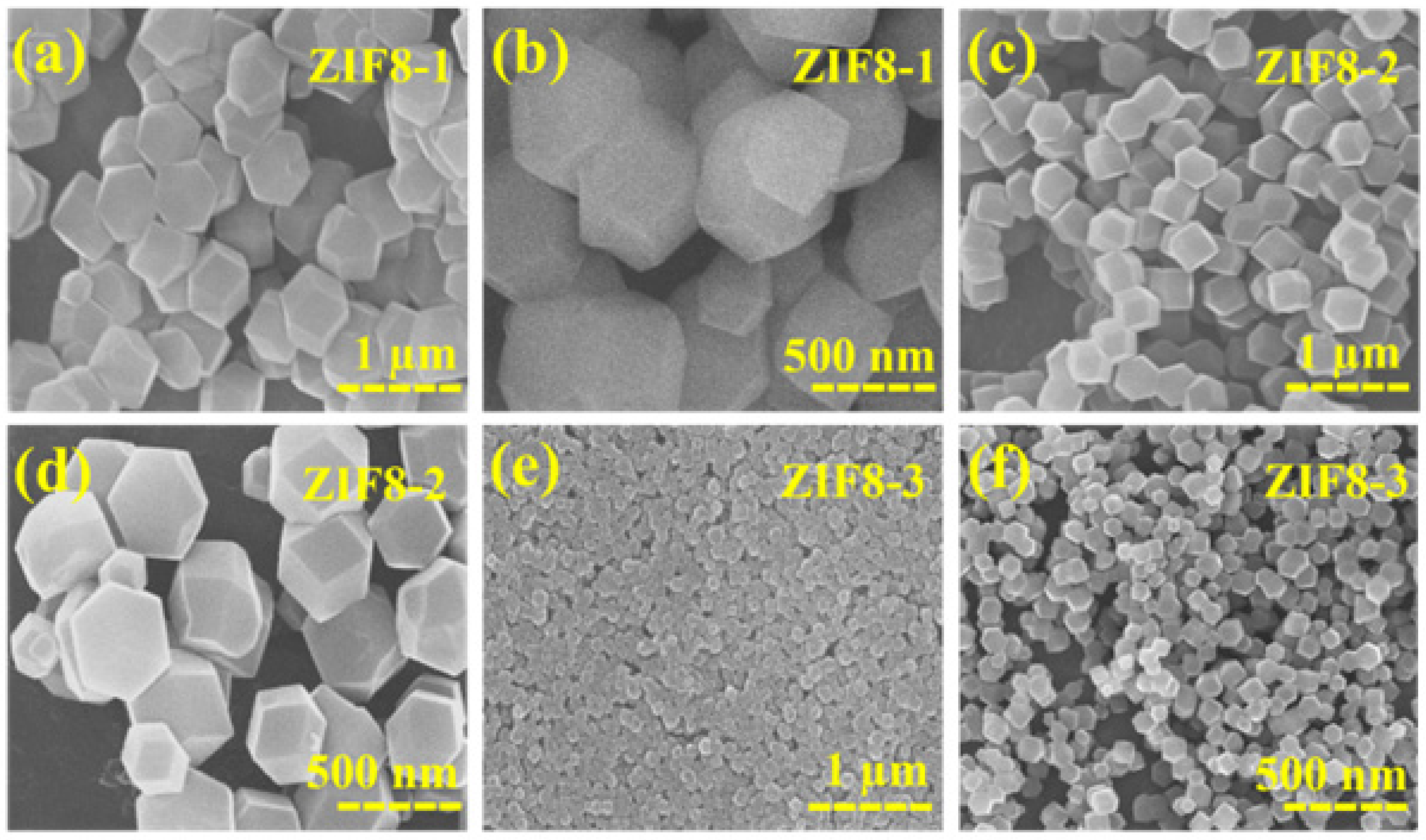



| Experiments | Sample | 2-MIM:Zn (NO3)2·6H2O | Crystal Size (nm) | Reaction Time (min) |
|---|---|---|---|---|
| 1 | ZIF8-1 | 1.10:1 | ~700–800 | 150 |
| 2 | ZIF8-2 | 1.10:1 | ~400–550 | 120 |
| 3 | ZIF8-3 | 1.10:1 | ~70–100 | 90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rabani, I.; Patil, S.A.; Tahir, M.S.; Afzal, F.; Lee, J.-W.; Im, H.; Seo, Y.-S.; Shrestha, N.K. Tunning the Zeolitic Imidazole Framework (ZIF8) through the Wet Chemical Route for the Hydrogen Evolution Reaction. Nanomaterials 2023, 13, 1610. https://doi.org/10.3390/nano13101610
Rabani I, Patil SA, Tahir MS, Afzal F, Lee J-W, Im H, Seo Y-S, Shrestha NK. Tunning the Zeolitic Imidazole Framework (ZIF8) through the Wet Chemical Route for the Hydrogen Evolution Reaction. Nanomaterials. 2023; 13(10):1610. https://doi.org/10.3390/nano13101610
Chicago/Turabian StyleRabani, Iqra, Supriya A. Patil, Muhammad Shoaib Tahir, Fatima Afzal, Je-Won Lee, Hyunsik Im, Young-Soo Seo, and Nabeen K. Shrestha. 2023. "Tunning the Zeolitic Imidazole Framework (ZIF8) through the Wet Chemical Route for the Hydrogen Evolution Reaction" Nanomaterials 13, no. 10: 1610. https://doi.org/10.3390/nano13101610







