Polydopamine Nanomaterials for Overcoming Current Challenges in Cancer Treatment
Abstract
1. Introduction
2. Various Types of PDA Nanostructures and Their Formation Method
2.1. PDA Core–Shell Structure
2.2. PDA Hollow Structure
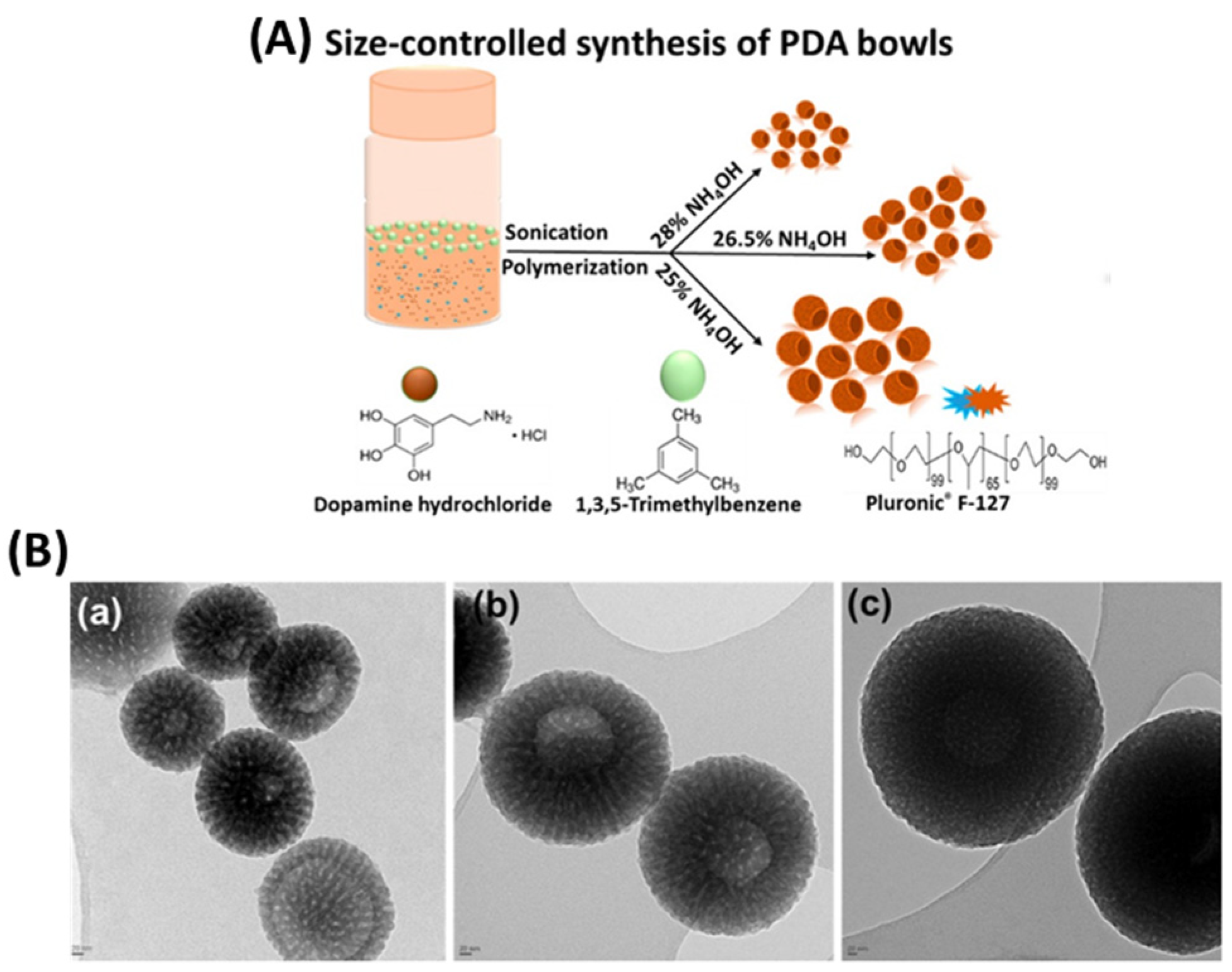
2.3. PDA Spheres
2.4. PDA Anisotropic-Shaped Nanoparticles
3. Potential of Polydopamine Nanoparticles for Cancer Treatment
3.1. PDA for Drug Delivery and Photothermal and Photodynamic Therapy
3.2. Efficiency of PDA as Image-Contrast Agent, and in Immunotherapy and Radiation Therapy
4. Conclusions and Future Direction
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Arruebo, M.; Vilaboa, N.; Sáez-Gutierrez, B.; Lambea, J.; Tres, A.; Valladares, M.; González-Fernández, A. Assessment of the evolution of cancer treatment therapies. Cancers 2011, 3, 3279–3330. [Google Scholar] [CrossRef][Green Version]
- Wang, X.; Zhang, H.; Chen, X. Drug resistance and combating drug resistance in cancer. Cancer Drug Resist. 2019, 2, 141–160. [Google Scholar] [CrossRef][Green Version]
- Pucci, C.; Martinelli, C.; Ciofani, G. Innovative approaches for cancer treatment: Current perspectives and new challenges. Ecancermedicalscience 2019, 13, 961. [Google Scholar] [CrossRef]
- Cheng, Z.; Li, M.; Dey, R.; Chen, Y. Nanomaterials for cancer therapy: Current progress and perspectives. J. Hematol. Oncol. 2021, 14, 85. [Google Scholar] [CrossRef]
- Debela, D.T.; Muzazu, S.G.; Heraro, K.D.; Ndalama, M.T.; Mesele, B.W.; Haile, D.C.; Kitui, S.K.; Manyazewal, T. New approaches and procedures for cancer treatment: Current perspectives. SAGE Open Med. 2021, 9, 20503121211034366. [Google Scholar] [CrossRef] [PubMed]
- Gavas, S.; Quazi, S.; Karpiński, T.M. Nanoparticles for Cancer Therapy: Current Progress and Challenges. Nanoscale Res. Lett. 2021, 16, 173. [Google Scholar] [CrossRef] [PubMed]
- Aslan, B.; Ozpolat, B.; Sood, A.K.; Lopez-Berestein, G. Nanotechnology in cancer therapy. J. Drug Target 2013, 21, 904–913. [Google Scholar] [CrossRef][Green Version]
- Navya, P.N.; Kaphle, A.; Srinivas, S.P.; Bhargava, S.K.; Rotello, V.M.; Daima, H.K. Current trends and challenges in cancer management and therapy using designer nanomaterials. Nano Converg. 2019, 6, 23. [Google Scholar] [CrossRef][Green Version]
- Pillai, G.; Ceballos-Coronel, M.L. Science and technology of the emerging nanomedicines in cancer therapy: A primer for physicians and pharmacists. SAGE Open Med. 2013, 1, 2050312113513759. [Google Scholar] [CrossRef][Green Version]
- Shukla, A.; Maiti, P. Nanomedicine and versatile therapies for cancer treatment. MedComm 2022, 3, e163. [Google Scholar] [CrossRef]
- Mundekkad, D.; Cho, W.C. Nanoparticles in Clinical Translation for Cancer Therapy. Int. J. Mol. Sci. 2022, 23, 1685. [Google Scholar] [CrossRef]
- Pandey, A.; Nikam, A.N.; Padya, B.S.; Kulkarni, S.; Fernandes, G.; Shreya, A.B.; García, M.C.; Caro, C.; Páez-Muñoz, J.M.; Dhas, N.; et al. Surface architectured black phosphorous nanoconstructs based smart and versatile platform for cancer theranostics. Coord. Chem. Rev. 2021, 435, 213826. [Google Scholar] [CrossRef]
- Cucinotto, I.; Fiorillo, L.; Gualtieri, S.; Arbitrio, M.; Ciliberto, D.; Staropoli, N.; Grimaldi, A.; Luce, A.; Tassone, P.; Caraglia, M.; et al. Nanoparticle albumin bound Paclitaxel in the treatment of human cancer: Nanodelivery reaches prime-time? J. Drug Deliv. 2013, 2013, 905091. [Google Scholar] [CrossRef]
- Ma, P.; Mumper, R.J. Paclitaxel Nano-Delivery Systems: A Comprehensive Review. J. Nanomed. Nanotechnol. 2013, 4, 1000164. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sajja, H.K.; East, M.P.; Mao, H.; Wang, Y.A.; Nie, S.; Yang, L. Development of multifunctional nanoparticles for targeted drug delivery and noninvasive imaging of therapeutic effect. Curr. Drug Discov. Technol. 2009, 6, 43–51. [Google Scholar] [CrossRef][Green Version]
- Srinivasan, M.; Rajabi, M.; Mousa, S.A. Multifunctional Nanomaterials and Their Applications in Drug Delivery and Cancer Therapy. Nanomaterials 2015, 5, 1690–1703. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Caro, C.; Gámez, F.; Quaresma, P.; Páez-Muñoz, J.M.; Domínguez, A.; Pearson, J.R.; Pernía Leal, M.; Beltrán, A.M.; Fernandez-Afonso, Y.; De la Fuente, J.M.; et al. Fe3O4-Au Core-Shell Nanoparticles as a Multimodal Platform for In Vivo Imaging and Focused Photothermal Therapy. Pharmaceutics 2021, 13, 416. [Google Scholar] [CrossRef]
- Bertrand, N.; Wu, J.; Xu, X.; Kamaly, N.; Farokhzad, O.C. Cancer nanotechnology: The impact of passive and active targeting in the era of modern cancer biology. Adv. Drug Deliv. Rev. 2014, 66, 2–25. [Google Scholar] [CrossRef][Green Version]
- Sriraman, S.K.; Aryasomayajula, B.; Torchilin, V.P. Barriers to drug delivery in solid tumors. Tissue Barriers 2014, 2, e29528. [Google Scholar] [CrossRef] [PubMed][Green Version]
- De Jong, W.H.; Borm, P.J. Drug delivery and nanoparticles:applications and hazards. Int. J. Nanomed. 2008, 3, 133–149. [Google Scholar] [CrossRef][Green Version]
- Matsumura, Y.; Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986, 46, 6387–6392. [Google Scholar] [PubMed]
- Maeda, H.; Bharate, G.Y.; Daruwalla, J. Polymeric drugs for efficient tumor-targeted drug delivery based on EPR-effect. Eur. J. Pharm. Biopharm. 2009, 71, 409–419. [Google Scholar] [CrossRef]
- van der Meel, R.; Lammers, T.; Hennink, W.E. Cancer nanomedicines: Oversold or underappreciated? Expert Opin. Drug Deliv. 2017, 14, 1–5. [Google Scholar] [CrossRef][Green Version]
- Wilhelm, S.; Tavares, A.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.; Chan, W. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016, 1, 16014. [Google Scholar] [CrossRef]
- Soetaert, F.; Korangath, P.; Serantes, D.; Fiering, S.; Ivkov, R. Cancer therapy with iron oxide nanoparticles: Agents of thermal and immune therapies. Adv. Drug Deliv. Rev. 2020, 163–164, 65–83. [Google Scholar] [CrossRef] [PubMed]
- Wu, J. The Enhanced Permeability and Retention (EPR) Effect: The Significance of the Concept and Methods to Enhance Its Application. J. Pers. Med. 2021, 11, 771. [Google Scholar] [CrossRef] [PubMed]
- Nichols, J.W.; Bae, Y.H. EPR: Evidence and fallacy. J. Control. Release 2014, 190, 451–464. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Mochida, A.; Choyke, P.L.; Kobayashi, H. Nanodrug Delivery: Is the Enhanced Permeability and Retention Effect Sufficient for Curing Cancer? Bioconjug. Chem. 2016, 27, 2225–2238. [Google Scholar] [CrossRef] [PubMed]
- Korangath, P.; Barnett, J.D.; Sharma, A.; Henderson, E.T.; Stewart, J.; Yu, S.H.; Kandala, S.K.; Yang, C.T.; Caserto, J.S.; Hedayati, M.; et al. Nanoparticle interactions with immune cells dominate tumor retention and induce T cell-mediated tumor suppression in models of breast cancer. Sci. Adv. 2020, 6, eaay1601. [Google Scholar] [CrossRef][Green Version]
- Bao, G.; Mitragotri, S.; Tong, S. Multifunctional nanoparticles for drug delivery and molecular imaging. Annu. Rev. Biomed. Eng. 2013, 15, 253–282. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Zhou, Y.; Liu, L.; Xu, Y.; Chen, Q.; Wang, Y.; Wu, S.; Deng, Y.; Zhang, J.; Shao, A. Nanoparticle-Based Drug Delivery in Cancer Therapy and Its Role in Overcoming Drug Resistance. Front. Mol. Biosci. 2020, 7, 193. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.; Yin, M.; Zhao, L.; Meng, F.; Luo, L. Recent progress on nanoparticle-based drug delivery systems for cancer therapy. Cancer Biol. Med. 2017, 14, 228–241. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ngwa, W.; Kumar, R.; Moreau, M.; Dabney, R.; Herman, A. Nanoparticle Drones to Target Lung Cancer with Radiosensitizers and Cannabinoids. Front. Oncol. 2017, 7, 208. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ridolfo, R.; Tavakoli, S.; Junnuthula, V.; Williams, D.S.; Urtti, A.; van Hest, J.C.M. Exploring the Impact of Morphology on the Properties of Biodegradable Nanoparticles and Their Diffusion in Complex Biological Medium. Biomacromolecules 2021, 22, 126–133. [Google Scholar] [CrossRef]
- Caldorera-Moore, M.; Guimard, N.; Shi, L.; Roy, K. Designer nanoparticles: Incorporating size, shape and triggered release into nanoscale drug carriers. Expert Opin. Drug Deliv. 2010, 7, 479–495. [Google Scholar] [CrossRef]
- Murugan, K.; Choonara, Y.E.; Kumar, P.; Bijukumar, D.; du Toit, L.C.; Pillay, V. Parameters and characteristics governing cellular internalization and trans-barrier trafficking of nanostructures. Int. J. Nanomed. 2015, 10, 2191–2206. [Google Scholar] [CrossRef][Green Version]
- Kohout, C.; Santi, C.; Polito, L. Anisotropic Gold Nanoparticles in Biomedical Applications. Int. J. Mol. Sci. 2018, 19, 3385. [Google Scholar] [CrossRef][Green Version]
- Jindal, A. The effect of particle shape on cellular interaction and drug delivery applications of micro- and nanoparticles. Int. J. Pharm. 2017, 532, 450–465. [Google Scholar] [CrossRef]
- Burrows, N.D.; Vartanian, A.M.; Abadeer, N.S.; Grzincic, E.M.; Jacob, L.M.; Lin, W.; Li, J.; Dennison, J.M.; Hinman, J.G.; Murphy, C.J. Anisotropic Nanoparticles and Anisotropic Surface Chemistry. J. Phys. Chem. Lett. 2016, 7, 632–641. [Google Scholar] [CrossRef]
- Acter, S.; Cho, J.; Kim, J.; Byun, A.; Park, K.H. Synthesis and Shape Control of Uniform Polymer Microparticles by Tailored Adsorption of Poly(ethylene oxide)- b -Poly(ε-caprolactone) Copolymer. Bull. Korean Chem. Soc. 2015, 36, 1467–1473. [Google Scholar] [CrossRef]
- Zhang, L.; Su, H.; Wang, H.; Li, Q.; Li, X.; Zhou, C.; Xu, J.; Chai, Y.; Liang, X.; Xiong, L.; et al. Tumor Chemo-Radiotherapy with Rod-Shaped and Spherical Gold Nano Probes: Shape and Active Targeting Both Matter. Theranostics 2019, 9, 1893–1908. [Google Scholar] [CrossRef] [PubMed]
- Guan, B.Y.; Zhang, S.L.; Lou, X.W. Realization of Walnut-Shaped Particles with Macro-/Mesoporous Open Channels through Pore Architecture Manipulation and Their Use in Electrocatalytic Oxygen Reduction. Angew. Chem. Int. Ed. 2018, 57, 6176–6180. [Google Scholar] [CrossRef] [PubMed]
- Guan, B.Y.; Yu, L.; Lou, X.W. Formation of Asymmetric Bowl-Like Mesoporous Particles via Emulsion-Induced Interface Anisotropic Assembly. J. Am. Chem. Soc. 2016, 138, 11306–11311. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, W.; Tong, W.; Gao, C. Enhanced Cellular Uptake of Bowl-like Microcapsules. ACS Appl. Mater. Interfaces 2016, 8, 11210–11214. [Google Scholar] [CrossRef]
- Acter, S.; Vidallon, M.L.P.; Crawford, S.; Tabor, R.F.; Teo, B.M. Efficient Cellular Internalization and Transport of Bowl-Shaped Polydopamine Particles. Part. Part. Syst. Charact. 2020, 37, 2000166. [Google Scholar] [CrossRef]
- Xie, X.; Liao, J.; Shao, X.; Li, Q.; Lin, Y. The Effect of shape on Cellular Uptake of Gold Nanoparticles in the forms of Stars, Rods, and Triangles. Sci. Rep. 2017, 7, 3827. [Google Scholar] [CrossRef][Green Version]
- Nangia, S.; Sureshkumar, R. Effects of Nanoparticle Charge and Shape Anisotropy on Translocation through Cell Membranes. Langmuir 2012, 28, 17666–17671. [Google Scholar] [CrossRef]
- Meyer, R.A.; Green, J.J. Shaping the future of nanomedicine: Anisotropy in polymeric nanoparticle design. WIREs Nanomed. Nanobiotechnol. 2016, 8, 191–207. [Google Scholar] [CrossRef][Green Version]
- Lu, Z.; Acter, S.; Teo, B.M.; Tabor, R.F. Synthesis and characterisation of polynorepinephrine-shelled microcapsules via an oil-in-water emulsion templating route. J. Mater. Chem. B 2021, 9, 9575–9582. [Google Scholar] [CrossRef]
- Lungu, I.I.; Grumezescu, A.M.; Volceanov, A.; Andronescu, E. Nanobiomaterials Used in Cancer Therapy: An Up-to-Date Overview. Molecules 2019, 24, 3547. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ball, V. Polydopamine Nanomaterials: Recent Advances in Synthesis Methods and Applications. Front. Bioeng. Biotechnol. 2018, 6, 109. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jin, A.; Wang, Y.; Lin, K.; Jiang, L. Nanoparticles modified by polydopamine: Working as “drug” carriers. Bioact. Mater. 2020, 5, 522–541. [Google Scholar] [CrossRef]
- Lu, Z.; Acter, S.; Teo, B.M.; Bishop, A.I.; Tabor, R.F.; Vidallon, M.L.P. Mesoporous, anisotropic nanostructures from bioinspired polymeric catecholamine neurotransmitters and their potential application as photoacoustic imaging agents. J. Mater. Chem. B 2022, 10, 9662–9670. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ai, K.; Lu, L. Polydopamine and Its Derivative Materials: Synthesis and Promising Applications in Energy, Environmental, and Biomedical Fields. Chem. Rev. 2014, 114, 5057–5115. [Google Scholar] [CrossRef]
- García-Pardo, J.; Novio, F.; Nador, F.; Cavaliere, I.; Suárez-García, S.; Lope-Piedrafita, S.; Candiota, A.P.; Romero-Gimenez, J.; Rodríguez-Galván, B.; Bové, J.; et al. Bioinspired Theranostic Coordination Polymer Nanoparticles for Intranasal Dopamine Replacement in Parkinson’s Disease. ACS Nano 2021, 15, 8592–8609. [Google Scholar] [CrossRef]
- Monge-Fuentes, V.; Biolchi Mayer, A.; Lima, M.R.; Geraldes, L.R.; Zanotto, L.N.; Moreira, K.G.; Martins, O.P.; Piva, H.L.; Felipe, M.S.S.; Amaral, A.C.; et al. Dopamine-loaded nanoparticle systems circumvent the blood-brain barrier restoring motor function in mouse model for Parkinson’s Disease. Sci. Rep. 2021, 11, 15185. [Google Scholar] [CrossRef]
- Cheng, W.; Zeng, X.; Chen, H.; Li, Z.; Zeng, W.; Mei, L.; Zhao, Y. Versatile Polydopamine Platforms: Synthesis and Promising Applications for Surface Modification and Advanced Nanomedicine. ACS Nano 2019, 13, 8537–8565. [Google Scholar] [CrossRef]
- Ochs, C.J.; Hong, T.; Such, G.K.; Cui, J.; Postma, A.; Caruso, F. Dopamine-Mediated Continuous Assembly of Biodegradable Capsules. Chem. Mater. 2011, 23, 3141–3143. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Zarei, M.; Hashemi, S.A. Polydopamine for Biomedical Application and Drug Delivery System. Med. Chem. 2018, 8, 218–229. [Google Scholar] [CrossRef]
- Zhu, Z.; Su, M. Polydopamine Nanoparticles for Combined Chemo- and Photothermal Cancer Therapy. Nanomaterials 2017, 7, 160. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Acter, S.; Jahan, N.; Vidallon, M.L.P.; Teo, B.M.; Tabor, R.F. Mesoporous Polydopamine Nanobowls Toward Combined Chemo- and Photothermal Cancer Therapy. Part. Part. Syst. Charact. 2022, 39, 2200015. [Google Scholar] [CrossRef]
- Acter, S.; Vidallon, M.L.P.; King, J.P.; Teo, B.M.; Tabor, R.F. Photothermally responsive Pickering emulsions stabilised by polydopamine nanobowls. J. Mater. Chem. B 2021, 9, 8962–8970. [Google Scholar] [CrossRef] [PubMed]
- Siciliano, G.; Monteduro, A.G.; Turco, A.; Primiceri, E.; Rizzato, S.; Depalo, N.; Curri, M.L.; Maruccio, G. Polydopamine-Coated Magnetic Iron Oxide Nanoparticles: From Design to Applications. Nanomaterials 2022, 12, 1145. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, U.; Wang, B.; Hu, P.; Rethore, J.; Aifantis, K.E. Polydopamine coated Si nanoparticles allow for improved mechanical and electrochemical stability. Electrochim. Acta 2021, 392, 138993. [Google Scholar] [CrossRef]
- Mrówczyński, R. Polydopamine-Based Multifunctional (Nano)materials for Cancer Therapy. ACS Appl. Mater. Interfaces 2018, 10, 7541–7561. [Google Scholar] [CrossRef]
- Black, K.; Yi, J.; Rivera, J.; Zelasko-Leon, D.; Messersmith, P. Polydopamine-enabled surface functionalization of gold nanorods for cancer cell targeting and thermal ablation. Nanomedicine 2012, 8, 17–28. [Google Scholar] [CrossRef][Green Version]
- Niu, G.; Zhao, L.; Wang, Y.; Jiang, Y. PDA/gold nanorod-based nanoparticles for synergistic genetic and photothermal combination therapy for cancer treatment. ChemPhysMater 2023, 2, 83–89. [Google Scholar] [CrossRef]
- You, Y.H.; Lin, Y.F.; Nirosha, B.; Chang, H.T.; Huang, Y.F. Polydopamine-coated gold nanostar for combined antitumor and antiangiogenic therapy in multidrug-resistant breast cancer. Nanotheranostics 2019, 3, 266–283. [Google Scholar] [CrossRef][Green Version]
- Khlebtsov, B.N.; Burov, A.M.; Pylaev, T.E.; Khlebtsov, N.G. Polydopamine-coated Au nanorods for targeted fluorescent cell imaging and photothermal therapy. Beilstein J. Nanotechnol. 2019, 10, 794–803. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhang, S.; Kang, N.; Huang, J.; Lv, X.; Wen, K.; Ye, S.; Chen, Z.; Zhou, X.; Ren, L. Polydopamine-Coated Manganese Carbonate Nanoparticles for Amplified Magnetic Resonance Imaging-Guided Photothermal Therapy. ACS Appl. Mater. Interfaces 2017, 9, 19296–19306. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lin, L.-S.; Cong, Z.-X.; Cao, J.-B.; Ke, K.-M.; Peng, Q.-L.; Gao, J.; Yang, H.-H.; Liu, G.; Chen, X. Multifunctional Fe3O4@Polydopamine Core–Shell Nanocomposites for Intracellular mRNA Detection and Imaging-Guided Photothermal Therapy. ACS Nano 2014, 8, 3876–3883. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Korchinski, D.J.; Taha, M.; Yang, R.; Nathoo, N.; Dunn, J.F. Iron Oxide as an MRI Contrast Agent for Cell Tracking. Magn. Reason. Insights 2015, 8, 15–29. [Google Scholar] [CrossRef][Green Version]
- Perlman, O.; Borodetsky, A.; Kauffmann, Y.; Shamay, Y.; Azhari, H.; Weitz, I.S. Gold/Copper@Polydopamine Nanocomposite for Contrast-Enhanced Dual Modal Computed Tomography–Magnetic Resonance Imaging. ACS Appl. Nano Mater. 2019, 2, 6124–6134. [Google Scholar] [CrossRef]
- Luo, D.; Wang, X.; Burda, C.; Basilion, J.P. Recent Development of Gold Nanoparticles as Contrast Agents for Cancer Diagnosis. Cancers 2021, 13, 1825. [Google Scholar] [CrossRef]
- Wang, W.; Tang, Z.; Zhang, Y.; Wang, Q.; Liang, Z.; Zeng, X. Mussel-Inspired Polydopamine: The Bridge for Targeting Drug Delivery System and Synergistic Cancer Treatment. Macromol. Biosci. 2020, 20, 2000222. [Google Scholar] [CrossRef]
- Carmignani, A.; Battaglini, M.; Sinibaldi, E.; Marino, A.; Vighetto, V.; Valentina, C.; Ciofani, G. In Vitro and Ex Vivo Investigation of the Effects of Polydopamine Nanoparticle Size on Their Antioxidant and Photothermal Properties: Implications for Biomedical Applications. ACS Appl. Nano Mater. 2022, 5, 1702–1713. [Google Scholar] [CrossRef]
- Liebscher, J.; Mrówczyński, R.; Scheidt, H.A.; Filip, C.; Hădade, N.D.; Turcu, R.; Bende, A.; Beck, S. Structure of Polydopamine: A Never-Ending Story? Langmuir 2013, 29, 10539–10548. [Google Scholar] [CrossRef]
- Acter, S.; Vidallon, M.L.P.; Crawford, S.; Tabor, R.F.; Teo, B.M. Bowl-Shaped Mesoporous Polydopamine Nanoparticles for Size-Dependent Endocytosis into HeLa Cells. ACS Appl. Nano Mater. 2021, 4, 9536–9546. [Google Scholar] [CrossRef]
- Xing, Y.; Zhang, J.; Chen, F.; Liu, J.; Cai, K. Mesoporous polydopamine nanoparticles with co-delivery function for overcoming multidrug resistance via synergistic chemo-photothermal therapy. Nanoscale 2017, 9, 8781–8790. [Google Scholar] [CrossRef]
- Sun, L.; Li, Q.; Zhang, L.; Xu, Z.; Kang, Y.; Xue, P. PEGylated Polydopamine Nanoparticles Incorporated with Indocyanine Green and Doxorubicin for Magnetically Guided Multimodal Cancer Therapy Triggered by Near-Infrared Light. ACS Appl. Nano Mater. 2018, 1, 325–336. [Google Scholar] [CrossRef]
- Zhao, Y.; Yeh, Y.; Liu, R.; You, J.; Qu, F. Facile deposition of gold nanoparticles on core–shell Fe3O4@polydopamine as recyclable nanocatalyst. Solid State Sci. 2015, 45, 9–14. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, J.; Wang, J.; Qi, X.; Rosenholm, J.M.; Cai, K. Polydopamine Coatings in Confined Nanopore Space: Toward Improved Retention and Release of Hydrophilic Cargo. J. Phys. Chem. C 2015, 119, 24512–24521. [Google Scholar] [CrossRef]
- Busa, P.; Koutavarapu, R.; Kuthati, Y. Polydopamine-Coated Copper-Substituted Mesoporous Silica Nanoparticles for Dual Cancer Therapy. Coatings 2022, 12, 60. [Google Scholar] [CrossRef]
- Dong, Z.; Gong, H.; Gao, M.; Zhu, W.; Sun, X.; Feng, L.; Fu, T.; Li, Y.; Liu, Z. Polydopamine Nanoparticles as a Versatile Molecular Loading Platform to Enable Imaging-guided Cancer Combination Therapy. Theranostics 2016, 6, 1031–1042. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, H.; Su, H.; Bi, X.; Bai, Y.; Chen, L.; Ge, D.; Shi, W.; Sun, Y. Polydopamine Nanocapsule: A Theranostic Agent for Photoacoustic Imaging and Chemo-Photothermal Synergistic Therapy. ACS Biomater. Sci. Eng. 2017, 3, 1799–1808. [Google Scholar] [CrossRef]
- Chen, X.; Yan, Y.; Müllner, M.; van Koeverden, M.P.; Noi, K.F.; Zhu, W.; Caruso, F. Engineering Fluorescent Poly(dopamine) Capsules. Langmuir 2014, 30, 2921–2925. [Google Scholar] [CrossRef]
- Wood, B.R.; Langford, S.J.; Cooke, B.M.; Lim, J.; Glenister, F.K.; Duriska, M.; Unthank, J.K.; McNaughton, D. Resonance Raman Spectroscopy Reveals New Insight into the Electronic Structure of β-Hematin and Malaria Pigment. J. Am. Chem. Soc. 2004, 126, 9233–9239. [Google Scholar] [CrossRef]
- Yuen, C.; Liu, Q. Magnetic field enriched surface enhanced resonance Raman spectroscopy for early malaria diagnosis. J. Biomed. Opt. 2012, 17, 017005. [Google Scholar] [CrossRef]
- Liu, X.; Cao, J.; Li, H.; Li, J.; Jin, Q.; Ren, K.; Ji, J. Mussel-Inspired Polydopamine: A Biocompatible and Ultrastable Coating for Nanoparticles in Vivo. ACS Nano 2013, 7, 9384–9395. [Google Scholar] [CrossRef]
- Zhou, X.; Chang, T.L.; Chen, S.; Liu, T.; Wang, H.; Liang, J.F. Polydopamine-Decorated Orlistat-Loaded Hollow Capsules with an Enhanced Cytotoxicity against Cancer Cell Lines. Mol. Pharm. 2019, 16, 2511–2521. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Douek, A.M.; Rozario, A.M.; Tabor, R.F.; Kaslin, J.; Follink, B.; Teo, B.M. Bioinspired polynorepinephrine nanoparticles as an efficient vehicle for enhanced drug delivery. J. Mater. Chem. B 2020, 8, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; He, X.; Zhang, J.; Chen, H.; Zhang, H.; Wang, Z. Controllable synthesis of polydopamine nanoparticles in microemulsions with pH-activatable properties for cancer detection and treatment. J. Mater. Chem. B 2015, 3, 6731–6739. [Google Scholar] [CrossRef] [PubMed]
- Di, J.; Gao, X.; Du, Y.; Zhang, H.; Gao, J.; Zheng, A. Size, shape, charge and “stealthy” surface: Carrier properties affect the drug circulation time in vivo. Asian J. Pharm. Sci. 2021, 16, 444–458. [Google Scholar] [CrossRef]
- Foroozandeh, P.; Aziz, A.A. Insight into Cellular Uptake and Intracellular Trafficking of Nanoparticles. Nanoscale Res. Lett. 2018, 13, 339. [Google Scholar] [CrossRef][Green Version]
- Chen, F.; Xing, Y.; Wang, Z.; Zheng, X.; Zhang, J.; Cai, K. Nanoscale Polydopamine (PDA) Meets π–π Interactions: An Interface-Directed Coassembly Approach for Mesoporous Nanoparticles. Langmuir 2016, 32, 12119–12128. [Google Scholar] [CrossRef]
- Ryu, J.H.; Messersmith, P.B.; Lee, H. Polydopamine Surface Chemistry: A Decade of Discovery. ACS Appl. Mater. Interfaces 2018, 10, 7523–7540. [Google Scholar] [CrossRef]
- Pada, A.K.; Desai, D.; Sun, K.; Prakirth Govardhanam, N.; Törnquist, K.; Zhang, J.; Rosenholm, J.M. Comparison of Polydopamine-Coated Mesoporous Silica Nanorods and Spheres for the Delivery of Hydrophilic and Hydrophobic Anticancer Drugs. Int. J. Mol. Sci. 2019, 20, 3408. [Google Scholar] [CrossRef][Green Version]
- Li, J.; Zhang, Z.; Deng, H.; Zheng, Z. Cinobufagin-Loaded and Folic Acid-Modified Polydopamine Nanomedicine Combined With Photothermal Therapy for the Treatment of Lung Cancer. Front. Chem. 2021, 9, 637754. [Google Scholar] [CrossRef]
- Hu, H.; Liu, X.; Hong, J.; Ye, N.; Xiao, C.; Wang, J.; Li, Z.; Xu, D. Mesoporous polydopamine-based multifunctional nanoparticles for enhanced cancer phototherapy. J. Colloid Interface Sci. 2022, 612, 246–260. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, K.; Hao, Y.; Yang, H.; Wang, J.; Zhang, Y.; Zhao, W.; Ma, S.; Mao, C. Polydopamine nanomotors loaded indocyanine green and ferric ion for photothermal and photodynamic synergistic therapy of tumor. J. Colloid Interface Sci. 2023, 633, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Chen, Y.; He, P.; Ma, Y.; Cai, Y.; Hou, X.; Zhang, G.; Zhang, X.; Wang, Z. Aggregation-Induced Emission (AIE) Photosensitizer Combined Polydopamine Nanomaterials for Organelle-Targeting Photodynamic and Photothermal Therapy by the Recognition of Sialic Acid. Adv. Healthc. Mater. 2022, 11, 2200242. [Google Scholar] [CrossRef] [PubMed]
- Ngwa, W.; Irabor, O.C.; Schoenfeld, J.D.; Hesser, J.; Demaria, S.; Formenti, S.C. Using immunotherapy to boost the abscopal effect. Nat. Rev. Cancer 2018, 18, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Altundal, Y.; Moreau, M.; Sajo, E.; Kumar, R.; Ngwa, W. Potential for enhancing external beam radiotherapy for lung cancer using high-Z nanoparticles administered via inhalation. Phys. Med. Biol. 2015, 60, 7035–7043. [Google Scholar] [CrossRef][Green Version]
- Hao, Y.; Yasmin-Karim, S.; Moreau, M.; Sinha, N.; Sajo, E.; Ngwa, W. Enhancing radiotherapy for lung cancer using immunoadjuvants delivered in situ from new design radiotherapy biomaterials: A preclinical study. Phys. Med. Biol. 2016, 61, N697–N707. [Google Scholar] [CrossRef][Green Version]
- Yasmin-Karim, S.; Bruck, P.T.; Moreau, M.; Kunjachan, S.; Chen, G.Z.; Kumar, R.; Grabow, S.; Dougan, S.K.; Ngwa, W. Radiation and Local Anti-CD40 Generate an Effective in situ Vaccine in Preclinical Models of Pancreatic Cancer. Front. Immunol. 2018, 9, 2030. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y.; Wang, Y.; Wei, C.; Deng, Y.; Chen, H.; Shen, J.; Ke, H. Biomineralized iron oxide–polydopamine hybrid nanodots for contrast-enhanced T1-weighted magnetic resonance imaging and photothermal tumor ablation. J. Mater. Chem. B 2021, 9, 1781–1786. [Google Scholar] [CrossRef]
- Nkanga, C.I.; Ortega-Rivera, O.A.; Steinmetz, N.F. Photothermal immunotherapy of melanoma using TLR-7 agonist laden tobacco mosaic virus with polydopamine coat. Nanomedicine 2022, 44, 102573. [Google Scholar] [CrossRef]
- Wang, N.; Yang, Y.; Wang, X.; Tian, X.; Qin, W.; Wang, X.; Liang, J.; Zhang, H.; Leng, X. Polydopamine as the Antigen Delivery Nanocarrier for Enhanced Immune Response in Tumor Immunotherapy. ACS Biomater. Sci. Eng. 2019, 5, 2330–2342. [Google Scholar] [CrossRef]
- Zhu, M.; Shi, Y.; Shan, Y.; Guo, J.; Song, X.; Wu, Y.; Wu, M.; Lu, Y.; Chen, W.; Xu, X.; et al. Recent developments in mesoporous polydopamine-derived nanoplatforms for cancer theranostics. J. Nanobiotechnol. 2021, 19, 387. [Google Scholar] [CrossRef]
- Wang, S.; Lin, Q.; Chen, J.; Gao, H.; Fu, D.; Shen, S. Biocompatible polydopamine-encapsulated gadolinium-loaded carbon nanotubes for MRI and color mapping guided photothermal dissection of tumor metastasis. Carbon 2017, 112, 53–62. [Google Scholar] [CrossRef]
- Mueller, R.; Moreau, M.; Yasmin-Karim, S.; Protti, A.; Tillement, O.; Berbeco, R.; Hesser, J.; Ngwa, W. Imaging and Characterization of Sustained Gadolinium Nanoparticle Release from Next Generation Radiotherapy Biomaterial. Nanomaterials 2020, 10, 2249. [Google Scholar] [CrossRef]
- Mao, W.; Hu, C.; Zheng, H.; Xie, J.; Shi, X.; Du, Y.; Wang, F. A Functionalized Polydopamine Theranostic Nanoprobe for Efficient Imaging of miRNA-21 and In Vivo Synergetic Cancer Therapy. Mol. Ther.-Nucleic Acids 2020, 22, 27–37. [Google Scholar] [CrossRef]
- Chen, R.; Zhu, C.; Fan, Y.; Feng, W.; Wang, J.; Shang, E.; Zhou, Q.; Chen, Z. Polydopamine-Based Multifunctional Platform for Combined Photothermal Therapy, Chemotherapy, and Immunotherapy in Malignant Tumor Treatment. ACS Appl. Bio Mater. 2019, 2, 874–883. [Google Scholar] [CrossRef]
- Dorsey, J.F.; Sun, L.; Joh, D.Y.; Witztum, A.; Kao, G.D.; Alonso-Basanta, M.; Avery, S.; Hahn, S.M.; Al Zaki, A.; Tsourkas, A. Gold nanoparticles in radiation research: Potential applications for imaging and radiosensitization. Transl. Cancer Res. 2013, 2, 280–291. [Google Scholar] [CrossRef]
- Yasmin-Karim, S.; Wood, J.; Wirtz, J.; Moreau, M.; Bih, N.; Swanson, W.; Muflam, A.; Ainsworth, V.; Ziberi, B.; Ngwa, W. Optimizing In Situ Vaccination During Radiotherapy. Front. Oncol. 2021, 11, 711078. [Google Scholar] [CrossRef]
- Wood, J.; Yasmin-Karim, S.; Mueller, R.; Viswanathan, A.N.; Ngwa, W. Single Radiotherapy Fraction with Local Anti-CD40 Therapy Generates Effective Abscopal Responses in Mouse Models of Cervical Cancer. Cancers 2020, 12, 1026. [Google Scholar] [CrossRef][Green Version]










Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acter, S.; Moreau, M.; Ivkov, R.; Viswanathan, A.; Ngwa, W. Polydopamine Nanomaterials for Overcoming Current Challenges in Cancer Treatment. Nanomaterials 2023, 13, 1656. https://doi.org/10.3390/nano13101656
Acter S, Moreau M, Ivkov R, Viswanathan A, Ngwa W. Polydopamine Nanomaterials for Overcoming Current Challenges in Cancer Treatment. Nanomaterials. 2023; 13(10):1656. https://doi.org/10.3390/nano13101656
Chicago/Turabian StyleActer, Shahinur, Michele Moreau, Robert Ivkov, Akila Viswanathan, and Wilfred Ngwa. 2023. "Polydopamine Nanomaterials for Overcoming Current Challenges in Cancer Treatment" Nanomaterials 13, no. 10: 1656. https://doi.org/10.3390/nano13101656
APA StyleActer, S., Moreau, M., Ivkov, R., Viswanathan, A., & Ngwa, W. (2023). Polydopamine Nanomaterials for Overcoming Current Challenges in Cancer Treatment. Nanomaterials, 13(10), 1656. https://doi.org/10.3390/nano13101656







