Impacts of Nanobubbles in Pore Water on Heavy Metal Pollutant Release from Contaminated Soil Columns
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Heavy Metal Contaminated Soil
2.2. Generation and Characterization of NBs
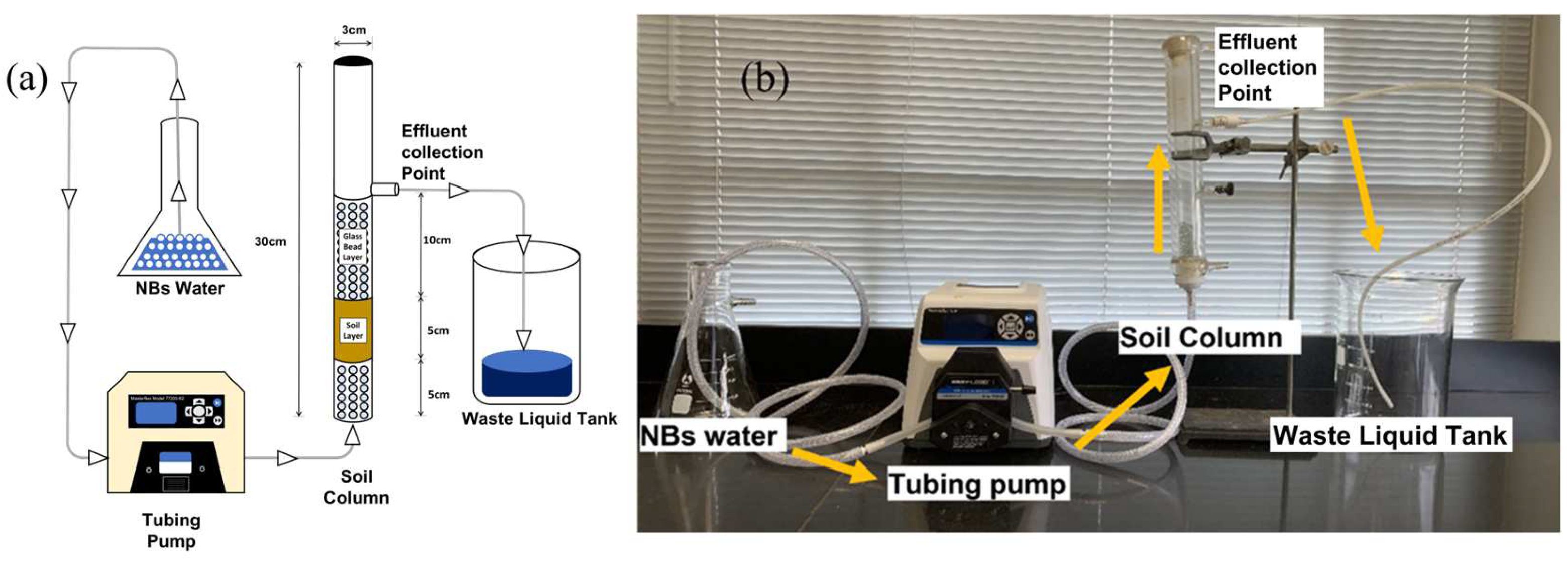
2.3. Column Setup and Characterization under a Bubble Water Flow
2.4. Evaluation of the Metal Release from the Spiked Soil under a Bubble Water Flow
2.5. Modeling Analysis of the Metal Leaching Kinetics
3. Results and Discussions
3.1. Soil Characterization
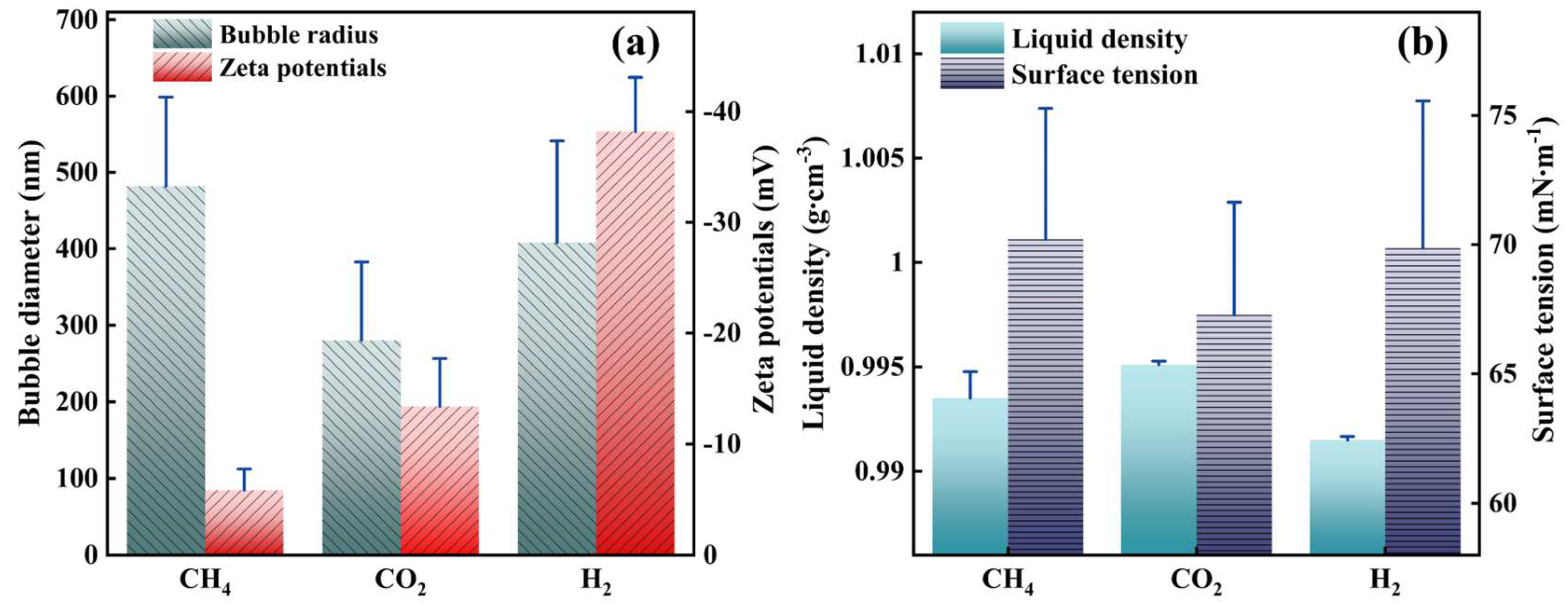
3.2. Bubble Characterization
3.3. Effects of Nanobubble Water on Soil Structures and Soil Chemistries
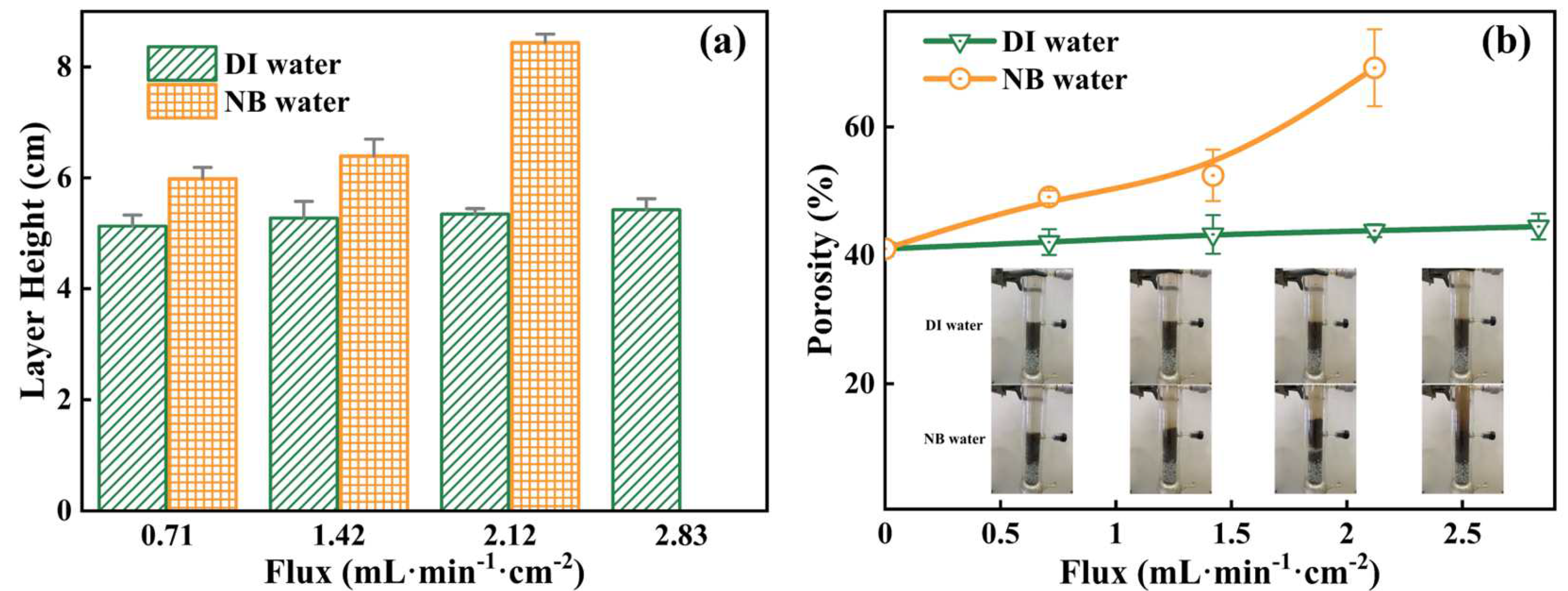
3.3.1. Soil Fluidization
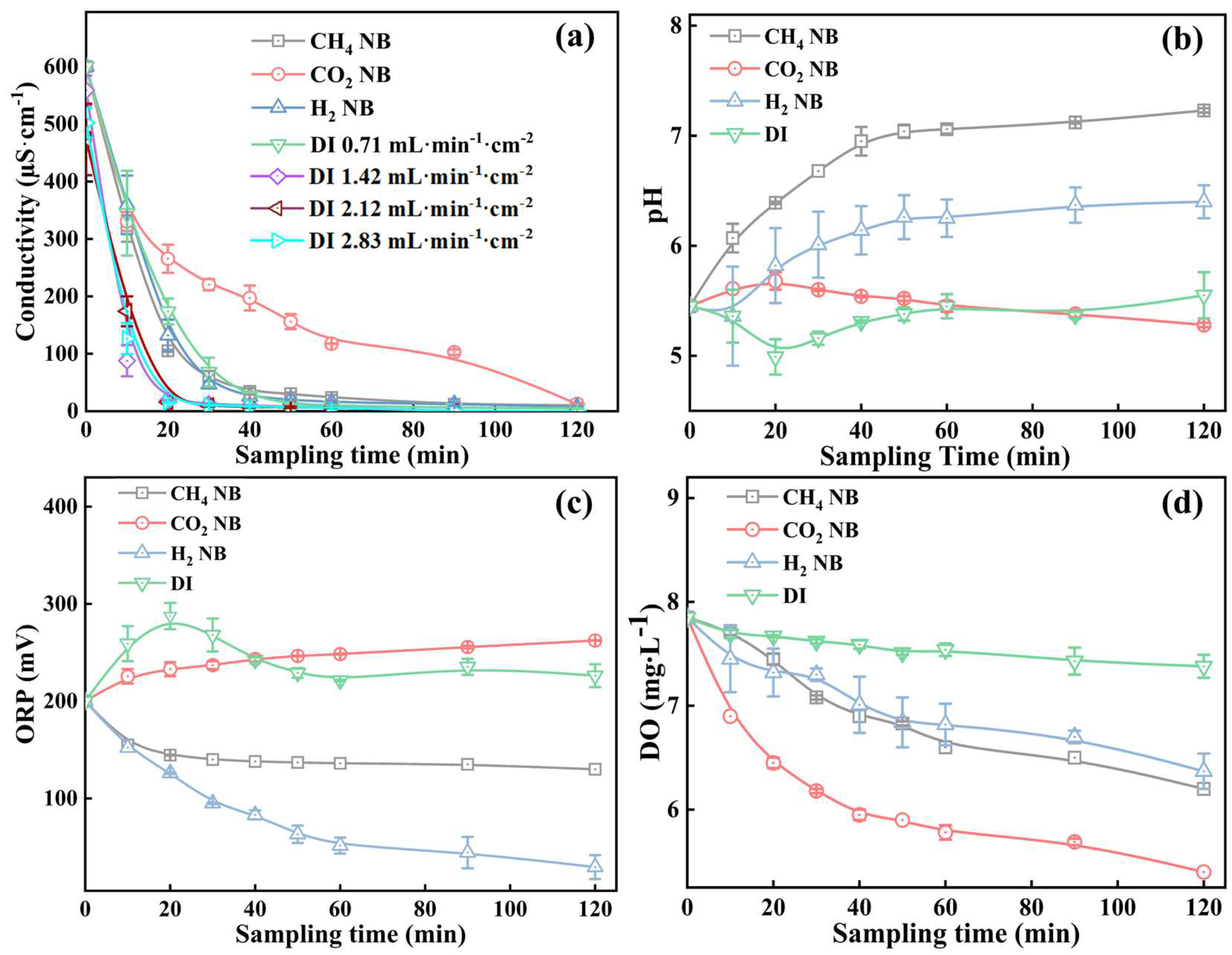
3.3.2. Water Chemistries of the Soil Elute
3.4. Release of Soil Heavy Metals under the Soil Washing by Different NB Water
3.5. Leaching Kinetics Modeling Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lai, Y.; Xia, X.; Dong, J.; Lin, W.; Mou, X.; Zhao, P.; Jiang, X.; Li, Z.; Tong, Y.; Zhao, Y. Equilibrium state of PAHs in bottom sediment–water–suspended sediment system of a large river considering freely dissolved concentrations. J. Environ. Qual. 2015, 44, 823–832. [Google Scholar] [CrossRef] [PubMed]
- Viana, P.; Yin, K.; Rockne, K. Comparison of direct benthic flux to ebullition-facilitated flux of polycyclic aromatic hydrocarbons and heavy metals measured in the field. J. Soils Sediments 2018, 18, 1729–1742. [Google Scholar] [CrossRef]
- Loganathan, B.G.; Lam, P.K.-S. Global Contamination Trends of Persistent Organic Chemicals; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Jung, H. Nutrients and heavy metals contamination in an urban estuary of northern new jersey. Geosciences 2017, 7, 108. [Google Scholar] [CrossRef]
- Yuan, Q. Experimental and Modeling Studies of Contaminant Transport in Capped Sediments during Gas Bubble Ebullition. Ph.D. Dissertation, Louisiana State University, Baton Rouge, LA, USA, 2007. [Google Scholar]
- Birks, S.; Cho, S.; Taylor, E.; Yi, Y.; Gibson, J. Characterizing the PAHs in surface waters and snow in the Athabasca region: Implications for identifying hydrological pathways of atmospheric deposition. Sci. Total Environ. 2017, 603, 570–583. [Google Scholar] [CrossRef] [PubMed]
- Huttunen, J.T.; Lappalainen, K.M.; Saarijärvi, E.; Väisänen, T.; Martikainen, P.J. A novel sediment gas sampler and a subsurface gas collector used for measurement of the ebullition of methane and carbon dioxide from a eutrophied lake. Sci. Total Environ. 2001, 266, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Valsaraj, K.T.; Reible, D.D. A model for contaminant and sediment transport via gas ebullition through a sediment cap. Environ. Eng. Sci. 2009, 26, 1381–1391. [Google Scholar] [CrossRef]
- Yuan, Q.; Valsaraj, K.T.; Reible, D.D.; Willson, C.S. A laboratory study of sediment and contaminant release during gas ebullition. J. Air Waste Manag. Assoc. 2007, 57, 1103–1111. [Google Scholar] [CrossRef]
- Wang, C.; Xu, D.; Bai, L.; Bai, C.; Huang, L.; Jiang, H. Important Role of Biogas and Secretions in the Formation of Biological Fluid Sediment under Cyanobacterial Bloom Biomass Degradation. ACS EST Water 2023, 3, 773–782. [Google Scholar] [CrossRef]
- Malyan, S.K.; Singh, O.; Kumar, A.; Anand, G.; Singh, R.; Singh, S.; Yu, Z.; Kumar, J.; Fagodiya, R.K.; Kumar, A. Greenhouse gases trade-off from ponds: An overview of emission process and their driving factors. Water 2022, 14, 970. [Google Scholar] [CrossRef]
- Ohgaki, K.; Khanh, N.Q.; Joden, Y.; Tsuji, A.; Nakagawa, T. Physicochemical approach to nanobubble solutions. Chem. Eng. Sci. 2010, 65, 1296–1300. [Google Scholar] [CrossRef]
- Razavi, N.R.; Ridal, J.J.; de Wit, W.; Hickey, M.B.; Campbell, L.M.; Hodson, P.V. Ebullition rates and mercury concentrations in St. Lawrence river sediments and a benthic invertebrate. Environ. Toxicol. Chem. 2013, 32, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Delwiche, K.; Gu, J.; Hemond, H.; Preheim, S.P. Vertical transport of sediment-associated metals and cyanobacteria by ebullition in a stratified lake. Biogeosciences 2020, 17, 3135–3147. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Koopmans, G.F.; Bonten, L.T.; Song, J.; Luo, Y.; Temminghoff, E.J.; Comans, R.N. Temporal variability in trace metal solubility in a paddy soil not reflected in uptake by rice (Oryza sativa L.). Environ. Geochem. Health 2016, 38, 1355–1372. [Google Scholar] [CrossRef] [PubMed]
- Pardue, J.H.; Patrick, W.H. Changes in metal speciation following alteration of sediment redox status. In Metal Contaminated Aquatic Sediments; Routledge: Oxfordshire, UK, 2018; pp. 169–185. [Google Scholar]
- Pan, Y.; Koopmans, G.F.; Bonten, L.T.; Song, J.; Luo, Y.; Temminghoff, E.J.; Comans, R.N. Influence of pH on the redox chemistry of metal (hydr) oxides and organic matter in paddy soils. J. Soils Sediments 2014, 14, 1713–1726. [Google Scholar] [CrossRef]
- Yang, C.; Jing, Y.; Zhang, F.; Shen, S. Effects of fine bubble aeration on sediment-water interface on release potential of heavy metals in the sediment from a heavily polluted urban river. J. Tongji Univ. 2015, 43, 6. [Google Scholar]
- Viana, P.Z.; Yin, K.; Rockne, K.J. Field measurements and modeling of ebullition-facilitated flux of heavy metals and polycyclic aromatic hydrocarbons from sediments to the water column. Environ. Sci. Technol. 2012, 46, 12046–12054. [Google Scholar] [CrossRef]
- Elrahmani, A.; Al-Raoush, R.I.; Abugazia, H.; Seers, T. Pore-scale simulation of fine particles migration in porous media using coupled CFD-DEM. Powder Technol. 2022, 398, 117130. [Google Scholar] [CrossRef]
- McLinn, E.L.; Stolzenburg, T.R. Ebullition-facilitated transport of manufactured gas plant tar from contaminated sediment. Environ. Toxicol. Chem. Int. J. 2009, 28, 2298–2306. [Google Scholar] [CrossRef]
- Brusseau, M.L. Assessing the potential contributions of additional retention processes to PFAS retardation in the subsurface. Sci. Total Environ. 2018, 613, 176–185. [Google Scholar] [CrossRef]
- Potapova, M.; Desianti, N.; Enache, M. Potential effects of sediment contaminants on diatom assemblages in coastal lagoons of New Jersey and New York States. Mar. Pollut. Bull. 2016, 107, 453–458. [Google Scholar] [CrossRef]
- Zhai, X.; Li, Z.; Huang, B.; Luo, N.; Huang, M.; Zhang, Q.; Zeng, G. Remediation of multiple heavy metal-contaminated soil through the combination of soil washing and in situ immobilization. Sci. Total Environ. 2018, 635, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Khaled Abdella Ahmed, A.; Sun, C.; Hua, L.; Zhang, Z.; Zhang, Y.; Marhaba, T.; Zhang, W. Colloidal Properties of Air, Oxygen, and Nitrogen Nanobubbles in Water: Effects of Ionic Strength, Natural Organic Matters, and Surfactants. Environ. Eng. Sci. 2018, 35, 720–727. [Google Scholar] [CrossRef]
- Berry, J.D.; Neeson, M.J.; Dagastine, R.R.; Chan, D.Y.; Tabor, R.F. Measurement of surface and interfacial tension using pendant drop tensiometry. J. Colloid Interface Sci. 2015, 454, 226–237. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Xia, Z. Application of ozone micro-nano-bubbles to groundwater remediation. J. Hazard. Mater. 2018, 342, 446–453. [Google Scholar] [CrossRef]
- Avnimelech, Y.; Ritvo, G.; Meijer, L.E.; Kochba, M. Water content, organic carbon and dry bulk density in flooded sediments. Aquac. Eng. 2001, 25, 25–33. [Google Scholar] [CrossRef]
- Tanner, C.C.; Adams, D.D.; Downes, M.T. Methane emissions from constructed wetlands treating agricultural wastewaters. J. Environ. Qual. 1997, 26, 1056–1062. [Google Scholar] [CrossRef]
- Rothfuss, F.; Conrad, R. Effect of gas bubbles on the diffusive flux of methane in anoxic paddy soil. Limnol. Oceanogr. 1998, 43, 1511–1518. [Google Scholar] [CrossRef]
- Henry, P.; Thomas, M.; Clennell, M.B. Formation of natural gas hydrates in marine sediments: 2. Thermodynamic calculations of stability conditions in porous sediments. J. Geophys. Res. Solid Earth 1999, 104, 23005–23022. [Google Scholar] [CrossRef]
- Himmelheber, D.; Hughes, J. Complete tetrachloroethene dechlorination in Anacostia River sediment. In Proceedings of the SETAC 26th Annual Meeting in North America, Baltmore, MD, USA, 13–17 November 2005; pp. 13–17. [Google Scholar]
- Costanza, J.; Abriola, L.M.; Pennell, K.D. Aqueous Film-Forming Foams Exhibit Greater Interfacial Activity than PFOA, PFOS, or FOSA. Environ. Sci. Technol. 2020, 54, 13590–13597. [Google Scholar] [CrossRef]
- Fu, Y.; Tian, Z.; Amoozegar, A.; Heitman, J. Measuring dynamic changes of soil porosity during compaction. Soil Tillage Res. 2019, 193, 114–121. [Google Scholar] [CrossRef]
- Xue, S.; Marhaba, T.; Zhang, W. Nanobubble watering affects nutrient release and soil characteristics. ACS Agric. Sci. Technol. 2022, 2, 453–461. [Google Scholar] [CrossRef]
- Kretzschmar, R.; Barmettler, K.; Grolimund, D.; Yan, Y.-D.; Borkovec, M.; Sticher, H. Experimental determination of colloid deposition rates and collision efficiencies in natural porous media. Water Resour. Res. 1997, 33, 1129–1137. [Google Scholar] [CrossRef]
- Tufenkji, N.; Redman, J.A.; Elimelech, M. Interpreting Deposition Patterns of Microbial Particles in Laboratory-Scale Column Experiments. Environ. Sci. Technol. 2003, 37, 8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wei, S.; Dong, S.; Wei, W.; Zhang, Y. Effects of sodium dodecyl sulfate and solution chemistry on retention and transport of biogenic nano-hydroxyapatite in saturated porous media. Colloids Surf. A Physicochem. Eng. Asp. 2023, 661, 130956. [Google Scholar] [CrossRef]
- Yang, J.Y.; Yang, X.E.; He, Z.L.; Li, T.Q.; Shentu, J.L.; Stoffella, P.J. Effects of pH, organic acids, and inorganic ions on lead desorption from soils. Environ. Pollut. 2006, 143, 9–15. [Google Scholar] [CrossRef]
- Riffaldi, R.; Levi-Minzi, R.; Soldatini, G. Pb adsorption by soils. Water Air Soil Pollut. 1976, 6, 10. [Google Scholar] [CrossRef]
- Somrani, A.; Shabani, M.; Mohamed, Z.; Ghaffour, N.; Seibel, F.; Briao, V.B.; Pontié, M. Transforming an end-of-life reverse osmosis membrane in a cationic exchange membrane and its application in a fungal microbial fuel cell. Ionics 2021, 27, 3169–3184. [Google Scholar] [CrossRef]
- Nguyen, D.; Wu, Z.; Shrestha, S.; Lee, P.-H.; Raskin, L.; Khanal, S.K. Intermittent micro-aeration: New strategy to control volatile fatty acid accumulation in high organic loading anaerobic digestion. Water Res. 2019, 166, 115080. [Google Scholar] [CrossRef]
- Wang, X.; Yuan, T.; Lei, Z.; Kobayashi, M.; Adachi, Y.; Shimizu, K.; Lee, D.-J.; Zhang, Z. Supplementation of O2-containing gas nanobubble water to enhance methane production from anaerobic digestion of cellulose. Chem. Eng. J. 2020, 398, 125652. [Google Scholar] [CrossRef]
- Asada, R.; Saitoh, Y.; Miwa, N. Effects of hydrogen-rich water bath on visceral fat and skin blotch, with boiling-resistant hydrogen bubbles. Med. Gas Res. 2019, 9, 68. [Google Scholar] [CrossRef]
- Bu, H.; Wang, W.; Song, X.; Zhang, Q. Characteristics and source identification of dissolved trace elements in the Jinshui River of the South Qinling Mts., China. Environ. Sci. Pollut. Res. Int. 2015, 22, 14248–14257. [Google Scholar] [CrossRef] [PubMed]
- Borggaard, O.K.; Holm, P.E.; Strobel, B.W. Potential of dissolved organic matter (DOM) to extract As, Cd, Co, Cr, Cu, Ni, Pb and Zn from polluted soils: A review. Geoderma 2019, 343, 235–246. [Google Scholar] [CrossRef]
- Li, S.; Wu, J.; Huo, Y.; Zhao, X.; Xue, L. Profiling multiple heavy metal contamination and bacterial communities surrounding an iron tailing pond in Northwest China. Sci. Total Environ. 2021, 752, 141827. [Google Scholar] [CrossRef]
- Stoppa, F.; Schiazza, M.; Pellegrini, J.; Ambrosio, F.A.; Rosatelli, G.; D’Orsogna, M.R. Phthalates, heavy metals and PAHs in an overpopulated coastal region: Inferences from Abruzzo, central Italy. Marine pollution bulletin 2017, 125, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Church, C.; Spargo, J.; Fishel, S. Strong acid extraction methods for “total phosphorus” in soils: EPA Method 3050B and EPA Method 3051. Agric. Environ. Lett. 2017, 2, 160037. [Google Scholar] [CrossRef]
- EPA. Method 3051A Microwave Assisted Acid Digestion of Sediments, Sludges, Soils, and Oils; EPA: Washington, DC, USA, 1996. [Google Scholar]
- Teng-Fei, S.; Xiang, L.; Lei, C.; Tao, X.; Ce-Hui, M.; Yan-Wen, L.; Quan-Ying, C.; Guo-Cheng, H.; De-Chun, H. Research progresses of determination of perfluorinated compounds in environmental water and solid samples. Chin. J. Anal. Chem. 2017, 45, 601–610. [Google Scholar]
- Bashforth, F.; Adams, J.C. An Attempt to Test the Theories of Capillary Action by Comparing the Theoretical and Measured Forms of Drops of Fluid; University Press: Oxford, UK, 1883. [Google Scholar]
- Morita, A.; Carastan, D.; Demarquette, N. Influence of drop volume on surface tension evaluated using the pendant drop method. Colloid Polym. Sci. 2002, 280, 857–864. [Google Scholar] [CrossRef]
- Wen, C.Y.; Yu, Y.H. Mechanics of fluidization. Chem. Eng. Prog. Symp. Ser. 1966, 62, 100–111. [Google Scholar]
- New York State Department of Environmental Conservation. Guidelines for Conducting Bird and Bat Studies at Commercial Wind Energy Projects; Division of Fish Wildlife and Marine Resources, Ed.: Albany, NY, USA, 2009. [Google Scholar]
- Fernandez, J.F.; Jastorff, B.; Stormann, R.; Stolte, S.; Thoming, J. Thinking in Terms of Structure-Activity-Relationships (T-SAR): A Tool to Better Understand Nanofiltration Membranes. Membranes 2011, 1, 162–183. [Google Scholar] [CrossRef]
- Kim, H.; Rao, P.S.C.; Annable, M.D. Determination of effective air-water interfacial area in partially saturated porous media using surfactant adsorption. Water Resour. Res. 1997, 33, 2705–2711. [Google Scholar] [CrossRef]
- Nakamura, K.; Yasutaka, T.; Kuwatani, T.; Komai, T. Development of a predictive model for lead, cadmium and fluorine soil-water partition coefficients using sparse multiple linear regression analysis. Chemosphere 2017, 186, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Ding, G.; Peijnenburg, W.J.G.M. Physicochemical Properties and Aquatic Toxicity of Poly- and Perfluorinated Compounds. Crit. Rev. Environ. Sci. Technol. 2013, 43, 598–678. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Song, Z.; Sugita, K.; Xue, S.; Zhang, W. Impacts of Nanobubbles in Pore Water on Heavy Metal Pollutant Release from Contaminated Soil Columns. Nanomaterials 2023, 13, 1671. https://doi.org/10.3390/nano13101671
Zhang Y, Song Z, Sugita K, Xue S, Zhang W. Impacts of Nanobubbles in Pore Water on Heavy Metal Pollutant Release from Contaminated Soil Columns. Nanomaterials. 2023; 13(10):1671. https://doi.org/10.3390/nano13101671
Chicago/Turabian StyleZhang, Yihan, Zimu Song, Kosuke Sugita, Shan Xue, and Wen Zhang. 2023. "Impacts of Nanobubbles in Pore Water on Heavy Metal Pollutant Release from Contaminated Soil Columns" Nanomaterials 13, no. 10: 1671. https://doi.org/10.3390/nano13101671
APA StyleZhang, Y., Song, Z., Sugita, K., Xue, S., & Zhang, W. (2023). Impacts of Nanobubbles in Pore Water on Heavy Metal Pollutant Release from Contaminated Soil Columns. Nanomaterials, 13(10), 1671. https://doi.org/10.3390/nano13101671





