Gene Therapy Using Efficient Direct Lineage Reprogramming Technology for Neurological Diseases
Abstract
:1. Introduction
2. Generation of Induced Neurons via Cell Fate Conversion
2.1. Acceleration of the Direct Neuronal Reprogramming Process
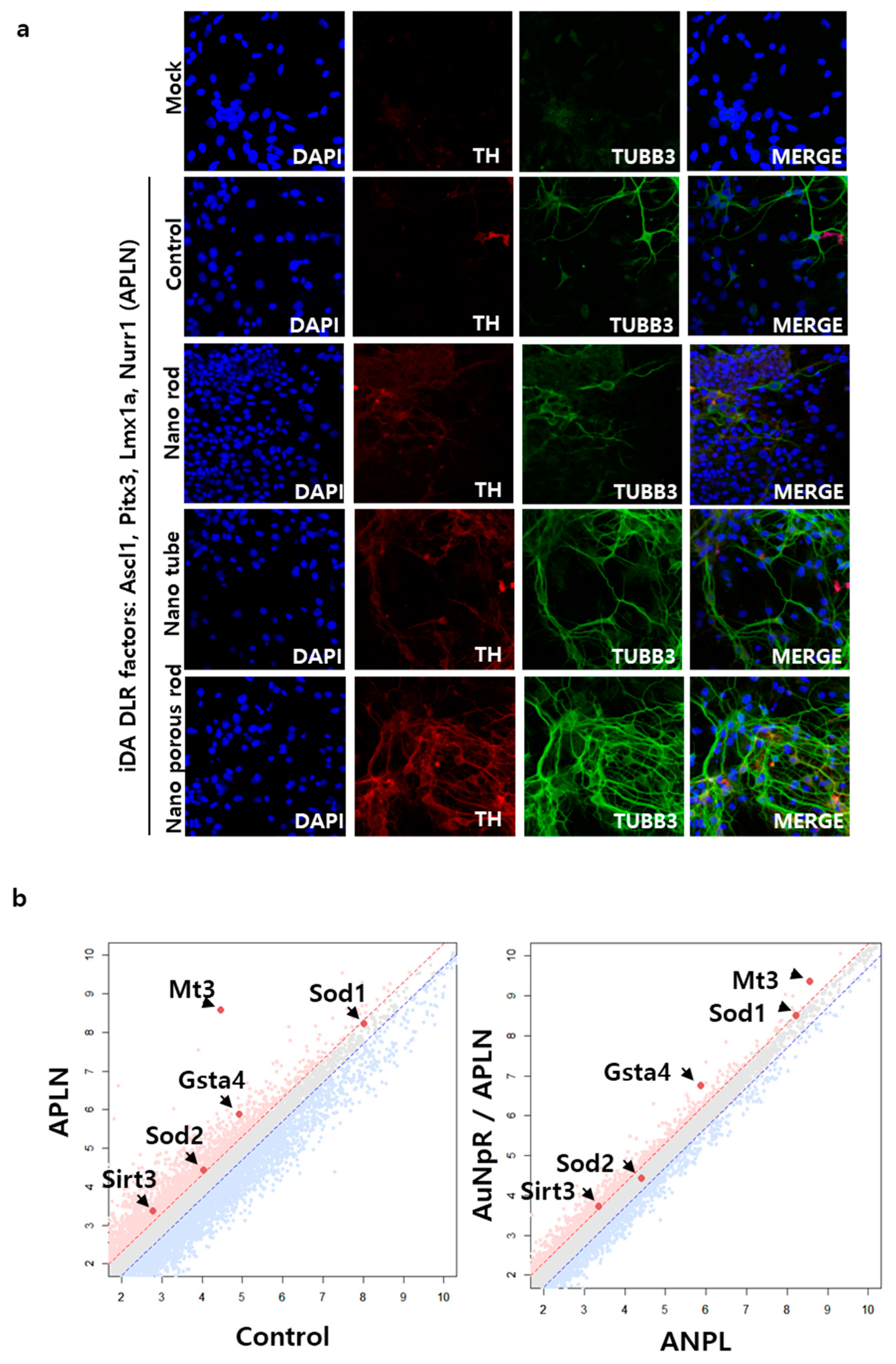
2.2. The Role of AuNpRs in the Direct Neuronal Reprogramming Process
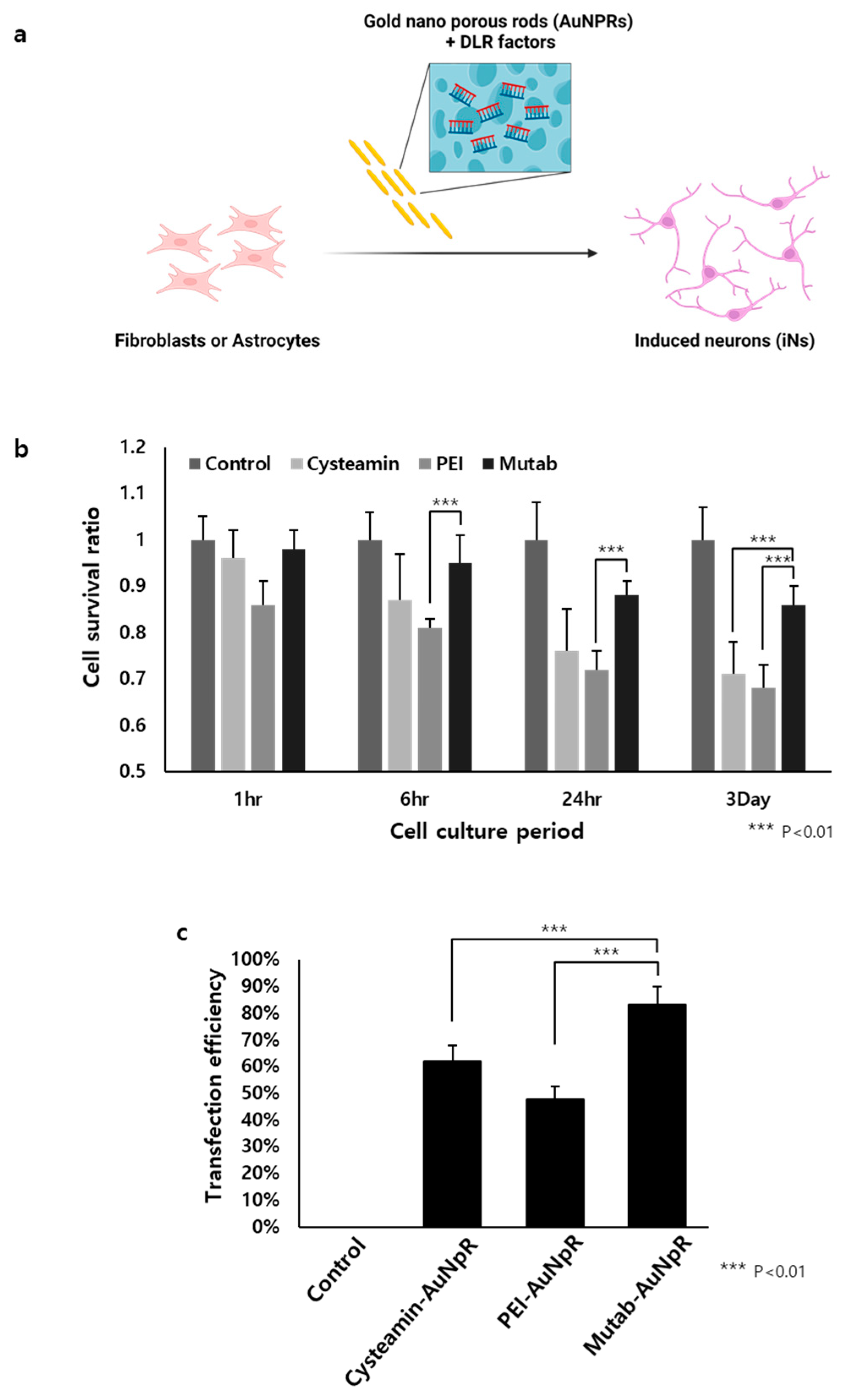
2.3. The Role of Biocompatible Materials as Delivery Cargo for DLR
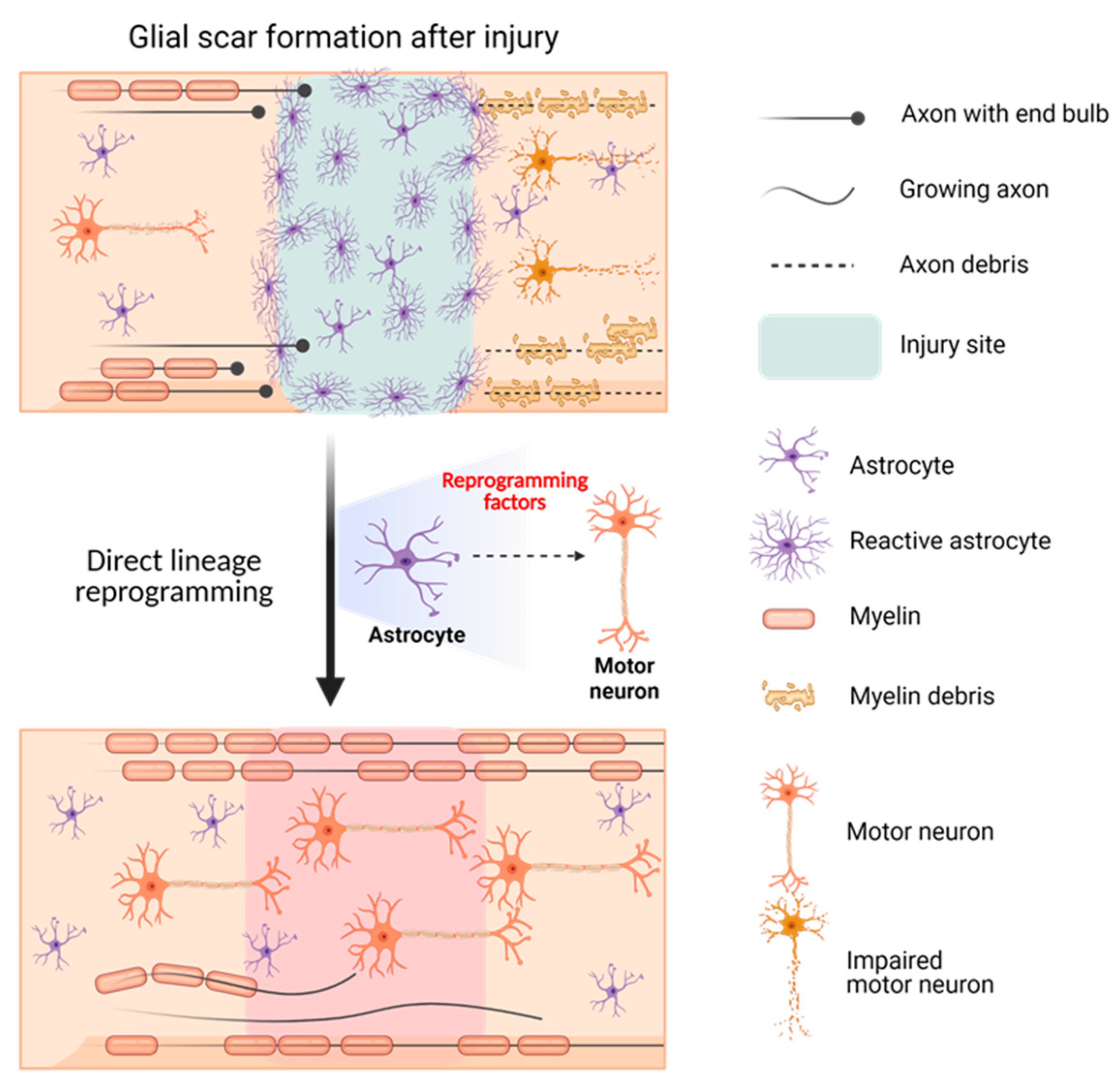
2.4. Gene Therapy for SCI Using DLR Technology
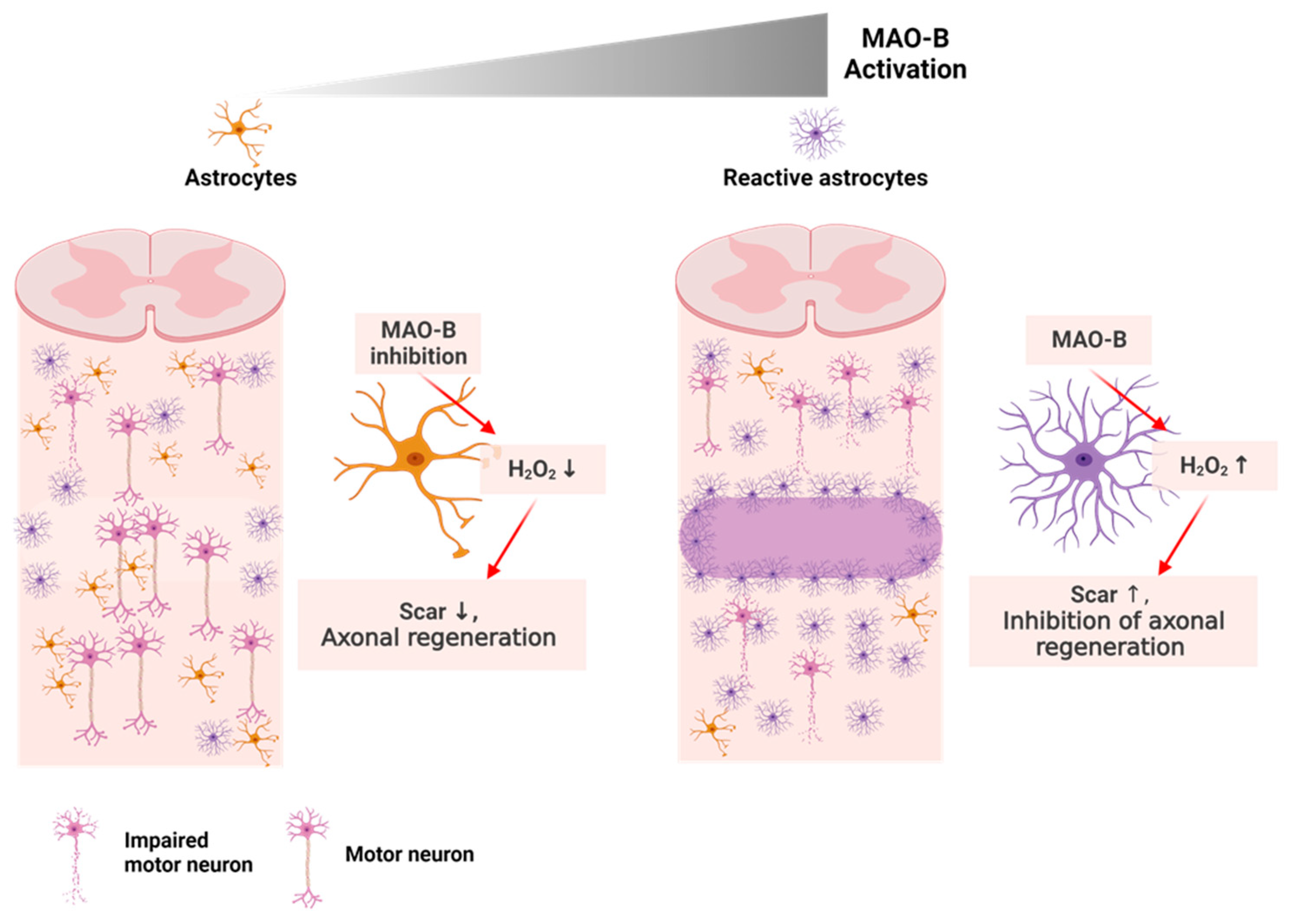
2.4.1. SCI: The Incurable Neurological Disorder
2.4.2. The Potential Therapeutic Method for SCI: AAV Gene Therapy Using DLR Technology
3. Therapeutic Insights and Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Vierbuchen, T.; Ostermeier, A.; Pang, Z.P.; Kokubu, Y.; Südhof, T.C.; Wernig, M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature 2010, 463, 1035–1041. [Google Scholar] [CrossRef] [PubMed]
- Son, E.Y.; Ichida, J.K.; Wainger, B.J.; Toma, J.S.; Rafuse, V.F.; Woolf, C.J.; Eggan, K. Conversion of mouse and human fibroblasts into functional spinal motor neurons. Cell Stem Cell 2011, 9, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Victor, M.B.; Richner, M.; Olsen, H.E.; Lee, S.W.; Monteys, A.M.; Ma, C.; Huh, C.J.; Zhang, B.; Davidson, B.L.; Yang, X.W. Striatal neurons directly converted from Huntington’s disease patient fibroblasts recapitulate age-associated disease phenotypes. Nat. Neurosci. 2018, 21, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.; Lee, E.; Kim, H.Y.; Youn, D.-H.; Jung, J.; Kim, H.; Chang, Y.; Lee, W.; Shin, J.; Baek, S. Electromagnetized gold nanoparticles mediate direct lineage reprogramming into induced dopamine neurons in vivo for Parkinson’s disease therapy. Nat. Nanotechnol. 2017, 12, 1006–1014. [Google Scholar] [CrossRef] [PubMed]
- Colasante, G.; Lignani, G.; Rubio, A.; Medrihan, L.; Yekhlef, L.; Sessa, A.; Massimino, L.; Giannelli, S.G.; Sacchetti, S.; Caiazzo, M.; et al. Rapid Conversion of Fibroblasts into Functional Forebrain GABAergic Interneurons by Direct Genetic Reprogramming. Cell Stem Cell 2015, 17, 719–734. [Google Scholar] [CrossRef]
- Hong, S.; Chung, S.; Leung, K.; Hwang, I.; Moon, J.; Kim, K.S. Functional roles of Nurr1, Pitx3, and Lmx1a in neurogenesis and phenotype specification of dopamine neurons during in vitro differentiation of embryonic stem cells. Stem Cells Dev. 2014, 23, 477–487. [Google Scholar] [CrossRef]
- Colasante, G.; Rubio, A.; Massimino, L.; Broccoli, V. Direct Neuronal Reprogramming Reveals Unknown Functions for Known Transcription Factors. Front. Neurosci. 2019, 13, 283. [Google Scholar] [CrossRef]
- Vignoles, R.; Lentini, C.; D’Orange, M.; Heinrich, C. Direct Lineage Reprogramming for Brain Repair: Breakthroughs and Challenges. Trends Mol. Med. 2019, 25, 897–914. [Google Scholar] [CrossRef]
- Zhang, Y.; Xie, X.; Hu, J.; Afreen, K.S.; Zhang, C.L.; Zhuge, Q.; Yang, J. Prospects of Directly Reprogrammed Adult Human Neurons for Neurodegenerative Disease Modeling and Drug Discovery: iN vs. IPSCs Models. Front. Neurosci. 2020, 14, 546484. [Google Scholar] [CrossRef]
- Guo, Z.; Zhang, L.; Wu, Z.; Chen, Y.; Wang, F.; Chen, G. In vivo direct reprogramming of reactive glial cells into functional neurons after brain injury and in an Alzheimer’s disease model. Cell Stem Cell 2014, 14, 188–202. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Ma, N.-X.; Pei, Z.-F.; Wu, Z.; Do-Monte, F.H.; Keefe, S.; Yellin, E.; Chen, M.S.; Yin, J.-C.; Lee, G. A NeuroD1 AAV-based gene therapy for functional brain repair after ischemic injury through in vivo astrocyte-to-neuron conversion. Mol. Ther. 2020, 28, 217–234. [Google Scholar] [CrossRef] [PubMed]
- Di Val Cervo, P.R.; Romanov, R.A.; Spigolon, G.; Masini, D.; Martin-Montanez, E.; Toledo, E.M.; La Manno, G.; Feyder, M.; Pifl, C.; Ng, Y.H.; et al. Induction of functional dopamine neurons from human astrocytes in vitro and mouse astrocytes in a Parkinson’s disease model. Nat. Biotechnol. 2017, 35, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Lee, E.; Kim, J.; Kwon, Y.-W.; Kwon, Y.; Kim, J. Efficient in vivo direct conversion of fibroblasts into cardiomyocytes using a nanoparticle-based gene carrier. Biomaterials 2019, 192, 500–509. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Xu, Z.; Zhong, P.; Ren, Y.; Liang, G.; Schilling, H.A.; Hu, Z.; Zhang, Y.; Wang, X.; Chen, S. Cell cycle and p53 gate the direct conversion of human fibroblasts to dopaminergic neurons. Nat. Commun. 2015, 6, 10100. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Seo, J.; Lee, J.S.; Shin, S.; Park, H.J.; Min, S.; Cheong, E.; Lee, T.; Cho, S.W. Triboelectric nanogenerator accelerates highly efficient nonviral direct conversion and in vivo reprogramming of fibroblasts to functional neuronal cells. Adv. Mater. 2016, 28, 7365–7374. [Google Scholar] [CrossRef]
- Lujan, E.; Chanda, S.; Ahlenius, H.; Südhof, T.C.; Wernig, M. Direct conversion of mouse fibroblasts to self-renewing, tripotent neural precursor cells. Proc. Natl. Acad. Sci. USA 2012, 109, 2527–2532. [Google Scholar] [CrossRef]
- Victor, M.B.; Richner, M.; Hermanstyne, T.O.; Ransdell, J.L.; Sobieski, C.; Deng, P.-Y.; Klyachko, V.A.; Nerbonne, J.M.; Yoo, A.S. Generation of human striatal neurons by microRNA-dependent direct conversion of fibroblasts. Neuron 2014, 84, 311–323. [Google Scholar] [CrossRef]
- Xue, Y.; Ouyang, K.; Huang, J.; Zhou, Y.; Ouyang, H.; Li, H.; Wang, G.; Wu, Q.; Wei, C.; Bi, Y. Direct conversion of fibroblasts to neurons by reprogramming PTB-regulated microRNA circuits. Cell 2013, 152, 82–96. [Google Scholar] [CrossRef]
- Yoo, A.S.; Sun, A.X.; Li, L.; Shcheglovitov, A.; Portmann, T.; Li, Y.; Lee-Messer, C.; Dolmetsch, R.E.; Tsien, R.W.; Crabtree, G.R. MicroRNA-mediated conversion of human fibroblasts to neurons. Nature 2011, 476, 228–231. [Google Scholar] [CrossRef]
- Ifkovits, J.L.; Addis, R.C.; Epstein, J.A.; Gearhart, J.D. Inhibition of TGFβ signaling increases direct conversion of fibroblasts to induced cardiomyocytes. PLoS ONE 2014, 9, e89678. [Google Scholar] [CrossRef] [PubMed]
- Ladewig, J.; Mertens, J.; Kesavan, J.; Doerr, J.; Poppe, D.; Glaue, F.; Herms, S.; Wernet, P.; Kögler, G.; Müller, F.-J. Small molecules enable highly efficient neuronal conversion of human fibroblasts. Nat. Methods 2012, 9, 575–578. [Google Scholar] [CrossRef]
- Yoo, J.; Noh, M.; Kim, H.; Jeon, N.L.; Kim, B.-S.; Kim, J. Nanogrooved substrate promotes direct lineage reprogramming of fibroblasts to functional induced dopaminergic neurons. Biomaterials 2015, 45, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Liu, S. Epigenetics advancing personalized nanomedicine in cancer therapy. Adv. Drug Deliv. Rev. 2012, 64, 1532–1543. [Google Scholar] [CrossRef] [PubMed]
- Sylvestre, J.-P.; Tang, M.-C.; Furtos, A.; Leclair, G.; Meunier, M.; Leroux, J.-C. Nanonization of megestrol acetate by laser fragmentation in aqueous milieu. J. Control. Release 2011, 149, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Kaneda, Y. Virosome: A novel vector to enable multi-modal strategies for cancer therapy. Adv. Drug Deliv. Rev. 2012, 64, 730–738. [Google Scholar] [CrossRef]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [PubMed]
- Yoo, J.; Kim, J.; Baek, S.; Park, Y.; Im, H.; Kim, J. Cell reprogramming into the pluripotent state using graphene based substrates. Biomaterials 2014, 35, 8321–8329. [Google Scholar] [CrossRef]
- Lee, S.; Shim, H.S.; Park, H.J.; Chang, Y.; Han, Y.-E.; Oh, S.-J.; Lee, W.; Im, H.; Seol, Y.; Ryu, H. Elongated nanoporous Au networks improve somatic cell direct conversion into induced dopaminergic neurons for Parkinson’s disease therapy. Acta Biomater. 2022, 151, 561–575. [Google Scholar] [CrossRef]
- Orellana, D.I.; Santambrogio, P.; Rubio, A.; Yekhlef, L.; Cancellieri, C.; Dusi, S.; Giannelli, S.G.; Venco, P.; Mazzara, P.G.; Cozzi, A. Coenzyme A corrects pathological defects in human neurons of PANK 2-associated neurodegeneration. EMBO Mol. Med. 2016, 8, 1197–1211. [Google Scholar] [CrossRef]
- Chang, Y.; Yoo, J.; Kim, J.; Hwang, Y.; Shim, G.; Oh, Y.K.; Kim, J. Electromagnetized Graphene Facilitates Direct Lineage Reprogramming into Dopaminergic Neurons. Adv. Funct. Mater. 2021, 31, 2105346. [Google Scholar] [CrossRef]
- Baek, S.; Oh, J.; Song, J.; Choi, H.; Yoo, J.; Park, G.Y.; Han, J.; Chang, Y.; Park, H.; Kim, H. Generation of Integration-Free Induced Neurons Using Graphene Oxide-Polyethylenimine. Small 2017, 13, 1601993. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Cho, B.; Kim, S.; Kim, J. Direct conversion of fibroblasts to osteoblasts as a novel strategy for bone regeneration in elderly individuals. Exp. Mol. Med. 2019, 51, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Cho, B.; Lee, E.; Kim, J.; Yoo, J.; Sung, J.-S.; Kwon, Y.; Kim, J. Electromagnetized gold nanoparticles improve neurogenesis and cognition in the aged brain. Biomaterials 2021, 278, 121157. [Google Scholar] [CrossRef]
- Yoo, J.; Chang, Y.; Kim, H.; Baek, S.; Choi, H.; Jeong, G.-J.; Shin, J.; Kim, H.; Kim, B.-S.; Kim, J. Efficient direct lineage reprogramming of fibroblasts into induced cardiomyocytes using nanotopographical cues. J. Biomed. Nanotechnol. 2017, 13, 269–279. [Google Scholar] [CrossRef]
- Wang, Q.; Song, Y.; Chen, J.; Li, Q.; Gao, J.; Tan, H.; Zhu, Y.; Wang, Z.; Li, M.; Yang, H. Direct in vivo reprogramming with non-viral sequential targeting nanoparticles promotes cardiac regeneration. Biomaterials 2021, 276, 121028. [Google Scholar] [CrossRef]
- Barbara, R.; Belletti, D.; Pederzoli, F.; Masoni, M.; Keller, J.; Ballestrazzi, A.; Vandelli, M.A.; Tosi, G.; Grabrucker, A.M. Novel Curcumin loaded nanoparticles engineered for Blood-Brain Barrier crossing and able to disrupt Abeta aggregates. Int. J. Pharm. 2017, 526, 413–424. [Google Scholar] [CrossRef]
- Ye, Y.; Sun, Y.; Zhao, H.; Lan, M.; Gao, F.; Song, C.; Lou, K.; Li, H.; Wang, W. A novel lactoferrin-modified beta-cyclodextrin nanocarrier for brain-targeting drug delivery. Int. J. Pharm. 2013, 458, 110–117. [Google Scholar] [CrossRef]
- Pandey, S.; Thakur, M.; Mewada, A.; Anjarlekar, D.; Mishra, N.; Sharon, M. Carbon dots functionalized gold nanorod mediated delivery of doxorubicin: Tri-functional nano-worms for drug delivery, photothermal therapy and bioimaging. J. Mater. Chem. B 2013, 1, 4972–4982. [Google Scholar] [CrossRef]
- Zadpoor, A.A. Bone tissue regeneration: The role of scaffold geometry. Biomater. Sci. 2015, 3, 231–245. [Google Scholar] [CrossRef]
- Pati, F.; Jang, J.; Ha, D.H.; Won Kim, S.; Rhie, J.W.; Shim, J.H.; Kim, D.H.; Cho, D.W. Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat. Commun. 2014, 5, 3935. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Kong, L.; Zhu, S. Reprogramming cell fates by small molecules. Protein Cell 2017, 8, 328–348. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Jeong, J.; Choi, D. Small-molecule-mediated reprogramming: A silver lining for regenerative medicine. Exp. Mol. Med. 2020, 52, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Su, S.C.; Wang, H.; Cheng, A.W.; Cassady, J.P.; Lodato, M.A.; Lengner, C.J.; Chung, C.-Y.; Dawlaty, M.M.; Tsai, L.-H. Functional integration of dopaminergic neurons directly converted from mouse fibroblasts. Cell Stem Cell 2011, 9, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Gao, L.; Guan, W.; Mao, J.; Hu, W.; Qiu, B.; Zhao, J.; Yu, Y.; Pei, G. Direct conversion of astrocytes into neuronal cells by drug cocktail. Cell Res. 2015, 25, 1269–1272. [Google Scholar] [CrossRef]
- Park, S.J.; Shin, H.; Won, C.; Min, D.H. Non-viral, direct neuronal reprogramming from human fibroblast using a polymer-functionalized nanodot. Nanomedicine 2021, 32, 102316. [Google Scholar] [CrossRef]
- Colella, P.; Ronzitti, G.; Mingozzi, F. Emerging issues in AAV-mediated in vivo gene therapy. Mol. Ther. Methods Clin. Dev. 2018, 8, 87–104. [Google Scholar]
- Chen, Y.; Zhi, S.; Liu, W.; Wen, J.; Hu, S.; Cao, T.; Sun, H.; Li, Y.; Huang, L.; Liu, Y. Development of Highly Efficient Dual-AAV Split Adenosine Base Editor for In Vivo Gene Therapy. Small Methods 2020, 4, 2000309. [Google Scholar] [CrossRef]
- Flotte, T.R.; Cataltepe, O.; Puri, A.; Batista, A.R.; Moser, R.; McKenna-Yasek, D.; Douthwright, C.; Gernoux, G.; Blackwood, M.; Mueller, C. AAV gene therapy for Tay-Sachs disease. Nat. Med. 2022, 28, 251–259. [Google Scholar] [CrossRef]
- Yu, T.W.; Bodamer, O. A solid start for gene therapy in Tay–Sachs disease. Nat. Med. 2022, 28, 236–237. [Google Scholar] [CrossRef]
- Bowles, D.E.; McPhee, S.W.; Li, C.; Gray, S.J.; Samulski, J.J.; Camp, A.S.; Li, J.; Wang, B.; Monahan, P.E.; Rabinowitz, J.E.; et al. Phase 1 gene therapy for Duchenne muscular dystrophy using a translational optimized AAV vector. Molecules 2012, 20, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Tai, P.W.; Gao, G. Adeno-associated virus vector as a platform for gene therapy delivery. Nat. Rev. Drug Discov. 2019, 18, 358–378. [Google Scholar] [CrossRef] [PubMed]
- Gil-Farina, I.; Fronza, R.; Kaeppel, C.; Lopez-Franco, E.; Ferreira, V.; D’avola, D.; Benito, A.; Prieto, J.; Petry, H.; Gonzalez-Aseguinolaza, G. Recombinant AAV integration is not associated with hepatic genotoxicity in nonhuman primates and patients. Mol. Ther. 2016, 24, 1100–1105. [Google Scholar] [CrossRef] [PubMed]
- Paneda, A.; Lopez-Franco, E.; Kaeppel, C.; Unzu, C.; Gil-Royo, A.G.; D’Avola, D.; Beattie, S.G.; Olagüe, C.; Ferrero, R.; Sampedro, A. Safety and liver transduction efficacy of rAAV5-cohPBGD in nonhuman primates: A potential therapy for acute intermittent porphyria. Hum. Gene Ther. 2013, 24, 1007–1017. [Google Scholar] [CrossRef]
- Goater, J.; Müller, R.; Kollias, G.; Firestein, G.S.; Sanz, I.; O’Keefe, R.J.; Schwarz, E.M. Empirical advantages of adeno associated viral vectors in vivo gene therapy for arthritis. J. Rheumatol. 2000, 27, 983–989. [Google Scholar]
- Yom-Tov, N.; Guy, R.; Offen, D. Extracellular vesicles over adeno-associated viruses: Advantages and limitations as drug delivery platforms in precision medicine. Adv. Drug Deliv. Rev. 2022, 190, 114535. [Google Scholar] [CrossRef]
- Mendell, J.R.; Al-Zaidy, S.A.; Rodino-Klapac, L.R.; Goodspeed, K.; Gray, S.J.; Kay, C.N.; Boye, S.L.; Boye, S.E.; George, L.A.; Salabarria, S. Current clinical applications of in vivo gene therapy with AAVs. Mol. Ther. 2021, 29, 464–488. [Google Scholar] [CrossRef]
- Bennett, J.; Wellman, J.; Marshall, K.A.; McCague, S.; Ashtari, M.; DiStefano-Pappas, J.; Elci, O.U.; Chung, D.C.; Sun, J.; Wright, J.F. Safety and durability of effect of contralateral-eye administration of AAV2 gene therapy in patients with childhood-onset blindness caused by RPE65 mutations: A follow-on phase 1 trial. Lancet 2016, 388, 661–672. [Google Scholar] [CrossRef]
- Xie, Q.; Bu, W.; Bhatia, S.; Hare, J.; Somasundaram, T.; Azzi, A.; Chapman, M.S. The atomic structure of adeno-associated virus (AAV-2), a vector for human gene therapy. Proc. Natl. Acad. Sci. USA 2002, 99, 10405–10410. [Google Scholar] [CrossRef]
- Mendell, J.R.; Al-Zaidy, S.; Shell, R.; Arnold, W.D.; Rodino-Klapac, L.R.; Prior, T.W.; Lowes, L.; Alfano, L.; Berry, K.; Church, K.; et al. Single-Dose Gene-Replacement Therapy for Spinal Muscular Atrophy. N. Engl. J. Med. 2017, 377, 1713–1722. [Google Scholar] [CrossRef]
- Nagahara, A.H.; Wilson, B.R.; Ivasyk, I.; Kovacs, I.; Rawalji, S.; Bringas, J.R.; Pivirotto, P.J.; Sebastian, W.S.; Samaranch, L.; Bankiewicz, K.S.; et al. MR-guided delivery of AAV2-BDNF into the entorhinal cortex of non-human primates. Gene 2018, 25, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Kells, A.P.; Eberling, J.; Su, X.; Pivirotto, P.; Bringas, J.; Hadaczek, P.; Narrow, W.C.; Bowers, W.J.; Federoff, H.J.; Forsayeth, J.; et al. Regeneration of the MPTP-lesioned dopaminergic system after convection-enhanced delivery of AAV2-GDNF. J. Neurosci. 2010, 30, 9567–9577. [Google Scholar] [CrossRef] [PubMed]
- Maguire, A.M.; Simonelli, F.; Pierce, E.A.; Pugh, E.N., Jr.; Mingozzi, F.; Bennicelli, J.; Banfi, S.; Marshall, K.A.; Testa, F.; Surace, E.M.; et al. Safety and efficacy of gene transfer for Leber’s congenital amaurosis. N. Engl. J. Med. 2008, 358, 2240–2248. [Google Scholar] [CrossRef] [PubMed]
- Cideciyan, A.V.; Hauswirth, W.W.; Aleman, T.S.; Kaushal, S.; Schwartz, S.B.; Boye, S.L.; Windsor, E.A.; Conlon, T.J.; Sumaroka, A.; Pang, J.J.; et al. Human RPE65 gene therapy for Leber congenital amaurosis: Persistence of early visual improvements and safety at 1 year. Hum. Gene 2009, 20, 999–1004. [Google Scholar] [CrossRef]
- Baum, B.J.; Alevizos, I.; Zheng, C.; Cotrim, A.P.; Liu, S.; McCullagh, L.; Goldsmith, C.M.; Burbelo, P.D.; Citrin, D.E.; Mitchell, J.B.; et al. Early responses to adenoviral-mediated transfer of the aquaporin-1 cDNA for radiation-induced salivary hypofunction. Proc. Natl. Acad. Sci. USA 2012, 109, 19403–19407. [Google Scholar] [CrossRef]
- MacLaren, R.E.; Groppe, M.; Barnard, A.R.; Cottriall, C.L.; Tolmachova, T.; Seymour, L.; Clark, K.R.; During, M.J.; Cremers, F.P.; Black, G.C.; et al. Retinal gene therapy in patients with choroideremia: Initial findings from a phase 1/2 clinical trial. Lancet 2014, 383, 1129–1137. [Google Scholar] [CrossRef]
- Heier, J.S.; Kherani, S.; Desai, S.; Dugel, P.; Kaushal, S.; Cheng, S.H.; Delacono, C.; Purvis, A.; Richards, S.; Le-Halpere, A.; et al. Intravitreous injection of AAV2-sFLT01 in patients with advanced neovascular age-related macular degeneration: A phase 1, open-label trial. Lancet 2017, 390, 50–61. [Google Scholar] [CrossRef]
- Marks, W.J., Jr.; Ostrem, J.L.; Verhagen, L.; Starr, P.A.; Larson, P.S.; Bakay, R.A.; Taylor, R.; Cahn-Weiner, D.A.; Stoessl, A.J.; Olanow, C.W.; et al. Safety and tolerability of intraputaminal delivery of CERE-120 (adeno-associated virus serotype 2-neurturin) to patients with idiopathic Parkinson’s disease: An open-label, phase I trial. Lancet Neurol. 2008, 7, 400–408. [Google Scholar] [CrossRef]
- Weleber, R.G.; Pennesi, M.E.; Wilson, D.J.; Kaushal, S.; Erker, L.R.; Jensen, L.; McBride, M.T.; Flotte, T.R.; Humphries, M.; Calcedo, R. Results at 2 years after gene therapy for RPE65-deficient Leber congenital amaurosis and severe early-childhood–onset retinal dystrophy. Ophthalmology 2016, 123, 1606–1620. [Google Scholar] [CrossRef]
- Kang, M.S.; Lee, S.Y.; Kim, K.S.; Han, D.-W. State of the art biocompatible gold nanoparticles for cancer theragnosis. Pharmaceutics 2020, 12, 701. [Google Scholar] [CrossRef]
- Tournebize, J.; Boudier, A.; Sapin-Minet, A.; Maincent, P.; Leroy, P.; Schneider, R.l. Role of gold nanoparticles capping density on stability and surface reactivity to design drug delivery platforms. ACS Appl. Mater. Interfaces 2012, 4, 5790–5799. [Google Scholar] [CrossRef]
- Amendola, V.; Pilot, R.; Frasconi, M.; Maragò, O.M.; Iatì, M.A. Surface plasmon resonance in gold nanoparticles: A review. J. Phys. Condens. Matter. 2017, 29, 203002. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Lee, S.; Yoo, S.; Jung, I.; Lee, S.; Kim, J.; Son, J.; Kim, J.-E.; Kim, J.-M.; Nam, J.-M. Enormous Enhancement in Single-Particle Surface-Enhanced Raman Scattering with Size-Controllable Au Double Nanorings. Chem. Mater. 2022, 34, 2197–2205. [Google Scholar] [CrossRef]
- Lee, S.; Lee, S.; Kim, J.-M.; Son, J.; Cho, E.; Yoo, S.; Hilal, H.; Nam, J.-M.; Park, S. Au nanolenses for near-field focusing. Chem. Sci. 2021, 12, 6355–6361. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lee, S.; Son, J.; Kim, J.M.; Lee, J.; Yoo, S.; Haddadnezhad, M.; Shin, J.; Kim, J.; Nam, J.M. Web-above-a-Ring (WAR) and Web-above-a-Lens (WAL): Nanostructures for Highly Engineered Plasmonic-Field Tuning and SERS Enhancement. Small 2021, 17, 2101262. [Google Scholar] [CrossRef]
- Lee, S.; Jung, I.; Son, J.; Lee, S.; Park, M.; Kim, J.-E.; Park, W.; Lee, J.; Nam, J.-M.; Park, S. Heterogeneous Component Au (Outer)–Pt (Middle)–Au (Inner) Nanorings: Synthesis and Vibrational Characterization on Middle Pt Nanorings with Surface-Enhanced Raman Scattering. ACS Nano. 2022, 16, 11259–11267. [Google Scholar] [CrossRef]
- Douglas, K.L.; Tabrizian, M. Effect of experimental parameters on the formation of alginate–chitosan nanoparticles and evaluation of their potential application as DNA carrier. J. Biomater. Sci. Polym. Ed. 2005, 16, 43–56. [Google Scholar] [CrossRef]
- Lee, Y.S.; Kim, S.W. Bioreducible polymers for therapeutic gene delivery. J. Control. Release 2014, 190, 424–439. [Google Scholar] [CrossRef]
- Yin, H.; Kanasty, R.L.; Eltoukhy, A.A.; Vegas, A.J.; Dorkin, J.R.; Anderson, D.G. Non-viral vectors for gene-based therapy. Nat. Rev. Genet. 2014, 15, 541–555. [Google Scholar] [CrossRef]
- Gao, X.; Huang, L. A novel cationic liposome reagent for efficient transfection of mammalian cells. Biochem. Biophys. Res. Commun. 1991, 179, 280–285. [Google Scholar] [CrossRef]
- Thomsen, L.B.; Larsen, A.B.; Lichota, J.; Moos, T. Nanoparticle-derived non-viral genetic transfection at the blood-brain barrier to enable neuronal growth factor delivery by secretion from brain endothelium. Curr. Med. Chem. 2011, 18, 3330–3334. [Google Scholar] [CrossRef]
- Ma, Y.; Jiang, L.; Mei, Y.; Song, R.; Tian, D.; Huang, H. Colorimetric sensing strategy for mercury (II) and melamine utilizing cysteamine-modified gold nanoparticles. Analyst 2013, 138, 5338–5343. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Min, S.-H.; Kim, M.-N.; Lee, D.-C.; Lim, M.-J.; Yeom, Y.-I. Alginate/PEI/DNA polyplexes: A new gene delivery system. Yao Xue Xue Bao Acta Pharm. Sin. 2006, 41, 439–445. [Google Scholar]
- Corpuz, R.D.; Ishida, Y.; Nguyen, M.T.; Yonezawa, T. Synthesis of positively charged photoluminescent bimetallic Au–Ag nanoclusters by double-target sputtering method on a biocompatible polymer matrix. Langmuir 2017, 33, 9144–9150. [Google Scholar] [CrossRef] [PubMed]
- Sarhan, F.; Saif, D.; Saif, A. An overview of traumatic spinal cord injury: Part 1. aetiology and pathophysiology. Br. J. Neurosci. Nurs. 2012, 8, 319–325. [Google Scholar] [CrossRef]
- Mothe, A.J.; Tator, C.H. Advances in stem cell therapy for spinal cord injury. J. Clin. Investig. 2012, 122, 3824–3834. [Google Scholar] [CrossRef]
- Leal-Filho, M.B. Spinal cord injury: From inflammation to glial scar. Surg. Neurol. Int. 2011, 2, 112. [Google Scholar] [CrossRef]
- Yuan, Y.-M.; He, C. The glial scar in spinal cord injury and repair. Neurosci. Bull. 2013, 29, 421–435. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, S.; Liu, C.; Han, X.; Gu, X.; Zhou, S. Deciphering glial scar after spinal cord injury. Burn. Trauma 2021, 9, tkab035. [Google Scholar] [CrossRef]
- Chun, H.; Lim, J.; Park, K.D.; Lee, C.J. Inhibition of monoamine oxidase B prevents reactive astrogliosis and scar formation in stab wound injury model. Glia 2022, 70, 354–367. [Google Scholar] [CrossRef]
- Lee, J.; Kim, J.-G.; Hong, S.; Kim, Y.S.; Ahn, S.; Kim, R.; Chun, H.; Park, K.D.; Jeong, Y.; Kim, D.-E. Intravital imaging of cerebral microinfarct reveals an astrocyte reaction led to glial scar. bioRxiv 2021. [Google Scholar] [CrossRef]
- Cho, J.-H.; Tsai, M.-J. The role of BETA2/NeuroD1 in the development of the nervous system. Mol. Neurobiol. 2004, 30, 35–47. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.; Chen, F.; Song, N.; Xie, J. In vivo Direct Conversion of Astrocytes to Neurons Maybe a Potential Alternative Strategy for Neurodegenerative Diseases. Front. Aging Neurosci. 2021, 474, 689276. [Google Scholar] [CrossRef]
- Liang, X.G.; Tan, C.; Wang, C.K.; Tao, R.R.; Huang, Y.J.; Ma, K.F.; Fukunaga, K.; Huang, M.Z.; Han, F. Myt1l induced direct reprogramming of pericytes into cholinergic neurons. CNS Neurosci. Ther. 2018, 24, 801–809. [Google Scholar] [CrossRef]
- Caiazzo, M.; Dell’Anno, M.T.; Dvoretskova, E.; Lazarevic, D.; Taverna, S.; Leo, D.; Sotnikova, T.D.; Menegon, A.; Roncaglia, P.; Colciago, G. Direct generation of functional dopaminergic neurons from mouse and human fibroblasts. Nature 2011, 476, 224–227. [Google Scholar] [CrossRef]
- Thiebes, K.P.; Nam, H.; Cambronne, X.A.; Shen, R.; Glasgow, S.M.; Cho, H.-H.; Kwon, J.-S.; Goodman, R.H.; Lee, J.W.; Lee, S. miR-218 is essential to establish motor neuron fate as a downstream effector of Isl1–Lhx3. Nat. Commun. 2015, 6, 7718. [Google Scholar] [CrossRef]
- De Gregorio, R.; Pulcrano, S.; De Sanctis, C.; Volpicelli, F.; Guatteo, E.; Von Oerthel, L.; Latagliata, E.C.; Esposito, R.; Piscitelli, R.M.; Perrone-Capano, C. miR-34b/c regulates Wnt1 and enhances mesencephalic dopaminergic neuron differentiation. Stem Cell Rep. 2018, 10, 1237–1250. [Google Scholar] [CrossRef]
- Yang, J.; Brown, M.E.; Zhang, H.; Martinez, M.; Zhao, Z.; Bhutani, S.; Yin, S.; Trac, D.; Xi, J.J.; Davis, M.E. High-throughput screening identifies microRNAs that target Nox2 and improve function after acute myocardial infarction. Am. J. Physiol. Heart Circ. Physiol. 2017, 312, H1002–H1012. [Google Scholar] [CrossRef]
- Sun, D. The potential of endogenous neurogenesis for brain repair and regeneration following traumatic brain injury. Neural Regen. Res. 2014, 9, 688. [Google Scholar] [CrossRef]


| Species | Starting Cells | Target Cells | Materials | Efficiency | Ref. |
|---|---|---|---|---|---|
| Ms/Hu | Fibroblasts | Neurons | Small molecules (CHIR, LDN, AA) | ~35% | [22] |
| Ms/Hu | Fibroblasts | Neurons | miRNA | ~40% | [4] |
| Ms/hu | Fibroblasts | Dopaminergic neurons | Electromagnetized gold nanoparticles | ~55% | [5] |
| Ms | Fibroblasts | Dopaminergic neurons | Electromagnetized graphene nanosheet | ~20% | [31] |
| Ms/Hu | Fibroblasts | Dopaminergic neurons | Elongated nanoporous gold nanorod | ~40% | [29] |
| Hu | Fibroblasts | Neurons | Polymer-functionalized Nanodot | ~40% | [46] |
| No. | Name | Application | Phase | Status | Identifier | Ref. |
|---|---|---|---|---|---|---|
| 1 | AAV-SMN1 | Muscular Atrophy, Spinal | Phase 4 | Active, not recruiting, | NCT05073133 | [60] |
| 2 | AAV2-BDNF | Alzheimer’s disease | Phase 1 | Recruiting | NCT05040217 | [61] |
| 3 | AAV2-GDNF | Parkinson’s disease | Phase 1 | Recruiting | NCT04167540 | [62] |
| 4 | AAV2-hRPE65v2 | Inherited retinal dystrophy | - | Active, not recruiting | NCT03602820 | [58,63] |
| 5 | AAV2/5-RPGR | X-linked retinitis pigmentosa | Phase 1/2 | Completed | NCT03252847 | [69] |
| 6 | rAAV2.REP1 | Choroideremia | Phase 2 | Completed | NCT02671539 | [58] |
| 7 | AAV2-REP1 | Choroideremia | Phase 2 | Completed | NCT02553135 | [64] |
| 8 | AAV2-hAQP1 | Squamous cell head and neck cancer Radiation induced xerostomia Salivary hypofunction | Phase 1 | Recruiting | NCT02446249 | [65] |
| 9 | AAV2-hCHM | Choroideremia | Phase 1/2 | Active, not recruiting | NCT02341807 | [66] |
| 10 | rAAV2.REP1 | Choroideremia | Phase 1/2 | Completed | NCT02077361 | [58] |
| 11 | AAV2-GDNF | Parkinson’s disease | Phase 1 | Completed | NCT01621581 | [62] |
| 12 | AAV2-sFLT01 | Macular degeneration | Phase 1 | Completed | NCT01024998 | [67] |
| 13 | AAV2-NTN | Parkinson’s disease | Phase 1 | Completed | NCT00252850 | [68] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Y.; Lee, S.; Kim, J.; Kim, C.; Shim, H.S.; Lee, S.E.; Park, H.J.; Kim, J.; Lee, S.; Lee, Y.K.; et al. Gene Therapy Using Efficient Direct Lineage Reprogramming Technology for Neurological Diseases. Nanomaterials 2023, 13, 1680. https://doi.org/10.3390/nano13101680
Chang Y, Lee S, Kim J, Kim C, Shim HS, Lee SE, Park HJ, Kim J, Lee S, Lee YK, et al. Gene Therapy Using Efficient Direct Lineage Reprogramming Technology for Neurological Diseases. Nanomaterials. 2023; 13(10):1680. https://doi.org/10.3390/nano13101680
Chicago/Turabian StyleChang, Yujung, Sungwoo Lee, Jieun Kim, Chunggoo Kim, Hyun Soo Shim, Seung Eun Lee, Hyeok Ju Park, Jeongwon Kim, Soohyun Lee, Yong Kyu Lee, and et al. 2023. "Gene Therapy Using Efficient Direct Lineage Reprogramming Technology for Neurological Diseases" Nanomaterials 13, no. 10: 1680. https://doi.org/10.3390/nano13101680





