Surface Adsorption Mechanism between Lead(II,IV) and Nanomaghemite Studied on Polluted Water Samples Collected from the Peruvian Rivers Mantaro and Cumbaza
Abstract
1. Introduction
2. Materials and Methods
2.1. Adsorbent Synthesis
2.2. Characterization
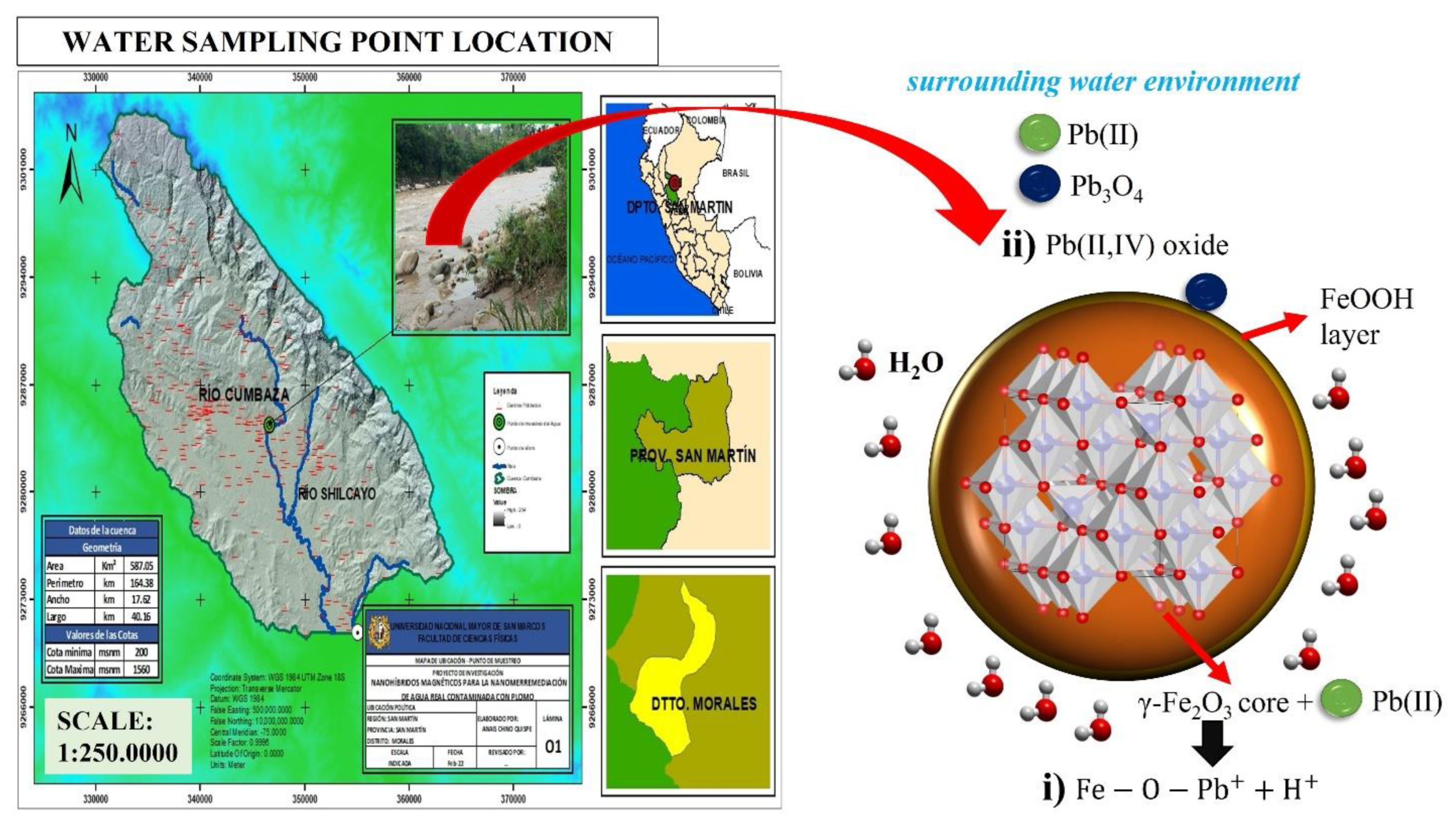
2.3. X-ray Photoelectron Spectroscopy before and after Heavy Metal Adsorption Experiments in Real Waters
2.4. Adsorption Kinetic Protocol in Real Waters
3. Results and Discussion
3.1. X-ray and Rietveld Analysis
3.2. Textural Properties
3.3. IR and µ-Raman Analysis
3.3.1. Characterization of Sample RG1 (“before Burning Protocol”)
3.3.2. Characterization of Sample RG1 (“after Burning Protocol”)
3.4. Pb Removal Efficiency in Real Waters
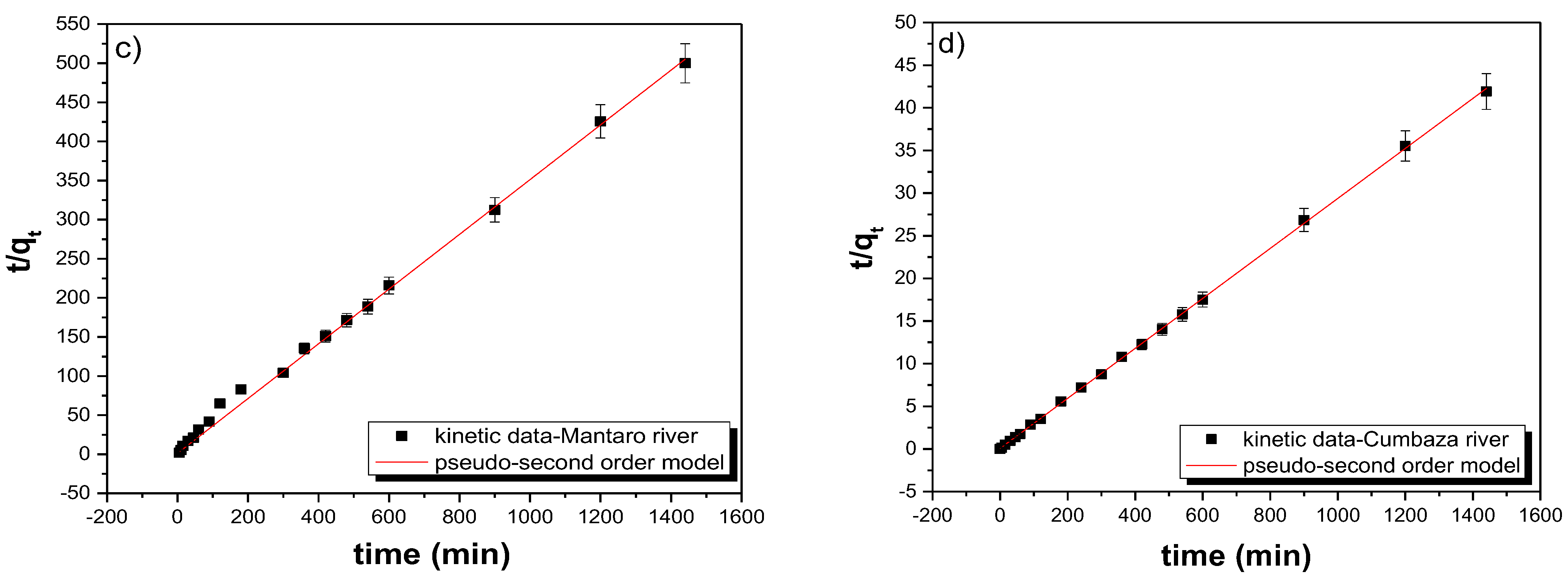
3.5. Kinetic Adsorption Models
3.6. Effect of Adsorbent Dose on Pb Removal
3.7. pH Monitoring during the Adsorption Kinetic and Adsorbent Dose Processes
3.8. Main Experimental Results Obtained after Adsorption Analysis
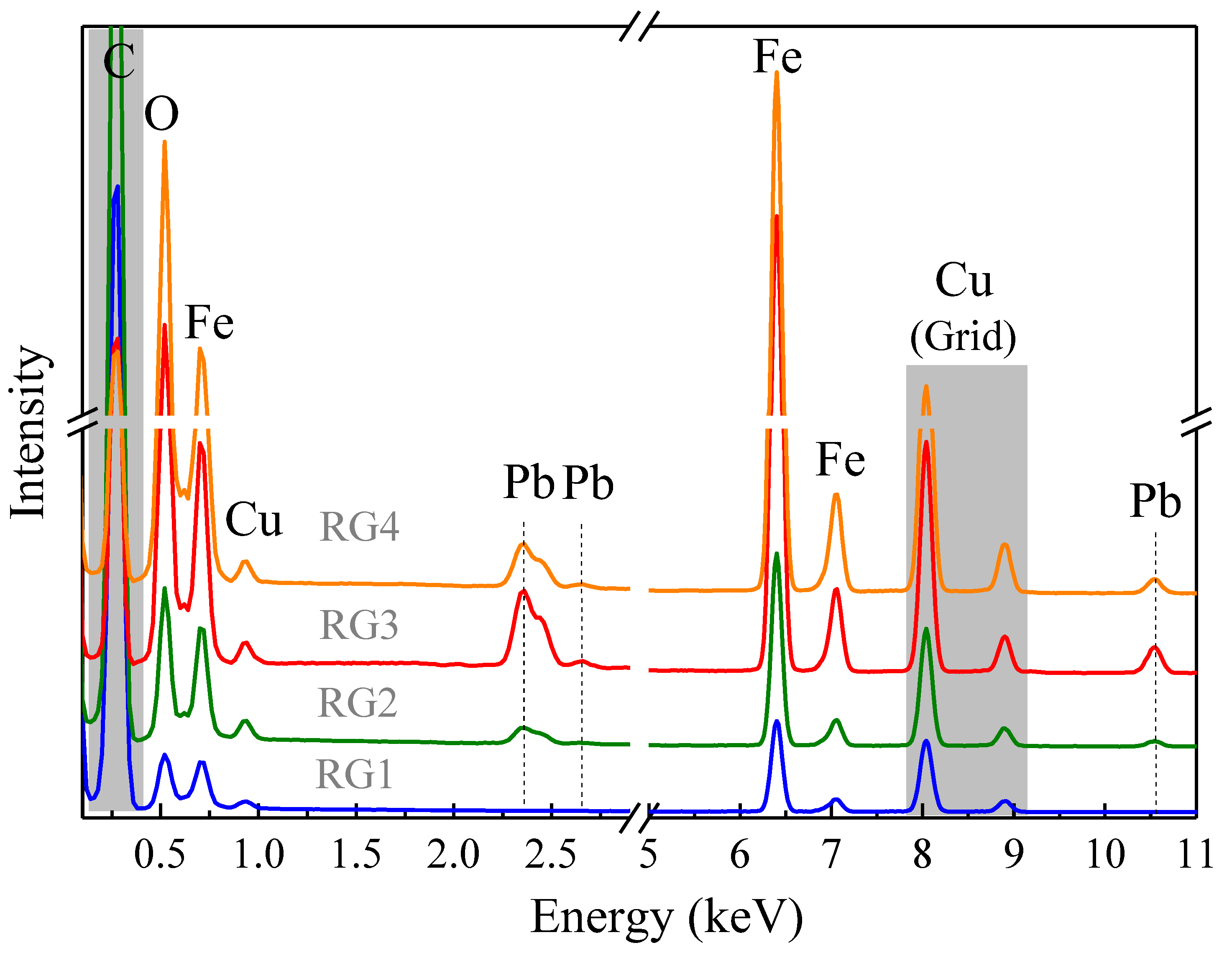
3.8.1. TEM and EDS Analysis
3.8.2. Magnetic Measurements
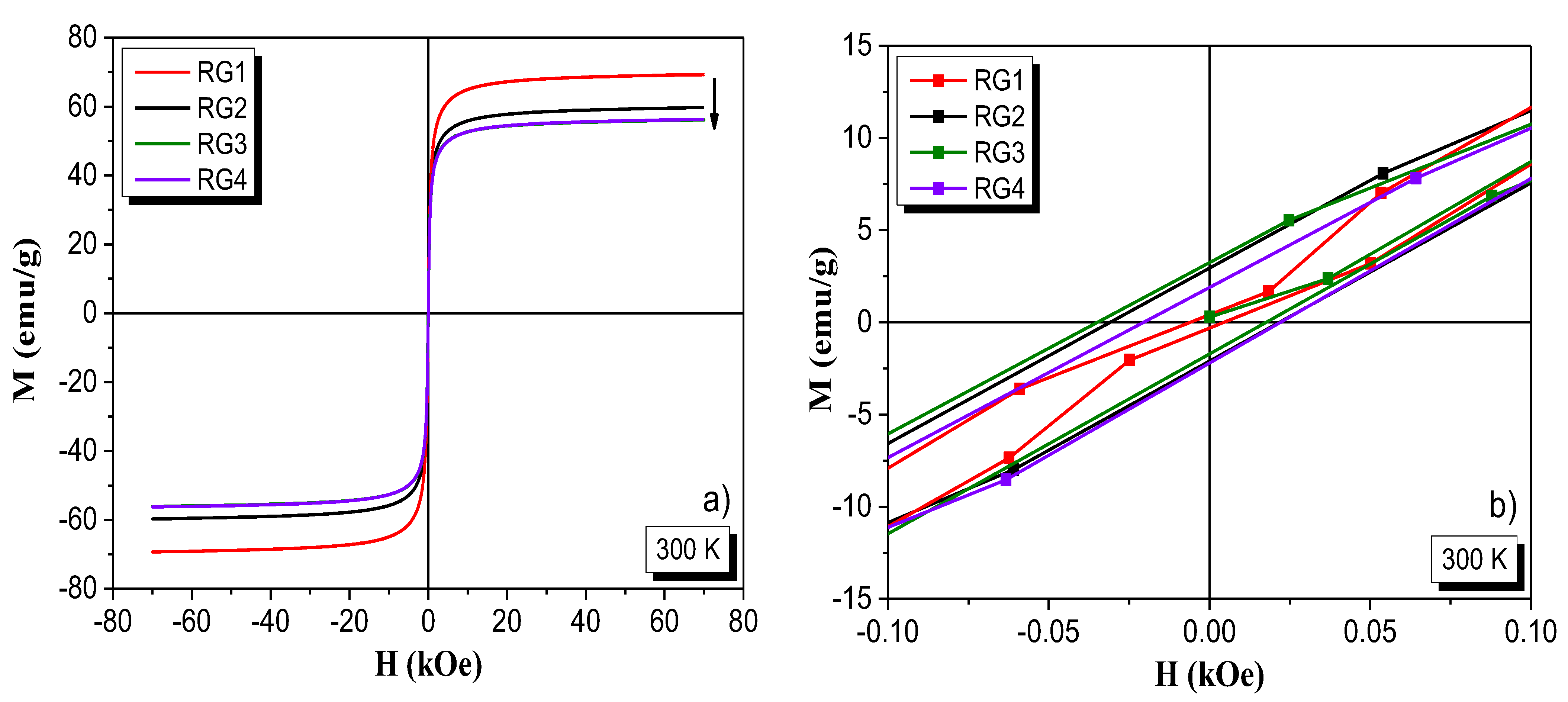
VSM Analysis
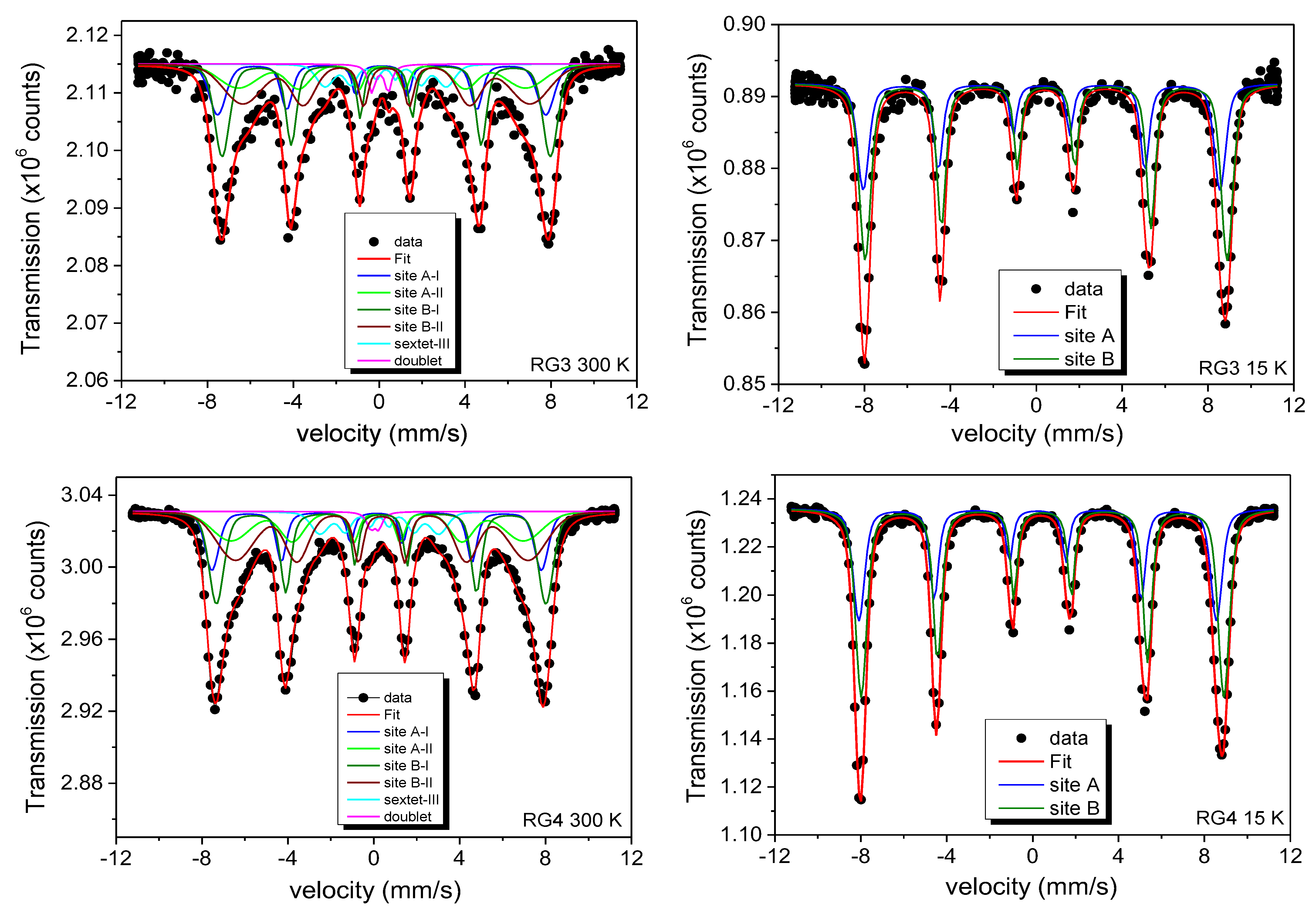
57Fe Mössbauer Spectroscopy Analysis
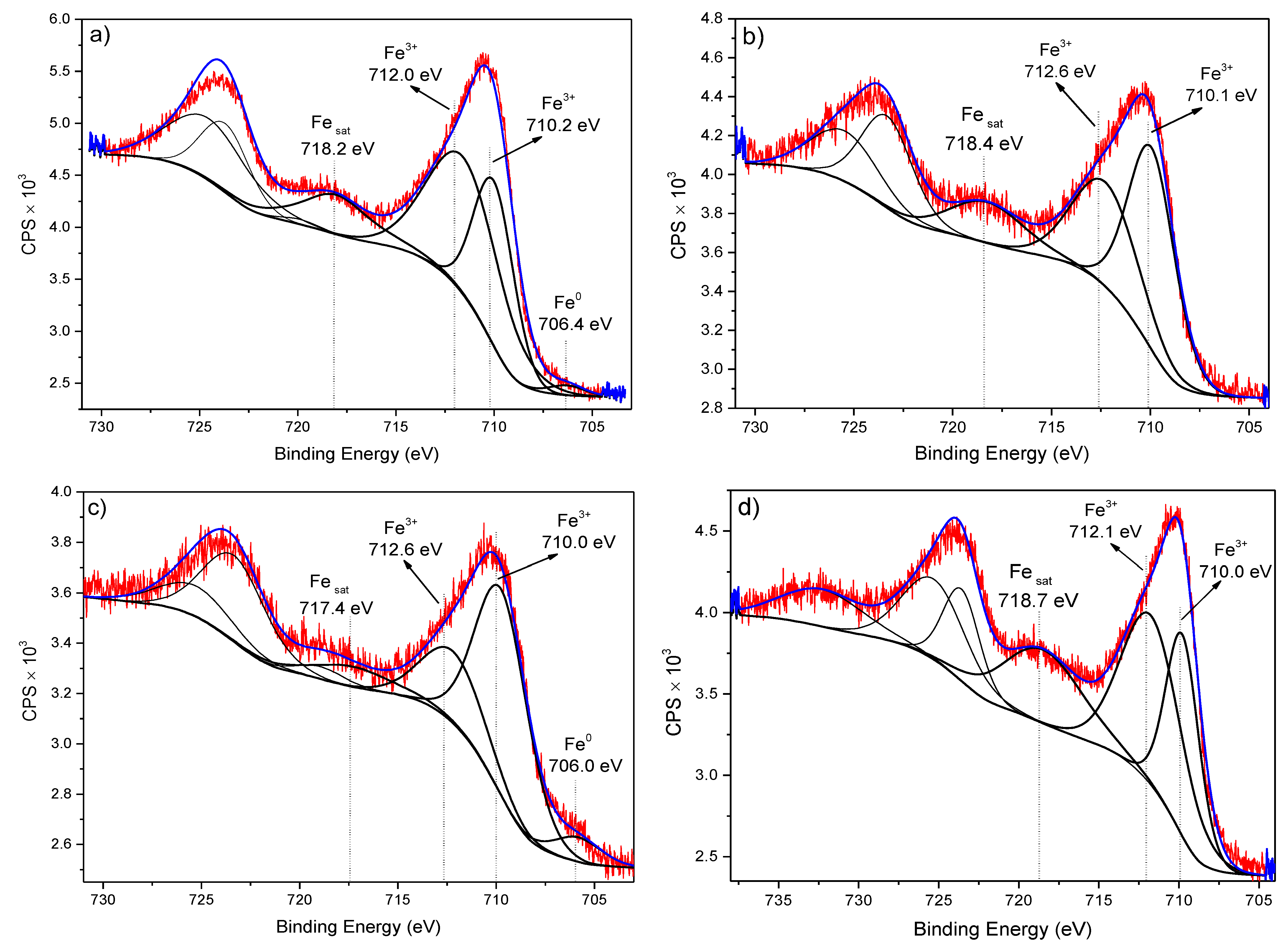
3.8.3. XPS Analysis (Adsorption Mechanism)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Y.; Wang, P.; Gojenko, B.; Yu, J.; Lezhang, W.; Luo, D.; Xiao, T. A review of water pollution arising from agriculture and mining activities in Central Asia: Facts, causes and effects. Environ. Pollut. 2021, 291, 118209. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Pesantes, A.; Peña Carpio, E.; Vitvar, T.; Koepke, R.; Menéndez-Aguado, J.M. A Comparative Study of Mining Control in Latin America. Mining 2021, 1, 6–18. [Google Scholar] [CrossRef]
- Quispe Yana, R.; Quispe, G.B.; Betancur, H.N.C.; Cáceres, S.H.; Mendoza, A.P.C.; Miranda, P.S.Y. Concentración de metales pesados: Cromo, cadmio y plomo en los sedimentos superficiales en el río Coata, Perú. Rev. Boliv. Quim. 2019, 36, 83–90. [Google Scholar]
- Jarvis, P.; Fawell, J. Lead in drinking water–an ongoing public health concern? Curr. Opin. Environ. Sci. Health 2021, 20, 100239. [Google Scholar] [CrossRef]
- Naranjo, V.I.; Hendricks, M.; Jones, K.S. Lead toxicity in children: An unremitting public health problem. Pediatr. Neurol. 2020, 113, 51–55. [Google Scholar] [CrossRef]
- Vaziri, N.D.; Gonick, H.C. Cardiovascular effects of lead exposure. Indian J. Med. Res. 2008, 128, 426–435. [Google Scholar]
- Sanders, T.; Liu, Y.; Buchner, V.; Tchounwou, P.B. Neurotoxic effects and biomarkers of lead exposure: A review. Rev. Environ. Health 2009, 24, 15–46. [Google Scholar] [CrossRef]
- Liu, T.; Lawluvy, Y.; Shi, Y.; Ighalo, J.O.; He, Y.; Zhang, Y.; Yap, P.S. Adsorption of cadmium and lead from aqueous solution using modified biochar: A review. J. Environ. Chem. Eng. 2022, 10, 106502. [Google Scholar] [CrossRef]
- Jjagwe, J.; Olupot, P.W.; Menya, E.; Kalibbala, H.M. Synthesis and application of Granular activated carbon from biomass waste materials for water treatment: A review. J. Bioresour. Bioprod. 2021, 6, 292–322. [Google Scholar] [CrossRef]
- Obey, G.; Adelaide, M.; Ramaraj, R. Biochar derived from non-customized matamba fruit shell as an adsorbent for wastewater treatment. J. Bioresour. Bioprod. 2022, 7, 109–115. [Google Scholar] [CrossRef]
- Thakur, A.K.; Singh, R.; Pullela, R.T.; Pundir, V. Green adsorbents for the removal of heavy metals from Wastewater: A review. Mater Today Proc. 2022, 57, 1468–1472. [Google Scholar] [CrossRef]
- Ramos-Guivar, J.A.; Flores-Cano, D.A.; Passamani, E.C. Differentiating nanomaghemite and nanomagnetite and discussing their importance in arsenic and lead removal from contaminated effluents: A critical review. Nanomaterials 2021, 11, 2310. [Google Scholar] [CrossRef] [PubMed]
- Mollideno Romero, L.F. Propuesta de un Sistema Natural Utilizando la Cáscara de Banano Para la Remoción de Plomo II. Bachelor’s Thesis, Universidad del Valle de Guatemala, Guatemala, Guatemala, 2021. [Google Scholar]
- Pinargote Arauz, M.E.; Vera Ramos, M.V. Adsorción de Plomo Mediante la Utilización del Exoesqueleto de Camarón y Cascarilla de Arroz en Aguas Residuales de la Industria Fabricante de Baterías. Bachelor’s Thesis, Escuela Superior Politécnica Agropecuaria de Manabí Manuel Félix López, Calceta, Ecuador, 2021. [Google Scholar]
- Ramos-Guivar, J.A.; Taipe, K.; Schettino, M.A.; Silva, E.; Torres, M.A.M.; Passamani, E.C.; Litterst, F.J. Improved removal capacity and equilibrium time of maghemite nanoparticles growth in zeolite type 5A for Pb (II) adsorption. Nanomaterials 2020, 10, 1668. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Guivar, J.A.; Lopez, E.O.; Greneche, J.M.; Litterst, F.J.; Passamani, E.C. Effect of EDTA organic coating on the spin canting behavior of maghemite nanoparticles for lead (II) adsorption. Appl. Surf. Sci. 2021, 538, 148021. [Google Scholar] [CrossRef]
- Chen, B.; Chen, S.; Zhao, H.; Liu, Y.; Long, F.; Pan, X. A versatile β-cyclodextrin and polyethyleneimine bi-functionalized magnetic nanoadsorbent for simultaneous capture of methyl orange and Pb(II) from complex wastewater. Chemosphere 2019, 216, 605–616. [Google Scholar] [CrossRef]
- Oyem, H.H.; Oyem, I.M.; Usese, A.I. Iron, manganese, cadmium, chromium, zinc and arsenic groundwater contents of Agbor and Owa communities of Nigeria. Springerplus 2015, 4, 104. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Niemark, A.V.; Olivier, J.P.; Rodríguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Vidal-Vidal, J.; Rivas, J.; López-Quintela, M.A. Synthesis of monodisperse maghemite nanoparticles by the microemulsion method. Colloids Surf. A Physicochem. Eng. Asp. 2006, 288, 44–51. [Google Scholar] [CrossRef]
- Jing, Z.; Wu, S. Synthesis, characterization and magnetic properties of γ-Fe2O3 nanoparticles via a non-aqueous medium. J. Solid State Chem. 2004, 177, 1213–1218. [Google Scholar] [CrossRef]
- Chamritski, I.; Burns, G. Infrared-and Raman-active phonons of magnetite, maghemite, and hematite: A computer simulation and spectroscopic study. J. Phys. Chem. B 2005, 109, 4965–4968. [Google Scholar] [CrossRef]
- Ramos-Guivar, J.A.; Gonzalez-Gonzalez, J.C.; Litterst, F.J.; Passamani, E.C. Rietveld Refinement, μ-Raman, X-ray Photoelectron, and Mössbauer Studies of Metal Oxide-Nanoparticles Growth on Multiwall Carbon Nanotubes and Graphene Oxide. Cryst. Growth Des. 2021, 21, 2128–2141. [Google Scholar] [CrossRef]
- El Mendili, Y.; Bardeau, J.F.; Randrianantoandro, N.; Grasset, F.; Greneche, J.M. Insights into the mechanism related to the phase transition from γ-Fe2O3 to α-Fe2O3 nanoparticles induced by thermal treatment and laser irradiation. J. Phys. Chem. C 2012, 116, 23785–23792. [Google Scholar] [CrossRef]
- Flores-Cano, D.A.; Checca-Huaman, N.R.; Castro-Merino, I.L.; Pinotti, C.N.; Passamani, E.C.; Litterst, F.J.; Ramos-Guivar, J.A. Progress toward Room-Temperature Synthesis and Functionalization of Iron oxide Nanoparticles. Int. J. Mol. Sci. 2022, 23, 8279. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Guivar, J.A.; Zarria-Romero, J.Y.; Castro-Merino, I.L.; Greneche, J.M.; Passamani, E.C. Improvement of the thermal stability of nanomaghemite by functionalization with type 5A zeolite and magnetic properties studied by in-field 57Fe Mössbauer measurements. J. Magn. Magn. Mater. 2022, 552, 169241. [Google Scholar] [CrossRef]
- Soler, M.A.G.; Alcantara, G.B.; Soares, F.Q.; Viali, W.R.; Sartoratto, P.P.C.; Fernandez, J.R.L.; da Silva, S.W.; Garg, V.K.; Oliveira, A.C.; Morais, P.C. Study of molecular surface coating on the stability of maghemite nanoparticles. Surf. Sci. 2007, 601, 3921–3925. [Google Scholar] [CrossRef]
- Kumari, M.; Pittman, C.U., Jr.; Mohan, D. Heavy metals [chromium (VI) and lead (II)] removal from water using mesoporous magnetite (Fe3O4) nanospheres. J. Colloid Interface Sci. 2015, 442, 120–132. [Google Scholar] [CrossRef]
- Roca, A.G.; Niznansky, D.; Poltierova-Vejpravova, J.; Bittova, B.; González-Fernández, M.A.; Serna, C.J.; Morales, M.P. Magnetite nanoparticles with no surface spin canting. J. Appl. Phys. 2009, 105, 114309. [Google Scholar] [CrossRef]
- Cornell, R.M.; Schwertmann, U. The Iron Oxides; Wiley-VCH: Weinheim, Germany, 2003. [Google Scholar]
- Mørup, S.; Tronc, E. Superparamagnetic relaxation of weakly interacting particles. Phys. Rev. Lett. 1994, 72, 3278. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. App. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Hawn, D.D.; DeKoven, B.M. Deconvolution as a correction for photoelectron inelastic energy losses in the core level XPS spectra of iron oxides. Surf. Interface Anal. 1987, 10, 63–74. [Google Scholar] [CrossRef]
- Radu, T.; Iacovita, C.; Benea, D.; Turcu, R. X-ray photoelectron spectroscopy characterization of iron oxide nanoparticles. Appl. Surf. Sci. 2017, 405, 337–343. [Google Scholar] [CrossRef]
- McIntyre, N.S.; Zetaruk, D.G. X-ray Photoelectron Spectroscopy Studies of Iron Oxides. Anal. Chem. 1977, 49, 1521–1529. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-ray Photoelectron Spectroscopy; Perkin-Elmer Corporation Physical Electronics Division: Eden Prairie, MN, USA, 1992. [Google Scholar]
- Morgan, W.E.; Van Wazer, J.R. Binding Energy Shifts in the X-ray Photoelectron Spectra of a Series of Related Group IV-a Compounds. J. Phys. Chem. 1973, 77, 964–969. [Google Scholar] [CrossRef]
- Beamson, G.; Briggs, D. High Resolution XPS of Organic Polymers the Scienta ESCA300 Database; John Wiley & Sons: New York, NY, USA, 1992. [Google Scholar]
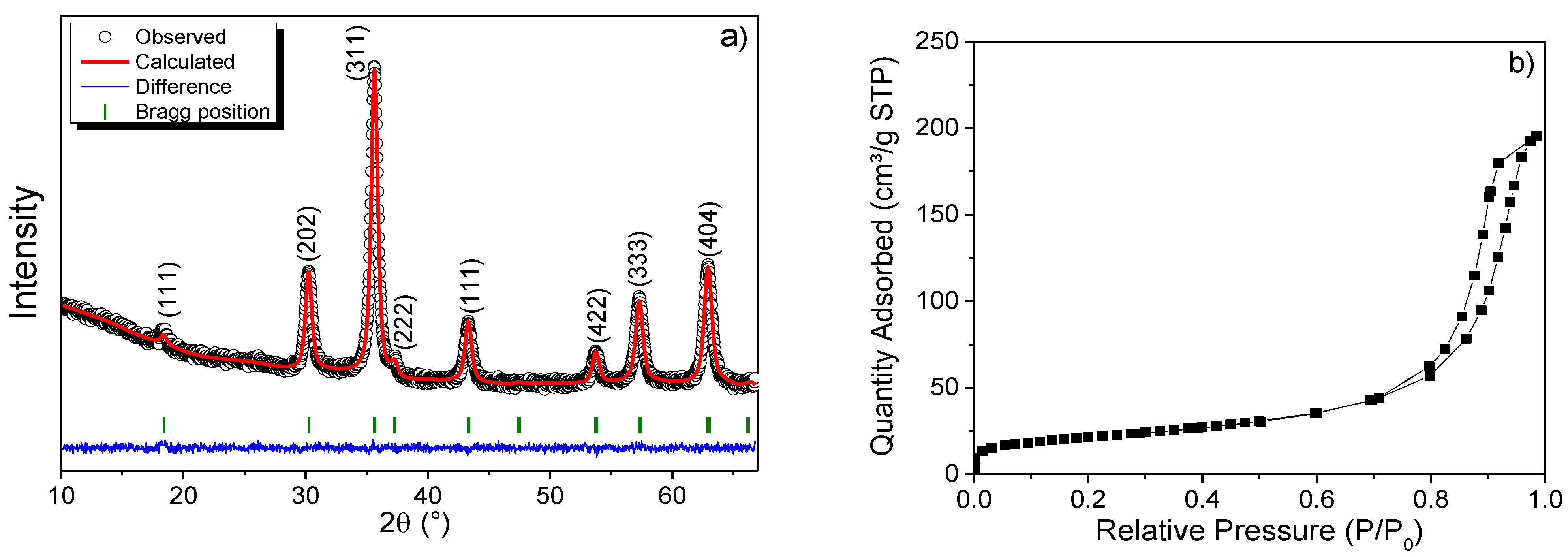
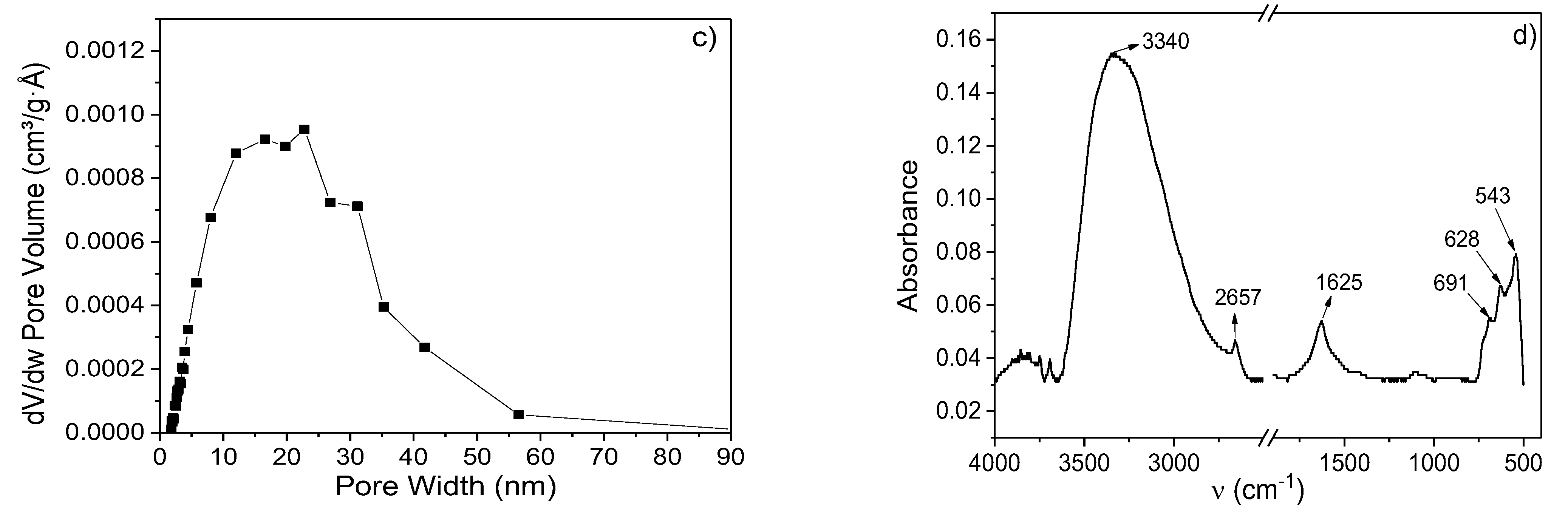
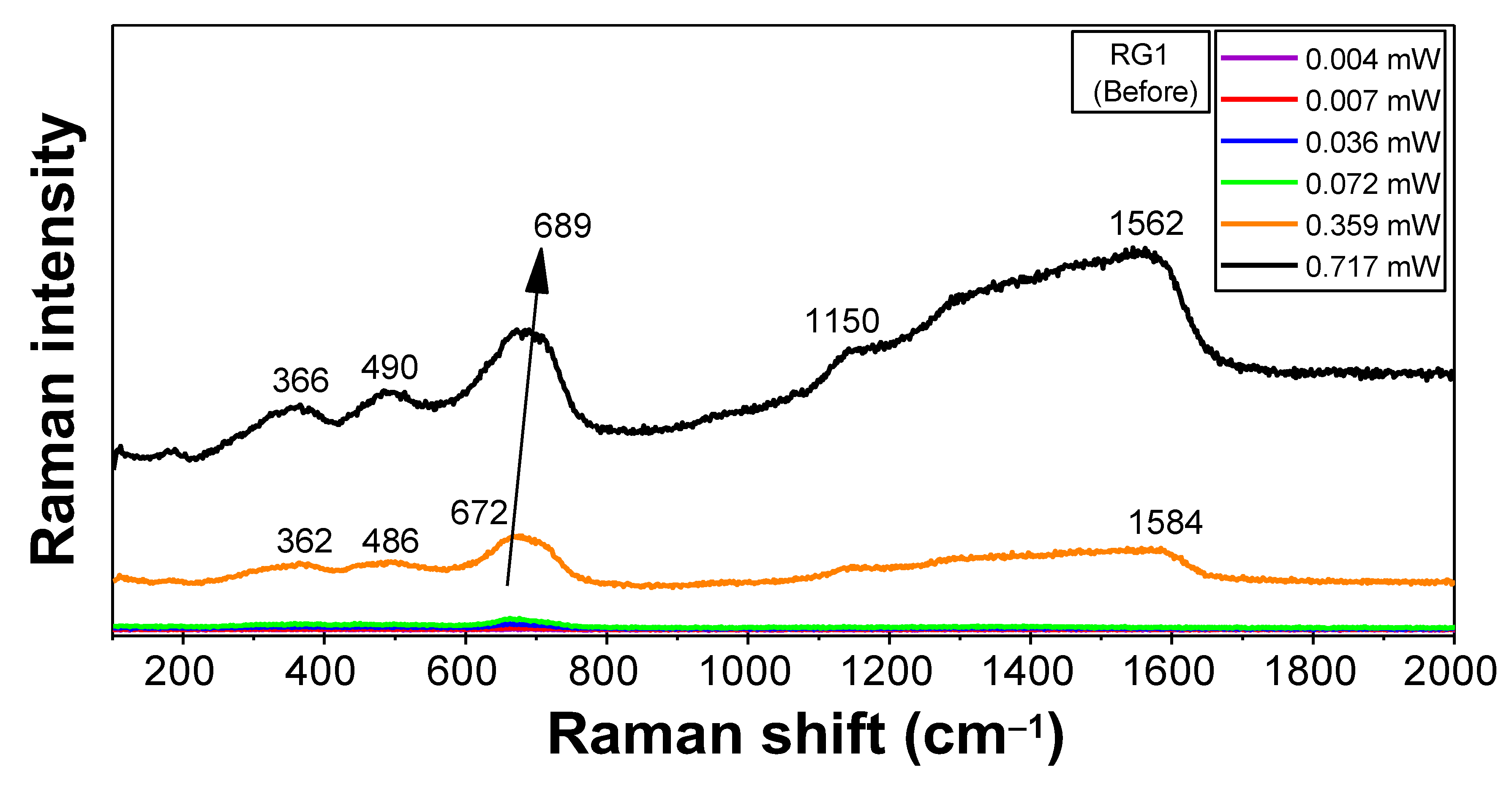



| Sample | Atoms | Atomic Positions |
Thermal Parameters |
Lattice Parameters | Statistical Parameters | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| γ-Fe2O3 | Fe-A | 0.12500 | 0.12500 | 0.12500 | 0.80137 | a = b = c = 8.339 α = β = γ = 90° | Rp = 256.0 % Rwp = 71.4 % Rexp = 68.6 % χ2 = 1.04 |
| Fe-B | 0.50000 | 0.50000 | 0.50000 | 1.87565 | |||
| O | 0.24955 | 0.24930 | 0.24930 | 0.55897 | |||
| Sample | Cumbaza River | Mantaro River |
|---|---|---|
| Intercept | 0.07273 | 1.27702 |
| Slope | 0.02931 | 0.34992 |
| R2 | 0.99977 | 0.99947 |
| qe (mg g−1) | 34.2 | 2.7 |
| k2 (mg g−1 min−1) 10−3 | 12 | 96 |
| Samples | <D> (nm) | Dm (nm) | Standard Deviation | Asymmetry |
|---|---|---|---|---|
| RG1 | 12.65 | 11.74 | 3.5 | 0.85 |
| RG2 | 17.07 | 15.21 | 5.98 | 1.09 |
| RG3 | 16.31 | 14.73 | 5.36 | 1.02 |
| RG4 | 14.29 | 12.37 | 5.63 | 1.24 |
| Sample | Fe (% at) | O (% at) | Pb (% at) |
|---|---|---|---|
| RG1 | 31.2 | 68.8 | - |
| RG2 | 30.2 | 68.8 | 0.9 |
| RG3 | 31.6 | 67.0 | 1.4 |
| RG4 | 29.0 | 70.4 | 0.6 |
| R.A.A. (%) | CS(vs Fe) (mm/s) | B (T) | σ(T) | Q (mm/s) | W (mm/s) | |
|---|---|---|---|---|---|---|
| RG1 17K | ||||||
| A | 38 (2) | 0.303 (5) | 51.8 (2) | 1.1 (1) | 0.00 (2) | 0.39 (1) |
| B | 62 (2) | 0.514 (5) | 52.6 (2) | 1.0 (1) | 0.00 (2) | 0.39 (1) |
| RG3 15K | ||||||
| A | 38 (2) | 0.359 (5) | 51.6 (2) | 1.1 (1) | 0.00 (2) | 0.37 (1) |
| B | 62 (2) | 0.469 (5) | 52.4 (2) | 1.1 (1) | 0.00 (2) | 0.37 (1) |
| RG4 15K | ||||||
| A | 38 (2) | 0.344 (5) | 51.8 (2) | 1.1 (1) | 0.00 (2) | 0.37 (1) |
| B | 62 (2) | 0.569 (5) | 52.5 (2) | 1.0 (1) | 0.00 (2) | 0.37 (1) |
| R.A.A. (%) | CS(vs Fe) (mm/s) | B (T) | σ (T) | Q (mm/s) | W (mm/s) | |
|---|---|---|---|---|---|---|
| RG1 300 K | ||||||
| I,A | 19 (2) | 0.20 (1) | 47.6 (1) | 1.2 (1) | 0.00 (1) | 0.37 (1) |
| I,B | 32 (2) | 0.41 (1) | 47.6 (1) | 1.8 (1) | 0.00 (1) | 0.37 (1) |
| II,A | 16 (2) | 0.22 (1) | 42.0 (1) | 4.5 (2) | 0.00 (1) | 0.37 (1) |
| II,B | 27 (2) | 0.43 (1) | 42.0 (1) | 4.5 (2) | 0.00 (1) | 0.37 (1) |
| III | 5 (2) | 0.30 (4) | 17.4 (3) | 1.0 (2) | −0.17 (5) | 0.37 (1) |
| “impurity“ | 2 (2) | 0.15 (4) | 0.8 (1) | 0.4 (1) | ||
| RG3 300 K | ||||||
| I,A | 17 (2) | 0.23 (1) | 47.5 (1) | 1.9 (1) | 0.00 (1) | 0.37 (1) |
| I,B | 28 (2) | 0.44 (1) | 47.5 (1) | 1.6 (1) | 0.00 (1) | 0.37 (1) |
| II,A | 17 (2) | 0.24 (1) | 41.6 (1) | 5.1 (2) | 0.00 (1) | 0.37 (1) |
| II,B | 28 (2) | 0.45 (1) | 41.6 (1) | 5.1 (2) | 0.00 (1) | 0.37 (1) |
| III | 8 (2) | 0.42 (4) | 17.5 (3) | 1.9 (2) | −0.01 (5) | 0.37 (1) |
| “impurity“ | 2 (2) | 0.15 (4) | 0.8 (1) | 0.4 (1) | ||
| RG4 300 K | ||||||
| I,A | 16 (2) | 0.24 (1) | 47.7 (1) | 1.4 (1) | 0.00 (2) | 0.37 (1) |
| I,B | 26 (2) | 0.45 (1) | 47.7 (1) | 1.6 (1) | 0.00 (2) | 0.37 (1) |
| II,A | 19 (2) | 0.27 (1) | 42.4 (1) | 4.9 (2) | 0.00 (2) | 0.37 (1) |
| II,B | 31 (2) | 0.48 (1) | 42.4 (1) | 4.9 (2) | 0.00 (2) | 0.37 (1) |
| III | 7 (2) | 0.37 (4) | 17.2 (3) | 2.0 (2) | −0.01 (2) | 0.37 (1) |
| “impurity“ | 1 (1) | 0.15 (4) | 0.4 (1) | 0.4 (1) |
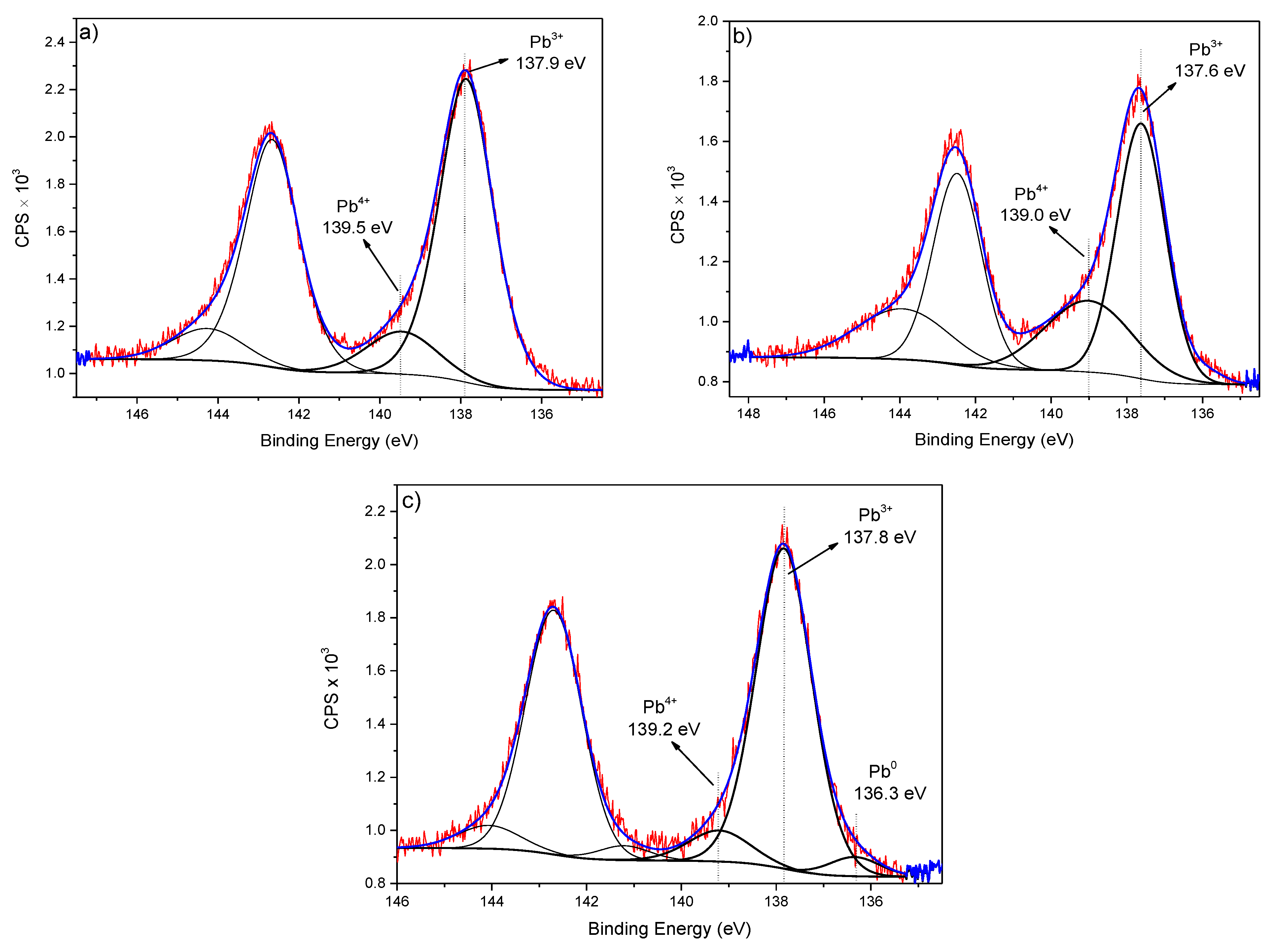
| Sample | Level | BE (eV) | at.% | Bond Type | Ref. |
|---|---|---|---|---|---|
| RG1 | O 1s | 529.5 | 50.4 | O-Metal | [35,36] |
| O 1s | 531.2 | 17.9 | OH-Metal | [32] | |
| O 1s | 533.0 | 11.8 | OH-Organic | [38] | |
| Fe 2p3/2 | 706.4 | 0.5 | Fe0 | [36] | |
| Fe 2p3/2 | 710.2 | 8.1 | Fe3+ (Fe2O3) | [32] | |
| Fe 2p3/2 | 712.0 | 11.4 | Fe3+ (FeOOH) | [35,36] | |
| RG2 | O 1s | 529.4 | 32.6 | O-Metal | [35,36] |
| O 1s | 531.4 | 27.8 | OH-Metal | [32] | |
| O 1s | 533.0 | 24.2 | OH-Organic | [38] | |
| Fe 2p3/2 | 710.1 | 6.3 | Fe3+ (Fe2O3) | [32] | |
| Fe 2p3/2 | 712.6 | 4.6 | Fe3+(FeOOH) | ||
| Pb 4f7/2 | 137.9 | 3.8 | Pb2+ (PbOx) | [36,37] | |
| Pb 4f7/2 | 139.5 | 0.7 | Pb4+ (complex) | [37] | |
| RG3 | O 1s | 529.1 | 22.0 | O-Metal | [35,36] |
| O 1s | 531.7 | 51.6 | OH-Metal | [32] | |
| O 1s | 533.5 | 13.6 | OH-Organic | [38] | |
| Fe 2p3/2 | 706.0 | 0.6 | Fe0 | [36] | |
| Fe 2p3/2 | 710.0 | 5.9 | Fe2+ (Fe2O3) | [32] | |
| Fe 2p3/2 | 712.6 | 2.5 | Fe3+(FeOOH) | ||
| Pb 4f7/2 | 137.6 | 2.5 | Pb2+ (PbOx) | [36,37] | |
| Pb 4f7/2 | 139.0 | 1.2 | Pb4+ (complex) | [37] | |
| RG4 | O 1s | 529.4 | 36.5 | O-Metal | [35,36] |
| O 1s | 530.8 | 6.2 | O-Organic | [36,38] | |
| O 1s | 531.8 | 12.5 | OH-Metal | [32] | |
| O 1s | 533.2 | 16.6 | OH-Organic | [38] | |
| Fe 2p3/2 | 710.0 | 5.9 | Fe3+ (Fe2O3) | [32] | |
| Fe 2p3/2 | 712.1 | 8.9 | Fe3+ (FeOOH) | [36] | |
| Si 2p3/2 | 100.5 | 10.1 | Si-C | [36] | |
| Pb 4f7/2 | 136.3 | 0.2 | Pb0 | [36] | |
| Pb 4f7/2 | 137.8 | 2.8 | Pb2+ (PbOx) | [36,37] | |
| Pb 4f7/2 | 139.2 | 0.3 | Pb4+ (complex) | [37] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos-Guivar, J.A.; Checca-Huaman, N.-R.; Litterst, F.J.; Passamani, E.C. Surface Adsorption Mechanism between Lead(II,IV) and Nanomaghemite Studied on Polluted Water Samples Collected from the Peruvian Rivers Mantaro and Cumbaza. Nanomaterials 2023, 13, 1684. https://doi.org/10.3390/nano13101684
Ramos-Guivar JA, Checca-Huaman N-R, Litterst FJ, Passamani EC. Surface Adsorption Mechanism between Lead(II,IV) and Nanomaghemite Studied on Polluted Water Samples Collected from the Peruvian Rivers Mantaro and Cumbaza. Nanomaterials. 2023; 13(10):1684. https://doi.org/10.3390/nano13101684
Chicago/Turabian StyleRamos-Guivar, Juan A., Noemi-Raquel Checca-Huaman, F. Jochen Litterst, and Edson C. Passamani. 2023. "Surface Adsorption Mechanism between Lead(II,IV) and Nanomaghemite Studied on Polluted Water Samples Collected from the Peruvian Rivers Mantaro and Cumbaza" Nanomaterials 13, no. 10: 1684. https://doi.org/10.3390/nano13101684
APA StyleRamos-Guivar, J. A., Checca-Huaman, N.-R., Litterst, F. J., & Passamani, E. C. (2023). Surface Adsorption Mechanism between Lead(II,IV) and Nanomaghemite Studied on Polluted Water Samples Collected from the Peruvian Rivers Mantaro and Cumbaza. Nanomaterials, 13(10), 1684. https://doi.org/10.3390/nano13101684







