Advancements in Perovskite Nanocrystal Stability Enhancement: A Comprehensive Review
Abstract
1. Introduction
2. Factors Influencing the Stability of PNCs
2.1. Structure Instability
2.2. Surface Instability
2.3. External Factors
3. Strategies to Enhance the Stability of PNCs
3.1. Encapsulation
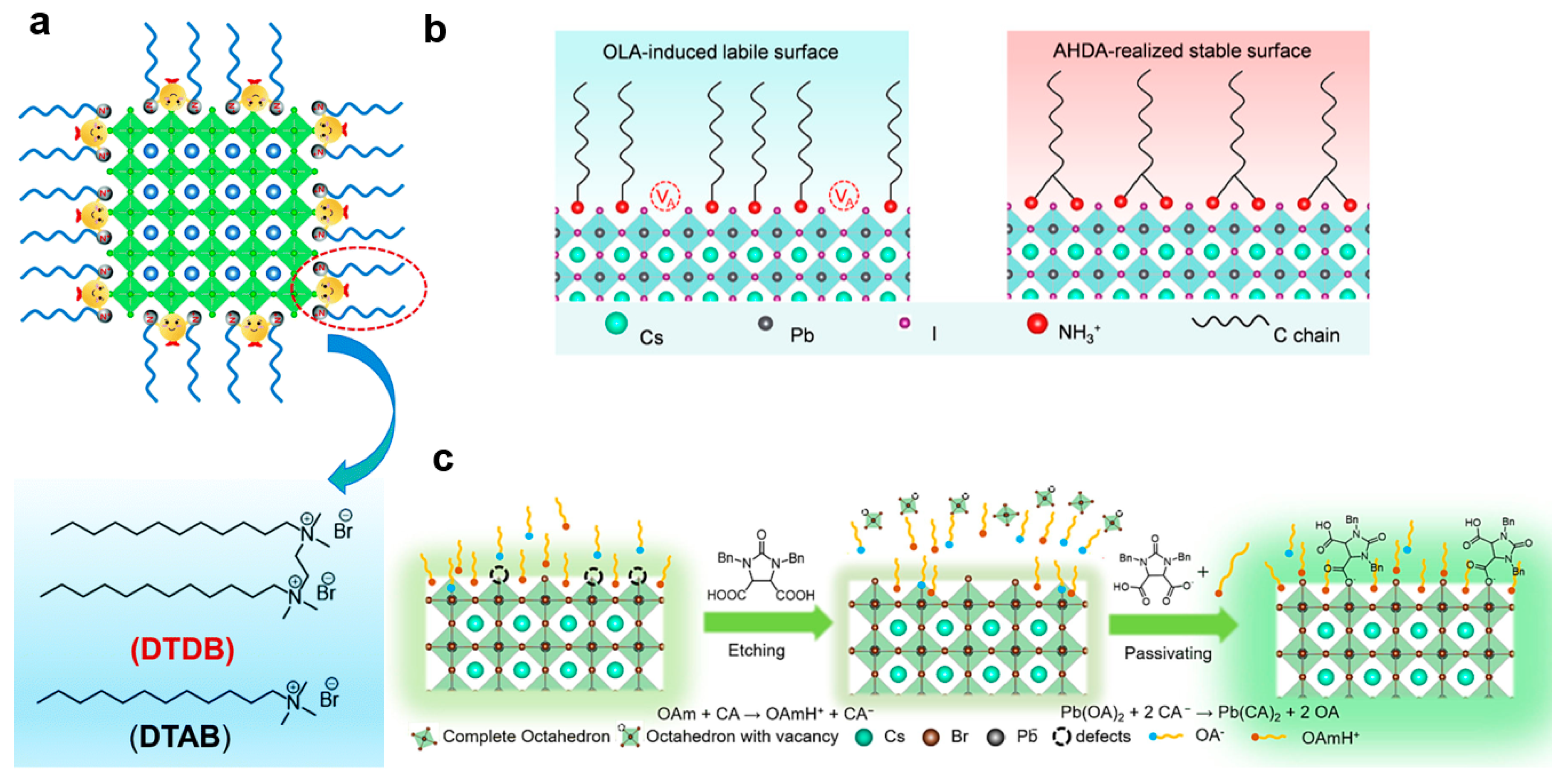
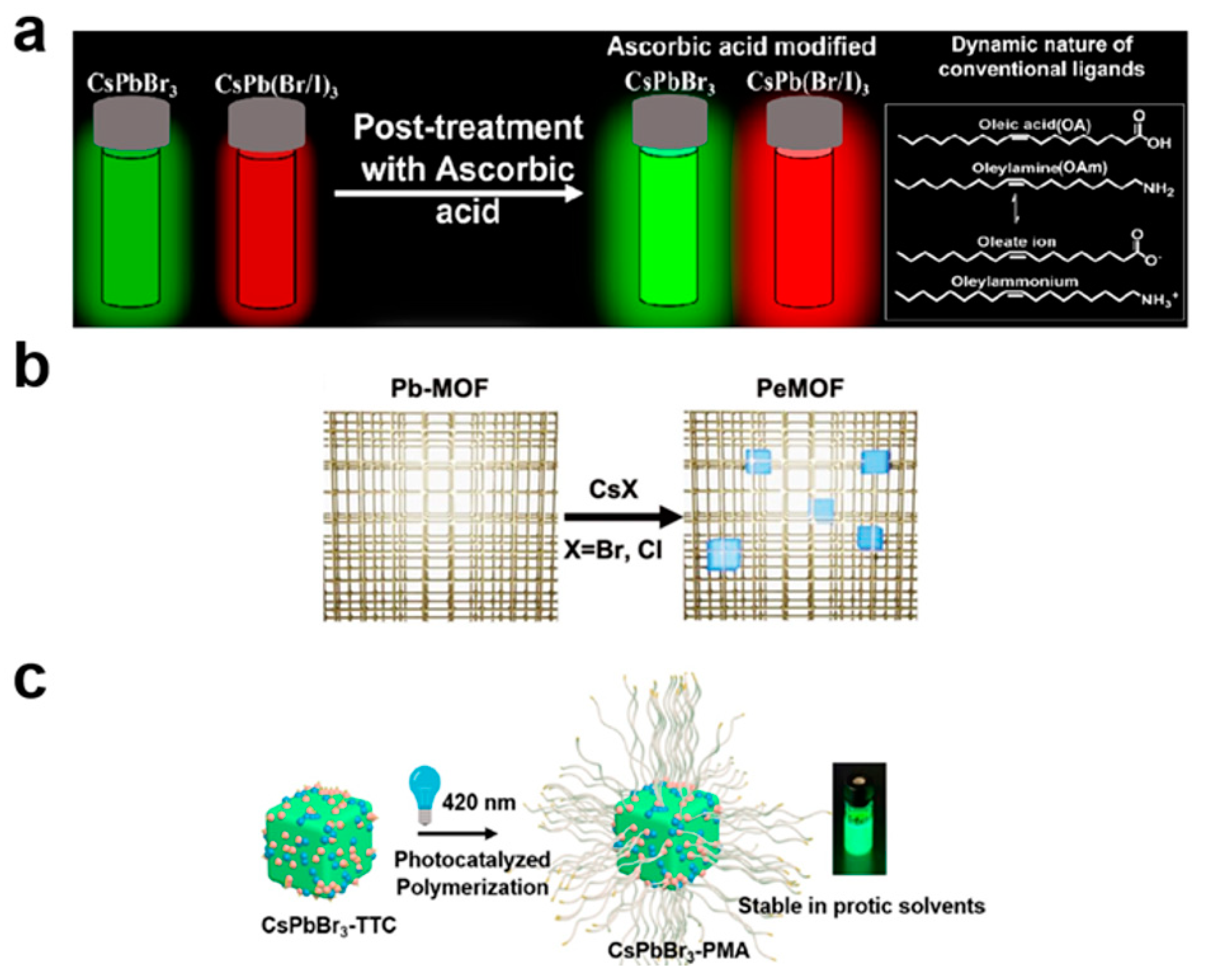
3.2. Ligand Engineering
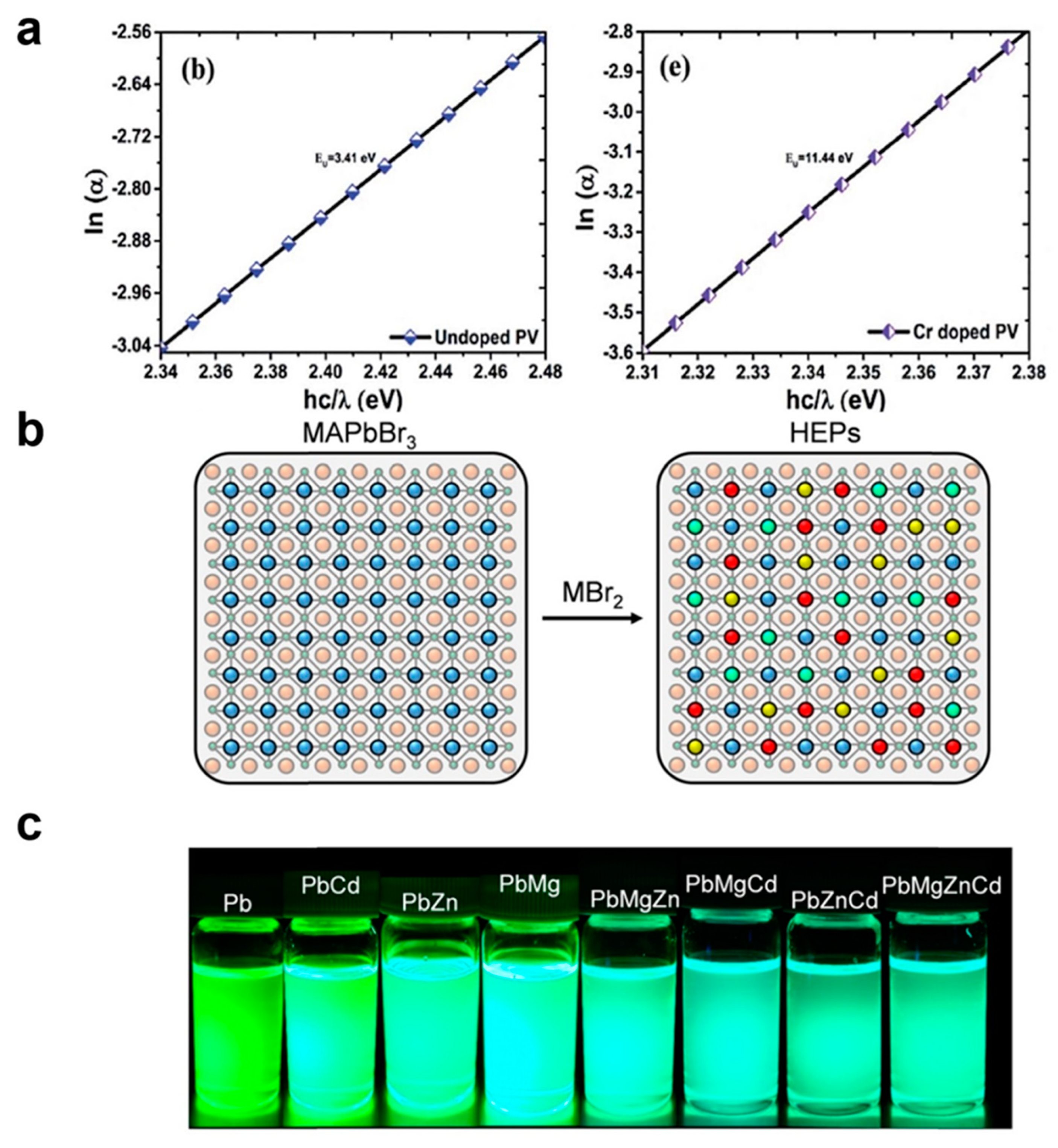
3.3. Metal Cation Dopants
3.4. Optimization of the Fabrication Process
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goldschmidt, V.M. Die gesetze der krystallochemie. Naturwissenschaften 1926, 14, 477–485. [Google Scholar] [CrossRef]
- Lin, N.; Gong, Y.; Wang, R.; Wang, Y.; Zhang, X. Critical review of perovskite-based materials in advanced oxidation system for wastewater treatment: Design, applications and mechanisms. J. Hazard. Mater. 2022, 424, 127637. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ji, X.; Yao, H.; Fan, Q.; Yu, B.; Li, J. Review on efficiency improvement effort of perovskite solar cell. Sol. Energy 2022, 233, 421–434. [Google Scholar] [CrossRef]
- Yusoff, A.R.b.M.; Nazeeruddin, M.K. Low-dimensional perovskites: From synthesis to stability in perovskite solar cells. Adv. Energy Mater. 2018, 8, 1702073. [Google Scholar] [CrossRef]
- Kumar, N.S.; Naidu, K.C.B. A review on perovskite solar cells (PSCs), materials and applications. J. Mater. 2021, 7, 940–956. [Google Scholar]
- Fakharuddin, A.; Gangishetty, M.K.; Abdi-Jalebi, M.; Chin, S.-H.; bin Mohd Yusoff, A.R.; Congreve, D.N.; Tress, W.; Deschler, F.; Vasilopoulou, M.; Bolink, H.J. Perovskite light-emitting diodes. Nat. Electron. 2022, 5, 203–216. [Google Scholar] [CrossRef]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef]
- Im, J.-H.; Lee, C.-R.; Lee, J.-W.; Park, S.-W.; Park, N.-G. 6.5% efficient perovskite quantum-dot-sensitized solar cell. Nanoscale 2011, 3, 4088–4093. [Google Scholar] [CrossRef]
- Burschka, J.; Pellet, N.; Moon, S.-J.; Humphry-Baker, R.; Gao, P.; Nazeeruddin, M.K.; Grätzel, M. Sequential deposition as a route to high-performance perovskite-sensitized solar cells. Nature 2013, 499, 316–319. [Google Scholar] [CrossRef]
- Lim, K.G.; Kim, H.B.; Jeong, J.; Kim, H.; Kim, J.Y.; Lee, T.W. Boosting the power conversion efficiency of perovskite solar cells using self-organized polymeric hole extraction layers with high work function. Adv. Mater. 2014, 26, 6461–6466. [Google Scholar] [CrossRef]
- Zhou, H.; Chen, Q.; Li, G.; Luo, S.; Song, T.-b.; Duan, H.-S.; Hong, Z.; You, J.; Liu, Y.; Yang, Y. Interface engineering of highly efficient perovskite solar cells. Science 2014, 345, 542–546. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.S.; Noh, J.H.; Jeon, N.J.; Kim, Y.C.; Ryu, S.; Seo, J.; Seok, S.I. High-performance photovoltaic perovskite layers fabricated through intramolecular exchange. Science 2015, 348, 1234–1237. [Google Scholar] [CrossRef] [PubMed]
- Jeon, N.J.; Noh, J.H.; Yang, W.S.; Kim, Y.C.; Ryu, S.; Seo, J.; Seok, S.I. Compositional engineering of perovskite materials for high-performance solar cells. Nature 2015, 517, 476–480. [Google Scholar] [CrossRef]
- Yu, J.C.; Kim, D.B.; Baek, G.; Lee, B.R.; Jung, E.D.; Lee, S.; Chu, J.H.; Lee, D.K.; Choi, K.J.; Cho, S. High-Performance Planar Perovskite Optoelectronic Devices: A Morphological and Interfacial Control by Polar Solvent Treatment. Adv. Mater. 2015, 27, 3492–3500. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wu, Y.; Yue, Y.; Liu, J.; Zhang, W.; Yang, X.; Chen, H.; Bi, E.; Ashraful, I.; Grätzel, M. Efficient and stable large-area perovskite solar cells with inorganic charge extraction layers. Science 2015, 350, 944–948. [Google Scholar] [CrossRef]
- Bi, D.; Yi, C.; Luo, J.; Décoppet, J.-D.; Zhang, F.; Zakeeruddin, S.M.; Li, X.; Hagfeldt, A.; Grätzel, M. Polymer-templated nucleation and crystal growth of perovskite films for solar cells with efficiency greater than 21%. Nat. Energy 2016, 1, 16142. [Google Scholar] [CrossRef]
- Sahli, F.; Werner, J.; Kamino, B.A.; Brauninger, M.; Monnard, R.; Paviet-Salomon, B.; Barraud, L.; Ding, L.; Diaz Leon, J.J.; Sacchetto, D.; et al. Fully textured monolithic perovskite/silicon tandem solar cells with 25.2% power conversion efficiency. Nat. Mater. 2018, 17, 820–826. [Google Scholar] [CrossRef]
- Park, J.; Kim, J.; Yun, H.-S.; Paik, M.J.; Noh, E.; Mun, H.J.; Kim, M.G.; Shin, T.J.; Seok, S.I. Controlled growth of perovskite layers with volatile alkylammonium chlorides. Nature 2023, 616, 724–730. [Google Scholar] [CrossRef]
- Schmidt, L.C.; Pertegas, A.; Gonzalez-Carrero, S.; Malinkiewicz, O.; Agouram, S.; Minguez Espallargas, G.; Bolink, H.J.; Galian, R.E.; Perez-Prieto, J. Nontemplate synthesis of CH3NH3PbBr3 perovskite nanoparticles. J. Am. Chem. Soc. 2014, 136, 850–853. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Song, J.; Xiao, L.; Zeng, H.; Sun, H. All-inorganic colloidal perovskite quantum dots: A new class of lasing materials with favorable characteristics. Adv. Mater. 2015, 27, 7101–7108. [Google Scholar] [CrossRef]
- Huang, H.; Susha, A.S.; Kershaw, S.V.; Hung, T.F.; Rogach, A.L. Control of emission color of high quantum yield CH3NH3PbBr3 perovskite quantum dots by precipitation temperature. Adv. Sci. 2015, 2, 1500194. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wu, Z.; Shao, J.; Yao, D.; Gao, H.; Liu, Y.; Yu, W.; Zhang, H.; Yang, B. CsPb x Mn1–x Cl3 perovskite quantum dots with high mn substitution ratio. ACS Nano 2017, 11, 2239–2247. [Google Scholar] [CrossRef]
- Zhu, H.; Fu, Y.; Meng, F.; Wu, X.; Gong, Z.; Ding, Q.; Gustafsson, M.V.; Trinh, M.T.; Jin, S.; Zhu, X. Lead halide perovskite nanowire lasers with low lasing thresholds and high quality factors. Nat. Mater. 2015, 14, 636–642. [Google Scholar] [CrossRef]
- Liu, P.; He, X.; Ren, J.; Liao, Q.; Yao, J.; Fu, H. Organic–inorganic hybrid perovskite nanowire laser arrays. ACS Nano 2017, 11, 5766–5773. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Poddar, S.; Lin, Y.; Long, Z.; Zhang, D.; Zhang, Q.; Shu, L.; Qiu, X.; Kam, M.; Javey, A. A biomimetic eye with a hemispherical perovskite nanowire array retina. Nature 2020, 581, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Urban, J.J.; Yun, W.S.; Gu, Q.; Park, H. Synthesis of single-crystalline perovskite nanorods composed of barium titanate and strontium titanate. J. Am. Chem. Soc. 2002, 124, 1186–1187. [Google Scholar] [CrossRef]
- Aharon, S.; Etgar, L. Two dimensional organometal halide perovskite nanorods with tunable optical properties. Nano Lett. 2016, 16, 3230–3235. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhou, W.; Zhong, Y.; Bu, Y.; Chen, X.; Zhong, Q.; Liu, M.; Shao, Z. A perovskite nanorod as bifunctional electrocatalyst for overall water splitting. Adv. Energy Mater. 2017, 7, 1602122. [Google Scholar] [CrossRef]
- Ebina, Y.; Sasaki, T.; Harada, M.; Watanabe, M. Restacked perovskite nanosheets and their Pt-loaded materials as photocatalysts. Chem. Mater. 2002, 14, 4390–4395. [Google Scholar] [CrossRef]
- Li, P.; Chen, Y.; Yang, T.; Wang, Z.; Lin, H.; Xu, Y.; Li, L.; Mu, H.; Shivananju, B.N.; Zhang, Y. Two-dimensional CH3NH3PbI3 perovskite nanosheets for ultrafast pulsed fiber lasers. ACS Appl. Mater. Interfaces 2017, 9, 12759–12765. [Google Scholar] [CrossRef]
- Yang, S.; Niu, W.; Wang, A.L.; Fan, Z.; Chen, B.; Tan, C.; Lu, Q.; Zhang, H. Ultrathin two-dimensional organic–inorganic hybrid perovskite nanosheets with bright, tunable photoluminescence and high stability. Angew. Chem. 2017, 129, 4316–4319. [Google Scholar] [CrossRef]
- Lv, L.; Xu, Y.; Fang, H.; Luo, W.; Xu, F.; Liu, L.; Wang, B.; Zhang, X.; Yang, D.; Hu, W. Generalized colloidal synthesis of high-quality, two-dimensional cesium lead halide perovskite nanosheets and their applications in photodetectors. Nanoscale 2016, 8, 13589–13596. [Google Scholar] [CrossRef] [PubMed]
- Sichert, J.A.; Tong, Y.; Mutz, N.; Vollmer, M.; Fischer, S.; Milowska, K.Z.; García Cortadella, R.; Nickel, B.; Cardenas-Daw, C.; Stolarczyk, J.K. Quantum size effect in organometal halide perovskite nanoplatelets. Nano Lett. 2015, 15, 6521–6527. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, P.; Arveson, S.M.; Tisdale, W.A. Colloidal organohalide perovskite nanoplatelets exhibiting quantum confinement. J. Phys. Chem. Lett. 2015, 6, 1911–1916. [Google Scholar] [CrossRef]
- Ravi, V.K.; Santra, P.K.; Joshi, N.; Chugh, J.; Singh, S.K.; Rensmo, H.; Ghosh, P.; Nag, A. Origin of the substitution mechanism for the binding of organic ligands on the surface of CsPbBr3 perovskite nanocubes. J. Phys. Chem. Lett. 2017, 8, 4988–4994. [Google Scholar] [CrossRef]
- Li, G.; Wang, H.; Zhu, Z.; Chang, Y.; Zhang, T.; Song, Z.; Jiang, Y. Shape and phase evolution from CsPbBr 3 perovskite nanocubes to tetragonal CsPb 2 Br 5 nanosheets with an indirect bandgap. Chem. Commun. 2016, 52, 11296–11299. [Google Scholar] [CrossRef]
- Wei, X.; Liu, H.; Zhang, Z.; Xu, W.; Huang, W.; Luo, L.-B.; Liu, J. Low-temperature architecture of a cubic-phase CsPbBr 3 single crystal for ultrasensitive weak-light photodetectors. Chem. Commun. 2021, 57, 7798–7801. [Google Scholar] [CrossRef]
- Fu, H. Colloidal metal halide perovskite nanocrystals: A promising juggernaut in photovoltaic applications. J. Mater. Chem. A 2019, 7, 14357–14379. [Google Scholar] [CrossRef]
- Wang, A.; Yan, X.; Zhang, M.; Sun, S.; Yang, M.; Shen, W.; Pan, X.; Wang, P.; Deng, Z. Controlled synthesis of lead-free and stable perovskite derivative Cs2SnI6 nanocrystals via a facile hot-injection process. Chem. Mater. 2016, 28, 8132–8140. [Google Scholar] [CrossRef]
- Parobek, D.; Dong, Y.; Qiao, T.; Son, D.H. Direct hot-injection synthesis of Mn-doped CsPbBr3 nanocrystals. Chem. Mater. 2018, 30, 2939–2944. [Google Scholar] [CrossRef]
- Han, D.; Imran, M.; Zhang, M.; Chang, S.; Wu, X.-g.; Zhang, X.; Tang, J.; Wang, M.; Ali, S.; Li, X. Efficient light-emitting diodes based on in situ fabricated FAPbBr3 nanocrystals: The enhancing role of the ligand-assisted reprecipitation process. ACS Nano 2018, 12, 8808–8816. [Google Scholar] [CrossRef] [PubMed]
- Minh, D.N.; Kim, J.; Hyon, J.; Sim, J.H.; Sowlih, H.H.; Seo, C.; Nam, J.; Eom, S.; Suk, S.; Lee, S. Room-temperature synthesis of widely tunable formamidinium lead halide perovskite nanocrystals. Chem. Mater. 2017, 29, 5713–5719. [Google Scholar] [CrossRef]
- Huang, H.; Zhao, F.; Liu, L.; Zhang, F.; Wu, X.-g.; Shi, L.; Zou, B.; Pei, Q.; Zhong, H. Emulsion synthesis of size-tunable CH3NH3PbBr3 quantum dots: An alternative route toward efficient light-emitting diodes. ACS Appl. Mater. Interfaces 2015, 7, 28128–28133. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Khan, K.; Mahmood, A.; Murtaza, G.; Akhtar, M.N.; Ali, I.; Shahid, M.; Shakir, I.; Warsi, M.F. Nanocrystalline La1−xSrxCo1−yFeyO3 perovskites fabricated by the micro-emulsion route for high frequency response devices fabrications. Ceram. Int. 2014, 40, 13211–13216. [Google Scholar] [CrossRef]
- Jang, D.M.; Kim, D.H.; Park, K.; Park, J.; Lee, J.W.; Song, J.K. Ultrasound synthesis of lead halide perovskite nanocrystals. J. Mater. Chem. C 2016, 4, 10625–10629. [Google Scholar] [CrossRef]
- Moghtada, A.; Ashiri, R. Enhancing the formation of tetragonal phase in perovskite nanocrystals using an ultrasound assisted wet chemical method. Ultrason. Sonochem. 2016, 33, 141–149. [Google Scholar] [CrossRef]
- Li, Y.; Huang, H.; Xiong, Y.; Kershaw, S.V.; Rogach, A.L. Revealing the formation mechanism of CsPbBr3 perovskite nanocrystals produced via a slowed-down microwave-assisted synthesis. Angew. Chem. Int. Ed. 2018, 57, 5833–5837. [Google Scholar] [CrossRef]
- Pan, Q.; Hu, H.; Zou, Y.; Chen, M.; Wu, L.; Yang, D.; Yuan, X.; Fan, J.; Sun, B.; Zhang, Q. Microwave-assisted synthesis of high-quality “all-inorganic” CsPbX 3 (X = Cl, Br, I) perovskite nanocrystals and their application in light emitting diodes. J. Mater. Chem. C 2017, 5, 10947–10954. [Google Scholar] [CrossRef]
- Chen, M.; Zou, Y.; Wu, L.; Pan, Q.; Yang, D.; Hu, H.; Tan, Y.; Zhong, Q.; Xu, Y.; Liu, H. Solvothermal synthesis of high-quality all-inorganic cesium lead halide perovskite nanocrystals: From nanocube to ultrathin nanowire. Adv. Funct. Mater. 2017, 27, 1701121. [Google Scholar] [CrossRef]
- Caruntu, D.; Rostamzadeh, T.; Costanzo, T.; Parizi, S.S.; Caruntu, G. Solvothermal synthesis and controlled self-assembly of monodisperse titanium-based perovskite colloidal nanocrystals. Nanoscale 2015, 7, 12955–12969. [Google Scholar] [CrossRef]
- Zhai, W.; Lin, J.; Li, Q.; Zheng, K.; Huang, Y.; Yao, Y.; He, X.; Li, L.; Yu, C.; Liu, C. Solvothermal synthesis of ultrathin cesium lead halide perovskite nanoplatelets with tunable lateral sizes and their reversible transformation into Cs4PbBr6 nanocrystals. Chem. Mater. 2018, 30, 3714–3721. [Google Scholar] [CrossRef]
- Lignos, I.; Stavrakis, S.; Nedelcu, G.; Protesescu, L.; deMello, A.J.; Kovalenko, M.V. Synthesis of cesium lead halide perovskite nanocrystals in a droplet-based microfluidic platform: Fast parametric space mapping. Nano Lett. 2016, 16, 1869–1877. [Google Scholar] [CrossRef]
- Epps, R.W.; Felton, K.C.; Coley, C.W.; Abolhasani, M. Automated microfluidic platform for systematic studies of colloidal perovskite nanocrystals: Towards continuous nano-manufacturing. Lab A Chip 2017, 17, 4040–4047. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Du, X.-Y.; Zhang, Y.-W.; Chen, S. In situ fabrication of halide perovskite nanocrystals embedded in polymer composites via microfluidic spinning microreactors. J. Mater. Chem. C 2017, 5, 9398–9404. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, C.; Zheng, W.; Zhang, Q.; Bao, Z.; Kong, L.; Li, L. Large-scale synthesis of highly luminescent perovskite nanocrystals by template-assisted solid-state reaction at 800 °C. Chem. Mater. 2019, 32, 308–314. [Google Scholar] [CrossRef]
- Vila-Liarte, D.; Kotov, N.A.; Liz-Marzán, L.M. Template-assisted self-assembly of achiral plasmonic nanoparticles into chiral structures. Chem. Sci. 2022, 13, 595–610. [Google Scholar] [CrossRef] [PubMed]
- Anuradha, T. Template-assisted sol-gel synthesis of nanocrystalline BaTiO3. E-J. Chem. 2014, 7, 894–898. [Google Scholar] [CrossRef]
- Wang, S.; Yousefi Amin, A.A.; Wu, L.; Cao, M.; Zhang, Q.; Ameri, T. Perovskite Nanocrystals: Synthesis, Stability, and Optoelectronic Applications. Small Struct. 2021, 2, 2000124. [Google Scholar] [CrossRef]
- Nie, W.; Tsai, H. Perovskite nanocrystals stabilized in metal–organic frameworks for light emission devices. J. Mater. Chem. A 2022, 10, 19518–19533. [Google Scholar] [CrossRef]
- Shamsi, J.; Urban, A.S.; Imran, M.; De Trizio, L.; Manna, L. Metal halide perovskite nanocrystals: Synthesis, post-synthesis modifications, and their optical properties. Chem. Rev. 2019, 119, 3296–3348. [Google Scholar] [CrossRef]
- Fan, Q.; Biesold-McGee, G.V.; Ma, J.; Xu, Q.; Pan, S.; Peng, J.; Lin, Z. Lead-free halide perovskite nanocrystals: Crystal structures, synthesis, stabilities, and optical properties. Angew. Chem. Int. Ed. 2020, 59, 1030–1046. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Liu, W.; Zhang, Q. Perovskite-based nanocrystals: Synthesis and applications beyond solar cells. Small Methods 2018, 2, 1700380. [Google Scholar] [CrossRef]
- Nedelcu, G.; Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Grotevent, M.J.; Kovalenko, M.V. Fast anion-exchange in highly luminescent nanocrystals of cesium lead halide perovskites (CsPbX3, X = Cl, Br, I). Nano Lett. 2015, 15, 5635–5640. [Google Scholar] [CrossRef] [PubMed]
- Akkerman, Q.A.; D’Innocenzo, V.; Accornero, S.; Scarpellini, A.; Petrozza, A.; Prato, M.; Manna, L. Tuning the optical properties of cesium lead halide perovskite nanocrystals by anion exchange reactions. J. Am. Chem. Soc. 2015, 137, 10276–10281. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Dong, Q.; Gundogdu, K.; So, F. Metal halide perovskites for laser applications. Adv. Funct. Mater. 2021, 31, 2010144. [Google Scholar] [CrossRef]
- Stylianakis, M.M.; Maksudov, T.; Panagiotopoulos, A.; Kakavelakis, G.; Petridis, K. Inorganic and hybrid perovskite based laser devices: A review. Materials 2019, 12, 859. [Google Scholar] [CrossRef]
- Huang, S.; Zhengzheng, L.; Juan, D.; Yuxin, L. Review of Perovskite Micro-and Nano-Lasers. Laser Optoelectron. Prog. 2020, 57, 071602. [Google Scholar] [CrossRef]
- Swarnkar, A.; Chulliyil, R.; Ravi, V.K.; Irfanullah, M.; Chowdhury, A.; Nag, A. Colloidal CsPbBr3 perovskite nanocrystals: Luminescence beyond traditional quantum dots. Angew. Chem. 2015, 127, 15644–15648. [Google Scholar] [CrossRef]
- Lin, K.; Xing, J.; Quan, L.N.; de Arquer, F.P.G.; Gong, X.; Lu, J.; Xie, L.; Zhao, W.; Zhang, D.; Yan, C. Perovskite light-emitting diodes with external quantum efficiency exceeding 20 per cent. Nature 2018, 562, 245–248. [Google Scholar] [CrossRef]
- Stranks, S.D.; Eperon, G.E.; Grancini, G.; Menelaou, C.; Alcocer, M.J.; Leijtens, T.; Herz, L.M.; Petrozza, A.; Snaith, H.J. Electron-hole diffusion lengths exceeding 1 micrometer in an organometal trihalide perovskite absorber. Science 2013, 342, 341–344. [Google Scholar] [CrossRef]
- Xing, G.; Mathews, N.; Sun, S.; Lim, S.S.; Lam, Y.M.; Gratzel, M.; Mhaisalkar, S.; Sum, T.C. Long-range balanced electron- and hole-transport lengths in organic-inorganic CH3NH3PbI3. Science 2013, 342, 344–347. [Google Scholar] [CrossRef] [PubMed]
- Green, M.A.; Ho-Baillie, A.; Snaith, H.J. The emergence of perovskite solar cells. Nat. Photonics 2014, 8, 506–514. [Google Scholar] [CrossRef]
- Oku, T. Crystal Structures of CH3NH3PbI3 and Related Perovskite Compounds Used for Solar Cells. In Solar Cells—New Approaches and Reviews; InTech: Rijeka, Croatia, 2015. [Google Scholar]
- Huang, Y.; Yin, W.-J.; He, Y. Intrinsic Point Defects in Inorganic Cesium Lead Iodide Perovskite CsPbI3. J. Phys. Chem. C 2018, 122, 1345–1350. [Google Scholar] [CrossRef]
- Sutton, R.J.; Filip, M.R.; Haghighirad, A.A.; Sakai, N.; Wenger, B.; Giustino, F.; Snaith, H.J. Cubic or Orthorhombic? Revealing the Crystal Structure of Metastable Black-Phase CsPbI3 by Theory and Experiment. ACS Energy Lett. 2018, 3, 1787–1794. [Google Scholar] [CrossRef]
- Yang, R.X.; Tan, L.Z. Understanding size dependence of phase stability and band gap in CsPbI3 perovskite nanocrystals. J. Chem. Phys. 2020, 152, 034702. [Google Scholar] [CrossRef] [PubMed]
- De Roo, J.; Ibáñez, M.; Geiregat, P.; Nedelcu, G.; Walravens, W.; Maes, J.; Martins, J.C.; Van Driessche, I.; Kovalenko, M.V.; Hens, Z. Highly dynamic ligand binding and light absorption coefficient of cesium lead bromide perovskite nanocrystals. ACS Nano 2016, 10, 2071–2081. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Li, Z.; Wang, B.; Zhu, N.; Zhang, C.; Kong, L.; Zhang, Q.; Shan, A.; Li, L. Morphology evolution and degradation of CsPbBr3 nanocrystals under blue light-emitting diode illumination. ACS Appl. Mater. Interfaces 2017, 9, 7249–7258. [Google Scholar] [CrossRef]
- Aristidou, N.; Eames, C.; Sanchez-Molina, I.; Bu, X.; Kosco, J.; Islam, M.S.; Haque, S.A. Fast oxygen diffusion and iodide defects mediate oxygen-induced degradation of perovskite solar cells. Nat. Commun. 2017, 8, 15218. [Google Scholar] [CrossRef]
- Hoke, E.T.; Slotcavage, D.J.; Dohner, E.R.; Bowring, A.R.; Karunadasa, H.I.; McGehee, M.D. Reversible photo-induced trap formation in mixed-halide hybrid perovskites for photovoltaics. Chem. Sci. 2015, 6, 613–617. [Google Scholar] [CrossRef]
- Kulbak, M.; Gupta, S.; Kedem, N.; Levine, I.; Bendikov, T.; Hodes, G.; Cahen, D. Cesium enhances long-term stability of lead bromide perovskite-based solar cells. J. Phys. Chem. Lett. 2016, 7, 167–172. [Google Scholar] [CrossRef]
- Dualeh, A.; Gao, P.; Seok, S.I.; Nazeeruddin, M.K.; Grätzel, M. Thermal behavior of methylammonium lead-trihalide perovskite photovoltaic light harvesters. Chem. Mater. 2014, 26, 6160–6164. [Google Scholar] [CrossRef]
- Gąsiorowski, M.; Dasgupta, S.; Bychto, L.; Ahmad, T.; Szymak, P.; Wojciechowski, K.; Patryn, A. Analysis of Perovskite Solar Cell Degradation over Time Using NIR Spectroscopy—A Novel Approach. Energies 2022, 15, 5397. [Google Scholar] [CrossRef]
- Zhang, T.; Meng, X.; Bai, Y.; Xiao, S.; Hu, C.; Yang, Y.; Chen, H.; Yang, S. Profiling the organic cation-dependent degradation of organolead halide perovskite solar cells. J. Mater. Chem. A 2017, 5, 1103–1111. [Google Scholar] [CrossRef]
- Zhidkov, I.S.; Poteryaev, A.I.; Kukharenko, A.I.; Finkelstein, L.D.; Cholakh, S.O.; Akbulatov, A.F.; Troshin, P.A.; Chueh, C.-C.; Kurmaev, E.Z. XPS evidence of degradation mechanism in CH3NH3PbI3 hybrid perovskite. J. Phys. Condens. Matter 2019, 32, 095501. [Google Scholar] [CrossRef] [PubMed]
- Balazs, A.C.; Emrick, T.; Russell, T.P. Nanoparticle polymer composites: Where two small worlds meet. Science 2006, 314, 1107–1110. [Google Scholar] [CrossRef]
- Dirin, D.N.; Protesescu, L.; Trummer, D.; Kochetygov, I.V.; Yakunin, S.; Krumeich, F.; Stadie, N.P.; Kovalenko, M.V. Harnessing defect-tolerance at the nanoscale: Highly luminescent lead halide perovskite nanocrystals in mesoporous silica matrixes. Nano Lett. 2016, 16, 5866–5874. [Google Scholar] [CrossRef]
- Avugadda, S.K.; Castelli, A.; Dhanabalan, B.; Fernandez, T.; Silvestri, N.; Collantes, C.; Baranov, D.; Imran, M.; Manna, L.; Pellegrino, T.; et al. Highly Emitting Perovskite Nanocrystals with 2-Year Stability in Water through an Automated Polymer Encapsulation for Bioimaging. ACS Nano 2022, 16, 13657–13666. [Google Scholar] [CrossRef]
- Talianov, P.M.; Yakubova, A.A.; Bukreeva, A.; Masharin, M.; Eliseev, I.E.; Zelenkov, L.; Muslimov, A.R.; Bukatin, A.; Gordeeva, A.; Kudryavtseva, V.; et al. Incorporation of Perovskite Nanocrystals into Polymer Matrix for Enhanced Stability in Biological Media: In Vitro and In Vivo Studies. ACS Appl. Bio Mater. 2022, 5, 2411–2420. [Google Scholar] [CrossRef]
- Chen, J.; Huang, X.; Xu, Z.; Chi, Y. Alcohol-Stable Perovskite Nanocrystals and Their In Situ Capsulation with Polystyrene. ACS Appl. Mater. Interfaces 2022, 14, 33703–33711. [Google Scholar] [CrossRef]
- Laishram, D.; Zeng, S.; Alam, K.M.; Kalra, A.P.; Cui, K.; Kumar, P.; Sharma, R.K.; Shankar, K. Air- and water-stable halide perovskite nanocrystals protected with nearly-monolayer carbon nitride for CO2 photoreduction and water splitting. Appl. Surf. Sci. 2022, 592, 153276. [Google Scholar] [CrossRef]
- Cao, Y.; Shao, Y.; Zhang, J.; Chen, C.; Wang, Q. The photothermal stability study of silica-coated CsPbBr3 perovskite nanocrystals. J. Solid State Chem. 2022, 311, 123086. [Google Scholar] [CrossRef]
- Huangfu, C.; Wang, Y.; Wang, Z.; Hu, Q.; Feng, L. A stable and humidity resistant NH3 sensor based on luminous CsPbBr3 perovskite nanocrystals. Talanta 2023, 253, 124070. [Google Scholar] [CrossRef]
- Azar, M.H.; Mohammadi, M.; Rezaei, N.T.; Aynehband, S.; Simchi, A. Effect of silica encapsulation on the stability and photoluminescence emission of FAPbI3 nanocrystals for white-light-emitting perovskite diodes. J. Alloys Compd. 2022, 907, 164465. [Google Scholar] [CrossRef]
- Gao, D.; Zhang, Y.; Lyu, B.; Guo, X.; Hou, Y.; Ma, J.; Yu, B.; Chen, S. Encapsulation of Pb-Free CsSnCl(3) Perovskite Nanocrystals with Bone Gelatin: Enhanced Stability and Application in Fe(3+) Sensing. Inorg. Chem. 2022, 61, 6547–6554. [Google Scholar] [CrossRef]
- Wang, S.; Wang, H.; Zhang, D.; Dou, Y.; Li, W.; Cao, F.; Yin, L.; Wang, L.; Zhang, Z.-J.; Zhang, J.; et al. Perovskite nanocrystals-polymer composites with a micro/nano structured superhydrophobic surface for stable and efficient white light-emitting diodes. Chem. Eng. J. 2022, 437, 135303. [Google Scholar] [CrossRef]
- Karabel Ocal, S.; Kiremitler, N.B.; Yazici, A.F.; Celik, N.; Mutlugun, E.; Onses, M.S. Natural Wax-Stabilized Perovskite Nanocrystals as Pen-on-Paper Inks and Doughs. ACS Appl. Nano Mater. 2022, 5, 6201–6212. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, L.; Li, B.; Xu, Y. Improved stability of all-inorganic perovskite nanocrystals in hierarchical ZSM-5 zeolites for multimodal applications. Chem. Eng. J. 2022, 437, 135290. [Google Scholar] [CrossRef]
- Udayabhaskararao, T.; Houben, L.; Cohen, H.; Menahem, M.; Pinkas, I.; Avram, L.; Wolf, T.; Teitelboim, A.; Leskes, M.; Yaffe, O. A mechanistic study of phase transformation in perovskite nanocrystals driven by ligand passivation. Chem. Mater. 2018, 30, 84–93. [Google Scholar] [CrossRef]
- Krieg, F.; Ochsenbein, S.T.; Yakunin, S.; Ten Brinck, S.; Aellen, P.; Süess, A.; Clerc, B.; Guggisberg, D.; Nazarenko, O.; Shynkarenko, Y. Colloidal CsPbX3 (X = Cl, Br, I) nanocrystals 2.0: Zwitterionic capping ligands for improved durability and stability. ACS Energy Lett. 2018, 3, 641–646. [Google Scholar] [CrossRef]
- Akhil, S.; Dutt, V.V.; Mishra, N. Amine-Free Synthesis of Colloidal Cesium Lead Halide Perovskite Nanocrystals. ChemNanoMat 2021, 7, 342–353. [Google Scholar] [CrossRef]
- Pan, A.; He, B.; Fan, X.; Liu, Z.; Urban, J.J.; Alivisatos, A.P.; He, L.; Liu, Y. Insight into the ligand-mediated synthesis of colloidal CsPbBr3 perovskite nanocrystals: The role of organic acid, base, and cesium precursors. ACS Nano 2016, 10, 7943–7954. [Google Scholar] [CrossRef] [PubMed]
- Alivisatos, A.P. Semiconductor clusters, nanocrystals, and quantum dots. Science 1996, 271, 933–937. [Google Scholar] [CrossRef]
- Talapin, D.V.; Lee, J.-S.; Kovalenko, M.V.; Shevchenko, E.V. Prospects of colloidal nanocrystals for electronic and optoelectronic applications. Chem. Rev. 2010, 110, 389–458. [Google Scholar] [CrossRef]
- Burda, C.; Chen, X.; Narayanan, R.; El-Sayed, M.A. Chemistry and properties of nanocrystals of different shapes. Chem. Rev. 2005, 105, 1025–1102. [Google Scholar] [CrossRef] [PubMed]
- Manna, L.; Scher, E.C.; Alivisatos, A.P. Synthesis of soluble and processable rod-, arrow-, teardrop-, and tetrapod-shaped CdSe nanocrystals. J. Am. Chem. Soc. 2000, 122, 12700–12706. [Google Scholar] [CrossRef]
- Kovalenko, M.V.; Scheele, M.; Talapin, D.V. Colloidal nanocrystals with molecular metal chalcogenide surface ligands. Science 2009, 324, 1417–1420. [Google Scholar] [CrossRef] [PubMed]
- Seth, S.; Ahmed, T.; De, A.; Samanta, A. Tackling the defects, stability, and photoluminescence of CsPbX3 perovskite nanocrystals. ACS Energy Lett. 2019, 4, 1610–1618. [Google Scholar] [CrossRef]
- Xu, K.; Vickers, E.T.; Luo, B.; Wang, Q.; A’Lester, C.A.; Wang, H.; Cherrette, V.; Li, X.; Zhang, J.Z. Room temperature synthesis of cesium lead bromide perovskite magic sized clusters with controlled ratio of carboxylic acid and benzylamine capping ligands. Sol. Energy Mater. Sol. Cells 2020, 208, 110341. [Google Scholar] [CrossRef]
- Kazes, M.; Udayabhaskararao, T.; Dey, S.; Oron, D. Effect of surface ligands in perovskite nanocrystals: Extending in and reaching out. Acc. Chem. Res. 2021, 54, 1409–1418. [Google Scholar] [CrossRef]
- Quarta, D.; Imran, M.; Capodilupo, A.L.; Petralanda, U.; van Beek, B.; De Angelis, F.; Manna, L.; Infante, I.; De Trizio, L.; Giansante, C. Stable Ligand Coordination at the Surface of Colloidal CsPbBr3 Nanocrystals. J. Phys. Chem. Lett. 2019, 10, 3715–3726. [Google Scholar] [CrossRef]
- Jia, D.; Chen, J.; Yu, M.; Liu, J.; Johansson, E.M.; Hagfeldt, A.; Zhang, X. Dual passivation of CsPbI3 perovskite nanocrystals with amino acid ligands for efficient quantum dot solar cells. Small 2020, 16, 2001772. [Google Scholar] [CrossRef] [PubMed]
- Koh, W.-K.; Park, S.; Ham, Y. Phosphonic acid stabilized colloidal CsPbX3 (X = Br, I) perovskite nanocrystals and their surface chemistry. ChemistrySelect 2016, 1, 3479–3482. [Google Scholar] [CrossRef]
- Xu, K.; Vickers, E.T.; Rao, L.; Lindley, S.A.; Allen, A.L.C.; Luo, B.; Li, X.; Zhang, J.Z. Synergistic Surface Passivation of CH3NH3PbBr3 Perovskite Quantum Dots with Phosphonic Acid and (3-Aminopropyl) triethoxysilane. Chem. Eur. J. 2019, 25, 5014–5021. [Google Scholar] [CrossRef]
- Ruan, L.; Shen, W.; Wang, A.; Xiang, A.; Deng, Z. Alkyl-thiol ligand-induced shape-and crystalline phase-controlled synthesis of stable perovskite-related CsPb2Br5 nanocrystals at room temperature. J. Phys. Chem. Lett. 2017, 8, 3853–3860. [Google Scholar] [CrossRef] [PubMed]
- Levchuk, I.; Herre, P.; Brandl, M.; Osvet, A.; Hock, R.; Peukert, W.; Schweizer, P.; Spiecker, E.; Batentschuk, M.; Brabec, C.J. Ligand-assisted thickness tailoring of highly luminescent colloidal CH 3 NH 3 PbX 3 (X = Br and I) perovskite nanoplatelets. Chem. Commun. 2017, 53, 244–247. [Google Scholar] [CrossRef]
- Park, J.K.; Heo, J.H.; Kim, B.W.; Im, S.H. Synthesis of post-processable metal halide perovskite nanocrystals via modified ligand-assisted re-precipitation method and their applications to self-powered panchromatic photodetectors. J. Ind. Eng. Chem. 2020, 92, 167–173. [Google Scholar] [CrossRef]
- Li, Y.; Cai, M.; Shen, M.; Cai, Y.; Xie, R.-J. Bidentate aliphatic quaternary ammonium ligand-stabilized CsPbBr3 perovskite nanocrystals with high PLQY (92.3%) and superior stability. J. Mater. Chem. C 2022, 10, 8356–8363. [Google Scholar] [CrossRef]
- Cai, Y.; Zhang, P.; Bai, W.; Lu, L.; Wang, L.; Chen, X.; Xie, R.-J. Synthesizing Bright CsPbBr3 Perovskite Nanocrystals with High Purification Yields and Their Composites with In Situ-Polymerized Styrene for Light-Emitting Diode Applications. ACS Sustain. Chem. Eng. 2022, 10, 7385–7393. [Google Scholar] [CrossRef]
- Cai, Y.; Li, W.; Tian, D.; Shi, S.; Chen, X.; Gao, P.; Xie, R.J. Organic Sulfonium-Stabilized High-Efficiency Cesium or Methylammonium Lead Bromide Perovskite Nanocrystals. Angew. Chem. Int. Ed. 2022, 61, e202209880. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Zhang, X.; Bing, Q.; Xiong, Y.; Yang, F.; Liu, H.; Liu, J.-Y.; Zhang, H.; Zheng, W.; Rogach, A.L.; et al. Surface Stabilization of Colloidal Perovskite Nanocrystals via Multi-amine Chelating Ligands. ACS Energy Lett. 2022, 7, 1963–1970. [Google Scholar] [CrossRef]
- Zhao, Y.; Zeng, H.; Cai, X.; Zhou, Y.; Jiang, Y.; Zheng, X.; Li, L.; Zhang, S.; Luo, L.; Li, W.; et al. Enhancement and recovery of photoluminescence and stability by multifunctional etching ligands treatment for perovskite nanocrystals. J. Energy Chem. 2023, 76, 495–502. [Google Scholar] [CrossRef]
- Pan, Q.; Hu, J.; Fu, J.; Lin, Y.; Zou, C.; Di, D.; Wang, Y.; Zhang, Q.; Cao, M. Ultrahigh Stability of Perovskite Nanocrystals by Using Semiconducting Molecular Species for Displays. ACS Nano 2022, 16, 12253–12261. [Google Scholar] [CrossRef] [PubMed]
- Shu, Y.; Wang, Y.; Guan, J.; Ji, Z.; Xu, Q.; Hu, X. Amphiphilic Polymer Ligand-Assisted Synthesis of Highly Luminescent and Stable Perovskite Nanocrystals for Sweat Fluorescent Sensing. Anal. Chem. 2022, 94, 5415–5424. [Google Scholar] [CrossRef]
- Min, X.; Xie, Q.; Wang, Z.; Wang, X.; Chen, M. Improving the stability and optical properties of CsPbI3 perovskite nanocrystals by 1-Octadecanethiol through surface modification. Mater. Chem. Phys. 2022, 276, 125404. [Google Scholar] [CrossRef]
- Jin, H.; Yeong Park, G.; Kyong Kim, M.; Cha, J.; Seok Ham, D.; Kim, M. Eco-friendly Solvent-Processible and highly luminescent perovskite nanocrystals with polymer zwitterions for Air-Stable optoelectronics. Chem. Eng. J. 2023, 459, 141531. [Google Scholar] [CrossRef]
- Liu, M.; Ma, L.; Xie, K.; Zeng, P.; Wei, S.; Zhang, F.; Li, C.; Wang, F. Efficiently Improved Photoluminescence in Cesium Lead Halide Perovskite Nanocrystals by Using Bis(trifluoromethane)sulfonimide. J. Phys. Chem. Lett. 2022, 13, 1519–1525. [Google Scholar] [CrossRef] [PubMed]
- Zang, S.; Xu, G.; Yang, R.; Zhu, Q.; Liu, H. Stabilization and Fluorescence Enhancement of CsPbX3 (X = Cl, Br, I) Perovskite Nanocrystals with Tungstosilicic Acid. J. Phys. Chem. C 2022, 126, 13032–13042. [Google Scholar] [CrossRef]
- Ahmad, R.; Zdražil, L.; Kalytchuk, S.; Naldoni, A.; Mohammadi, E.; Schmuki, P.; Zboril, R.; Kment, Š. Robust dual cationic ligand for stable and efficient warm-white light emission in lead-free double perovskite nanocrystals. Appl. Mater. Today 2022, 26, 101288. [Google Scholar] [CrossRef]
- Das Adhikari, S.; Guria, A.K.; Pradhan, N. Insights of doping and the photoluminescence properties of Mn-doped perovskite nanocrystals. J. Phys. Chem. Lett. 2019, 10, 2250–2257. [Google Scholar] [CrossRef]
- Pan, G.; Bai, X.; Yang, D.; Chen, X.; Jing, P.; Qu, S.; Zhang, L.; Zhou, D.; Zhu, J.; Xu, W. Doping lanthanide into perovskite nanocrystals: Highly improved and expanded optical properties. Nano Lett. 2017, 17, 8005–8011. [Google Scholar] [CrossRef]
- Guria, A.K.; Dutta, S.K.; Adhikari, S.D.; Pradhan, N. Doping Mn2+ in lead halide perovskite nanocrystals: Successes and challenges. ACS Energy Lett. 2017, 2, 1014–1021. [Google Scholar] [CrossRef]
- Behera, R.K.; Dutta, A.; Ghosh, D.; Bera, S.; Bhattacharyya, S.; Pradhan, N. Doping the smallest Shannon radii transition metal ion Ni (II) for stabilizing α-CsPbI3 perovskite nanocrystals. J. Phys. Chem. Lett. 2019, 10, 7916–7921. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Peng, B.; Bravić, I.; Yu, Y.; Dong, Y.; Liang, R.; Ou, Q.; Monserrat, B.; Zhang, S. Highly efficient blue-emitting CsPbBr3 perovskite nanocrystals through neodymium doping. Adv. Sci. 2020, 7, 2001698. [Google Scholar] [CrossRef] [PubMed]
- Mir, W.J.; Sheikh, T.; Arfin, H.; Xia, Z.; Nag, A. Lanthanide doping in metal halide perovskite nanocrystals: Spectral shifting, quantum cutting and optoelectronic applications. NPG Asia Mater. 2020, 12, 9. [Google Scholar] [CrossRef]
- Mir, W.J.; Mahor, Y.; Lohar, A.; Jagadeeswararao, M.; Das, S.; Mahamuni, S.; Nag, A. Postsynthesis doping of Mn and Yb into CsPbX3 (X= Cl, Br, or I) perovskite nanocrystals for downconversion emission. Chem. Mater. 2018, 30, 8170–8178. [Google Scholar] [CrossRef]
- Mohapatra, A.; Kar, M.R.; Bhaumik, S. Suppression of halide migration and improved stability in double-coated cesium lead halide perovskite nanocrystals for application in down-conversion white-light-emitting diodes. J. Alloys Compd. 2022, 927, 166972. [Google Scholar] [CrossRef]
- Nazim, M.; Parwaz Khan, A.A.; Khan, F.; Cho, S.K.; Ahmad, R. Insertion of metal cations into hybrid organometallic halide perovskite nanocrystals for enhanced stability: Eco-friendly synthesis, lattice strain engineering, and defect chemistry studies. Nanoscale Adv. 2022, 4, 2729–2743. [Google Scholar] [CrossRef]
- Deng, J.; Cui, Y.; Jiang, Z.; Du, R.; Yang, L.; Zhang, X.; He, R.; Zhang, J. Transition metal(ii) ion doping of CsPb2Br5/CsPbBr3 perovskite nanocrystals enables high luminescence efficiency and stability. J. Mater. Chem. C 2022, 10, 18336–18342. [Google Scholar] [CrossRef]
- Solari, S.F.; Poon, L.N.; Worle, M.; Krumeich, F.; Li, Y.T.; Chiu, Y.C.; Shih, C.J. Stabilization of Lead-Reduced Metal Halide Perovskite Nanocrystals by High-Entropy Alloying. J. Am. Chem. Soc. 2022, 144, 5864–5870. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Shao, H.; Wu, X.; Chen, W.; Zhu, J.; Dong, B.; Xu, L.; Xu, W.; Hu, J.; Zhou, M.; et al. Aluminum-doped lead-free double perovskite Cs2AgBiCl6 nanocrystals with ultrahigh stability towards white light emitting diodes. Mater. Res. Bull. 2022, 147, 111645. [Google Scholar] [CrossRef]
- Paul, S.; Samanta, A. N-bromosuccinimide as bromide precursor for direct synthesis of stable and highly luminescent green-emitting perovskite nanocrystals. ACS Energy Lett. 2019, 5, 64–69. [Google Scholar] [CrossRef]
- Imran, M.; Caligiuri, V.; Wang, M.; Goldoni, L.; Prato, M.; Krahne, R.; De Trizio, L.; Manna, L. Benzoyl halides as alternative precursors for the colloidal synthesis of lead-based halide perovskite nanocrystals. J. Am. Chem. Soc. 2018, 140, 2656–2664. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarty, A.; Satija, S.; Gangwar, U.; Sapra, S. Precursor-mediated synthesis of shape-controlled colloidal CsPbBr3 perovskite nanocrystals and their nanofiber-directed self-assembly. Chem. Mater. 2019, 32, 721–733. [Google Scholar] [CrossRef]
- Yoon, Y.J.; Lee, K.T.; Lee, T.K.; Kim, S.H.; Shin, Y.S.; Walker, B.; Park, S.Y.; Heo, J.; Lee, J.; Kwak, S.K. Reversible, full-color luminescence by post-treatment of perovskite nanocrystals. Joule 2018, 2, 2105–2116. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, C.; Zhang, Y.; Wu, H.; Ji, C.; Chuai, Y.; Wang, P.; Wen, S.; Zhang, C.; Yu, W.W. Bright perovskite nanocrystal films for efficient light-emitting devices. J. Phys. Chem. Lett. 2016, 7, 4602–4610. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, N. Alkylammonium Halides for Facet Reconstruction and Shape Modulation in Lead Halide Perovskite Nanocrystals. Acc. Chem. Res. 2021, 54, 1200–1208. [Google Scholar] [CrossRef]
- Tsai, H.; Shrestha, S.; Vilá, R.A.; Huang, W.; Liu, C.; Hou, C.-H.; Huang, H.-H.; Wen, X.; Li, M.; Wiederrecht, G. Bright and stable light-emitting diodes made with perovskite nanocrystals stabilized in metal–organic frameworks. Nat. Photonics 2021, 15, 843–849. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, B.; Li, W.; Huang, S.; Kong, L.; Li, Z.; Li, L. Conversion of invisible metal-organic frameworks to luminescent perovskite nanocrystals for confidential information encryption and decryption. Nat. Commun. 2017, 8, 1138. [Google Scholar] [CrossRef]
- Zhang, D.; Zhou, W.; Liu, Q.; Xia, Z. CH3NH3PbBr3 perovskite nanocrystals encapsulated in lanthanide metal–organic frameworks as a photoluminescence converter for anti-counterfeiting. ACS Appl. Mater. Interfaces 2018, 10, 27875–27884. [Google Scholar] [CrossRef]
- Ryu, U.; Jee, S.; Park, J.-S.; Han, I.K.; Lee, J.H.; Park, M.; Choi, K.M. Nanocrystalline titanium metal–organic frameworks for highly efficient and flexible perovskite solar cells. ACS Nano 2018, 12, 4968–4975. [Google Scholar] [CrossRef]
- Jeon, N.J.; Noh, J.H.; Kim, Y.C.; Yang, W.S.; Ryu, S.; Seok, S.I. Solvent engineering for high-performance inorganic–organic hybrid perovskite solar cells. Nat. Mater. 2014, 13, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Cheng, T.; Wang, F.; Dai, S.; Tan, Z.a. Morphology Engineering for High-Performance and Multicolored Perovskite Light-Emitting Diodes with Simple Device Structures. Small 2016, 12, 4412–4420. [Google Scholar] [CrossRef] [PubMed]
- Di, D.; Musselman, K.P.; Li, G.; Sadhanala, A.; Ievskaya, Y.; Song, Q.; Tan, Z.-K.; Lai, M.L.; MacManus-Driscoll, J.L.; Greenham, N.C. Size-dependent photon emission from organometal halide perovskite nanocrystals embedded in an organic matrix. J. Phys. Chem. Lett. 2015, 6, 446–450. [Google Scholar] [CrossRef]
- Xin, Y.; Zhao, H.; Zhang, J. Highly stable and luminescent perovskite–polymer composites from a convenient and universal strategy. ACS Appl. Mater. Interfaces 2018, 10, 4971–4980. [Google Scholar] [CrossRef] [PubMed]
- Akhil, S.; Palabathuni, M.; Biswas, S.; Singh, R.; Mishra, N. Highly Stable Amine-Free CsPbBr3 Perovskite Nanocrystals for Perovskite-Based Display Applications. ACS Appl. Nano Mater. 2022, 5, 13561–13572. [Google Scholar] [CrossRef]
- Paul, S.; Samanta, A. Phase-Stable and Highly Luminescent CsPbI(3) Perovskite Nanocrystals with Suppressed Photoluminescence Blinking. J. Phys. Chem. Lett. 2022, 13, 5742–5750. [Google Scholar] [CrossRef]
- Mahankudo, S.K.; Das, A.; Mishra, K.; Barai, M.; Mal, P.; Ghosh, S. Synthesis of Cs/Methylammonium/Formamidinium PbBr3 Perovskite Nanocrystals with Green Emissions: Implications for Display Applications. ACS Appl. Nano Mater. 2022, 5, 4360–4366. [Google Scholar] [CrossRef]
- Xiong, J.; Wang, Y.; Xue, Q.; Wu, X. Synthesis of highly stable dispersions of nanosized copper particles using L-ascorbic acid. Green Chem. 2011, 13, 900–904. [Google Scholar] [CrossRef]
- Umer, A.; Naveed, S.; Ramzan, N.; Rafique, M.S.; Imran, M. A green method for the synthesis of copper nanoparticles using L-ascorbic acid. Matéria (Rio de janeiro) 2014, 19, 197–203. [Google Scholar] [CrossRef]
- Dutt, V.G.V.; Akhil, S.; Singh, R.; Palabathuni, M.; Mishra, N. High-Quality CsPbX3 (X = Cl, Br, or I) Perovskite Nanocrystals Using Ascorbic Acid Post-Treatment: Implications for Light-Emitting Applications. ACS Appl. Nano Mater. 2022, 5, 5972–5982. [Google Scholar] [CrossRef]
- Akhil, S.; Dutt, V.G.V.; Singh, R.; Mishra, N. Post-synthesis Treatment with Lead Bromide for Obtaining Near-Unity Photoluminescence Quantum Yield and Ultra-stable Amine-Free CsPbBr3 Perovskite Nanocrystals. J. Phys. Chem. C 2022, 126, 10742–10751. [Google Scholar] [CrossRef]
- Tsai, H.; Huang, H.H.; Watt, J.; Hou, C.H.; Strzalka, J.; Shyue, J.J.; Wang, L.; Nie, W. Cesium Lead Halide Perovskite Nanocrystals Assembled in Metal-Organic Frameworks for Stable Blue Light Emitting Diodes. Adv. Sci. 2022, 9, e2105850. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Ma, K.; Chakkamalayath, J.; Morsby, J.; Gao, H. In Situ Photocatalyzed Polymerization to Stabilize Perovskite Nanocrystals in Protic Solvents. ACS Energy Lett. 2022, 7, 610–616. [Google Scholar] [CrossRef]
- Baskoro, F.; Wong, H.Q.; Labasan, K.B.; Cho, C.-W.; Pao, C.-W.; Yang, P.-Y.; Chang, C.-C.; Chen, C.-I.; Chueh, C.-C.; Nie, W. An efficient and reversible battery anode electrode derived from a lead-based metal–organic framework. Energy Fuels 2021, 35, 9669–9682. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, Q.; Tong, Y.; Liu, X.; Zhao, J.; Peng, B.; Zeng, R.; Pan, S.; Zou, B.; Xiang, W. Tunable Green Light-Emitting CsPbBr(3) Based Perovskite-Nanocrystals-in-Glass Flexible Film Enables Production of Stable Backlight Display. J. Phys. Chem. Lett. 2022, 13, 4701–4709. [Google Scholar] [CrossRef]
- Tabassum, M.; Zia, Q.; Li, J.; Khawar, M.T.; Aslam, S.; Su, L. FAPbBr3 Perovskite Nanocrystals Embedded in Poly(L–lactic acid) Nanofibrous Membranes for Enhanced Air and Water Stability. Membranes 2023, 13, 279. [Google Scholar] [CrossRef]
- Liang, Z.-B.; Chen, X.; Liao, X.-J.; Li, J.-J.; Yang, Y.; Wang, C.-F.; Zheng, Y.-X.; Chen, S. Continuous production of stable chiral perovskite nanocrystals in electrospinning nanofibers to exhibit circularly polarized luminescence. J. Mater. Chem. C 2022, 10, 12644–12651. [Google Scholar] [CrossRef]
- Khurana, S.; Hassan, M.S.; Yadav, P.; Chonamada, T.D.; Das, M.R.; Santra, P.K.; Ghosh, D.; Sapra, S. Defect Passivation Results in the Stability of Cesium Lead Halide Perovskite Nanocrystals. J. Phys. Chem. C 2023, 127, 3355–3366. [Google Scholar] [CrossRef]
- Xu, F.; Chen, D.; Huang, D.; Xu, K.; Liang, S.; Hu, J.; Zhang, X.; Liu, L.; Xiong, F.; Zhu, H. Suppression of Photoinduced Phase Segregation in Mixed-Halide Perovskite Nanocrystals for Stable Light-Emitting Diodes. J. Phys. Chem. Lett. 2022, 13, 718–725. [Google Scholar] [CrossRef]
- Mahato, S.; Ghorai, A.; Mondal, A.; Srivastava, S.K.; Modak, M.; Das, S.; Ray, S.K. Atomic-Scale Imaging and Nano-Scale Mapping of Cubic alpha-CsPbI(3) Perovskite Nanocrystals for Inverted Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2022, 14, 9711–9723. [Google Scholar] [CrossRef]



| Perovskite | Dopant Metal Ion | Performance Stability |
|---|---|---|
| CsPbBr3 | Zn2+ [137] | Maintained 91% normalized PL intensity after 60 min heating |
| CsPbBr3 | Ni2+ [139] | Maintained 71.93% normalized PLQY after 60 days under water |
| MAPbBr3 | Zn2+, Mn2+ [140] | Maintained 92% normalized PL intensity after 30 days |
| Cs2AgBiCl6 | Al3+ [141] | Maintained 92% normalized PLQY after 240 days |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Lee, E.-C. Advancements in Perovskite Nanocrystal Stability Enhancement: A Comprehensive Review. Nanomaterials 2023, 13, 1707. https://doi.org/10.3390/nano13111707
Liu X, Lee E-C. Advancements in Perovskite Nanocrystal Stability Enhancement: A Comprehensive Review. Nanomaterials. 2023; 13(11):1707. https://doi.org/10.3390/nano13111707
Chicago/Turabian StyleLiu, Xuewen, and Eun-Cheol Lee. 2023. "Advancements in Perovskite Nanocrystal Stability Enhancement: A Comprehensive Review" Nanomaterials 13, no. 11: 1707. https://doi.org/10.3390/nano13111707
APA StyleLiu, X., & Lee, E.-C. (2023). Advancements in Perovskite Nanocrystal Stability Enhancement: A Comprehensive Review. Nanomaterials, 13(11), 1707. https://doi.org/10.3390/nano13111707






