Radiolysis-Assisted Direct Growth of Gold-Based Electrocatalysts for Glycerol Oxidation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Radiolytic Synthesis
2.3. Characterization Techniques
2.3.1. Cyclic Voltammetry
2.3.2. Electrochemical Impedance Spectroscopy (EIS)
2.3.3. Underpotential Deposition of Lead (Pb UPD)
2.3.4. Material Characterization
3. Results and Discussion
3.1. Characterization of the Gold-Based Electrocatalysts
3.1.1. SEM and EDX Analysis
3.1.2. Electrochemical Analysis
3.1.3. X-ray Photoelectron Spectroscopy
3.1.4. X-ray Diffraction
3.2. Electrocatalytic Performance towards Glycerol Oxidation
3.2.1. Cyclic Voltammetry Measurements in the Presence of Glycerol
3.2.2. Electrochemical Impedance Spectroscopy (EIS)
3.2.3. Inductively Coupled Plasma—Optical Emission Spectroscopy
3.2.4. Electrocatalytic Performance in an H-Type Cell
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coutanceau, C.; Baranton, S. Electrochemical conversion of alcohols for hydrogen production: A short overview. WIREs Energy Environ. 2016, 5, 388–400. [Google Scholar] [CrossRef]
- Houache, M.S.E.; Hughes, K.; Baranova, E.A. Study on catalyst selection for electrochemical valorization of glycerol. Sustain. Energy Fuels 2019, 3, 1892–1915. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, Y.; Lee, D.; Kim, J.-R.; Chae, H.-J.; Jeong, S.-Y.; Kim, B.-S.; Lee, J.; Huber, G.W.; Byun, J.; et al. Coproducing Value-Added Chemicals and Hydrogen with Electrocatalytic Glycerol Oxidation Technology: Experimental and Techno-Economic Investigations. ACS Sustain. Chem. Eng. 2017, 5, 6626–6634. [Google Scholar] [CrossRef]
- Talebian-Kiakalaieh, A.; Amin, N.A.S.; Rajaei, K.; Tarighi, S. Oxidation of bio-renewable glycerol to value-added chemicals through catalytic and electro-chemical processes. Appl. Energy 2018, 230, 1347–1379. [Google Scholar] [CrossRef]
- Ahmad, M.S.; Ab Rahim, M.H.; Alqahtani, T.M.; Witoon, T.; Lim, J.-W.; Cheng, C.K. A review on advances in green treatment of glycerol waste with a focus on electro-oxidation pathway. Chemosphere 2021, 276, 130128. [Google Scholar] [CrossRef]
- Tuleushova, N.; Holade, Y.; Cornu, D.; Tingry, S. Glycerol electro-reforming in alkaline electrolysis cells for the simultaneous production of value-added chemicals and pure hydrogen—Mini-review. Electrochem. Sci. Adv. 2022, 3, e2100174. [Google Scholar] [CrossRef]
- Fan, L.; Liu, B.; Liu, X.; Senthilkumar, N.; Wang, G.; Wen, Z. Recent Progress in Electrocatalytic Glycerol Oxidation. Energy Technol. 2020, 9, 2000804. [Google Scholar] [CrossRef]
- Alaba, P.A.; Lee, C.S.; Abnisa, F.; Aroua, M.K.; Cognet, P.; Pérès, Y.; Daud, W.M.A.W. A review of recent progress on electrocatalysts toward efficient glycerol electrooxidation. Rev. Chem. Eng. 2020, 37, 779–811. [Google Scholar] [CrossRef]
- Li, J.; Zhou, Z.; Xu, H.; Wang, C.; Hata, S.; Dai, Z.; Shiraishi, Y.; Du, Y. In situ nanopores enrichment of Mesh-like palladium nanoplates for bifunctional fuel cell reactions: A joint etching strategy. J. Colloid Interface Sci. 2022, 611, 523–532. [Google Scholar] [CrossRef]
- Weber, M.; Collot, P.; El Gaddari, H.; Tingry, S.; Bechelany, M.; Holade, Y. Enhanced Catalytic Glycerol Oxidation Activity Enabled by Activated-Carbon-Supported Palladium Catalysts Prepared through Atomic Layer Deposition. Chemelectrochem 2018, 5, 743–747. [Google Scholar] [CrossRef]
- Velázquez-Hernández, I.; Zamudio, E.; Rodríguez-Valadez, F.J.; García-Gómez, N.A.; Álvarez-Contreras, L.; Guerra-Balcázar, M.; Arjona, N. Electrochemical valorization of crude glycerol in alkaline medium for energy conversion using Pd, Au and PdAu nanomaterials. Fuel 2020, 262, 116556. [Google Scholar] [CrossRef]
- Li, S.; Lai, J.; Luque, R.; Xu, G. Designed multimetallic Pd nanosponges with enhanced electrocatalytic activity for ethylene glycol and glycerol oxidation. Energy Environ. Sci. 2016, 9, 3097–3102. [Google Scholar] [CrossRef]
- Cassani, A.; Tuleushova, N.; Wang, Q.; Guesmi, H.; Bonniol, V.; Cambedouzou, J.; Tingry, S.; Bechelany, M.; Cornu, D.; Holade, Y. Fe-modified Pd as an effective multifunctional electrocatalyst for catalytic oxygen reduction and glycerol oxidation reactions in alkaline media. ACS Appl. Energy Mater. 2021, 4, 9944–9960. [Google Scholar] [CrossRef]
- De Souza, M.B.C.; Yukuhiro, V.Y.; Vicente, R.A.; Pires, C.T.G.V.M.T.; Bott-Neto, J.L.; Fernandez, P.S. Pb- and Bi-Modified Pt Electrodes toward Glycerol Electrooxidation in Alkaline Media. Activity, Selectivity, and the Importance of the Pt Atoms Arrangement. ACS Catal. 2020, 10, 2131–2137. [Google Scholar] [CrossRef]
- Lima, C.C.; Rodrigues, M.V.; Neto, A.F.; Zanata, C.R.; Pires, C.T.; Costa, L.S.; Solla-Gullón, J.; Fernández, P.S. Highly active Ag/C nanoparticles containing ultra-low quantities of sub-surface Pt for the electrooxidation of glycerol in alkaline media. Appl. Catal. B Environ. 2020, 279, 119369. [Google Scholar] [CrossRef]
- Zhou, Y.; Shen, Y.; Xi, J. Seed-mediated synthesis of PtxAuy@Ag electrocatalysts for the selective oxidation of glycerol. Appl. Catal. B Environ. 2019, 245, 604–612. [Google Scholar] [CrossRef]
- Holade, Y.; Morais, C.; Arrii-Clacens, S.; Servat, K.; Napporn, T.W.; Kokoh, K.B. New preparation of PdNi/C and PdAg/C Nanocatalysts for glycerol electrooxidation in alkaline medium. Electrocatalysis 2013, 4, 167–178. [Google Scholar] [CrossRef]
- Ghosh, S.; Bysakh, S.; Basu, R.N. Bimetallic Pd96Fe4 nanodendrites embedded in graphitic carbon nanosheets as highly efficient anode electrocatalysts. Nanoscale Adv. 2019, 1, 3929–3940. [Google Scholar] [CrossRef]
- Li, J.; Wang, C.; Shang, H.; Wang, Y.; You, H.; Xu, H.; Du, Y. Metal-modified PtTe2 nanorods: Surface reconstruction for efficient methanol oxidation electrocatalysis. Chem. Eng. J. 2021, 424, 130319. [Google Scholar] [CrossRef]
- Zope, B.N.; Hibbitts, D.D.; Neurock, M.; Davis, R.J. Reactivity of the Gold/Water Interface During Selective Oxidation Catalysis. Science 2010, 330, 74–78. [Google Scholar] [CrossRef]
- Ottoni, C.; Da Silva, S.G.; De Souza, R.; Neto, A.O. Glycerol oxidation reaction using PdAu/C electrocatalysts. Ionics 2016, 22, 1167–1175. [Google Scholar] [CrossRef]
- Garcia, A.C.; Caliman, J.; Ferreira, E.B.; Tremiliosi-Filho, G.; Linares, J.J. Promotional effect of Ag on the catalytic activity of Au for glycerol electrooxidation in alkaline medium. Chemelectrochem 2015, 2, 1036–1041. [Google Scholar] [CrossRef]
- Thia, L.; Xie, M.; Liu, Z.; Ge, X.; Lu, Y.; Fong, W.E.; Wang, X. Copper-modified gold nanoparticles as highly selective catalysts for glycerol electro-oxidation in alkaline solution. Chemcatchem 2016, 8, 3272–3278. [Google Scholar] [CrossRef]
- Dai, C.; Sun, L.; Liao, H.; Khezri, B.; Webster, R.D.; Fisher, A.C.; Xu, Z.J. Electrochemical production of lactic acid from glycerol oxidation catalyzed by AuPt nanoparticles. J. Catal. 2017, 356, 14–21. [Google Scholar] [CrossRef]
- Boukil, R.; Tuleushova, N.; Cot, D.; Rebiere, B.; Bonniol, V.; Cambedouzou, J.; Tingry, S.; Cornu, D.; Holade, Y. Enhanced electrocatalytic activity and selectivity of glycerol oxidation triggered by nanoalloyed silver–gold nanocages directly grown on gas diffusion electrodes. J. Mater. Chem. A 2020, 8, 8848–8856. [Google Scholar] [CrossRef]
- Song, J.H.; Fan, S.J.; Yu, J.Y.; Ye, C.W.; Xu, C.W. Glycerol electrooxidation on au hollow spheres three-dimensional structure catalyst. Int. J. Electrochem. Sci. 2012, 7, 10842–10850. [Google Scholar]
- Li, Z.; Yan, Y.; Xu, S.-M.; Zhou, H.; Xu, M.; Ma, L.; Shao, M.; Kong, X.; Bin Wang, B.; Zheng, L.; et al. Alcohols electrooxidation coupled with H2 production at high current densities promoted by a cooperative catalyst. Nat. Commun. 2022, 13, 147. [Google Scholar] [CrossRef]
- Marshall, A.; Haverkamp, R. Production of hydrogen by the electrochemical reforming of glycerol–water solutions in a PEM electrolysis cell. Int. J. Hydrogen Energy 2008, 33, 4649–4654. [Google Scholar] [CrossRef]
- Padayachee, D.; Golovko, V.; Ingham, B.; Marshall, A.T. Influence of particle size on the electrocatalytic oxidation of glycerol over carbon-supported gold nanoparticles. Electrochim. Acta 2014, 120, 398–407. [Google Scholar] [CrossRef]
- Garcia, A.G.; Lopes, P.P.; Gomes, J.F.; Pires, C.; Ferreira, E.B.; Lucena, R.G.M.; Gasparotto, L.H.S.; Tremiliosi-Filho, G. Eco-friendly synthesis of bimetallic AuAg nanoparticles. New J. Chem. 2014, 38, 2865–2873. [Google Scholar] [CrossRef]
- Houache, M.S.E.; Safari, R.; Nwabara, U.O.; Rafaïdeen, T.; Botton, G.A.; Kenis, P.J.A.; Baranton, S.; Coutanceau, C.; Baranova, E.A. Selective Electrooxidation of Glycerol to Formic Acid over Carbon Supported Ni1–xMx (M = Bi, Pd, and Au) Nanocatalysts and Coelectrolysis of CO2. ACS Appl. Energy Mater. 2020, 3, 8725–8738. [Google Scholar] [CrossRef]
- Wang, L.; Mao, W.; Ni, D.; Di, J.; Wu, Y.; Tu, Y. Direct electrodeposition of gold nanoparticles onto indium/tin oxide film coated glass and its application for electrochemical biosensor. Electrochem. Commun. 2008, 10, 673–676. [Google Scholar] [CrossRef]
- Holade, Y.; Hickey, D.P.; Minteer, S.D. Halide-regulated growth of electrocatalytic metal nanoparticles directly onto a carbon paper electrode. J. Mater. Chem. A 2016, 4, 17154–17162. [Google Scholar] [CrossRef]
- Morandi, P.; Tuleushova, N.; Tingry, S.; Cambedouzou, J.; Minteer, S.D.; Cornu, D.; Holade, Y. Bromide-Regulated Anisotropic Growth of Desert-Rose-Like Nanostructured Gold onto Carbon Fiber Electrodes as Freestanding Electrocatalysts. ACS Appl. Energy Mater. 2020, 3, 7560–7571. [Google Scholar] [CrossRef]
- Huang, C.-J.; Chiu, P.-H.; Wang, Y.-H.; Yang, C.-F. Synthesis of the gold nanodumbbells by electrochemical method. J. Colloid Interface Sci. 2006, 303, 430–436. [Google Scholar] [CrossRef]
- Houache, M.S.; Cossar, E.; Ntais, S.; Baranova, E.A. Electrochemical modification of nickel surfaces for efficient glycerol electrooxidation. J. Power Sources 2018, 375, 310–319. [Google Scholar] [CrossRef]
- Arvinte, A.; Crudu, I.-A.; Doroftei, F.; Timpu, D.; Pinteala, M. Electrochemical codeposition of silver-gold nanoparticles on CNT-based electrode and their performance in electrocatalysis of dopamine. J. Electroanal. Chem. 2018, 829, 184–193. [Google Scholar] [CrossRef]
- Shendage, S.S.; Singh, A.S.; Nagarkar, J.M. One step electrochemical synthesis of bimetallic PdAu supported on nafion–graphene ribbon film for ethanol electrooxidation. Mater. Res. Bull. 2015, 70, 539–544. [Google Scholar] [CrossRef]
- Paiva, V.M.; Assis, K.L.d.S.C.; Archanjo, B.S.; Ferreira, D.R.; Senna, C.A.; Ribeiro, E.S.; Achete, C.A.; D’Elia, E. Electrochemical Analysis of Free Glycerol in Biodiesel Using Reduced Graphene Oxide and Gold/Palladium Core-Shell Nanoparticles Modified Glassy Carbon Electrode. Processes 2021, 9, 1389. [Google Scholar] [CrossRef]
- Hansen, H.E.; Fakhri, D.; Seland, F.; Sunde, S.; Burheim, O.S.; Pollet, B.G. Sonochemical Synthesis of Cu@Pt Bimetallic Nanoparticles. Molecules 2022, 27, 5281. [Google Scholar] [CrossRef]
- Saloga, P.E.J.; Kästner, C.; Thünemann, A.F. High-Speed but Not Magic: Microwave-Assisted Synthesis of Ultra-Small Silver Nanoparticles. Langmuir 2017, 34, 147–153. [Google Scholar] [CrossRef]
- Musza, K.; Szabados, M.; Ádám, A.A.; Kónya, Z.; Kukovecz, Á.; Sipos, P.; Pálinkó, I. Mechanochemically modified hydrazine reduction method for the synthesis of nickel nanoparticles and their catalytic activities in the Suzuki–Miyaura cross-coupling reaction. React. Kinet. Catal. Lett. 2018, 126, 857–868. [Google Scholar] [CrossRef]
- Abidi, W. Gold based Nanoparticles Generated by Radiolytic and Photolytic Methods. Recent Pat. Eng. 2010, 4, 170–188. [Google Scholar] [CrossRef]
- Gachard, E.; Remita, H.; Khatouri, J.; Keita, B.; Nadjo, L.; Belloni, J. Radiation-induced and chemical formation of gold clusters. New J. Chem. 1998, 22, 1257–1265. [Google Scholar] [CrossRef]
- Belloni, J.; Mostafavi, M.; Remita, H.; Marignier, J.-L.; Delcourt, M.-O. Radiation-induced synthesis of mono- and multi-metallic clusters and nanocolloids. New J. Chem. 1998, 22, 1239–1255. [Google Scholar] [CrossRef]
- De Freitas, L.F.; Varca, G.H.C.; Dos Santos Batista, J.G.; Lugão, A.B. An Overview of the Synthesis of Gold Nanoparticles Using Radiation Technologies. Nanomaterials 2018, 8, 939. [Google Scholar] [CrossRef]
- Holade, Y.; Lehoux, A.; Remita, H.; Kokoh, K.B.; Napporn, T.W. Au@Pt Core–Shell Mesoporous Nanoballs and Nanoparticles as Efficient Electrocatalysts toward Formic Acid and Glucose Oxidation. J. Phys. Chem. C 2015, 119, 27529–27539. [Google Scholar] [CrossRef]
- Ghosh, S.; Holade, Y.; Remita, H.; Servat, K.; Beaunier, P.; Hagège, A.; Kokoh, K.B.; Napporn, T.W. One-pot synthesis of reduced graphene oxide supported gold-based nanomaterials as robust nanocatalysts for glucose electrooxidation. Electrochim. Acta 2016, 212, 864–875. [Google Scholar] [CrossRef]
- Mackiewicz, N.; Surendran, G.; Remita, H.; Keita, B.; Zhang, G.; Nadjo, L.; Hagège, A.; Doris, E.; Mioskowski, C. Supramolecular Self-Assembly of Amphiphiles on Carbon Nanotubes: A Versatile Strategy for the Construction of CNT/Metal Nanohybrids, Application to Electrocatalysis. J. Am. Chem. Soc. 2008, 130, 8110–8111. [Google Scholar] [CrossRef] [PubMed]
- Holade, Y.; Servat, K.; Tingry, S.; Napporn, T.W.; Remita, H.; Cornu, D.; Kokoh, K.B. Advances in Electrocatalysis for Energy Conversion and Synthesis of Organic Molecules. ChemPhysChem 2017, 18, 2573–2605. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.F.; Geraldes, A.N.; Pino, E.S.; Neto, A.O.; Linardi, M.; Spinacé, E.V. PtRu/C Electrocatalysts Prepared Using Gamma and Electron Beam Irradiation for Methanol Electrooxidation. J. Nanomater. 2012, 2012, 6. [Google Scholar] [CrossRef]
- Lee, K.-P.; Gopalan, A.I.; Santhosh, P.; Lee, S.H.; Nho, Y.C. Gamma radiation induced distribution of gold nanoparticles into carbon nanotube-polyaniline composite. Compos. Sci. Technol. 2007, 67, 811–816. [Google Scholar] [CrossRef]
- Grabowska, E.; Zaleska, A.; Sorgues, S.; Kunst, M.; Etcheberry, A.; Colbeau-Justin, C.; Remita, H. Modification of Titanium(IV) Dioxide with Small Silver Nanoparticles: Application in Photocatalysis. J. Phys. Chem. C 2013, 117, 1955–1962. [Google Scholar] [CrossRef]
- Treguer, M.; de Cointet, C.; Remita, H.; Khatouri, J.; Mostafavi, M.; Amblard, J.; Belloni, J.; de Keyzer, R. Dose Rate Effects on Radiolytic Synthesis of Gold−Silver Bimetallic Clusters in Solution. J. Phys. Chem. B 1998, 102, 4310–4321. [Google Scholar] [CrossRef]
- Deng, L.; Hu, W.; Deng, H.; Xiao, S.; Tang, J. Au–Ag Bimetallic Nanoparticles: Surface Segregation and Atomic-Scale Structure. J. Phys. Chem. C 2011, 115, 11355–11363. [Google Scholar] [CrossRef]
- Le Gratiet, B.; Remita, H.; Picq, G.; Delcourt, M.O. CO-Stabilized Supported Pt Catalysts for Fuel Cells: Radiolytic Synthesis. J. Catal. 1996, 164, 36–43. [Google Scholar] [CrossRef]
- Orazem, M.E.; Tribollet, B. Electrochemical Impedance Spectroscopy, 2nd ed.; Electrochemical Society: Pennington, NJ, USA, 2017. [Google Scholar]
- Piella, J.; Bastús, N.G.; Puntes, V. Size-Controlled Synthesis of Sub-10-nanometer Citrate-Stabilized Gold Nanoparticles and Related Optical Properties. Chem. Mater. 2016, 28, 1066–1075. [Google Scholar] [CrossRef]
- Jans, H.; Jans, K.; Lagae, L.; Borghs, G.; Maes, G.; Huo, Q. Poly(acrylic acid)-stabilized colloidal gold nanoparticles: Synthesis and properties. Nanotechnology 2010, 21, 455702. [Google Scholar] [CrossRef]
- Meyre, M.-E.; Tréguer-Delapierre, M.; Faure, C. Radiation-Induced Synthesis of Gold Nanoparticles within Lamellar Phases. Formation of Aligned Colloidal Gold by Radiolysis. Langmuir 2008, 24, 4421–4425. [Google Scholar] [CrossRef]
- Olaya, A.R.S.; Zandersons, B.; Wittstock, G. Restructuring of nanoporous gold surface during electrochemical cycling in acidic and alkaline media. Chemelectrochem 2020, 7, 3670–3678. [Google Scholar] [CrossRef]
- Borkowska, Z.; Tymosiak-Zielinska, A.; Shul, G. Electrooxidation of methanol on polycrystalline and single crystal gold electrodes. Electrochim. Acta 2004, 49, 1209–1220. [Google Scholar] [CrossRef]
- Hernández, J.; Solla-Gullón, J.; Herrero, E.; Aldaz, A.; Feliu, J.M. Methanol oxidation on gold nanoparticles in alkaline media: Unusual electrocatalytic activity. Electrochim. Acta 2006, 52, 1662–1669. [Google Scholar] [CrossRef]
- Bard Allen, J.; Faulkner Larry, R. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; Wiley: New York, NY, USA, 2008. [Google Scholar]
- Espinoza, E.M.; Clark, J.A.; Soliman, J.; Derr, J.B.; Morales, M.; Vullev, V.I. Practical Aspects of Cyclic Voltammetry: How to Estimate Reduction Potentials When Irreversibility Prevails. J. Electrochem. Soc. 2019, 166, H3175–H3187. [Google Scholar] [CrossRef]
- Olaya, A.R.S.; Zandersons, B.; Wittstock, G. Effect of the residual silver and adsorbed lead anions towards the electrocatalytic methanol oxidation on nanoporous gold in alkaline media. Electrochim. Acta 2021, 383, 138348. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-ray Photoelectron Spectroscopy; John Wiley & Sons, Ltd.: Eden Prairie, MN, USA, 1992. [Google Scholar]
- Song, Z.; Li, W.; Niu, F.; Xu, Y.; Niu, L.; Yang, W.; Wang, Y.; Liu, J. A novel method to decorate Au clusters onto graphene via a mild co-reduction process for ultrahigh catalytic activity. J. Mater. Chem. A 2016, 5, 230–239. [Google Scholar] [CrossRef]
- Strohmeier, B.R. Copper/Silver/Gold Alloy by XPS. Surf. Sci. Spectra 1994, 3, 175–181. [Google Scholar] [CrossRef]
- Zhidkov, I.S.; Kurmaev, E.Z.; Condorelli, M.; Cholakh, S.O.; Boyarchenkov, A.S.; Fazio, E.; D’urso, L. X-ray Photoelectron Spectra of Ag-Au Colloidal Nanoparticles after Interaction with Linear Carbon Chains. Appl. Sci. 2021, 11, 685. [Google Scholar] [CrossRef]
- Yakimchuk, D.V.; Bundyukova, V.D.; Ustarroz, J.; Terryn, H.; Baert, K.; Kozlovskiy, A.L.; Zdorovets, M.V.; Khubezhov, S.A.; Trukhanov, A.V.; Trukhanov, S.V.; et al. Morphology and Microstructure Evolution of Gold Nanostructures in the Limited Volume Porous Matrices. Sensors 2020, 20, 4397. [Google Scholar] [CrossRef]
- Hoflund, G.B.; Weaver, J.F.; Epling, W.S. Ag2O XPS Spectra. Surf. Sci. Spectra 1994, 3, 157–162. [Google Scholar] [CrossRef]
- Fan, Y.; Bao, Y.; Song, Z.; Sun, Z.; Wang, D.; Han, D.; Niu, L. Controllable synthesis of coloured Ag0/AgCl with spectral analysis for photocatalysis. RSC Adv. 2018, 8, 24812–24818. [Google Scholar] [CrossRef]
- Villalobos-Noriega, J.M.A.; Rodríguez-León, E.; Rodríguez-Beas, C.; Larios-Rodríguez, E.; Plascencia-Jatomea, M.; Martínez-Higuera, A.; Acuña-Campa, H.; García-Galaz, A.; Mora-Monroy, R.; Alvarez-Cirerol, F.J.; et al. Au@Ag Core@Shell Nanoparticles Synthesized with Rumex hymenosepalus as Antimicrobial Agent. Nanoscale Res. Lett. 2021, 16, 118. [Google Scholar] [CrossRef]
- Trinh, N.D.; Nguyen, T.T.B.; Nguyen, T.H. Preparation and characterization of silver chloride nanoparticles as an antibacterial agent. Adv. Nat. Sci. Nanosci. Nanotechnol. 2015, 6, 045011. [Google Scholar] [CrossRef]
- Wang, J.; An, C.; Zhang, M.; Qin, C.; Ming, X.; Zhang, Q. Photochemical conversion of AgCl nanocubes to hybrid AgCl–Ag nanoparticles with high activity and long-term stability towards photocatalytic degradation of organic dyes. Can. J. Chem. 2012, 90, 858–864. [Google Scholar] [CrossRef]
- Hamelin, A.; Lipkowski, J. Underpotential deposition of lead on gold single crystal faces. J. Electroanal. Chem. Interfacial Electrochem. 1984, 171, 317–330. [Google Scholar] [CrossRef]
- Mayet, N.; Servat, K.; Kokoh, K.B.; Napporn, T.W. Probing the Surface of Noble Metals Electrochemically by Underpotential Deposition of Transition Metals. Surfaces 2019, 2, 257–276. [Google Scholar] [CrossRef]
- Bokshits, Y.; Osipovich, N.; Strel’tsov, E.; Shevchenko, G. Underpotential deposition of lead on silver and gold colloids. Colloids Surf. A Physicochem. Eng. Asp. 2004, 242, 79–83. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Basic potential step methods. In Electrochemical Methods: Fundamentals and Applications; Wiley: New York, NY, USA, 2001; pp. 156–225. [Google Scholar]
- Eliaz, N.; Gileadi, E. Physical Electrochemistry: Fundamentals, Techniques, and Applications, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2018. [Google Scholar]
- Li, D.; Batchelor-McAuley, C.; Compton, R.G. Some thoughts about reporting the electrocatalytic performance of nanomaterials. Appl. Mater. Today 2020, 18, 100404. [Google Scholar] [CrossRef]
- Palma, L.M.; Almeida, T.S.; Morais, C.; Napporn, T.W.; Kokoh, K.B.; De Andrade, A.R. Effect of Co-catalyst on the Selective Electrooxidation of Glycerol over Ruthenium-based Nanomaterials. Chemelectrochem 2016, 4, 39–45. [Google Scholar] [CrossRef]
- Yongprapat, S.; Therdthianwong, S. RuO2 promoted Au/C catalysts for alkaline direct alcohol fuel cells. Electrochim. Acta 2012, 83, 87–93. [Google Scholar] [CrossRef]
- Zhang, Z.; Xin, L.; Li, W. Supported gold nanoparticles as anode catalyst for anion-exchange membrane-direct glycerol fuel cell (AEM-DGFC). Int. J. Hydrogen Energy 2012, 37, 9393–9401. [Google Scholar] [CrossRef]
- Wang, H.; Thia, L.; Li, N.; Ge, X.; Liu, Z.; Wang, X. Selective electro-oxidation of glycerol over Au supported on extended poly(4-vinylpyridine) functionalized graphene. Appl. Catal. B Environ. 2015, 166, 25–31. [Google Scholar] [CrossRef]
- Qi, J.; Xin, L.; Chadderdon, D.J.; Qiu, Y.; Jiang, Y.; Benipal, N.; Liang, C.; Li, W. Electrocatalytic selective oxidation of glycerol to tartronate on Au/C anode catalysts in anion exchange membrane fuel cells with electricity cogeneration. Appl. Catal. B Environ. 2014, 154, 360–368. [Google Scholar] [CrossRef]
- Pittayaporn, N.; Therdthianwong, A. Au/C catalysts promoted with Ni for glycerol electrooxidation in alkaline media. J. Appl. Electrochem. 2018, 48, 251–262. [Google Scholar] [CrossRef]
- Zhang, J.-H.; Zhu, T.; Liang, Y.-J.; Zhang, C.-J.; Shi, S.-T.; Xu, C.-W. CeO2 promoted Au/C catalyst for glycerol electro-oxidation in alkaline medium. J. Energy Inst. 2016, 89, 325–329. [Google Scholar] [CrossRef]
- Han, J.; Kim, Y.; Jackson, D.H.; Jeong, K.-E.; Chae, H.-J.; Lee, K.-Y.; Kim, H.J. Role of Au-TiO2 interfacial sites in enhancing the electrocatalytic glycerol oxidation performance. Electrochem. Commun. 2018, 96, 16–21. [Google Scholar] [CrossRef]
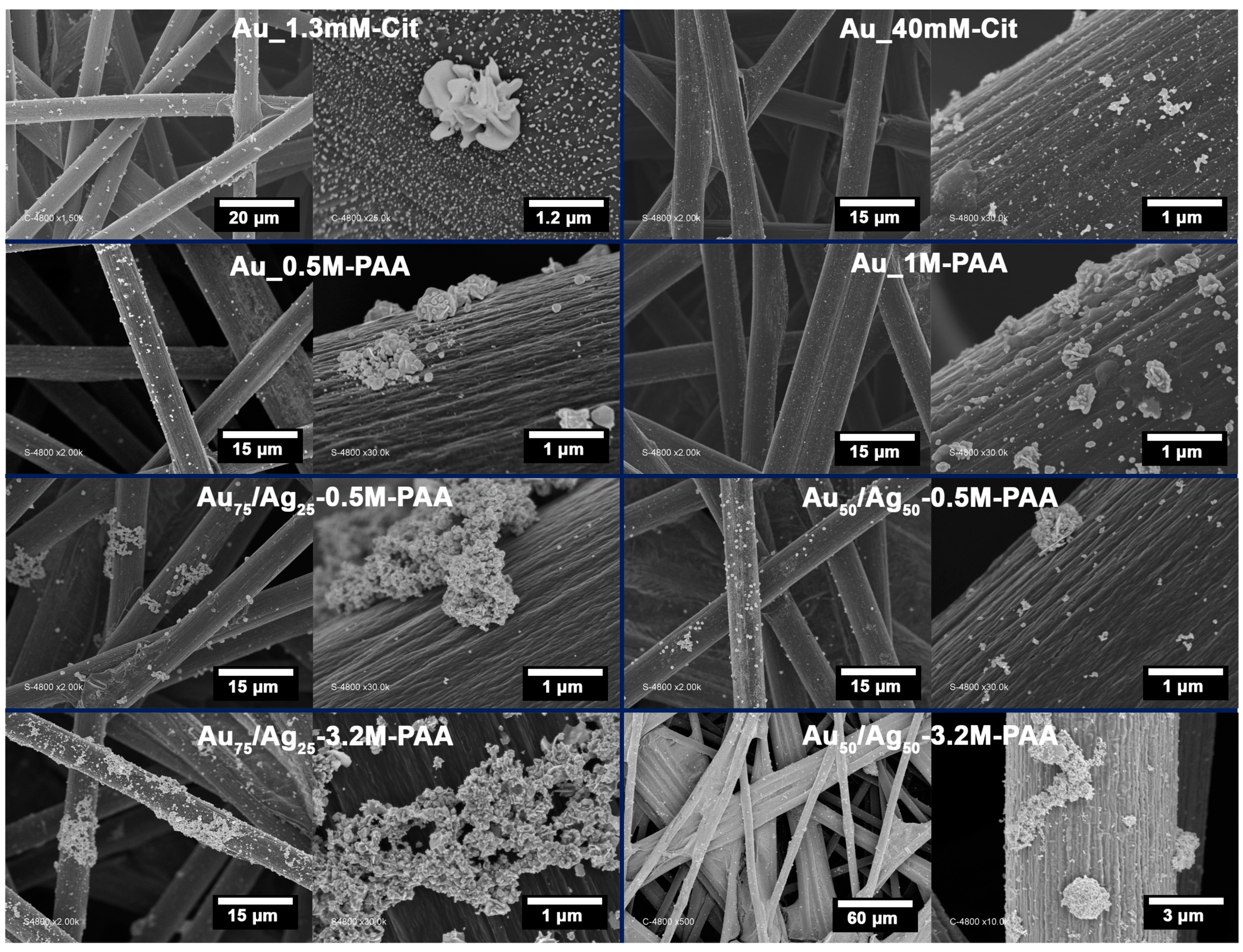





| [AuCl4−], mM | [Ag+], mM | Cit, mM | PAA, M | Sample Reference |
|---|---|---|---|---|
| 1.0 | 0 | 1.3 | 0 | Au_1.3mM-Cit |
| 1.0 | 0 | 40 | 0 | Au_40mM-Cit |
| 1.0 | 0 | 0 | 0.5 | Au_0.5M-PAA |
| 1.0 | 0 | 0 | 1.0 | Au_1M-PAA |
| 1.5 | 0.5 | 0 | 0.5 | Au75/Ag25_0.5M-PAA |
| 1.5 | 0.5 | 0 | 3.2 | Au75/Ag25_3.2M-PAA |
| 1.0 | 1.0 | 0 | 0.5 | Au50/Ag50_0.5M-PAA |
| 1.0 | 1.0 | 0 | 3.2 | Au50/Ag50_3.2M-PAA |
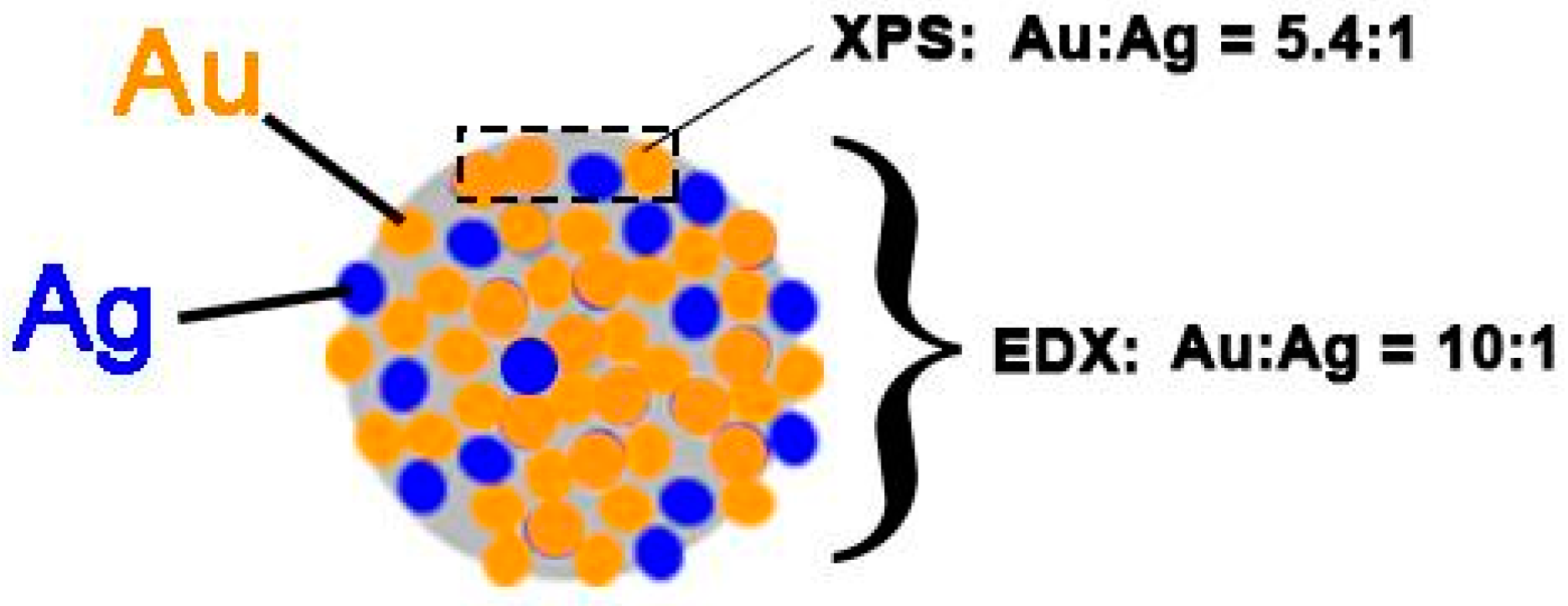
| Analyzed Samples | Au:Ag Atomic Ratios Determined By | |
|---|---|---|
| XPS | EDX | |
| Au75/Ag25-0.5M-PAA | 2.2:1 | 20:1 |
| Au50/Ag50-0.5M-PAA | 5.4:1 | 10:1 |
| Au50/Ag50-3.2M-PAA | 1.1:1 | 1.6:1 |
| Sample Reference | OCP vs. RHE | Rct, Ω·cm2 | Tafel Slope, mV·dec−1 | R2, % |
|---|---|---|---|---|
| Au_1.3mM-Cit | 0.66 | 77 | 170 | 99.0 |
| Au_40mM-Cit | 0.16 | 222 | 173 | 99.9 |
| Au_0.5M-PAA | 0.16 | 164 | 282 | 99.1 |
| Au_1M-PAA | 0.19 | 221 | 266 | 99.8 |
| Au75/Ag25-0.5M-PAA | 0.43 | 17 | 45 | 95.7 |
| Au75/Ag25-3.2M-PAA | 0.56 | 16 | 223 | 98.1 |
| Au50/Ag50-0.5M-PAA | 0.14 | 32 | 73 | 99.1 |
| Au50/Ag50-3.2M-PAA | 0.57 | 9 | 192 | 95.7 |
| Sample Reference | Au Loading, µg/cm2 | Ag Loading, µg/cm2 | Au:Ag Molar Ratio | Specific Peak Current Density, A·mg−1 |
|---|---|---|---|---|
| Au_1.3mM-Cit | 23.1 | - | - | 3.3 |
| Au_40mM-Cit | 20.0 | - | - | 2.4 |
| Au_0.5M-PAA | 12.2 | - | - | 5.2 |
| Au_1M-PAA | 11.3 | - | - | 8.0 |
| Au75/Ag25-0.5M-PAA | 73.0 | 2.5 | 16:1 | 1.5 |
| Au75/Ag25-3.2M-PAA | 71.7 | 4.9 | 8:1 | 1.1 |
| Au50/Ag50-0.5M-PAA | 40.9 | 2.3 | 10:1 | 2.4 |
| Au50/Ag50-3.2M-PAA | 102.6 | 28.8 | 2:1 | 0.6 |
| Ref. | Electrode | jp (mA cm−2) | Eonset (V vs. RHE) | Concentration of Glycerol (mol·L−1) | Selectivity |
|---|---|---|---|---|---|
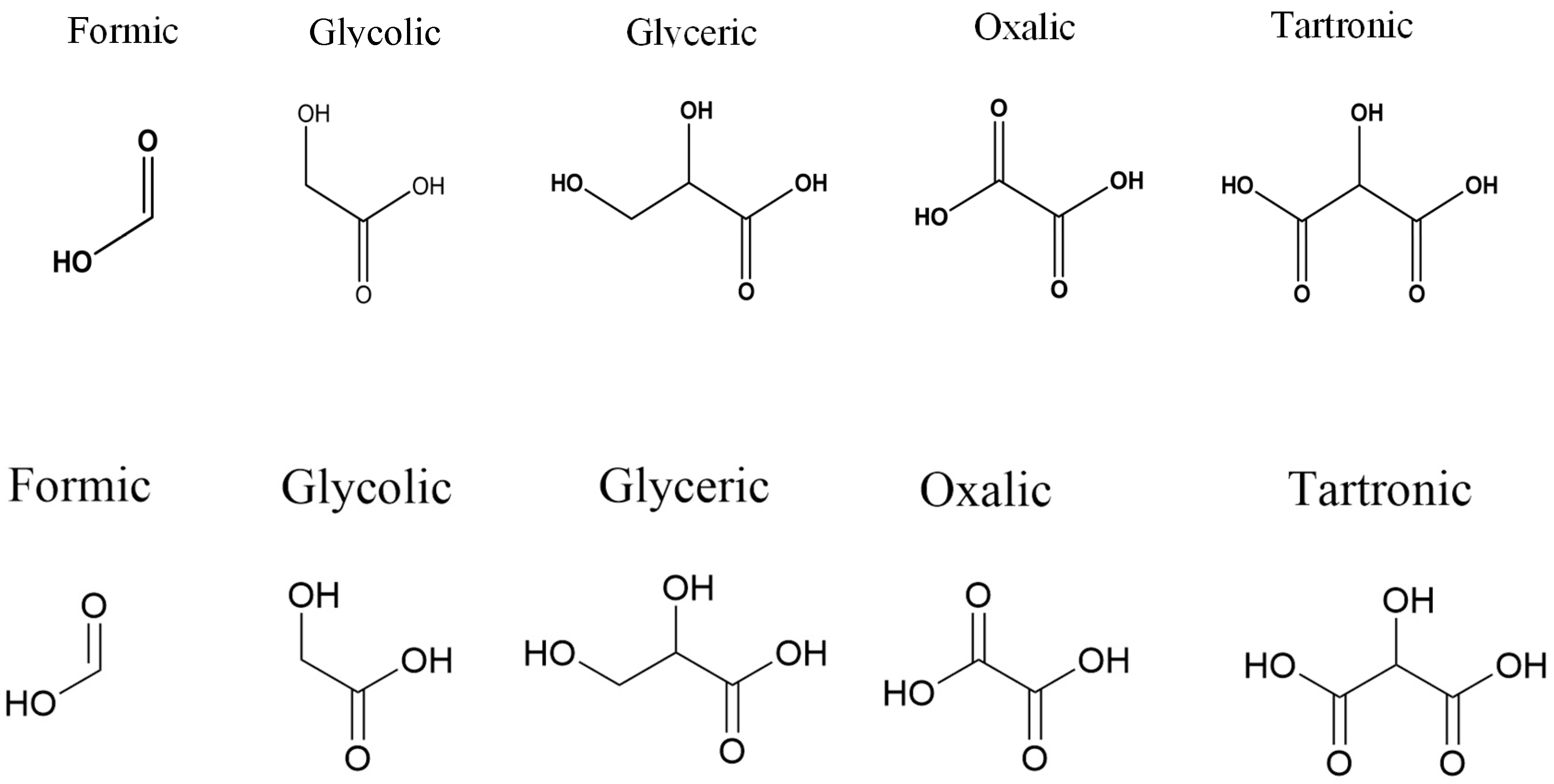
| Wang et al. [86] | Au-P4P/graphene | 70 | 0.74 | 0.5 | Glyceric acid ~45% /glycolic acid ~36% /formic acid ~19% |
| Han et al. [90] | TiO2-Au/C | 960 | 0.70 | 2.0 | Glyceric acid 65% /glycolic acid 22% |
| Garcia et al. [22] | Au3Ag/C | 181 | 0.60 | 1.0 | not analyzed |
| Pittayaporn et al. [88] | Au2/Ni1/C | 43 | 0.55 | 0.1 | not analyzed |
| Zhang et al. [89] | Au-CeO2/C | 75.4 | 0.50 | 1 | not analyzed |
| This work | Au_1.3mM-Cit | 75.6 | 0.65 | 0.1 | Formic acid 50%/glycolic acid 43% |
| Au_1mM-PAA | 90 | 0.60 | Formic acid 49%/glycolic acid 46% | ||
| Au75/Ag25_0.5M-PAA | 110 | 0.55 | Formic acid 56%/glycolic acid 41% | ||
| Au75/Ag25_3.2M-PAA | 83 | 0.55 | Formic acid 58%/glycolic acid 41% | ||
| Au50/Ag50-0.5M-PAA | 103 | 0.55 | Formic acid 64%/glycolic acid 35% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tuleushova, N.; Amanova, A.; Abdellah, I.; Benoit, M.; Remita, H.; Cornu, D.; Holade, Y.; Tingry, S. Radiolysis-Assisted Direct Growth of Gold-Based Electrocatalysts for Glycerol Oxidation. Nanomaterials 2023, 13, 1713. https://doi.org/10.3390/nano13111713
Tuleushova N, Amanova A, Abdellah I, Benoit M, Remita H, Cornu D, Holade Y, Tingry S. Radiolysis-Assisted Direct Growth of Gold-Based Electrocatalysts for Glycerol Oxidation. Nanomaterials. 2023; 13(11):1713. https://doi.org/10.3390/nano13111713
Chicago/Turabian StyleTuleushova, Nazym, Aisara Amanova, Ibrahim Abdellah, Mireille Benoit, Hynd Remita, David Cornu, Yaovi Holade, and Sophie Tingry. 2023. "Radiolysis-Assisted Direct Growth of Gold-Based Electrocatalysts for Glycerol Oxidation" Nanomaterials 13, no. 11: 1713. https://doi.org/10.3390/nano13111713
APA StyleTuleushova, N., Amanova, A., Abdellah, I., Benoit, M., Remita, H., Cornu, D., Holade, Y., & Tingry, S. (2023). Radiolysis-Assisted Direct Growth of Gold-Based Electrocatalysts for Glycerol Oxidation. Nanomaterials, 13(11), 1713. https://doi.org/10.3390/nano13111713







