Natural Cellulose-Based Multifunctional Nanofibers for the Effective Removal of Particulate Matter and Volatile Organic Compounds
Abstract
:1. Introduction
2. Material and Methods
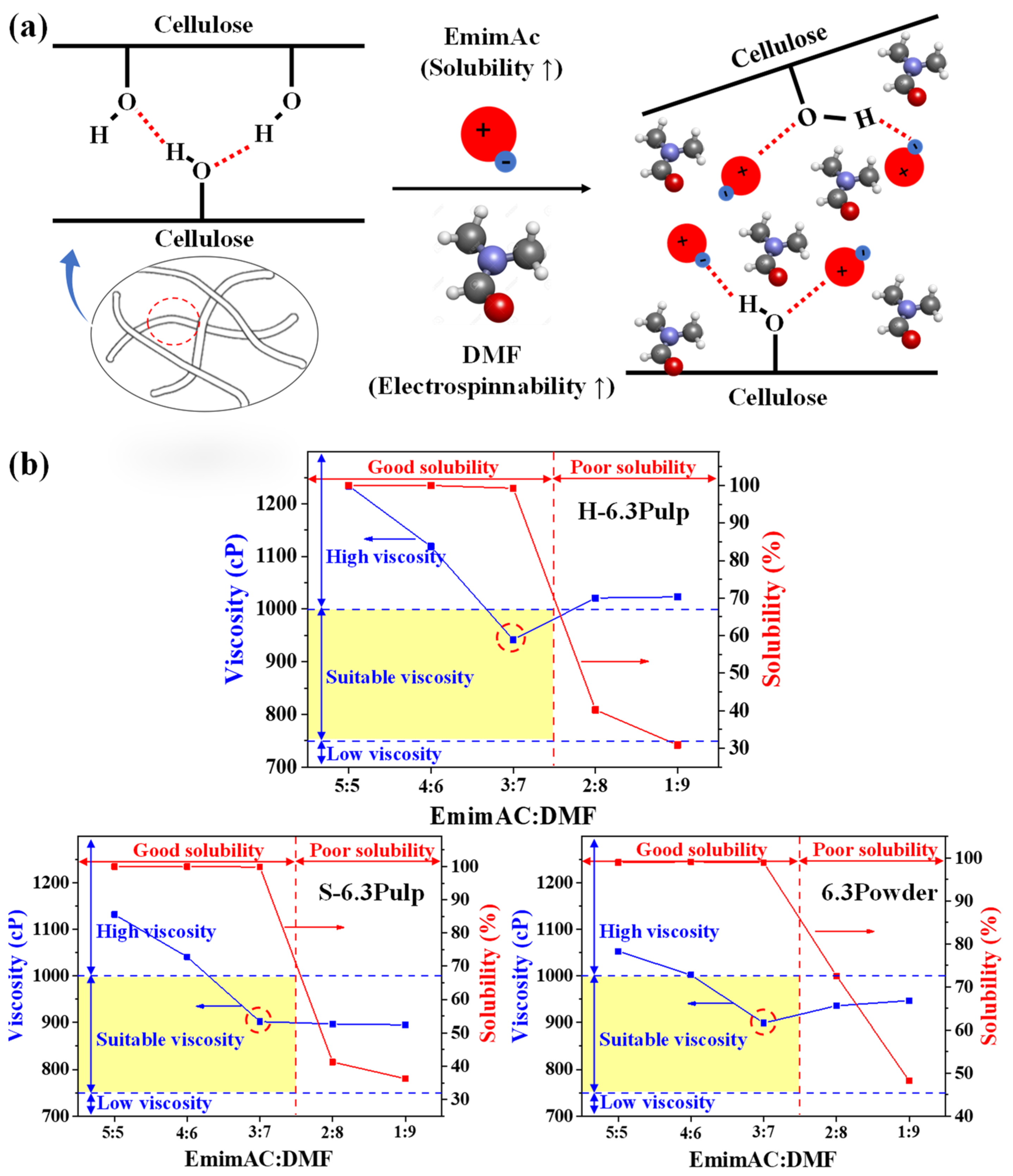
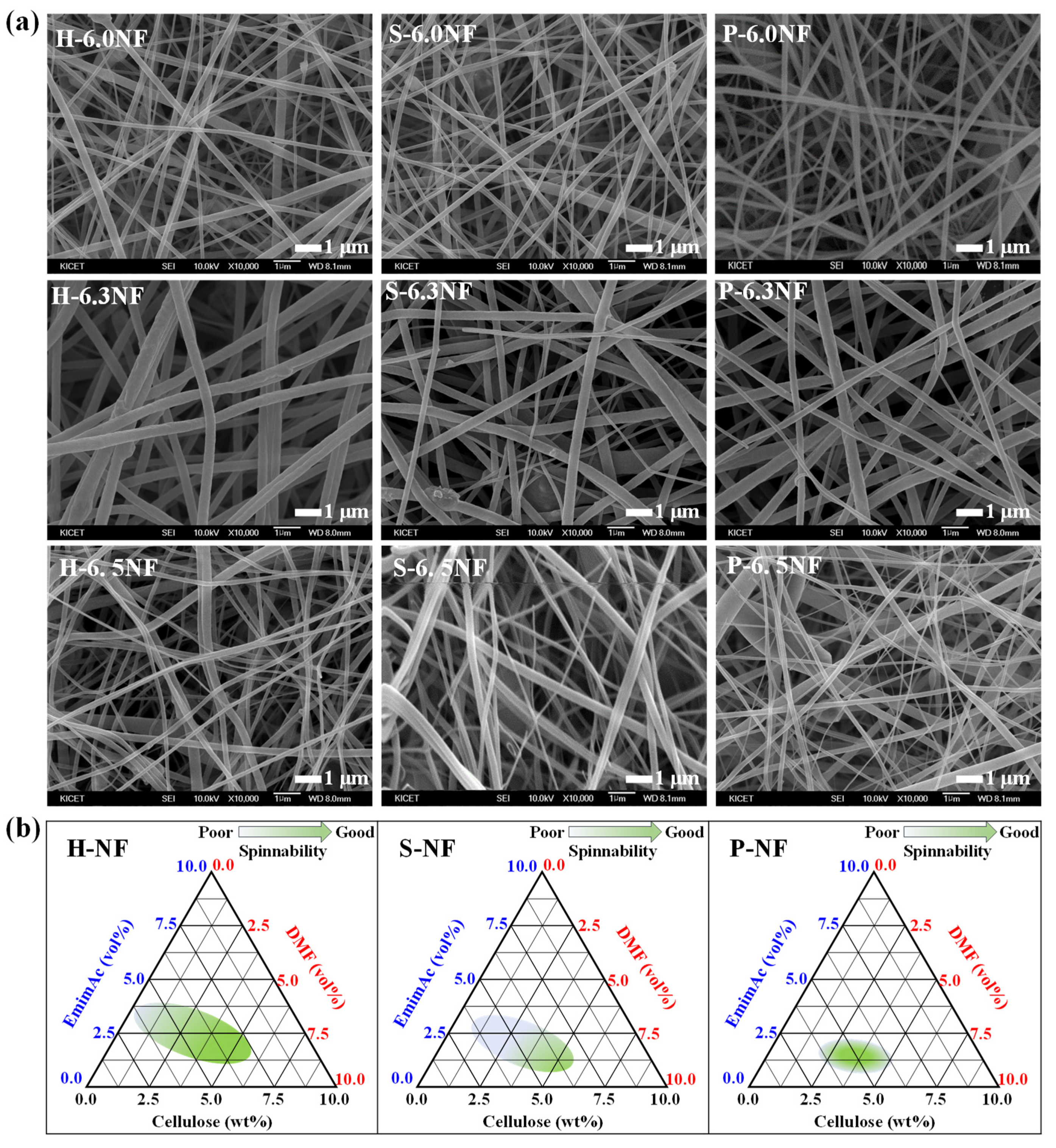
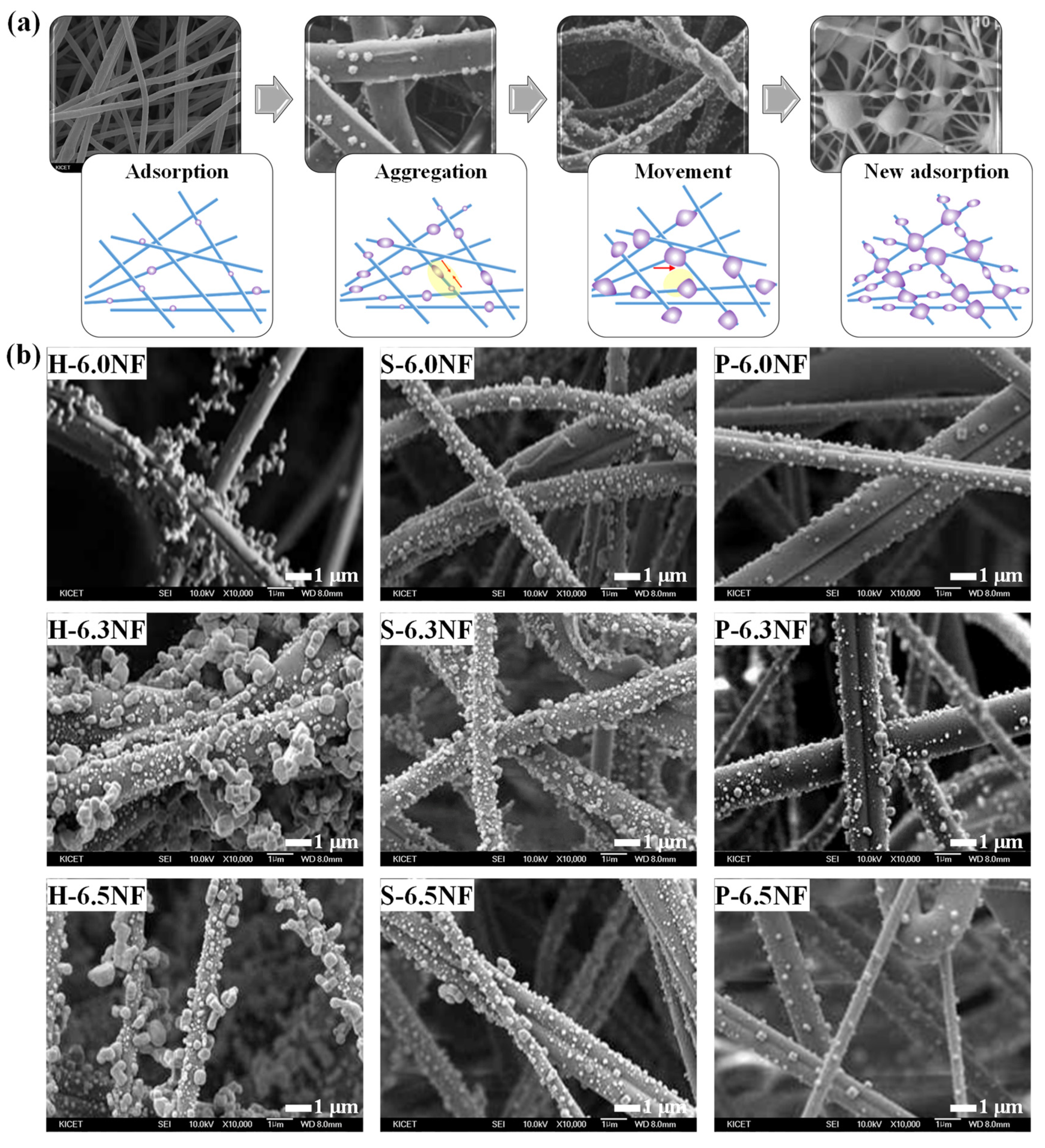
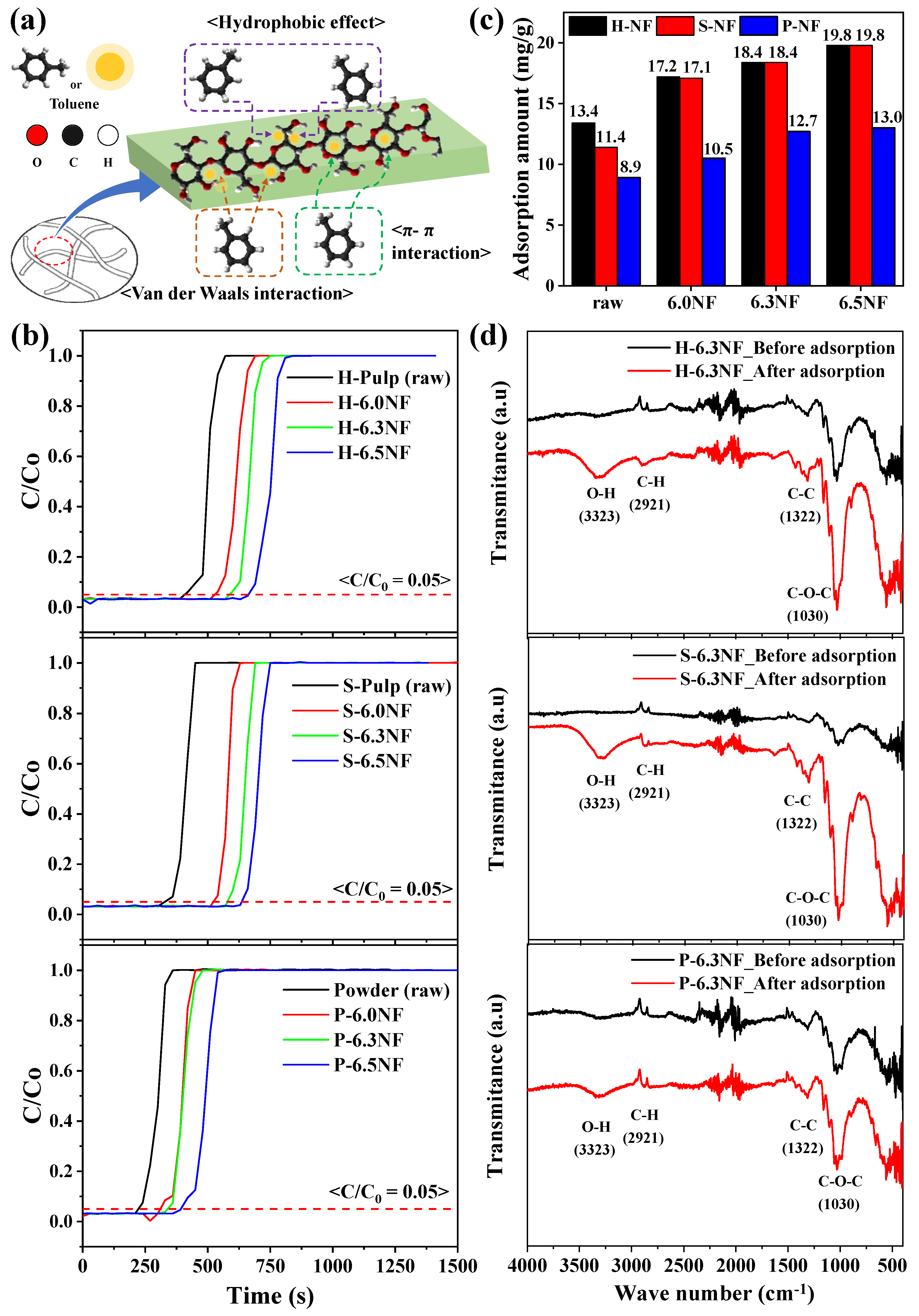
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Navas-Martín, M.Á.; Oteiza, I.; Cuerdo-Vilches, T. Dwelling in Times of COVID-19: An Analysis on Habitability and Environmental Factors of Spanish Housing. J. Build. Eng. 2022, 60, 105012. [Google Scholar] [CrossRef]
- Gustafson, P.; Östman, C.; Sällsten, G. Indoor Levels of Polycyclic Aromatic Hydrocarbons in Homes with or Without Wood Burning for Heating. Environ. Sci. Technol. 2008, 42, 5074–5080. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Sun, H.; Zhang, J.; Xu, Y.; Ding, Z. The Hematologic Effects of BTEX Exposure Among Elderly Residents in Nanjing: A Cross-Sectional Study. Environ. Sci. Pollut. Res. Int. 2019, 26, 10552–10561. [Google Scholar] [CrossRef] [PubMed]
- Pui, D.Y.H.; Chen, S.-C.; Zuo, Z. PM2.5 in China: Measurements, Sources, Visibility and Health Effects, and Mitigation. Particuology 2014, 13, 1–26. [Google Scholar] [CrossRef]
- Mallakpour, S.; Azadi, E.; Hussain, C.M. Fabrication of Air Filters with Advanced Filtration Performance for Removal of Viral Aerosols and Control the Spread of COVID-19. Adv. Colloid Interface Sci. 2022, 303, 102653. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liu, S.; Liu, J. Surface Modification of Coconut Shell Based Activated Carbon for the Improvement of Hydrophobic VOC Removal. J. Hazard. Mater. 2011, 192, 683–690. [Google Scholar] [CrossRef]
- Zhang, Y.; He, X.; Zhu, Z.; Wang, W.-N.; Chen, S.-C. Simultaneous Removal of VOCs and PM2.5 by Metal-Organic Framework Coated Electret Filter Media. J. Membr. Sci. 2021, 618, 118629. [Google Scholar] [CrossRef]
- Zhao, Y.; Low, Z.X.; Feng, S.; Zhong, Z.; Wang, Y.; Yao, Z. Multifunctional Hybrid Porous Filters with Hierarchical Structures for Simultaneous Removal of Indoor VOCs, Dusts and Microorganisms. Nanoscale. 2017, 9, 5433–5444. [Google Scholar] [CrossRef]
- Palmieri, S.; Pierpaoli, M.; Riderelli, L.; Qi, S.; Ruello, M.L. Preparation and Characterization of an Electrospun PLA-Cyclodextrins Composite for Simultaneous High-Efficiency PM and VOC Removal. J. Compos. Sci. 2020, 4, 79. [Google Scholar] [CrossRef]
- Karzar Jeddi, M.K.; Laitinen, O.; Liimatainen, H. Magnetic Superabsorbents Based on Nanocellulose Aerobeads for Selective Removal of Oils and Organic Solvents. Mater. Des. 2019, 183, 108115. [Google Scholar] [CrossRef]
- Ma, X.Y.D.; Ang, J.M.; Zhang, Y.; Zeng, Z.; Zhao, C.; Chen, F.; Ng, B.F.; Wan, M.P.; Wong, S.-C.; Li, Z.; et al. Highly Porous Polymer Nanofibrous Aerogels Cross-Linked via Spontaneous Inter-fiber Stereocomplexation and Their Potential for Capturing Ultrafine Airborne Particles. Polymer 2019, 179, 121649. [Google Scholar] [CrossRef]
- Wang, W.; Fang, Y.; Ni, X.; Wu, K.; Wang, Y.; Jiang, F.; Riffat, S.B. Fabrication and Characterization of a Novel Konjac Glucomannan-Based Air Filtration Aerogels Strengthened by Wheat Straw and Okara. Carbohydr. Polym. 2019, 224, 115129. [Google Scholar] [CrossRef] [PubMed]
- Awad, R.; Haghighat Mamaghani, A.H.; Boluk, Y.; Hashisho, Z. Synthesis and Characterization of Electrospun PAN-based Activated Carbon Nanofibers Reinforced with Cellulose Nanocrystals for Adsorption of VOCs. Chem. Eng. J. 2021, 410, 128412. [Google Scholar] [CrossRef]
- Saleem, H.; Trabzon, L.; Kilic, A.; Zaidi, S.J. Recent Advances in Nanofibrous Membranes: Production and Applications in Water Treatment and Desalination. Desalination 2020, 478, 114178. [Google Scholar] [CrossRef]
- Zhu, M.; Han, J.; Wang, F.; Shao, W.; Xiong, R.; Zhang, Q.; Pan, H.; Yang, Y.; Samal, S.K.; Zhang, F.; et al. Electrospun Nanofibers Membranes for Effective Air Filtration. Macromol. Mater. Eng. 2017, 302, 1600353. [Google Scholar] [CrossRef]
- Chen, G.; Hong, F.F.; Yuan, J.; Li, L.; Fang, M.; Wei, W.; Wang, X.; Wei, Y. Super Solvent of Cellulose with Extra High Solubility for Tunable Cellulose Structure with Versatile Application. Carbohydr. Polym. 2022, 296, 119917. [Google Scholar] [CrossRef]
- Lin, L.; Tsuchii, K. Dissolution Behavior of Cellulose in a Novel Cellulose Solvent. Carbohydr. Res. 2022, 511, 108490. [Google Scholar] [CrossRef]
- Yang, X.; Pu, Y.; Zhang, Y.; Liu, X.; Li, J.; Yuan, D.; Ning, X. Multifunctional Composite Membrane Based on BaTiO3@PU/PSA Nanofibers for High-Efficiency PM2.5 Removal. J. Hazard. Mater. 2020, 391, 122254. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, C.; Hsu, P.C.; Zhang, C.; Liu, N.; Zhang, J.; Lee, H.R.; Lu, Y.; Qiu, Y.; Chu, S.; et al. Nanofiber Air Filters with High-Temperature Stability for Efficient PM2.5 Removal from the Pollution Sources. Nano Lett. 2016, 16, 3642–3649. [Google Scholar] [CrossRef]
- Krugly, E.; Pauliukaityte, I.; Ciuzas, D.; Bulota, M.; Peciulyte, L.; Martuzevicius, D. Cellulose Electrospinning from Ionic Liquids: The Effects of Ionic Liquid Removal on the Fiber Morphology. Carbohydr. Polym. 2022, 285, 119260. [Google Scholar] [CrossRef]
- Zugenmaier, P. Crystalline Cellulose and Derivatives: Characterization and Structures; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar] [CrossRef]
- Uto, T.; Yamamoto, K.; Kadokawa, J.I. Cellulose Crystal Dissolution in Imidazolium-Based Ionic Liquids: A Theoretical Study. J. Phys. Chem. B 2018, 122, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Casasola, R.; Thomas, N.L.; Trybala, A.; Georgiadou, S. Electrospun Poly Lactic Acid (PLA) Fibres: Effect of Different Solvent Systems on Fibre Morphology and Diameter. Polymer 2014, 55, 4728–4737. [Google Scholar] [CrossRef]
- Li, B.; Liu, Y.; Wei, S.; Huang, Y.; Yang, S.; Xue, Y.; Xuan, H.; Yuan, H. A Solvent System Involved Fabricating Electrospun Polyurethane Nanofibers for Biomedical Applications. Polymers 2020, 12, 3038. [Google Scholar] [CrossRef] [PubMed]
- Wakai, F.; Akatsu, T. Anisotropic Viscosities and Shrinkage Rates in Sintering of Particles Arranged in a Simple Orthorhombic Structure. Acta Mater. 2010, 58, 1921–1929. [Google Scholar] [CrossRef]
- Rodrigues, B.V.M.; Ramires, E.C.; Santos, R.P.O.; Frollini, E.J. Ultrathin and Nanofibers via Room Temperature Electrospinning from Trifluoroacetic Acid Solutions of Untreated Lignocellulosic Sisal Fiber or Sisal Pulp. J. Appl. Polym. Sci. 2015, 132, 41826. [Google Scholar] [CrossRef]
- Liu, C.; Hsu, P.C.; Lee, H.W.; Ye, M.; Zheng, G.; Liu, N.; Li, W.; Cui, Y. Transparent Air Filter for High-Efficiency PM2.5 Capture. Nat. Commun. 2015, 6, 6205. [Google Scholar] [CrossRef]
- Cheng, T.; Li, J.; Ma, X.; Zhou, L.; Wu, H.; Yang, L. Alkylation Modified Pistachio Shell-Based Biochar to Promote the Adsorption of VOCs in High Humidity Environment. Environ. Pollut. 2022, 295, 118714. [Google Scholar] [CrossRef]
- Ece, M.Ş.; Kutluay, S. Comparative and Competitive Adsorption of Gaseous Toluene, Ethylbenzene, and Xylene onto Natural Cellulose-Modified Fe3O4 Nanoparticles. J. Environ. Chem. Eng. 2022, 10, 107389. [Google Scholar] [CrossRef]

| Adsorption Efficiency (%) | Pressure Drop | Air Permeability | PM2.5 Quality Factor | |||
|---|---|---|---|---|---|---|
| PM0.5 | PM1.0 | PM2.5 | (Pa) | (cm3/cm2/s) | (Pa–1) | |
| H-6.0NF | 85.36 | 88.14 | 89.12 | 98 | 277 | 0.24 |
| H-6.3NF | 92.25 | 97.25 | 97.38 | 105 | 284 | 0.28 |
| H-6.5NF | 88.25 | 93.22 | 95.25 | 97 | 270 | 0.23 |
| S-6.0NF | 83.24 | 87.15 | 92.36 | 96 | 274 | 0.22 |
| S-6.3NF | 86.25 | 88.83 | 94.21 | 102 | 283 | 0.27 |
| S-6.5NF | 83.12 | 89.12 | 95.31 | 93 | 269 | 0.21 |
| P-6.0NF | 74.15 | 76.81 | 79.17 | 88 | 281 | 0.19 |
| P-6.3NF | 75.43 | 77.22 | 81.21 | 99 | 277 | 0.25 |
| P-6.5NF | 73.25 | 76.22 | 80.05 | 86 | 264 | 0.18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, S.H.; Yun, J.S. Natural Cellulose-Based Multifunctional Nanofibers for the Effective Removal of Particulate Matter and Volatile Organic Compounds. Nanomaterials 2023, 13, 1720. https://doi.org/10.3390/nano13111720
Ji SH, Yun JS. Natural Cellulose-Based Multifunctional Nanofibers for the Effective Removal of Particulate Matter and Volatile Organic Compounds. Nanomaterials. 2023; 13(11):1720. https://doi.org/10.3390/nano13111720
Chicago/Turabian StyleJi, Sang Hyun, and Ji Sun Yun. 2023. "Natural Cellulose-Based Multifunctional Nanofibers for the Effective Removal of Particulate Matter and Volatile Organic Compounds" Nanomaterials 13, no. 11: 1720. https://doi.org/10.3390/nano13111720





