Novel Materials in Perovskite Solar Cells: Efficiency, Stability, and Future Perspectives
Abstract
1. Introduction
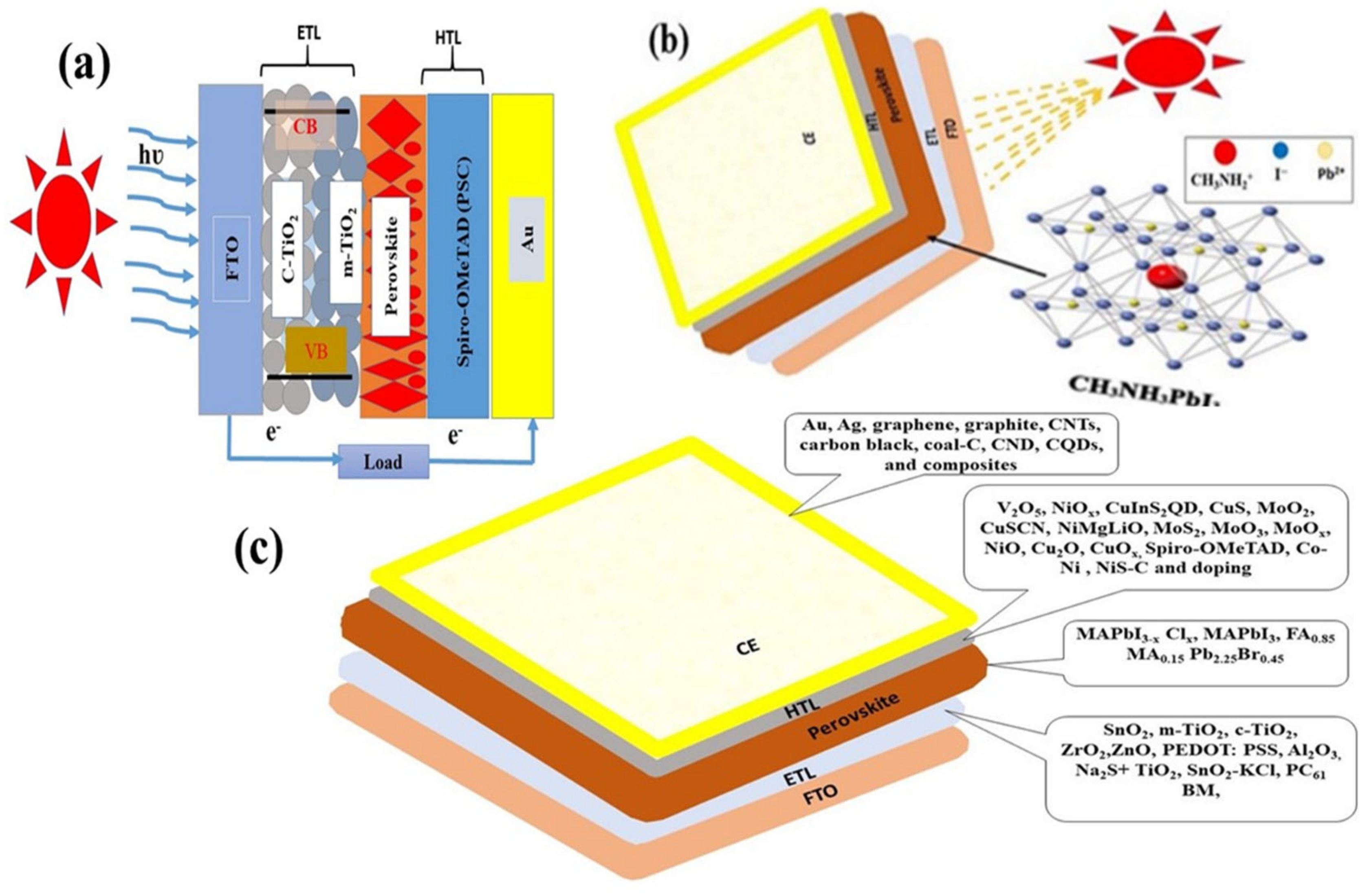
1.1. Perovskite Solar Cells
1.2. Working Mechanism of Perovskite Solar Cells
1.3. Generations of Solar Cells
2. Interfaces of Perovskite
2.1. Interface of Perovskite (PSC) and HTL
2.2. Interface of ETL and Perovskite
2.3. Quality of the Light Absorbing Layer for Stable and Efficient Perovskite Solar Cells
2.4. Effect of the Carbonaceous Counter Electrode (CE) on the Performance of Perovskite Solar Cells
3. Effect of Nanomaterials on the Performance of the Perovskite Solar Cells
4. Effect of Plasmon Nanoparticles on the Performance of Perovskite Solar Cells
5. Characterization of Perovskite Solar Cells
5.1. UV-Visible Spectroscopy/Photoluminescence Spectra in Terms of Efficiency
5.2. Electrochemical Impedance Spectroscopy (EIS) of Perovskite Solar Cells in Terms of Efficiency
5.3. X-ray Diffraction Analysis (XRD) of PSC in Terms of Efficiency
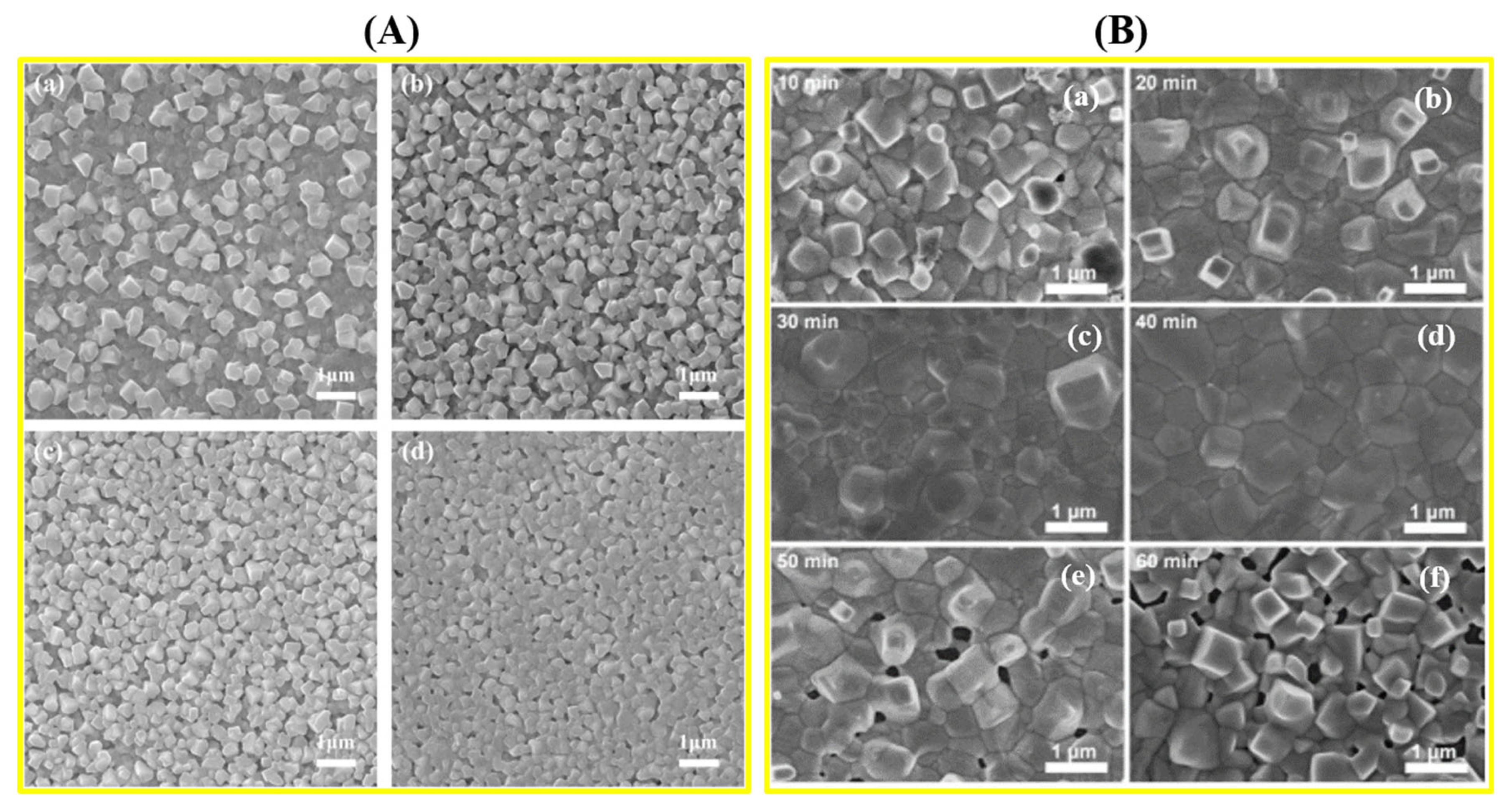
5.4. Morphological Study of Perovskite Crystals
5.5. Current-Voltage (J-V) Characteristics of Perovskite Solar Cells
6. Energy Alignment of Different Constituents of PSC
7. Current Trends and Commercialization of PSCs
8. Conclusions
Funding
Conflicts of Interest
References
- Park, N.G. Perovskite Solar Cells: An Emerging Photovoltaic Technology. Mater. Today 2015, 18, 65–72. [Google Scholar] [CrossRef]
- Hu, R.; Zhang, R.; Ma, Y.; Liu, W.; Chu, L.; Mao, W.; Zhang, J.; Yang, J.; Pu, Y.; Li, X. Enhanced Hole Transfer in Hole-Conductor-Free Perovskite Solar Cells via Incorporating CuS into Carbon Electrodes. Appl. Surf. Sci. 2018, 462, 840–846. [Google Scholar] [CrossRef]
- Green, M.A.; Yoshita, M.; Rauer, M.; Hohl-ebinger, E.D.D.J.; Kopidakis, N.; Bothe, K.; Hinken, D.; Hao, X. Solar Cell Efficiency Tables (Version 60). Prog. Photovolt. 2022, 30, 687–701. [Google Scholar] [CrossRef]
- Jiang, E.; Ai, Y.; Yan, J.; Li, N.; Lin, L.; Wang, Z.; Shou, C.; Yan, B.; Zeng, Y.; Sheng, J.; et al. Phosphate-Passivated SnO2 Electron Transport Layer for High-Performance Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2019, 11, 36727–36734. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zhang, Z.; Wang, L.; Chen, K.; Tao, L.; Zhang, Y.; Zhou, X. Commercial Carbon-Based All-Inorganic Perovskite Solar Cells with a High Efficiency of 13.81%: Interface Engineering and Photovoltaic Performance. ACS Appl. Energy Mater. 2021, 4, 3255–3264. [Google Scholar] [CrossRef]
- Yang, Y.; Pham, N.D.; Yao, D.; Fan, L.; Hoang, M.T.; Tiong, V.T.; Wang, Z.; Zhu, H.; Wang, H. Interface Engineering to Eliminate Hysteresis of Carbon-Based Planar Heterojunction Perovskite Solar Cells via CuSCN Incorporation. ACS Appl. Mater. Interfaces 2019, 11, 28431–28441. [Google Scholar] [CrossRef]
- Luo, Q.; Ma, H.; Hou, Q.; Li, Y.; Ren, J.; Dai, X.; Yao, Z.; Zhou, Y.; Xiang, L.; Du, H.; et al. All-Carbon-Electrode-Based Endurable Flexible Perovskite Solar Cells. Adv. Funct. Mater. 2018, 28, 1706777. [Google Scholar] [CrossRef]
- Al Katrib, M.; Planes, E.; Perrin, L. Effect of Chlorine Addition on the Performance and Stability of Electrodeposited Mixed Perovskite Solar Cells. Chem. Mater. 2022, 34, 2218–2230. [Google Scholar] [CrossRef]
- Cui, X.; Chen, Y.; Zhang, M.; Harn, Y.W.; Qi, J.; Gao, L.; Wang, Z.L.; Huang, J.; Yang, Y.; Lin, Z. Tailoring Carrier Dynamics in Perovskite Solar Cells via Precise Dimension and Architecture Control and Interfacial Positioning of Plasmonic Nanoparticles. Energy Environ. Sci. 2020, 13, 1743–1752. [Google Scholar] [CrossRef]
- Lee, T.C.; Yun, D.Q.; Zhao, D.W.; Yu, M.Y.; Zheng, L.L.; Li, M.; Dai, S.J.; Chen, D.C. Enhanced Efficiency and Stability of Planar Perovskite Solar Cells Using a Dual Electron Transport Layer of Gold Nanoparticles Embedded in Anatase TiO2 Films. ACS Appl. Energy Mater. 2020, 3, 9568–9575. [Google Scholar] [CrossRef]
- Ran, M.; Liu, N.; Yang, H.; Meng, R.; Chen, M.; Lu, H.; Yang, Y. Positive Effects in Perovskite Solar Cells Achieved Using Down-Conversion NaEuF4 Nanoparticles. Appl. Phys. Lett. 2020, 116, 113503. [Google Scholar] [CrossRef]
- Guo, M.; Liu, J.; Yuan, Y.; Zhang, Z.; Yin, S.; Leng, J.; Huang, N. CNTs/Cf Based Counter Electrode for Highly Efficient Hole-Transport-Material-Free Perovskite Solar Cells. J. Photochem. Photobiol. A Chem. 2020, 403, 112843. [Google Scholar] [CrossRef]
- Ramli, N.F.; Fahsyar, P.N.A.; Ludin, N.A.; Teridi, M.A.M.; Ibrahim, M.A.; Sepeai, S. Graphene Dispersion as a Passivation Layer for the Enhancement of Perovskite Solar Cell Stability. Mater. Chem. Phys. 2021, 257, 123798. [Google Scholar] [CrossRef]
- Zhao, Y.; Duan, J.; Wang, Y.; Yang, X.; Tang, Q. Precise Stress Control of Inorganic Perovskite Films for Carbon-Based Solar Cells with an Ultrahigh Voltage of 1.622 V. Nano Energy 2020, 67, 104286. [Google Scholar] [CrossRef]
- Li, J.; Jiu, T.; Chen, S.; Liu, L.; Yao, Q.; Bi, F.; Zhao, C.; Wang, Z.; Zhao, M.; Zhang, G.; et al. Graphdiyne as a Host Active Material for Perovskite Solar Cell Application. Nano Lett. 2018, 18, 6941–6947. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Zhou, Y.; Meng, F.; Li, Y.; Liu, A.; Li, Y.; Zhang, C.; Fan, M.; Wei, G.; Ma, T. Several Economical and Eco-Friendly Bio-Carbon Electrodes for Highly Efficient Perovskite Solar Cells. Carbon 2020, 162, 267–272. [Google Scholar] [CrossRef]
- Yang, F.; Liu, J.; Lu, Z.; Dai, P.; Nakamura, T.; Wang, S.; Chen, L.; Wakamiya, A.; Matsuda, K. Recycled Utilization of a Nanoporous Au Electrode for Reduced Fabrication Cost of Perovskite Solar Cells. Adv. Sci. 2020, 7, 1902474. [Google Scholar] [CrossRef][Green Version]
- Chen, D.; Fan, G.; Zhang, H.; Zhou, L.; Zhu, W.; Xi, H. Efficient Ni/Au Mesh Transparent Electrodes for ITO-Free Planar Perovskite Solar Cells. Nanomaterials 2019, 9, 932. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, J.; Li, J.; Zhou, Y.; Yu, C.; Hua, Y.; Yu, Y.; Li, R.; Lin, X.; Chen, R.; Wu, H.; et al. Tuning an Electrode Work Function Using Organometallic Complexes in Inverted Perovskite Solar Cells. J. Am. Chem. Soc. 2021, 143, 7759–7768. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, M.K.A. High-Performance Hole Conductor-Free Perovskite Solar Cell Using a Carbon Nanotube Counter Electrode. RSC Adv. 2020, 10, 35831–35839. [Google Scholar] [CrossRef]
- Guo, X.; Du, J.; Lin, Z.; Su, J.; Feng, L.; Zhang, J.; Hao, Y.; Chang, J. Enhanced Efficiency and Stability of Planar Perovskite Solar Cells Using SnO2:InCl3 Electron Transport Layer through Synergetic Doping and Passivation Approaches. Chem. Eng. J. 2021, 407, 127997. [Google Scholar] [CrossRef]
- Xiang, S.; Fu, Z.; Li, W.; Wei, Y.; Liu, J.; Liu, H.; Zhu, L.; Zhang, R.; Chen, H. Highly Air-Stable Carbon-Based α-CsPbI3 Perovskite Solar Cells with a Broadened Optical Spectrum. ACS Energy Lett. 2018, 3, 1824–1831. [Google Scholar] [CrossRef]
- Sun, P.P.; Bai, L.; Kripalani, D.R.; Zhou, K. A New Carbon Phase with Direct Bandgap and High Carrier Mobility as Electron Transport Material for Perovskite Solar Cells. npj Comput. Mater 2019, 5, 9. [Google Scholar] [CrossRef][Green Version]
- Bist, A.; Chatterjee, S. Review on Efficiency Enhancement Using Natural Extract Mediated Dye-Sensitized Solar Cell for Sustainable Photovoltaics. Energy Technol. 2021, 9, 2001058. [Google Scholar] [CrossRef]
- Khatibi, A.; Razi Astaraei, F.; Ahmadi, M.H. Generation and Combination of the Solar Cells: A Current Model Review. Energy Sci. Eng. 2019, 7, 305–322. [Google Scholar] [CrossRef][Green Version]
- Płaczek-Popko, E. Top PV Market Solar Cells 2016. Opto-Electron. Rev. 2017, 25, 55–64. [Google Scholar] [CrossRef]
- Omer, M.I.; Wang, X.; Tang, X. Determination of Dominant Recombination Site in Perovskite Solar Cells through Illumination-Side-Dependent Impedance Spectroscopy. Prog. Photovolt. Res. Appl. 2022, 30, 1228–1237. [Google Scholar] [CrossRef]
- Green, M.A. Third Generation Photovoltaics: Solar Cells for 2020 and beyond. Phys. E Low-Dimens. Syst. Nanostructures 2002, 14, 65–70. [Google Scholar] [CrossRef]
- Shao, S.; Loi, M.A. The Role of the Interfaces in Perovskite Solar Cells. Adv. Mater. Interfaces 2020, 7, 1901469. [Google Scholar] [CrossRef][Green Version]
- Guo, M.; Wei, C.; Liu, C.; Zhang, K.; Su, H.; Xie, K.; Zhai, P.; Zhang, J.; Liu, L. Composite Electrode Based on Single-Atom Ni Doped Graphene for Planar Carbon-Based Perovskite Solar Cells. Mater. Des. 2021, 209, 109972. [Google Scholar] [CrossRef]
- Meng, F.; Gao, L.; Liu, A.; Li, Y.; Ma, T. Interfacial Engineering Designed on CuSCN for Highly Efficient and Stable Carbon-Based Perovskite Solar Cells. Mater. Today Energy 2021, 21, 100801. [Google Scholar] [CrossRef]
- Liu, N.; Chu, L.; Ahmad, W.; Hu, R.; Luan, R.; Liu, W.; Yang, J.; Ma, Y.; Li, X. Low-Pressure Treatment of CuSCN Hole Transport Layers for Enhanced Carbon-Based Perovskite Solar Cells. J. Power Sources 2021, 499, 229970. [Google Scholar] [CrossRef]
- Lv, Y.; Jin, Y.; Cai, W.; Zhang, Z.; Zhou, X.; Chen, H. Air-Processed Carbon-Based Perovskite Solar Cells with Enhanced Efficiency and Stability: Effect of Temperature Control and Using CuSCN. J. Alloys Compd. 2020, 821, 153272. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, H.; Mei, Y.; Liu, H.; Wang, S.; Li, X. A Carbon Nanotube Bridging Method for Hole Transport Layer-Free Paintable Carbon-Based Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2019, 11, 916–923. [Google Scholar] [CrossRef]
- Zhu, W.; Chai, W.; Chen, D.; Ma, J.; Chen, D.; Xi, H.; Zhang, J.; Zhang, C.; Hao, Y. High-Efficiency (>14%) and Air-Stable Carbon- Based, All-Inorganic CsPbI2Br Perovskite Solar Cells through a Top-Seeded Growth Strategy. ACS Energy Lett. 2021, 6, 1500–1510. [Google Scholar] [CrossRef]
- Mariani, P.; Najafi, L.; Bianca, G.; Zappia, M.I.; Gabatel, L.; Agresti, A.; Pescetelli, S.; Di Carlo, A.; Bellani, S.; Bonaccorso, F. Low-Temperature Graphene-Based Paste for Large-Area Carbon Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2021, 13, 22368–22380. [Google Scholar] [CrossRef]
- Wu, C.; Wang, K.; Jiang, Y.; Yang, D.; Hou, Y.; Ye, T.; Han, C.S.; Chi, B.; Zhao, L.; Wang, S.; et al. All Electrospray Printing of Carbon-Based Cost-Effective Perovskite Solar Cells. Adv. Funct. Mater. 2021, 31, 2006803. [Google Scholar] [CrossRef]
- Xie, Y.; Cheng, J.; Liu, H.; Liu, J.; Maitituersun, B.; Ma, J.; Qiang, Y.; Shi, H.; Geng, C.; Li, Y.; et al. Co-Ni Alloy@carbon Aerogels for Improving the Efficiency and Air Stability of Perovskite Solar Cells and Its Hysteresis Mechanism. Carbon 2019, 154, 322–329. [Google Scholar] [CrossRef]
- Pitchaiya, S.; Natarajan, M.; Santhanam, A.; Ramakrishnan, V.M.; Asokan, V.; Palanichamy, P.; Rangasamy, B.; Sundaram, S.; Velauthapillai, D. Nickel Sulphide-Carbon Composite Hole Transporting Material for (CH3NH3PbI3) Planar Heterojunction Perovskite Solar Cell. Mater. Lett. 2018, 221, 283–288. [Google Scholar] [CrossRef]
- Meng, F.; Gao, L.; Yan, Y.; Cao, J.; Wang, N.; Wang, T.; Ma, T. Ultra-Low-Cost Coal-Based Carbon Electrodes with Seamless Interfacial Contact for Effective Sandwich-Structured Perovskite Solar Cells. Carbon 2019, 145, 290–296. [Google Scholar] [CrossRef]
- Cai, C.; Zhou, K.; Guo, H.; Pei, Y.; Hu, Z.; Zhang, J.; Zhu, Y. Enhanced Hole Extraction by NiO Nanoparticles in Carbon-Based Perovskite Solar Cells. Electrochim. Acta 2019, 312, 100–108. [Google Scholar] [CrossRef]
- Geng, C.; Xie, Y.; Wei, P.; Liu, H.; Qiang, Y.; Zhang, Y. An Efficient Co-NC Composite Additive for Enhancing Interface Performance of Carbon-Based Perovskite Solar Cells. Electrochim. Acta 2020, 358, 136883. [Google Scholar] [CrossRef]
- Li, X.; Tang, X.; Yang, Y.; Ye, T.; Wu, D.; Wang, H.; Li, J.; Wang, X. A Dopant-Free Polymer as Hole-Transporting Material for Highly Efficient and Stable Perovskite Solar Cells. Prog. Photovolt. Res. Appl. 2018, 26, 994–1002. [Google Scholar] [CrossRef]
- Spalla, M.; Perrin, L.; Planes, E.; Matheron, M.; Berson, S.; Flandin, L. Effect of the Hole Transporting/Active Layer Interface on the Perovskite Solar Cell Stability. ACS Appl. Energy Mater. 2020, 3, 3282–3292. [Google Scholar] [CrossRef]
- Lin, S.; Yang, B.; Qiu, X.; Yan, J.; Shi, J.; Yuan, Y.; Tan, W.; Liu, X.; Huang, H.; Gao, Y.; et al. Efficient and Stable Planar Hole-Transport-Material-Free Perovskite Solar Cells Using Low Temperature Processed SnO2 as Electron Transport Material. Org. Electron. 2018, 53, 235–241. [Google Scholar] [CrossRef]
- Galagan, Y. Perovskite Solar Cells: Toward Industrial-Scale Methods. J. Phys. Chem. Lett. 2018, 9, 4326–4335. [Google Scholar] [CrossRef]
- Sun, H.; Xie, D.; Song, Z.; Liang, C.; Xu, L.; Qu, X.; Yao, Y.; Li, D.; Zhai, H.; Zheng, K.; et al. Interface Defects Passivation and Conductivity Improvement in Planar Perovskite Solar Cells Using Na2S-Doped Compact TiO2 Electron Transport Layers. ACS Appl. Mater. Interfaces 2020, 12, 22853–22861. [Google Scholar] [CrossRef]
- Dong, Q.; Ho, C.H.Y.; Yu, H.; Salehi, A.; So, F. Defect Passivation by Fullerene Derivative in Perovskite Solar Cells with Aluminum-Doped Zinc Oxide as Electron Transporting Layer. Chem. Mater. 2019, 31, 6833–6840. [Google Scholar] [CrossRef]
- Qiang, Y.; Cheng, J.; Qi, Y.; Shi, H.; Liu, H.; Geng, C.; Xie, Y. Low-Temperature Preparation of HTM-Free SnO2-Based Planar Heterojunction Perovskite Solar Cells with Commercial Carbon as Counter Electrode. J. Alloys Compd. 2019, 809, 151817. [Google Scholar] [CrossRef]
- Li, F.; Shen, Z.; Weng, Y.; Lou, Q.; Chen, C.; Shen, L.; Guo, W.; Li, G. Novel Electron Transport Layer Material for Perovskite Solar Cells with Over 22% Efficiency and Long-Term Stability. Adv. Funct. Mater. 2020, 30, 2004933. [Google Scholar] [CrossRef]
- Zhu, P.; Gu, S.; Luo, X.; Gao, Y.; Li, S.; Zhu, J.; Tan, H. Simultaneous Contact and Grain-Boundary Passivation in Planar Perovskite Solar Cells Using SnO2-KCl Composite Electron Transport Layer. Adv. Energy Mater. 2020, 10, 1903083. [Google Scholar] [CrossRef]
- Wang, H.; Yang, F.; Li, N.; Kamarudin, M.A.; Qu, J.; Song, J.; Hayase, S.; Brabec, C.J. Efficient Surface Passivation and Electron Transport Enable Low Temperature-Processed Inverted Perovskite Solar Cells with Efficiency over 20%. ACS Sustain. Chem. Eng. 2020, 8, 8848–8856. [Google Scholar] [CrossRef]
- Fernandez-Delgado, O.; Chandrasekhar, P.S.; Cano-Sampaio, N.; Simon, Z.C.; Puente-Santiago, A.R.; Liu, F.; Castro, E.; Echegoyen, L. The Role of Fullerene Derivatives in Perovskite Solar Cells: Electron Transporting or Electron Extraction Layers? J. Mater. Chem. C 2021, 9, 10759–10767. [Google Scholar] [CrossRef]
- Tavakoli, M.M.; Tavakoli, R.; Yadav, P.; Kong, J. A Graphene/ZnO Electron Transfer Layer Together with Perovskite Passivation Enables Highly Efficient and Stable Perovskite Solar Cells. J. Mater. Chem. A 2019, 7, 679–686. [Google Scholar] [CrossRef][Green Version]
- Cao, J.J.; Wang, K.L.; Dong, C.; Li, X.M.; Yang, W.F.; Wang, Z.K. Bottom-Contact Passivation for High-Performance Perovskite Solar Cells Using TaCl5-Doped SnO2 as Electron-Transporting Layer. Org. Electron. 2021, 88, 105972. [Google Scholar] [CrossRef]
- Alias, N.; Ali Umar, A.; Malek, N.A.A.; Liu, K.; Li, X.; Abdullah, N.A.; Rosli, M.M.; Rahman, M.Y.A.; Shi, Z.; Zhang, X.; et al. Photoelectrical Dynamics Uplift in Perovskite Solar Cells by Atoms Thick 2D TiS2 layer Passivation of TiO2 nanograss Electron Transport Layer. ACS Appl. Mater. Interfaces 2021, 13, 3051–3061. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zuo, X.; He, Y.; Qian, F.; Zuo, S.; Zhang, Y.; Liang, L.; Chen, Z.; Zhao, K.; Liu, Z.; et al. Dual Passivation of Perovskite and SnO2 for High-Efficiency MAPbI3 Perovskite Solar Cells. Adv. Sci. 2021, 8, 2001466. [Google Scholar] [CrossRef]
- Chen, R.; Feng, Y.; Jing, L.; Wang, M.; Ma, H.; Bian, J.; Shi, Y. Low-Temperature Sprayed Carbon Electrode in Modular HTL-Free Perovskite Solar Cells: A Comparative Study on the Choice of Carbon Sources. J. Mater. Chem. C 2021, 9, 3546–3554. [Google Scholar] [CrossRef]
- Ji, J.; Liu, B.; Huang, H.; Wang, X.; Yan, L.; Qu, S.; Liu, X.; Jiang, H.; Duan, M.; Li, Y.; et al. Nondestructive Passivation of the TiO2 electron Transport Layer in Perovskite Solar Cells by the PEIE-2D MOF Interfacial Modified Layer. J. Mater. Chem. C 2021, 9, 7057–7064. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, S.; Seo, G.; Paek, S.; Cho, K.T.; Huckaba, A.J.; Calizzi, M.; Choi, D.W.; Park, J.S.; Lee, D.; et al. Efficient Planar Perovskite Solar Cells Using Passivated Tin Oxide as an Electron Transport Layer. Adv. Sci. 2018, 5, 1800130. [Google Scholar] [CrossRef]
- Lin, C.T.; Pont, S.; Kim, J.; Du, T.; Xu, S.; Li, X.; Bryant, D.; McLachlan, M.A.; Durrant, J.R. Passivation against Oxygen and Light Induced Degradation by the PCBM Electron Transport Layer in Planar Perovskite Solar Cells. Sustain. Energy Fuels 2018, 2, 1686–1692. [Google Scholar] [CrossRef][Green Version]
- Wang, J.; Datta, K.; Weijtens, C.H.L.; Wienk, M.M.; Janssen, R.A.J. Insights into Fullerene Passivation of SnO2 Electron Transport Layers in Perovskite Solar Cells. Adv. Funct. Mater. 2019, 29, 1905883. [Google Scholar] [CrossRef][Green Version]
- Sun, P.-P.; Kripalani, D.R.; Bai, L.; Chi, W.; Zhou, K. Pentadiamond: A Highly Efficient Electron Transport Layer for Perovskite Solar Cells. J. Phys. Chem. C 2021, 125, 5372–5379. [Google Scholar] [CrossRef]
- Maxim, A.A.; Sadyk, S.N.; Aidarkhanov, D.; Surya, C.; Ng, A.; Hwang, Y.H.; Atabaev, T.S.; Jumabekov, A.N. PMMA Thin Film with Embedded Carbon Quantum Dots for Post-Fabrication Improvement of Light Harvesting in Perovskite Solar Cells. Nanomaterials 2020, 10, 291. [Google Scholar] [CrossRef][Green Version]
- Zhang, Z.; Fan, W.; Wei, X.; Zhang, L.; Yang, Z.; Wei, Z.; Shen, T.; Si, H.; Qi, J. Promoted Performance of Carbon Based Perovskite Solar Cells by Environmentally Friendly Additives of CH3COONH4 and Zn(CH3COO)2. J. Alloys Compd. 2019, 802, 694–703. [Google Scholar] [CrossRef]
- Zhu, X.; Du, M.; Feng, J.; Wang, H.; Xu, Z.; Wang, L.; Zuo, S.; Wang, C.; Wang, Z.; Zhang, C.; et al. High-Efficiency Perovskite Solar Cells with Imidazolium-Based Ionic Liquid for Surface Passivation and Charge Transport. Angew. Chem.—Int. Ed. 2021, 60, 4238–4244. [Google Scholar] [CrossRef]
- Wang, X.; Qiu, Y.; Wang, L.; Zhang, T.; Zhu, L.; Shan, T.; Wang, Y.; Jiang, J.; Kong, L.; Zhong, H.; et al. Organic Nanocrystals Induced Surface Passivation towards High-Efficiency and Stable Perovskite Solar Cells. Nano Energy 2021, 89, 106445. [Google Scholar] [CrossRef]
- Jiang, X.; Chen, S.; Li, Y.; Zhang, L.; Shen, N.; Zhang, G.; Du, J.; Fu, N.; Xu, B. Direct Surface Passivation of Perovskite Film by 4-Fluorophenethylammonium Iodide toward Stable and Efficient Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2021, 13, 2558–2565. [Google Scholar] [CrossRef]
- Choi, H.; Liu, X.; Kim, H.I.; Kim, D.; Park, T.; Song, S. A Facile Surface Passivation Enables Thermally Stable and Efficient Planar Perovskite Solar Cells Using a Novel IDTT-Based Small Molecule Additive. Adv. Energy Mater. 2021, 11, 2003829. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhao, Y.; Zhang, X.; Yang, X.; Chen, Y.; Chu, Z.; Ye, Q.; Li, X.; Yin, Z.; You, J. Surface Passivation of Perovskite Film for Efficient Solar Cells. Nat. Photonics 2019, 13, 460–466. [Google Scholar] [CrossRef]
- Mahapatra, A.; Prochowicz, D.; Tavakoli, M.M.; Trivedi, S.; Kumar, P.; Yadav, P. A Review of Aspects of Additive Engineering in Perovskite Solar Cells. J. Mater. Chem. A 2020, 8, 27–54. [Google Scholar] [CrossRef]
- Qian, F.; Yuan, S.; Cai, Y.; Han, Y.; Zhao, H.; Sun, J.; Liu, Z.; Liu, S. Novel Surface Passivation for Stable FA0.85MA0.15PbI3 Perovskite Solar Cells with 21.6% Efficiency. Sol. RRL 2019, 3, 1900072. [Google Scholar] [CrossRef]
- Chen, S.C.; Wang, D.; Zheng, Q. Surface Passivation of All-Inorganic CsPbI2Br with a Fluorinated Organic Ammonium Salt for Perovskite Solar Cells with Efficiencies over 16%. Sol. RRL 2020, 4, 2000321. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, L.; Xu, Z.; Olthof, S.; Ren, X.; Liu, Y.; Yang, D.; Gao, F.; Liu, S. Efficient Perovskite Solar Cellsviasurface Passivation by a Multifunctional Small Organic Ionic Compound. J. Mater. Chem. A 2020, 8, 8313–8322. [Google Scholar] [CrossRef]
- Ezike, S.C.; Alabi, A.B.; Ossai, A.N.; Aina, A.O. Stability-Improved Perovskite Solar Cells through 4-Tertbutylpyridine Surface-Passivated Perovskite Layer Fabricated in Ambient Air. Opt. Mater. 2021, 112, 110753. [Google Scholar] [CrossRef]
- Xia, J.; Liang, C.; Mei, S.; Gu, H.; He, B.; Zhang, Z.; Liu, T.; Wang, K.; Wang, S.; Chen, S.; et al. Deep Surface Passivation for Efficient and Hydrophobic Perovskite Solar Cells. J. Mater. Chem. A 2021, 9, 2919–2927. [Google Scholar] [CrossRef]
- Han, W.; Ren, G.; Li, Z.; Dong, M.; Liu, C.; Guo, W. Improving the Performance of Perovskite Solar Cells by Surface Passivation. J. Energy Chem. 2020, 46, 202–207. [Google Scholar] [CrossRef]
- Kırbıyık, Ç.; Toprak, A.; Başlak, C.; Kuş, M.; Ersöz, M. Nitrogen-Doped CQDs to Enhance the Power Conversion Efficiency of Perovskite Solar Cells via Surface Passivation. J. Alloys Compd. 2020, 832, 154897. [Google Scholar] [CrossRef]
- Mahmud, M.A.; Duong, T.; Yin, Y.; Pham, H.T.; Walter, D.; Peng, J.; Wu, Y.; Li, L.; Shen, H.; Wu, N.; et al. Double-Sided Surface Passivation of 3D Perovskite Film for High-Efficiency Mixed-Dimensional Perovskite Solar Cells. Adv. Funct. Mater. 2020, 30, 1907962. [Google Scholar] [CrossRef]
- Mahmud, M.A.; Duong, T.; Yin, Y.; Peng, J.; Wu, Y.; Lu, T.; Pham, H.T.; Shen, H.; Walter, D.; Nguyen, H.T.; et al. In Situ Formation of Mixed-Dimensional Surface Passivation Layers in Perovskite Solar Cells with Dual-Isomer Alkylammonium Cations. Small 2020, 16, 2005022. [Google Scholar] [CrossRef]
- Ren, J.; Luo, Q.; Hou, Q.; Chen, H.; Liu, T.; He, H.; Wang, J.; Shao, Q.; Dong, M.; Wu, S.; et al. Suppressing Charge Recombination and Ultraviolet Light Degradation of Perovskite Solar Cells Using Silicon Oxide Passivation. ChemElectroChem 2019, 6, 3167–3174. [Google Scholar] [CrossRef]
- Omer, M.I.; Xizu, W.; Xiaohong, T. Enhancement of the Performance of Planar Perovskite Solar Cells by Active-Layer Surface/Interface Modification with Optimal Mixed Solvent-Antisolvent Post-Treatment. Org. Electron. 2022, 100, 106349. [Google Scholar] [CrossRef]
- Onwubiko, I.; Khan, W.S.; Subeshan, B.; Asmatulu, R. Investigating the Effects of Carbon-Based Counter Electrode Layers on the Efficiency of Hole-Transporter-Free Perovskite Solar Cells. Energy Ecol. Environ. 2020, 5, 141–152. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, X.; Lu, X.; Gao, X.; Gao, J.; Shui, L.; Wu, S.; Liu, J.M. Promoting the Hole Extraction with Co3O4 Nanomaterials for Efficient Carbon-Based CsPbI2Br Perovskite Solar Cells. Sol. RRL 2019, 3, 1800315. [Google Scholar] [CrossRef]
- Teng, P.; Han, X.; Li, J.; Xu, Y.; Kang, L.; Wang, Y.; Yang, Y.; Yu, T. An Elegant Face-Down Liquid-Space-Restricted Deposition of CsPbBr3 Films for Efficient Carbon-Based All-Inorganic Planar Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2018, 10, 9541–9546. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.; Liu, W.; Qin, Z.; Zhang, R.; Hu, R.; Yang, J.; Yang, J.; Li, X. Boosting Efficiency of Hole Conductor-Free Perovskite Solar Cells by Incorporating p-Type NiO Nanoparticles into Carbon Electrodes. Sol. Energy Mater. Sol. Cells 2018, 178, 164–169. [Google Scholar] [CrossRef]
- Raptis, D.; Stoichkov, V.; Meroni, S.M.P.; Pockett, A.; Worsley, C.A.; Carnie, M.; Worsley, D.A.; Watson, T. Enhancing Fully Printable Mesoscopic Perovskite Solar Cell Performance Using Integrated Metallic Grids to Improve Carbon Electrode Conductivity. Curr. Appl. Phys. 2020, 20, 619–627. [Google Scholar] [CrossRef]
- Hsu, H.L.; Hsiao, H.T.; Juang, T.Y.; Jiang, B.H.; Chen, S.C.; Jeng, R.J.; Chen, C.P. Carbon Nanodot Additives Realize High-Performance Air-Stable p–i–n Perovskite Solar Cells Providing Efficiencies of up to 20.2%. Adv. Energy Mater. 2018, 8, 1802323. [Google Scholar] [CrossRef]
- Gao, L.; Hu, J.; Meng, F.; Zhou, Y.; Li, Y.; Wei, G.; Ma, T. Comparison of Interfacial Bridging Carbon Materials for Effective Carbon-Based Perovskite Solar Cells. J. Colloid Interface Sci. 2020, 579, 425–430. [Google Scholar] [CrossRef]
- Li, S.; Li, Y.; Liu, K.; Chen, M.; Peng, W.; Yang, Y.; Li, X. Laser Fabricated Carbon Quantum Dots in Anti-Solvent for Highly Efficient Carbon-Based Perovskite Solar Cells. J. Colloid Interface Sci. 2021, 600, 691–700. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, S.; Zhang, H.; Feng, Y.; Tian, W.; Yan, Y.; Bian, J.; Wang, Y.; Jin, S.; Zakeeruddin, S.M.; et al. Efficient Stable Graphene-Based Perovskite Solar Cells with High Flexibility in Device Assembling: Via Modular Architecture Design. Energy Environ. Sci. 2019, 12, 3585–3594. [Google Scholar] [CrossRef]
- Passatorntaschakorn, W.; Bhoomanee, C.; Ruankham, P.; Gardchareon, A.; Songsiriritthigul, P.; Wongratanaphisan, D. Room-Temperature Carbon Electrodes with Ethanol Solvent Interlacing Process for Efficient and Stable Planar Hybrid Perovskite Solar Cells. Energy Rep. 2021, 7, 2493–2500. [Google Scholar] [CrossRef]
- Jiang, P.; Xiong, Y.; Xu, M.; Mei, A.; Sheng, Y.; Hong, L.; Jones, T.W.; Wilson, G.J.; Xiong, S.; Li, D.; et al. The Influence of the Work Function of Hybrid Carbon Electrodes on Printable Mesoscopic Perovskite Solar Cells. J. Phys. Chem. C 2018, 122, 16481–16487. [Google Scholar] [CrossRef]
- Jiang, P.; Jones, T.W.; Duffy, N.W.; Anderson, K.F.; Bennett, R.; Grigore, M.; Marvig, P.; Xiong, Y.; Liu, T.; Sheng, Y.; et al. Fully Printable Perovskite Solar Cells with Highly-Conductive, Low-Temperature, Perovskite-Compatible Carbon Electrode. Carbon 2018, 129, 830–836. [Google Scholar] [CrossRef]
- Zhong, Y.; Xu, L.; Li, C.; Zhang, B.; Wu, W. Needle Coke: A Predominant Carbon Black Alternative for Printable Triple Mesoscopic Perovskite Solar Cells. Carbon 2019, 153, 602–608. [Google Scholar] [CrossRef]
- Liu, H.; Xie, Y.; Wei, P.; Wang, W.; Chen, H.; Geng, C.; Qiang, Y. Interface Optimization of Hole-Conductor Free Perovskite Solar Cells Using Porous Carbon Materials Derived from Biomass Soybean Dregs as a Cathode. J. Alloys Compd. 2020, 842, 155851. [Google Scholar] [CrossRef]
- Su, G.; He, B.; Gong, Z.; Ding, Y.; Duan, J.; Zhao, Y.; Chen, H.; Tang, Q. Enhanced Charge Extraction in Carbon-Based All-Inorganic CsPbBr3 Perovskite Solar Cells by Dual-Function Interface Engineering. Electrochim. Acta 2019, 328, 135102. [Google Scholar] [CrossRef]
- Raminafshar, C.; Dracopoulos, V.; Mohammadi, M.R.; Lianos, P. Carbon Based Perovskite Solar Cells Constructed by Screen-Printed Components. Electrochim. Acta 2018, 276, 261–267. [Google Scholar] [CrossRef]
- Teixeira, C.O.; Andrade, L.; Mendes, A. Easy Processing Carbon Paper Electrode for Highly Efficient Perovskite Solar Cells. J. Power Sources 2020, 479, 229071. [Google Scholar] [CrossRef]
- Pant, B.; Saud, P.S.; Park, M.; Park, S.J.; Kim, H.Y. General One-Pot Strategy to Prepare Ag–TiO2 Decorated Reduced Graphene Oxide Nanocomposites for Chemical and Biological Disinfectant. J. Alloys Compd. 2016, 671, 51–59. [Google Scholar] [CrossRef]
- Pant, B.; Park, M.; Kim, H.Y.; Park, S.J. Ag-ZnO Photocatalyst Anchored on Carbon Nanofibers: Synthesis, Characterization, and Photocatalytic Activities. Synth. Met. 2016, 220, 533–537. [Google Scholar] [CrossRef]
- Pant, B.; Prasad Ojha, G.; Acharya, J.; Park, M. Ag3PO4-TiO2-Carbon Nanofiber Composite: An Efficient Visible-Light Photocatalyst Obtained from Eelectrospinning and Hydrothermal Methods. Sep. Purif. Technol. 2021, 276, 119400. [Google Scholar] [CrossRef]
- Pant, B.; Ojha, G.P.; Acharya, J.; Pant, H.R.; Park, M. Lokta Paper-Derived Free-Standing Carbon as a Binder-Free Electrode Material for High-Performance Supercapacitors. Sustain. Mater. Technol. 2022, 33, e00450. [Google Scholar] [CrossRef]
- Pant, B.; Park, M.; Park, S.J. TiO2 NPs Assembled into a Carbon Nanofiber Composite Electrode by a One-Step Electrospinning Process for Supercapacitor Applications. Polymers 2019, 11, 899. [Google Scholar] [CrossRef][Green Version]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, Applications and Toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Pant, B.; Pant, H.R.; Park, M.; Liu, Y.; Choi, J.W.; Barakat, N.A.M.; Kim, H.Y. Electrospun CdS-TiO2 Doped Carbon Nanofibers for Visible-Light-Induced Photocatalytic Hydrolysis of Ammonia Borane. Catal. Commun. 2014, 50, 63–68. [Google Scholar] [CrossRef]
- Wang, L.; Teles, M.P.R.; Arabkoohsar, A.; Yu, H.; Ismail, K.A.R.; Mahian, O.; Wongwises, S. A Holistic and State-of-the-Art Review of Nanotechnology in Solar Cells. Sustain. Energy Technol. Assess. 2022, 54, 102864. [Google Scholar] [CrossRef]
- Bist, A.; Ahmed, K.; Ishtiaque, S.; Saud, P.S. A Study of Dye-Sensitized Solar Cells Using Pomegranate Dye as Sensitizer with Two Different Concentrations in Terms of Solar Parameters. Pak. J. Chem. 2022, 12, 44–48. [Google Scholar]
- Gao, Y.; Wu, Y.; Lu, H.; Chen, C.; Liu, Y.; Bai, X.; Yang, L.; Yu, W.W.; Dai, Q.; Zhang, Y. CsPbBr3 Perovskite Nanoparticles as Additive for Environmentally Stable Perovskite Solar Cells with 20.46% Efficiency. Nano Energy 2019, 59, 517–526. [Google Scholar] [CrossRef]
- Chen, R.; Feng, Y.; Zhang, C.; Wang, M.; Jing, L.; Ma, C.; Bian, J.; Shi, Y. Carbon-Based HTL-Free Modular Perovskite Solar Cells with Improved Contact at Perovskite/Carbon Interfaces. J. Mater. Chem. C 2020, 8, 9262–9270. [Google Scholar] [CrossRef]
- Li, Z.; Wang, R.; Xue, J.; Xing, X.; Yu, C.; Huang, T.; Chu, J.; Wang, K.L.; Dong, C.; Wei, Z.; et al. Core-Shell ZnO@SnO2 Nanoparticles for Efficient Inorganic Perovskite Solar Cells. J. Am. Chem. Soc. 2019, 141, 17610–17616. [Google Scholar] [CrossRef]
- Tooghi, A.; Fathi, D.; Eskandari, M. Numerical Study of a Highly Efficient Light Trapping Nanostructure of Perovskite Solar Cell on a Textured Silicon Substrate. Sci. Rep. 2020, 10, 18699. [Google Scholar] [CrossRef]
- Zhou, L.; Zuo, Y.; Mallick, T.K.; Sundaram, S. Enhanced Efficiency of Carbon-Based Mesoscopic Perovskite Solar Cells through a Tungsten Oxide Nanoparticle Additive in the Carbon Electrode. Sci. Rep. 2019, 9, 8778. [Google Scholar] [CrossRef][Green Version]
- Sahai, S.; Varshney, A. Solar Absorbance Enhancement in Perovskite Solar Cells with the Inclusion of Copper Nanoparticles: An Architectural Study. Opt. Quantum Electron. 2021, 53, 111. [Google Scholar] [CrossRef]
- Subair, R.; Di Girolamo, D.; Bodik, M.; Nadazdy, V.; Li, B.; Nadazdy, P.; Markovic, Z.; Benkovicova, M.; Chlpik, J.; Kotlar, M.; et al. Effect of the Doping of PC61BM Electron Transport Layer with Carbon Nanodots on the Performance of Inverted Planar MAPbI3 Perovskite Solar Cells. Sol. Energy 2019, 189, 426–434. [Google Scholar] [CrossRef]
- Deng, W.; Yuan, Z.; Liu, S.; Yang, Z.; Li, J.; Wang, E.; Wang, X.; Li, J. Plasmonic Enhancement for High-Efficiency Planar Heterojunction Perovskite Solar Cells. J. Power Sources 2019, 432, 112–118. [Google Scholar] [CrossRef]
- Ghahremanirad, E.; Olyaee, S.; Hedayati, M. The Influence of Embedded Plasmonic Nanostructures on the Optical Absorption of Perovskite Solar Cells. Photonics 2019, 6, 37. [Google Scholar] [CrossRef][Green Version]
- Mohammadi, M.H.; Eskandari, M.; Fathi, D. Effects of the Location and Size of Plasmonic Nanoparticles (Ag and Au) in Improving the Optical Absorption and Efficiency of Perovskite Solar Cells. J. Alloys Compd. 2021, 877, 160177. [Google Scholar] [CrossRef]
- Yao, K.; Zhong, H.; Liu, Z.; Xiong, M.; Leng, S.; Zhang, J.; Xu, Y.X.; Wang, W.; Zhou, L.; Huang, H.; et al. Plasmonic Metal Nanoparticles with Core-Bishell Structure for High-Performance Organic and Perovskite Solar Cells. ACS Nano 2019, 13, 5397–5409. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhu, X.; Li, S.; Chen, M.; Liu, N.; Yang, H.; Ran, M.; Lu, H.; Yang, Y. Enhancing the Photovoltaic Performance of Perovskite Solar Cells Using Plasmonic Au@Pt@Au Core-Shell Nanoparticles. Nanomaterials 2019, 9, 1263. [Google Scholar] [CrossRef][Green Version]
- Behrouznejad, F.; Forouzandeh, M.; Khosroshahi, R.; Meraji, K.; Badrabadi, M.N.; Dehghani, M.; Li, X.; Zhan, Y.; Liao, Y.; Ning, Z.; et al. Effective Carbon Composite Electrode for Low-Cost Perovskite Solar Cell with Inorganic CuIn0.75Ga0.25S2 Hole Transport Material. Sol. RRL 2020, 4, 1900564. [Google Scholar] [CrossRef]
- Tao, H.; Li, Y.; Zhang, C.; Wang, K.; Tan, B.; Wang, J.; Tao, J. Efficiency Enhancement of Perovskite Solar Cells by Forming a Tighter Interface Contact of C/CH3NH3PbI3. J. Phys. Chem. Solids 2018, 123, 25–31. [Google Scholar] [CrossRef]
- Forouzandeh, M.; Behrouznejad, F.; Ghavaminia, E.; Khosroshahi, R.; Li, X.; Zhan, Y.; Liao, Y.; Ning, Z.; Taghavinia, N. Effect of Indium Ratio in CuInxGa1-XS2/Carbon Hole Collecting Electrode for Perovskite Solar Cells. J. Power Sources 2020, 475, 228658. [Google Scholar] [CrossRef]
- Kajal, P.; Lew, J.H.; Kanwat, A.; Rana, P.J.S.; Nutan, G.V.; Koh, T.M.; Mhaisalkar, S.G.; Powar, S.; Mathews, N. Unveiling the Role of Carbon Black in Printable Mesoscopic Perovskite Solar Cells. J. Power Sources 2021, 501, 230019. [Google Scholar] [CrossRef]
- Omer, M.I.; Wang, X.; Zhu, Q.; Tang, X. Identification of Asymmetric Interfacial Recombination in Perovskite Solar Cells through Impedance Spectroscopy. ACS Appl. Energy Mater. 2022, 5, 14760–14768. [Google Scholar] [CrossRef]
- Chu, Q.Q.; Sun, Z.; Ding, B.; Moon, K.S.; Yang, G.J.; Wong, C.P. Greatly Enhanced Power Conversion Efficiency of Hole-Transport-Layer-Free Perovskite Solar Cell via Coherent Interfaces of Perovskite and Carbon Layers. Nano Energy 2020, 77, 105110. [Google Scholar] [CrossRef]
- Barichello, J.; Vesce, L.; Matteocci, F.; Lamanna, E.; Di Carlo, A. The Effect of Water in Carbon-Perovskite Solar Cells with Optimized Alumina Spacer. Sol. Energy Mater. Sol. Cells 2019, 197, 76–83. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, H.; Zhang, Y.; Hu, R.; Jiang, M.; Zhang, R.; Tian, J.; Chu, L.; Zhang, J.; Xue, Q.; et al. Enhancing the Performance of Inverted Perovskite Solar Cells via Grain Boundary Passivation with Carbon Quantum Dots. ACS Appl. Mater. Interfaces 2019, 11, 3044–3052. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Ahmad, Z.; Zimmermann, I.; Martineau, D.; Shakoor, R.A.; Touati, F.; Riaz, K.; Al-Muhtaseb, S.A.; Nazeeruddin, M.K. Effect of Annealing Temperature on the Performance of Printable Carbon Electrodes for Perovskite Solar Cells. Org. Electron. 2019, 65, 375–380. [Google Scholar] [CrossRef]
- Singh, R.; Jun, H.K.; Arof, A.K. Activated Carbon as Back Contact for HTM-Free Mixed Cation Perovskite Solar Cell. Phase Transit. 2018, 91, 1268–1276. [Google Scholar] [CrossRef]
- Jiang, H.; Liu, X.; Chai, N.; Huang, F.; Peng, Y.; Zhong, J.; Zhang, Q.; Ku, Z.; Cheng, Y.B. Alleviate the J—V Hysteresis of Carbon-Based Perovskite Solar Cells via Introducing Additional Methylammonium Chloride into MAPbI3 Precursor. RSC Adv. 2018, 8, 35157–35161. [Google Scholar] [CrossRef][Green Version]
- Pitchaiya, S.; Eswaramoorthy, N.; Natarajan, M.; Santhanam, A.; Asokan, V.; Madurai Ramakrishnan, V.; Rangasamy, B.; Sundaram, S.; Ravirajan, P.; Velauthapillai, D. Perovskite Solar Cells: A Porous Graphitic Carbon Based Hole Transporter/Counter Electrode Material Extracted from an Invasive Plant Species Eichhornia Crassipes. Sci. Rep. 2020, 10, 6835. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zheng, X.; Troughton, J.; Gasparini, N.; Lin, Y.; Wei, M.; Hou, Y.; Liu, J.; Song, K.; Chen, Z.; Yang, C.; et al. Quantum Dots Supply Bulk- and Surface-Passivation Agents for Efficient and Stable Perovskite Solar Cells. Joule 2019, 3, 1963–1976. [Google Scholar] [CrossRef]
- Zhu, W.; Zhang, Z.; Chen, D.; Chai, W.; Chen, D.; Zhang, J.; Zhang, C.; Hao, Y. Interfacial Voids Trigger Carbon-Based, All-Inorganic CsPbIBr2 Perovskite Solar Cells with Photovoltage Exceeding 1.33 V. Nano-Micro Lett. 2020, 12, 87. [Google Scholar] [CrossRef][Green Version]
- Zeng, J.; Bi, L.; Cheng, Y.; Xu, B.; Jen, A.K.-Y. Self-Assembled Monolayer Enabling Improved Buried Interfaces in Blade-Coated Perovskite Solar Cells for High Efficiency and Stability. Nano Res. Energy 2022, 1, e9120004. [Google Scholar] [CrossRef]
- Terada, S.; Oku, T.; Suzuki, A.; Tachikawa, T.; Hasegawa, T.; Okita, M.; Fukunishi, S. Ethylammonium Bromide- and Potassium-Added CH3NH3PbI3 Perovskite Solar Cells. Photonics 2022, 9, 971. [Google Scholar] [CrossRef]
- Yang, H.Y.; Rho, W.Y.; Lee, S.K.; Kim, S.H.; Hahn, Y.B. TiO2 Nanoparticles/Nanotubes for Efficient Light Harvesting in Perovskite Solar Cells. Nanomaterials 2019, 9, 326. [Google Scholar] [CrossRef][Green Version]
- Sajid, S.; Alzahmi, S.; Salem, I.B.; Obaidat, I.M. Guidelines for Fabricating Highly Efficient Perovskite Solar Cells with Cu2O as the Hole Transport Material. Nanomaterials 2022, 12, 3315. [Google Scholar] [CrossRef]
- Yang, J.; Wang, J.; Yang, W.; Zhu, Y.; Feng, S.; Su, P.; Fu, W. Low-Temperature Processed Brookite Interfacial Modification for Perovskite Solar Cells with Improved Performance. Nanomaterials 2022, 12, 3653. [Google Scholar] [CrossRef]
- Kim, D.I.; Lee, J.W.; Jeong, R.H.; Boo, J.H. A High-efficiency and Stable Perovskite Solar Cell Fabricated in Ambient Air Using a Polyaniline Passivation Layer. Sci. Rep. 2022, 12, 697. [Google Scholar] [CrossRef]
- Li, Z.; Li, B.; Wu, X.; Sheppard, S.A.; Zhang, S.; Gao, D.; Long, N.J.; Zhu, Z. Organometallic-Functionalized Interfaces for Highly Efficient Inverted Perovskite Solar Cells. Science 2022, 376, 416–420. [Google Scholar] [CrossRef]
- Li, M.; Zhou, J.; Tan, L.; Li, H.; Liu, Y.; Jiang, C.; Ye, Y.; Ding, L.; Tress, W.; Yi, C. Multifunctional Succinate Additive for Flexible Perovskite Solar Cells with More than 23% Power-Conversion Efficiency. Innovation 2022, 3, 100310. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Han, Q.; Wang, L.; Yang, S.; Cai, X.; Zhang, C.; Ma, T. Surface Management for Carbon-Based CsPbI2Br Perovskite Solar Cell with 14% Power Conversion Efficiency. Sol. RRL 2021, 5, 2100404. [Google Scholar] [CrossRef]
- Duan, J.; Zhao, Y.; He, B.; Tang, Q. Simplified Perovskite Solar Cell with 4.1% Efficiency Employing Inorganic CsPbBr3 as Light Absorber. Small 2018, 14, 1704443. [Google Scholar] [CrossRef]
- Mabrouk, S.; Bahrami, B.; Elbohy, H.; Reza, K.M.; Gurung, A.; Liang, M.; Wu, F.; Wang, M.; Yang, S.; Qiao, Q. Synergistic Engineering of Hole Transport Materials in Perovskite Solar Cells. InfoMat 2020, 2, 928–941. [Google Scholar] [CrossRef]
- Li, X.; Tang, X.; Ye, T.; Wu, D.; Wang, H.; Wang, X. Fully Printable Organic and Perovskite Solar Cells with Transfer-Printed Flexible Electrodes. ACS Appl. Mater. Interfaces 2017, 9, 18730–18738. [Google Scholar] [CrossRef] [PubMed]
- Lemercier, T.; Perrin, L.; Berson, S.; Flandin, L.; Planes, E. Innovative PIN-Type Perovskite Solar Cells with 17% Efficiency: Processing and Characterization. Mater. Adv. 2021, 2, 7907–7921. [Google Scholar] [CrossRef]






| ETL Materials | Thickness | Sintering Temperature | Types of Solar Cells | Voc (mV) | Jsc (mA cm−2) | FF% | η% | Ref. |
|---|---|---|---|---|---|---|---|---|
| SnO2: ethanol (1:0) | 400 nm | 200 °C for 1 h | Planar | 860 | 20.84 | 38 | 6.78 | [49] |
| SnO2: ethanol (1:1) | 890 | 21.00 | 41 | 7.67 | ||||
| SnO2: ethanol (1:2) | 920 | 21.33 | 39 | 7.59 | ||||
| SnO2: ethanol (1:3) | 930 | 22.50 | 40 | 8.32 | ||||
| SnO2: ethanol (1:4) | 870 | 21.79 | 38 | 7.18 | ||||
| SnO2: ethanol (1:5) | 680 | 19.95 | 32 | 4.35 | ||||
| m-TiO2: ethanol (1:6) | 60 nm | 20 °C @ 10 min | Planar | 690 | 16.39 | 53.06 | 6.67 | [33] |
| 40 °C @ 10 min | 893 | 20.11 | 49.81 | 9.94 | ||||
| 60 °C @ 10 min | 964 | 18.89 | 54.94 | 11.12 | ||||
| 80 °C @ 10 min | 817 | 18.40 | 57.83 | 9.66 | ||||
| 100 °C @ 10 min | 851 | 14.40 | 53.55 | 7.29 | ||||
| Na2S + TiO2 (0.5% solution) | - | 450 °C @ 1 h | Planar | 1210 | 22.63 | 75.10 | 19.85 | [47] |
| Na2S + TiO2 (1% solution) | 1220 | 23.85 | 75.35 | 21.25 | ||||
| Na2S + TiO2 (2% solution) | 1210 | 23.90 | 72.53 | 19.90 | ||||
| Na2S + TiO2 (3% solution) | 1190 | 23.58 | 73.25 | 18.80 | ||||
| Pristine TiO2 | 1190 | 22.63 | 71.95 | 18.24 | ||||
| TiO2 doped with 1% NaCl | 1200 | 23.44 | 75.05 | 19.08 | ||||
| SnO2 (Reverse scan) | 40 nm | Dry the film @ 50 °C for 5 min | Planar | 1080 | 22.58 | 75.79 | 18.52 | [50] |
| SnO2 (Forward scan) | 1010 | 22.58 | 72.48 | 16.53 | ||||
| (CH3)2Sn(COOH)2 (Reverse Scan) | 1160 | 23.89 | 74.23 | 20.57 | ||||
| (CH3)2Sn(COOH)2 (forward Scan) | 1140 | 23.84 | 73.04 | 19.85 | ||||
| (CH3)2Sn(COOH)2 with KCl (Reverse Scan) | 300 nm | Annealed @ 120 °C for 15 min | 1180 | 23.93 | 78.23 | 22.09 | ||
| (CH3)2Sn(COOH)2 with KCl (forward Scan) | 1180 | 24.03 | 78.33 | 22.21 | ||||
| SnO2 (Reverse scan) | - | 150 °C for 30 min in air | Planar | 1077 | 24.0 | 77.9 | 20.2 | [51] |
| SnO2 (Forward scan) | 1017 | 24.0 | 75.4 | 18.4 | ||||
| SnO2-KCl (Reverse scan) | 1137 | 24.2 | 80.7 | 22.2 | ||||
| SnO2-KCl (Forward scan) | 1097 | 24.2 | 79.9 | 21.2 | ||||
| Pristin PCBM | No passivation | - | Inverted | 1080 | 22.7 | 70.0 | 17.3 | [52] |
| 2FBT2FPDI | 7 nm | 1100 | 23.5 | 72.5 | 18.8 | |||
| 2FBT2FPDI | 13 nm | 1100 | 23.9 | 77.2 | 20.3 | |||
| 2FBT2FPDI | 21 nm | 1110 | 22.8 | 72.0 | 18.2 | |||
| Pristine 2FBT2FPDI | - | 1100 | 22.5 | 67.8 | 16.8 | |||
| PC61 BM | 85 nm | 80 °C each for 15 min | Planar | 1033 | 22.05 | 70 | 15.43 | [53] |
| BPy-C60 | 980 | 22.31 | 67 | 14.59 | ||||
| BpAn-C60 | 989 | 22.05 | 59 | 12.92 | ||||
| BAn-C60 | 851 | 16.93 | 24 | 3.42 | ||||
| ZnO (forward scan) | 300 nm | 150 °C for 30 min in ambient air | Planar | 971 | 20.12 | 66.4 | 12.93 | [54] |
| ZnO (reverse scan) | 996 | 20.63 | 67.3 | 13.82 | ||||
| MLG/ZnO (forward) | 1114 | 22.74 | 77.1 | 19.54 | ||||
| MLG/ZnO (backward) | 1120 | 22.71 | 77.9 | 19.81 | ||||
| MLG/ZnO with passivation-(forward) | 1149 | 23.39 | 77.5 | 20.82 | ||||
| MLG/ZnO with passivation–(backward) | 1150 | 23.42 | 78.1 | 21.03 |
| Passivated Material | Percentage Initial Stability of Efficiency | Thermal Ageing (Humidity) | Time (Hours) | Percentage Final Stability of Efficiency | Ref. |
|---|---|---|---|---|---|
| Without BETAB | 90% | @ 60 °C | 200 | 85% | [67] |
| BETAB | 90% in air | 500 | 90% | ||
| Without F-PEAI | 93.3% | @ 60 °C | 720 | 70% | [68] |
| F-PEAI | 98.9% | 89.9% | |||
| IDTT-ThCz | 100% | @85 °C | 500 | 95% | [69] |
| PEAI | Stable | @85 °C | Above 500 | Stability declined after a few hours | [70] |
| CF3PEAI | 100% | 70–80% humidity | 528 | 98% | [71] |
| PSK/CuSCN/C | 100% | 75–85% | 240 | 98% | [6] |
| PSK/C-CuSCN | 100% | 91% |
| Carbon Material (CE) | ETLs | HTLs | Active Area (cm2) | Voc (mV) | Jsc (mA cm−2) | FF (%) | ɳ (%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| CNC | SnO2 | CNC | 1 | 936 | 15.34 | 33.28 | 4.78 | [58] |
| MWCNT | MWCNT | 1009 | 22.01 | 50.44 | 11.20 | |||
| Graphene | Graphene | 996 | 21.93 | 41.96 | 9.17 | |||
| Pure carbon(C) | c-TiO2, m-TiO2 | C | 0.16 | 1018 | 17.59 | 57.5 | 10.29 | [93] |
| NiO:Carbon (NiO-C) (1:10) | NiO-C | 1021 | 19.95 | 60 | 11.80 | |||
| NiO:Carbon (1:20) | NiO-C | 1035 | 20.93 | 61.12 | 13.26 | |||
| NiO:Carbon (1:30) | NiO-C | 1030 | 20.15 | 60.7 | 12.60 | |||
| Carbon (C) | c-TiO2, m-TiO2 | C | 0.14 | 930 | 16.14 | 62.69 | 9.36 | [2] |
| C-0.5% CuS | (C-CuS) | 920 | 16.62 | 63.92 | 10.14 | |||
| C-1% CuS | (C-CuS) | 980 | 18.26 | 63.23 | 11.28 | |||
| C-2% CuS | (C-CuS) | 880 | 16.67 | 57.87 | 8.49 | |||
| Coal-C coating | c-TiO2, m-TiO2 | Coal-C | 1.0 | 890 | 22.29 | 44 | 8.72 | [40] |
| Carbon Black (CB) | CB | 0.3 | 840 | 21.39 | 60 | 10.87 | ||
| Standard carbon electrode (std) | c-TiO2, m-TiO2, m-ZrO2 | 0.49 | 830 | 3.35 | 46.68 | 7.73 | [94] | |
| Std + carbon ink (SCI) | SCI | 840 | 3.53 | 43.44 | 7.70 | |||
| Std + carbon ink + Cu grid (SCI-Cu) | (SCI-Cu) | 840 | 3.65 | 59.18 | 11.05 | |||
| Std + carbon ink + Al grid (SCI-Ag) | (SCI-Ag) | 850 | 3.49 | 56.77 | 9.97 | |||
| CS-B | TiO2 | CS-B | 0.15 | 690 | 18.70 | 49 | 6.36 | [16] |
| PA-B | PA-B | 740 | 19.35 | 54 | 7.85 | |||
| PS-B | PS-B | 81 | 21.06 | 61 | 10.30 | |||
| BC-B | BC-B | 85 | 23.15 | 65 | 12.82 | |||
| Pristine | PCBM | NiOx | 0.06 | 1040 | 18.24 | 76.36 | 15.11 | [95] |
| 3% CND | 1040 | 19.38 | 73.94 | 15.86 | ||||
| 5% CND | 1060 | 20.13 | 77.34 | 16.94 | ||||
| 10% CND | 1010 | 18.68 | 74.55 | 15.32 | ||||
| Urea | 1050 | 21.57 | 74.98 | 17.76 | ||||
| 5% CND/Urea | 1070 | 22.74 | 76.92 | 19.50 | ||||
| CN/carbon | TiO2 | CN | 0.06 | 940 | 23.51 | 68 | 15.09 | [96] |
| AB/carbon | AB | 910 | 22.99 | 64 | 13.47 | |||
| NC/carbon | NC | 890 | 22.88 | 61 | 12.37 | |||
| GN/carbon | GN | 850 | 22.11 | 61 | 11.50 | |||
| EACQDs (pristine) | c-TiO2, m-TiO2, ZrO2 | Carbon quantum dots (CQDs) | 0.06 | 1005 | 21.65 | 55.86 | 12.15 | [97] |
| EACQDs (0.005 mg/mL) | 1016 | 22.49 | 60.91 | 13.92 | ||||
| EACQDs (0.01 mg/mL) | 1021 | 22.72 | 65.29 | 15.14 | ||||
| EACQDs (0.02 mg/mL) | 1002 | 22.35 | 61.86 | 13.87 | ||||
| EACQDs (0.05 mg/mL) | 9880 | 21.10 | 59.38 | 12.38 | ||||
| CB | SnO2 | Spiro-OMeTAD | 1.30 | 1040 | 21.11 | 64 | 14.05 | [98] |
| GS | 840 | 21.52 | 55 | 9.94 | ||||
| G | 1050 | 22.78 | 78 | 18.65 | ||||
| Carbon black (CB) | SnO2 | w/N-CQDs | 0.09 | 1622 | 7.87 | 80.1 | 10.71 | [14] |
| w/o N-CQDs | 1588 | 7.64 | 84.1 | 10.20 |
| ETL/Perovskite/CE Interface | (Rs) Ω | (Rrce) Ω | Voc (mV) | Jsc (mAcm−2) | FF (%) | η (%) | Ref. |
|---|---|---|---|---|---|---|---|
| Cf | 40.71 | 306.90 | 780 | 21.32 | 45 | 7.47 | [12] |
| CNTs | 32.84 | 203.90 | 800 | 22.98 | 49 | 8.93 | |
| CNTs/Cf | 27.84 | 102.30 | 770 | 23.60 | 65 | 11.80 | |
| O-MWCNTs (0.0 mg/mL) | 26.2 | 577 | 970 | 21.89 | 37.24 | 7.96 | [110] |
| O-MWCNTs (0.5 mg/mL) | 25.8 | 833 | 996 | 21.96 | 41.09 | 8.99 | |
| O-MWCNTs (1.0 mg/mL) | 28.8 | 93 | 841 | 19.42 | 32.58 | 5.28 | |
| VC | 11.1 | 2.8 | 940 | 20.87 | 63.77 | 12.55 | [124] |
| MC | 10.7 | 1.6 | 930 | 20.53 | 61.28 | 11.76 | |
| SP | 12.8 | 15.0 | 940 | 20.22 | 55.07 | 10.48 | |
| Au@Ag@SiO2 (0%) | 23.49 | 131.8 | 1033 | 20.63 | 70 | 15.41 | [116] |
| Au@Ag@SiO2 (0.5%) | 23.73 | 169.0 | 1046 | 21.02 | 71 | 16.12 | |
| Au@Ag@SiO2 (1.0%) | 27.68 | 184.1 | 1044 | 22.10 | 72 | 17.38 | |
| Au@Ag@SiO2 (1.5%) | 28.04 | 240.5 | 1048 | 21.15 | 71 | 16.15 |
| Substrate | ETL | Perovskite | HTL | CE | Voc (mV) | Jsc (mAcm−2) | FF (%) | ɳ (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| FTO | TiO2 | MAPbI2Br | NiO | Au | 720 | 23.28 | 50.85 | 8.53 | [139] |
| Cu2O | 1292 | 23.30 | 83.65 | 25.21 | |||||
| CuI | 1138 | 23.33 | 72 | 21.80 | |||||
| CuSCN | 950 | 23.30 | 59.91 | 13.35 | |||||
| FTO | TiO2 treated with TiCl4 (no annealing) | CH3NH3PbI3 | Spiro-OMeTAD | Ag | 947 | 19.16 | 49.87 | 9.09 | [140] |
| Annealed @ 150°C | 1016 | 21.57 | 64.65 | 14.16 | |||||
| Annealed @ 300°C | 975 | 19.99 | 53.45 | 10.42 | |||||
| Annealed @ 450°C | 990 | 20.49 | 58.91 | 11.98 | |||||
| FTO | TiO2, PANI was incorporated at TiO2/MAPbI3 Interface | MAPbI3 | Spiro-OMeTAD | Au | 960 | 17.95 | 62.1 | 10.7 | [141] |
| PANI/MAPbI3 (65 nm) | 990 | 23.71 | 64.2 | 15.1 | |||||
| PANI/MAPbI3 (100 nm) | 980 | 20.10 | 64.1 | 12.7 | |||||
| PANI/MAPbI3 (135 nm) | 980 | 13.40 | 63.2 | 11.0 | |||||
| PANI/MAPbI3 (170 nm) | 980 | 16.30 | 64.4 | 10.3 | |||||
| ITO | C60 | Cs0.05 (FA0.98MA0.02)0.95Pb(I0.98Br0.02)3 (control) | Poly (triaryl- amine) (PTAA) | Ag | 1130 | 25.25 | 80.45 | 23.0 | [142] |
| Cs0.05 (FA0.98MA0.02)0.95Pb(I0.98Br0.02)3/FcTc2 (1.0 mg/mL) | 1184 | 25.68 | 82.32 | 25.0 | |||||
| FTO | TiO2 | FA0.85MA0.15PbI3 (control) | Spiro-OMeTAD | Au | 1090 | 24.02 | 71.91 | 19.55 | [72] |
| FA0.85MA0.15PbI3/TPFPB | 1120 | 24.69 | 78.22 | 21.60 | |||||
| ITO | SnO2 | FAPbI3(control) | Spiro-OMeTAD | Au | 1136 | 26.27 | 78.49 | 23.42 | [143] |
| FAPbI3/MS | 1164 | 26.31 | 82.82 | 25.38 | |||||
| ITO | SnO2 | CsPbBrI2/BMIMBF4 (0 mg/mL) | Carbon | Carbon | 1140 | 14.33 | 69 | 11.37 | [137] |
| CsPbBrI2/BMIMBF4 (0.25 mg/mL) | 1270 | 14.21 | 72 | 13.09 | |||||
| CsPbBrI2/BMIMBF4 (0.50 mg/mL) | 1270 | 14.68 | 75 | 14.03 | |||||
| CsPbBrI2/BMIMBF4 (1.0 mg/mL) | 1270 | 14.30 | 71 | 12.88 | |||||
| CsPbBrI2/BMIMBF4 (2.5 mg/mL) | 1260 | 13.88 | 66 | 11.49 | |||||
| CsPbBrI2/BMIMBF4 (5 mg/mL) | 1250 | 13.78 | 58 | 10.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bist, A.; Pant, B.; Ojha, G.P.; Acharya, J.; Park, M.; Saud, P.S. Novel Materials in Perovskite Solar Cells: Efficiency, Stability, and Future Perspectives. Nanomaterials 2023, 13, 1724. https://doi.org/10.3390/nano13111724
Bist A, Pant B, Ojha GP, Acharya J, Park M, Saud PS. Novel Materials in Perovskite Solar Cells: Efficiency, Stability, and Future Perspectives. Nanomaterials. 2023; 13(11):1724. https://doi.org/10.3390/nano13111724
Chicago/Turabian StyleBist, Anup, Bishweshwar Pant, Gunendra Prasad Ojha, Jiwan Acharya, Mira Park, and Prem Singh Saud. 2023. "Novel Materials in Perovskite Solar Cells: Efficiency, Stability, and Future Perspectives" Nanomaterials 13, no. 11: 1724. https://doi.org/10.3390/nano13111724
APA StyleBist, A., Pant, B., Ojha, G. P., Acharya, J., Park, M., & Saud, P. S. (2023). Novel Materials in Perovskite Solar Cells: Efficiency, Stability, and Future Perspectives. Nanomaterials, 13(11), 1724. https://doi.org/10.3390/nano13111724










