Enhancing Functionalization of Health Care Textiles with Gold Nanoparticle-Loaded Hydroxyapatite Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Pre-Treatments
2.3. Textiles Functionalization
2.4. Design of Experiments (DoE)
2.5. UV-Visible Spectroscopy
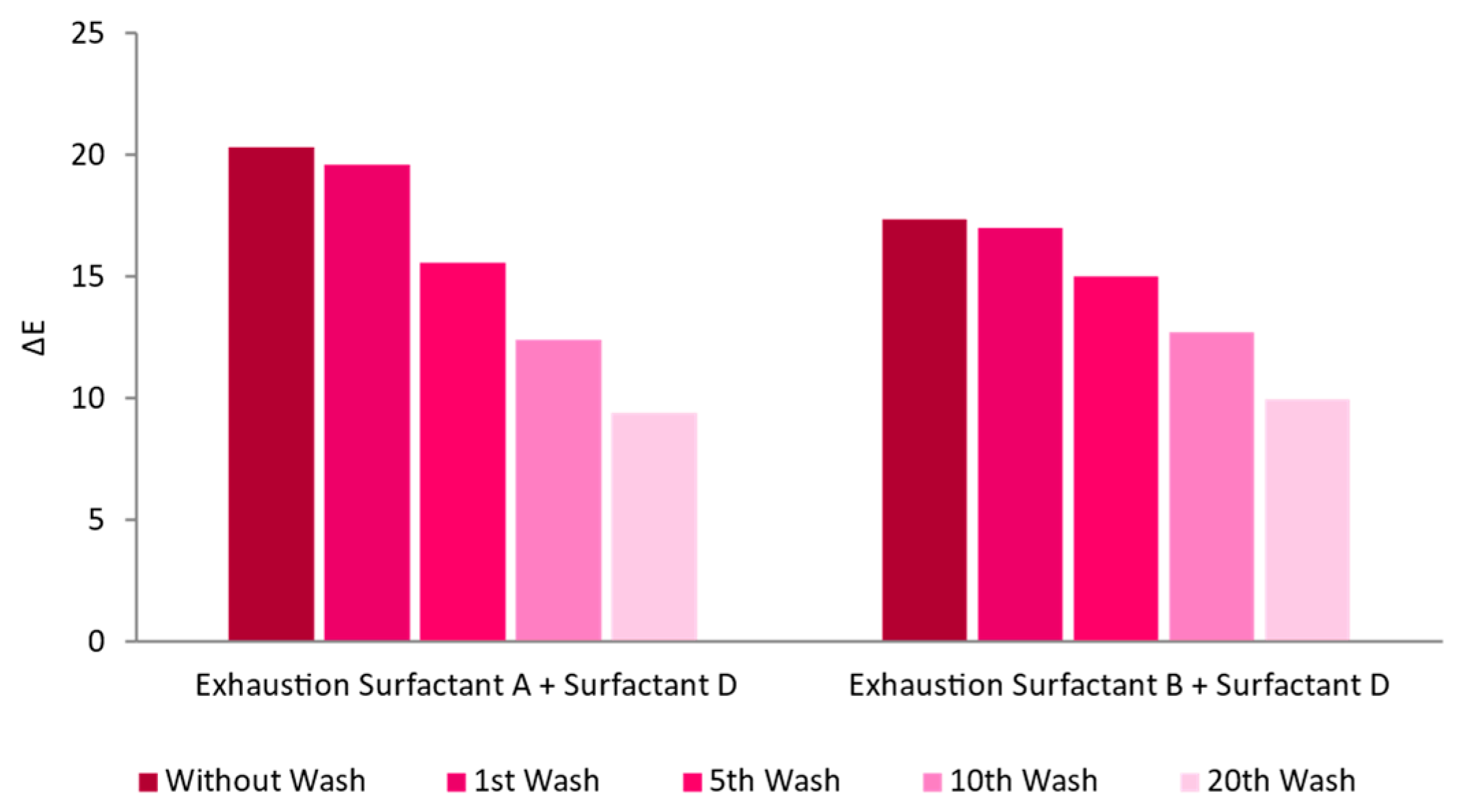
2.6. Washing Fastness
2.7. Atomic Absorption Spectroscopy (AAS)
2.8. Transmission Electron Microscopy (TEM) Analysis
2.9. Scanning Electron Microscope (SEM) and Energy-Dispersive X-ray Spectroscopy (EDS) Analysis
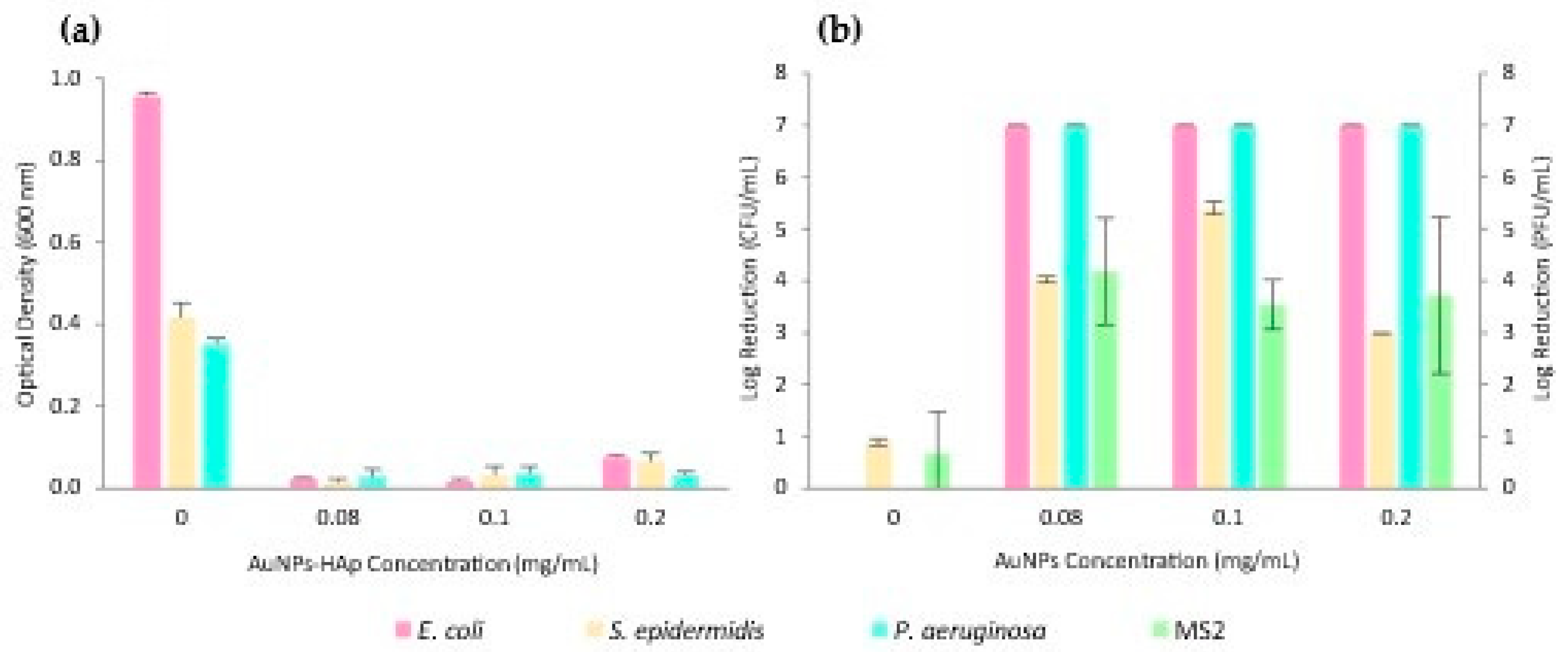
2.10. Antimicrobial Activity
3. Results
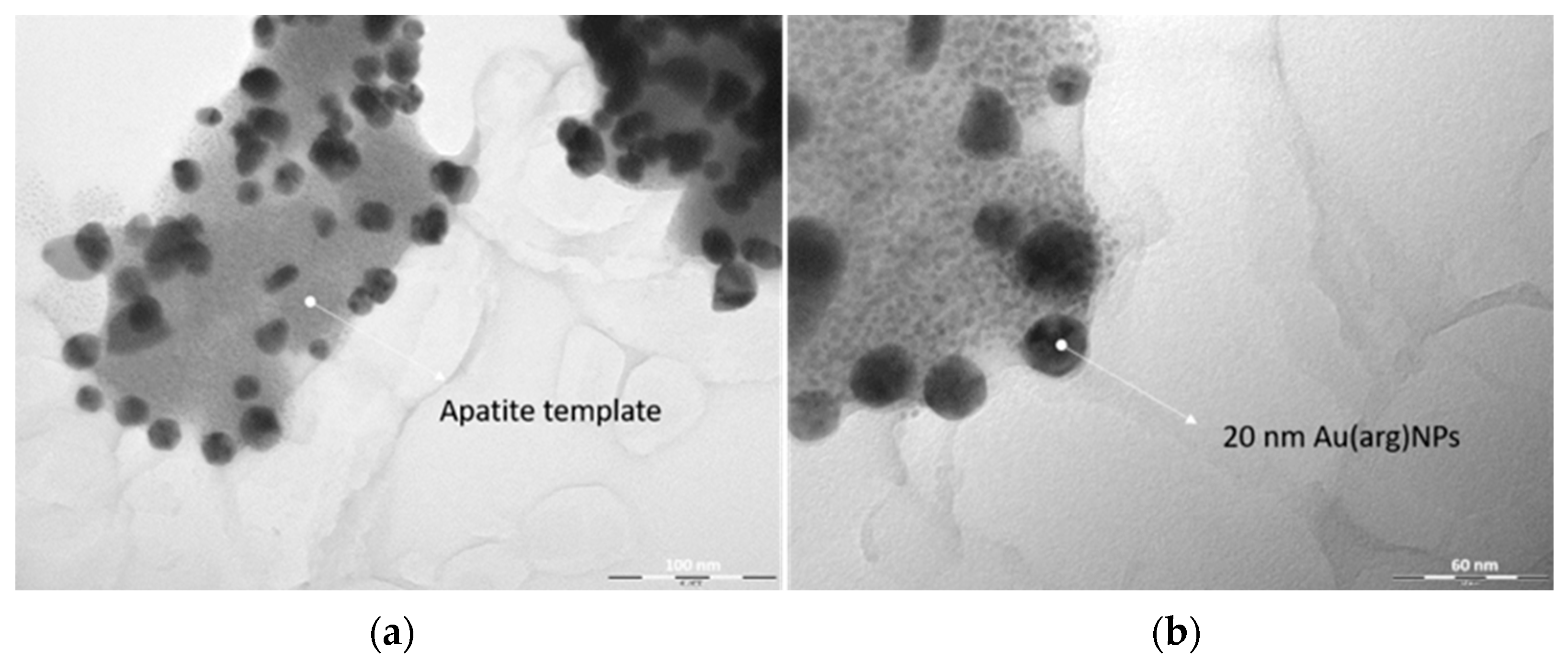
3.1. AuNPs-HAp Characterization
3.2. Textile Functionalization
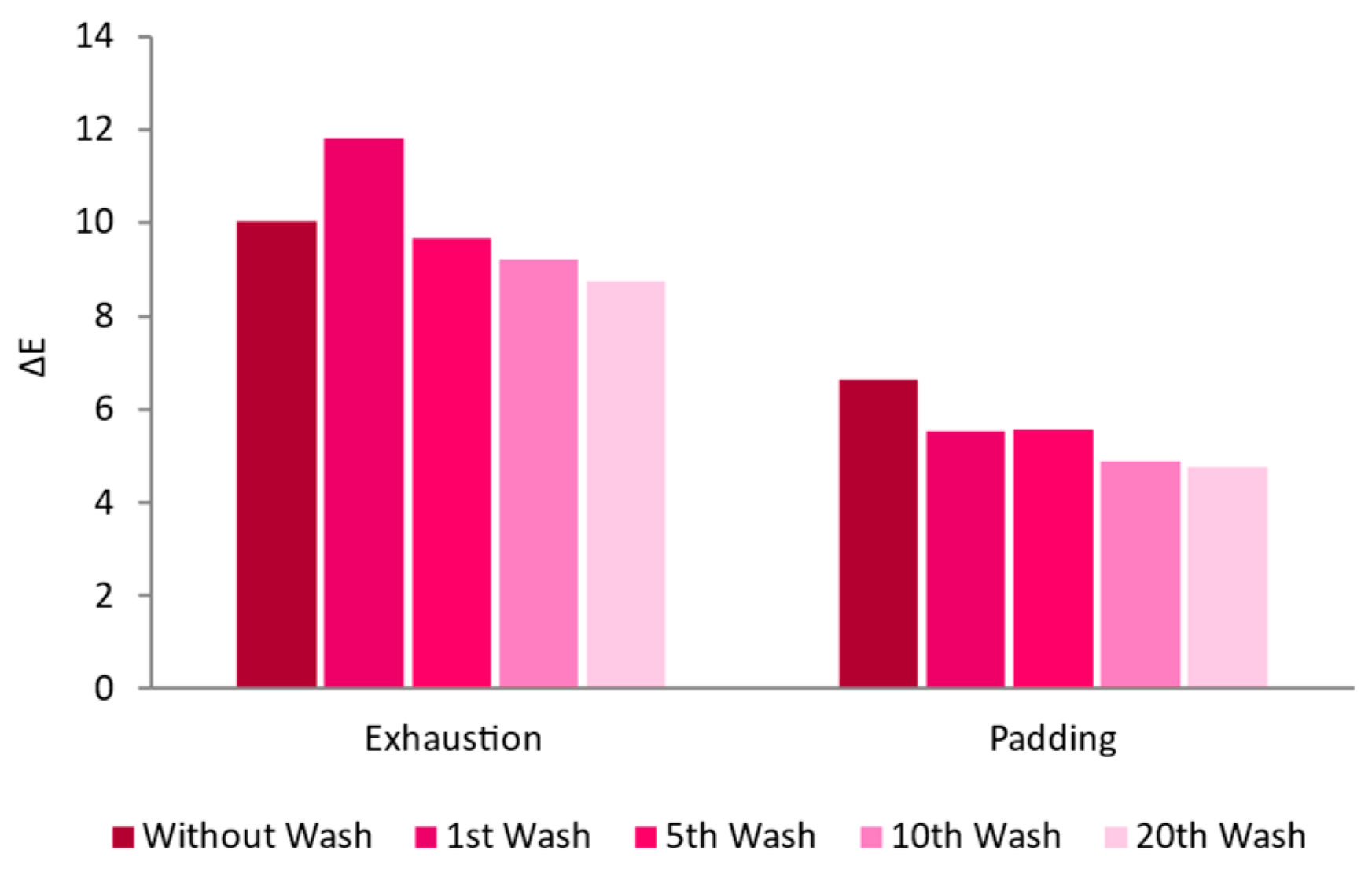
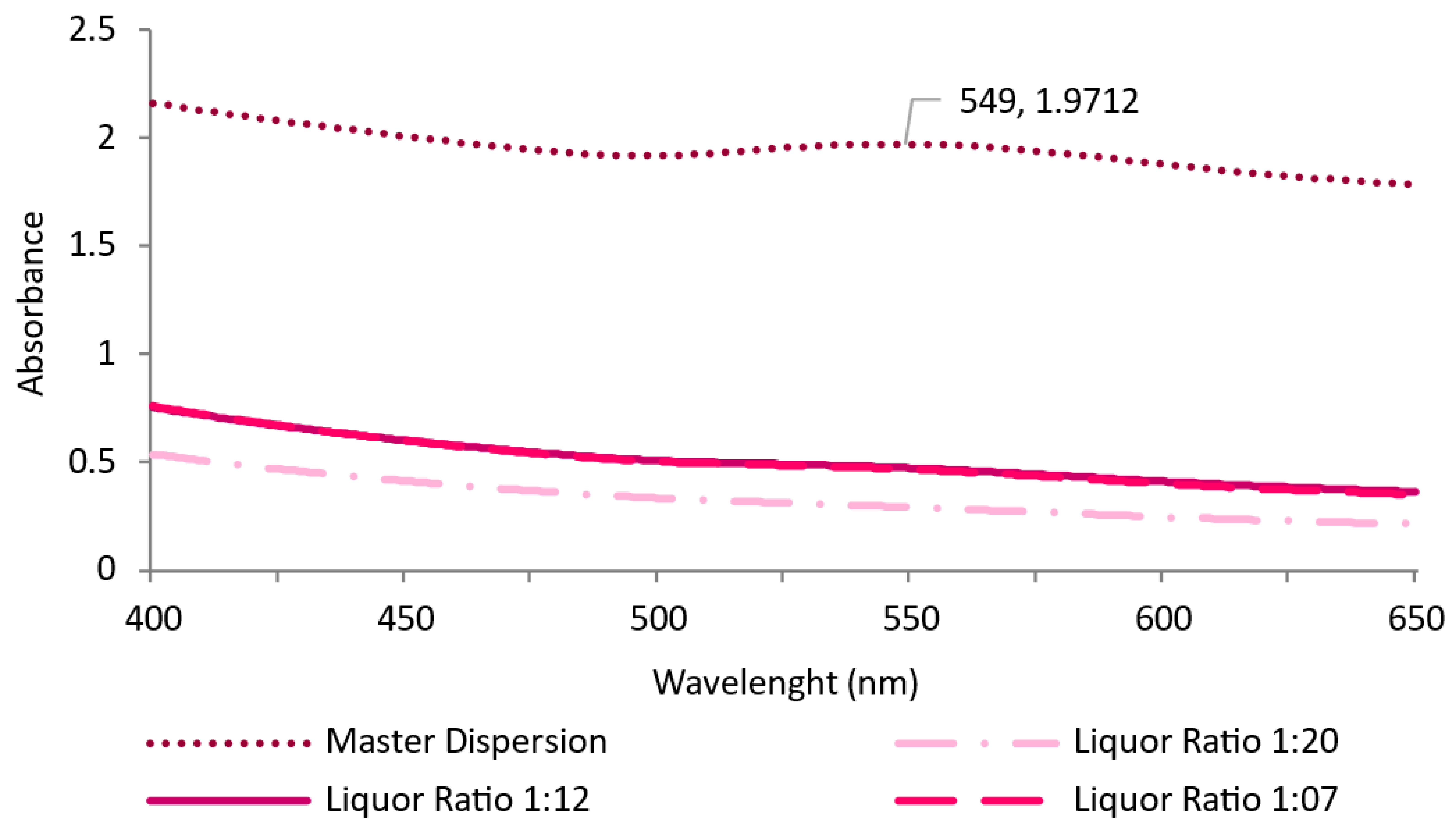
3.2.1. Surfactant Impact
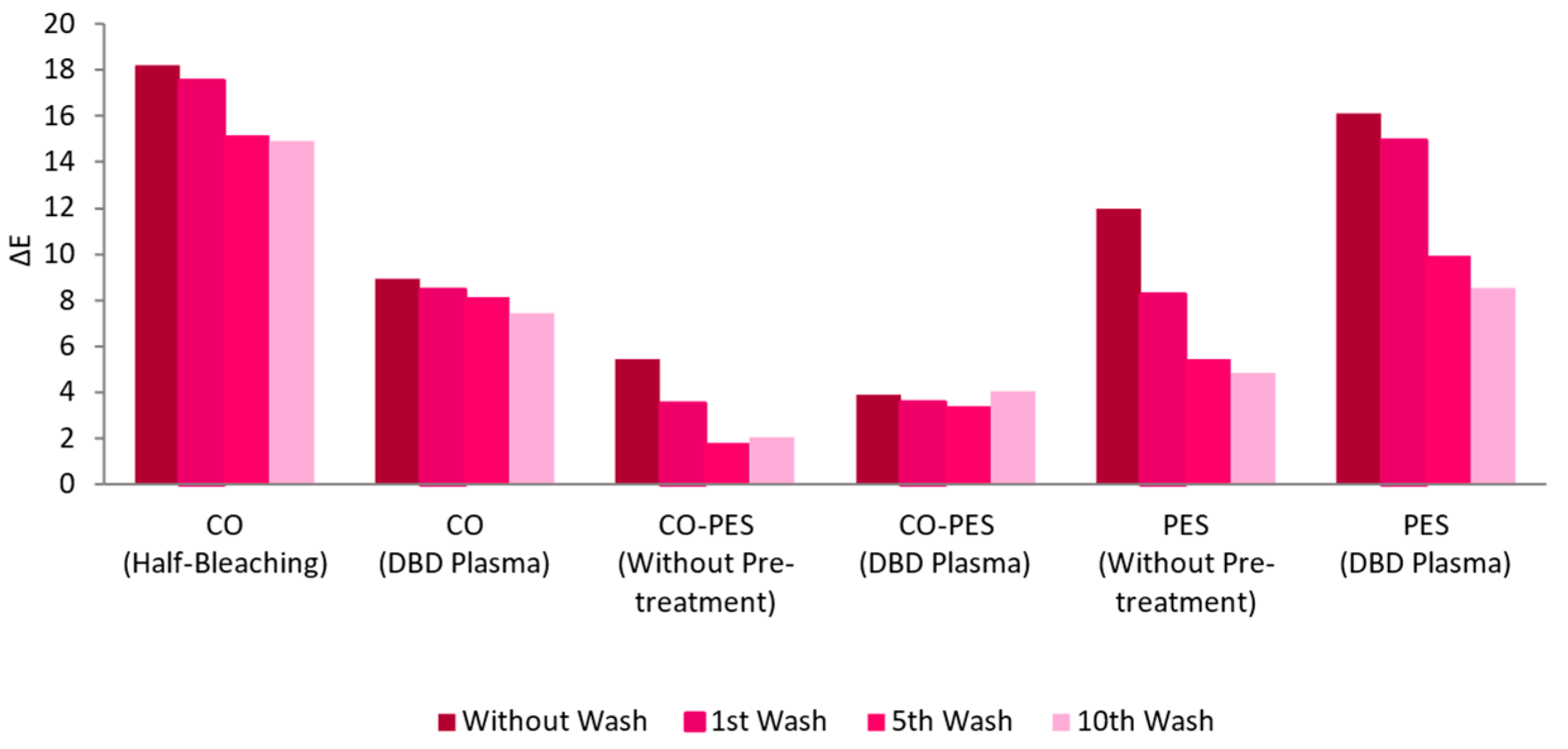
3.2.2. Pre-Treatments
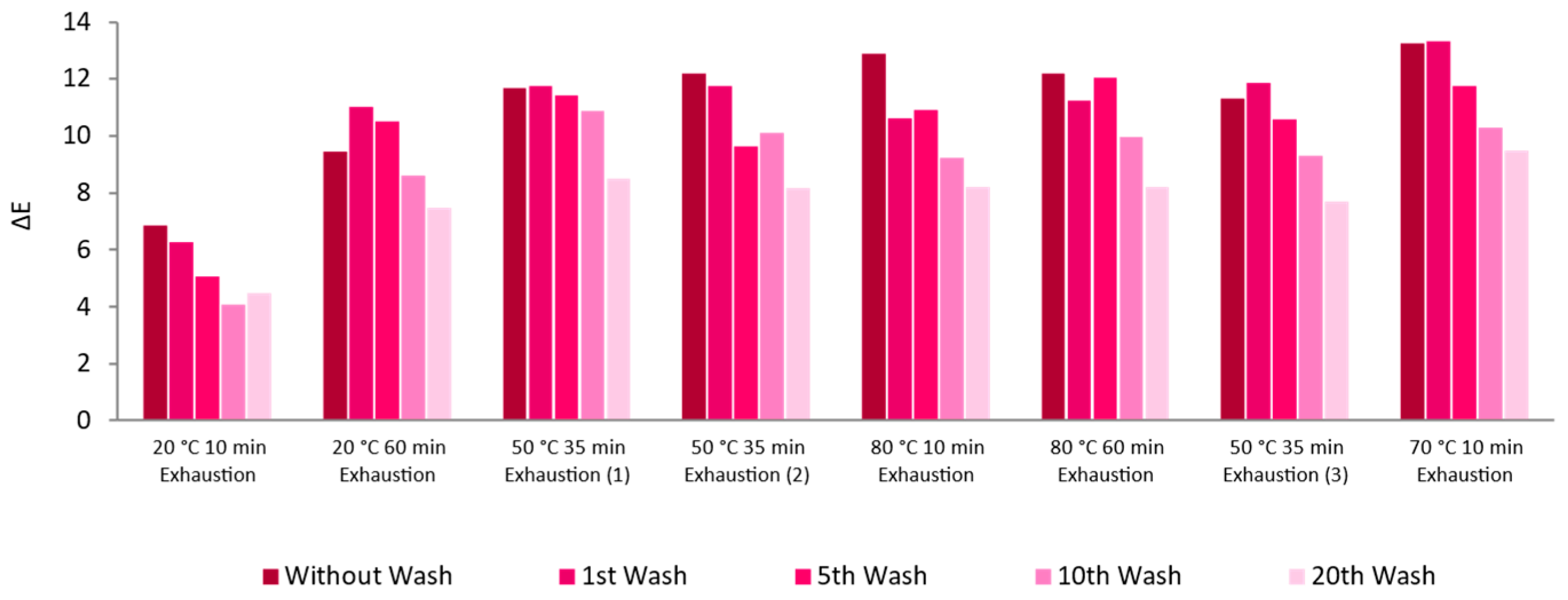
3.2.3. Functionalization Process
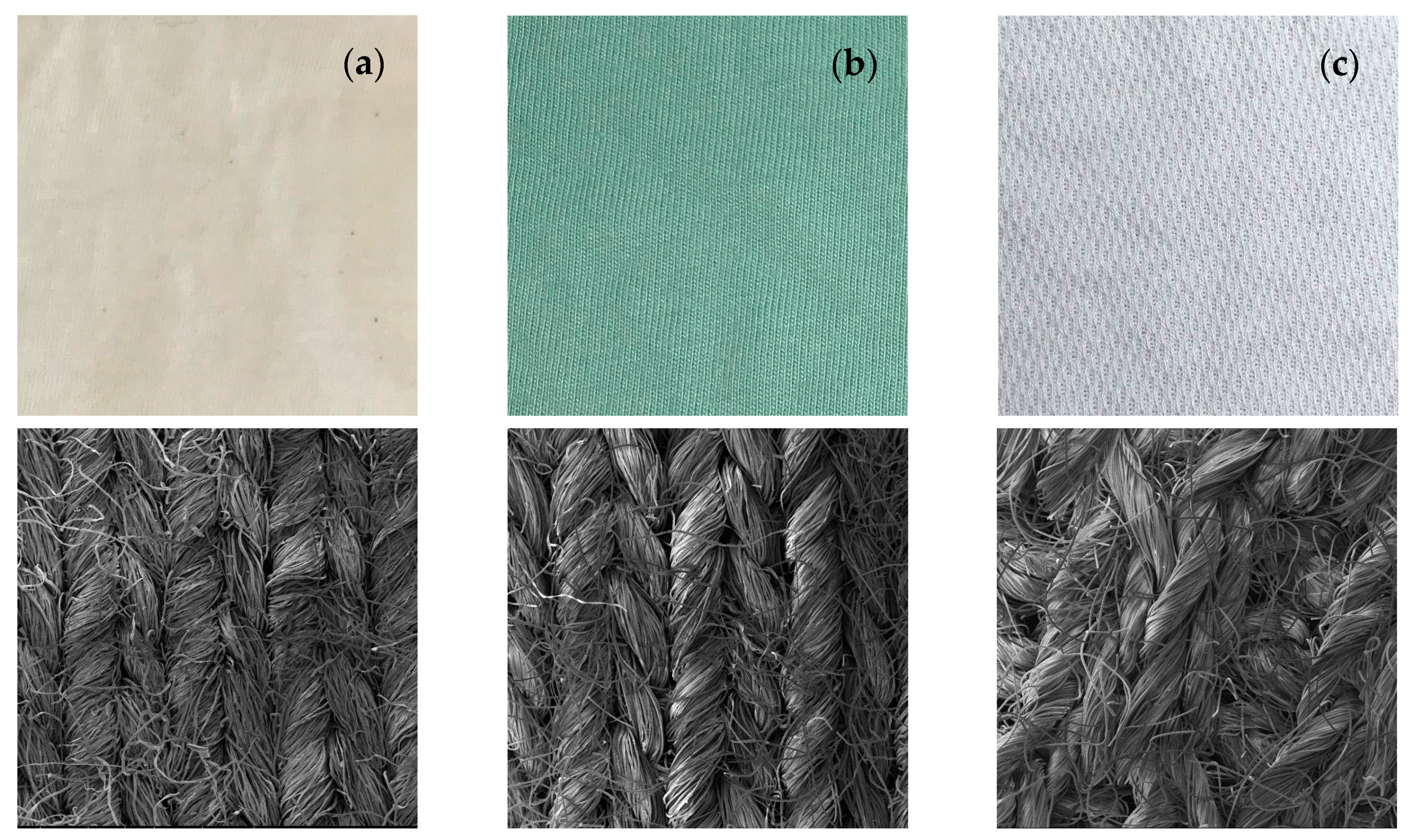
3.2.4. Textiles Characterization
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cheungpasitporn, W.; Serra-Burriel, M.; Keys, M.; Campillo-Artero, C.; Agodi, A.; Barchitta, M.; Gikas, A.; Palos, C.; López-Casasnovas, G. Impact of multi-drug resistant bacteria on economic and clinical outcomes of healthcare-associated infections in adults: Systematic review and meta-analysis. PLoS ONE 2020, 15, e0227139. [Google Scholar] [CrossRef]
- Dancer, S.J. Reducing the risk of COVID-19 transmission in hospitals: Focus on additional infection control strategies. Surgery 2021, 39, 752–758. [Google Scholar] [CrossRef]
- Afraz, N. Antimicrobial finishes for Textiles. Curr. Trends Fash. Technol. Text. Eng. 2019, 4, 87–94. [Google Scholar] [CrossRef]
- Saleem, H.; Zaidi, S. Sustainable Use of Nanomaterials in Textiles and Their Environmental Impact. Materials 2020, 13, 5134. [Google Scholar] [CrossRef]
- Costa, N.; Hoogendijk, E.O.; Mounié, M.; Bourrel, R.; Rolland, Y.; Vellas, B.; Molinier, L.; Cesari, M. Additional Cost Because of Pneumonia in Nursing Home Residents: Results from the Incidence of Pneumonia and Related Consequences in Nursing Home Resident Study. J. Am. Med. Dir. Assoc. 2017, 18, 453.e7–453.e12. [Google Scholar] [CrossRef]
- Yeargin, T.; Buckley, D.; Fraser, A.; Jiang, X. The survival and inactivation of enteric viruses on soft surfaces: A systematic review of the literature. Am. J. Infect. Control 2016, 44, 1365–1373. [Google Scholar] [CrossRef] [PubMed]
- Sanders, D.; Grunden, A.; Dunn, R.R. A review of clothing microbiology: The history of clothing and the role of microbes in textiles. Biol. Lett. 2021, 17, 20200700. [Google Scholar] [CrossRef]
- Pachiayappan, K.M.; Prakash, C.; Kumar, V. Influence of process variables on antimicrobial properties of cotton knitted fabrics. J. Nat. Fibers 2018, 17, 313–325. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, P. Application of Knitting Structure Textiles in Medical Areas. Autex Res. J. 2018, 18, 181–191. [Google Scholar] [CrossRef]
- Masood, R.; Jamshaid, H.; Khubaib, M.A. Development of knitted vest fabrics for human body thermoregulation. J. Therm. Anal. Calorim. 2019, 139, 159–167. [Google Scholar] [CrossRef]
- Thadepalli, S. Review of multifarious applications of polymers in medical and health care textiles. Mater. Today Proc. 2022, 55, 330–336. [Google Scholar] [CrossRef]
- Fernandes, M.; Padrão, J.; Ribeiro, A.I.; Fernandes, R.D.V.; Melro, L.; Nicolau, T.; Mehravani, B.; Alves, C.; Rodrigues, R.; Zille, A. Polysaccharides and Metal Nanoparticles for Functional Textiles: A Review. Nanomaterials 2022, 12, 1006. [Google Scholar] [CrossRef]
- Porto, R.C.T.; Uchôa, P.Z.; Peschel, L.T.; Justi, B.; Koslowski, L.A.D.; Nogueira, A.L. Nanopartículas de óxido de zinco sintetizadas pelo método poliol: Caracterização e avaliação da atividade antibacteriana. Matéria 2018, 22. [Google Scholar] [CrossRef]
- Arakawa, F.S.; Shimabuku, Q.L.; Silveira, C.; Bazana, S.D.L.; Bueno, M.R.; Moreti, L.D.O.R.; Camacho, F.P.; Heidemann, G.; Bergamasco, R. SÍntese De Nanopartículas Metálicas E Óxidos Metálicos Suportadas Em Carvão Ativado Para Remoção De Escherichia Coli Da Água. E-Xacta 2015, 8, 87–98. [Google Scholar] [CrossRef]
- Raza, Z.A.; Taqi, M.; Tariq, M.R. Antibacterial agents applied as antivirals in textile-based PPE: A narrative review. J. Text. Inst. 2021, 113, 515–526. [Google Scholar] [CrossRef]
- Saidin, S.; Jumat, M.A.; Mohd Amin, N.A.A.; Saleh Al-Hammadi, A.S. Organic and inorganic antibacterial approaches in combating bacterial infection for biomedical application. Mater. Sci. Eng. C 2021, 118, 111382. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.I.; Senturk, D.; Silva, K.S.; Modic, M.; Cvelbar, U.; Dinescu, G.; Mitu, B.; Nikiforov, A.; Leys, C.; Kuchakova, I.; et al. Efficient silver nanoparticles deposition method on DBD plasma-treated polyamide 6,6 for antimicrobial textiles. IOP Conf. Ser. Mater. Sci. Eng. 2018, 460, 012007. [Google Scholar] [CrossRef]
- Said, M.M.; Rehan, M.; El-Sheikh, S.M.; Zahran, M.K.; Abdel-Aziz, M.S.; Bechelany, M.; Barhoum, A. Multifunctional Hydroxyapatite/Silver Nanoparticles/Cotton Gauze for Antimicrobial and Biomedical Applications. Nanomaterials 2021, 11, 429. [Google Scholar] [CrossRef] [PubMed]
- Rebelo, R.; Padrão, J.; Fernandes, M.M.; Carvalho, S.; Henriques, M.; Zille, A.; Fangueiro, R. Aging Effect on Functionalized Silver-Based Nanocoating Braided Coronary Stents. Coatings 2020, 10, 1234. [Google Scholar] [CrossRef]
- Yougbaré, S.; Mutalik, C.; Okoro, G.; Lin, I.H.; Krisnawati, D.I.; Jazidie, A.; Nuh, M.; Chang, C.-C.; Kuo, T.-R. Emerging Trends in Nanomaterials for Antibacterial Applications. Int. J. Nanomed. 2021, 16, 5831–5867. [Google Scholar] [CrossRef]
- Tortella, G.R.; Rubilar, O.; Durán, N.; Diez, M.C.; Martínez, M.; Parada, J.; Seabra, A.B. Silver nanoparticles: Toxicity in model organisms as an overview of its hazard for human health and the environment. J. Hazard. Mater. 2020, 390, 121974. [Google Scholar] [CrossRef] [PubMed]
- Haslinger, S.; Ye, Y.; Rissanen, M.; Hummel, M.; Sixta, H. Cellulose Fibers for High-Performance Textiles Functionalized with Incorporated Gold and Silver Nanoparticles. ACS Sustain. Chem. Eng. 2019, 8, 649–658. [Google Scholar] [CrossRef]
- Almeida, L.; Ramos, D. Health and safety concerns of textiles with nanomaterials. IOP Conf. Ser. Mater. Sci. Eng. 2017, 254, 102002. [Google Scholar] [CrossRef]
- Xu, Q.; Ke, X.; Shen, L.; Ge, N.; Zhang, Y.; Fu, F.; Liu, X. Surface modification by carboxymethy chitosan via pad-dry-cure method for binding Ag NPs onto cotton fabric. Int. J. Biol. Macromol. 2018, 111, 796–803. [Google Scholar] [CrossRef] [PubMed]
- Marković, D.; Radoičić, M.; Barudžija, T.; Radetić, M. Modification of PET and PA fabrics with alginate and copper oxides nanoparticles. Compos. Interfaces 2021, 28, 1171–1187. [Google Scholar] [CrossRef]
- Naebe, M.; Haque, A.N.M.A.; Haji, A. Plasma-Assisted Antimicrobial Finishing of Textiles: A Review. Engineering 2022, 12, 145–163. [Google Scholar] [CrossRef]
- Vukomanovic, M.; Skapin, S.D.; Suvorov, D. Functionalized Hydroxyapatite/Gold Composites as “Green” Materials with Antibacterial Activity and the Process for Preparing and Use Thereof. EP 2 863 751 B1, 8 May 2013. [Google Scholar]
- Gao, J.; Fan, G.; Yang, L.; Cao, X.; Zhang, P.; Li, F. Oxidative Esterification of Methacrolein to Methyl Methacrylate over Gold Nanoparticles on Hydroxyapatite. ChemCatChem 2017, 9, 1230–1241. [Google Scholar] [CrossRef]
- Franco, D.; Calabrese, G.; Guglielmino, S.P.P.; Conoci, S. Metal-Based Nanoparticles: Antibacterial Mechanisms and Biomedical Application. Microorganisms 2022, 10, 1778. [Google Scholar] [CrossRef]
- Ramachandran, R.; Krishnaraj, C.; Kumar, V.K.A.; Harper, S.L.; Kalaichelvan, T.P.; Yun, S.-I. In vivo toxicity evaluation of biologically synthesized silver nanoparticles and gold nanoparticles on adult zebrafish: A comparative study. 3 Biotech 2018, 8, 441. [Google Scholar] [CrossRef]
- Mehravani, B.; Ribeiro, A.I.; Cvelbar, U.; Padrão, J.; Zille, A. In Situ Synthesis of Copper Nanoparticles on Dielectric Barrier Discharge Plasma-Treated Polyester Fabrics at Different Reaction pHs. ACS Appl. Polym. Mater. 2022, 4, 3908–3918. [Google Scholar] [CrossRef]
- Ribeiro, A.I.; Shvalya, V.; Cvelbar, U.; Silva, R.; Marques-Oliveira, R.; Remião, F.; Felgueiras, H.P.; Padrão, J.; Zille, A. Stabilization of Silver Nanoparticles on Polyester Fabric Using Organo-Matrices for Controlled Antimicrobial Performance. Polymers 2022, 14, 1138. [Google Scholar] [CrossRef]
- ISO 105-C06; Textiles—Tests for Colour Fastness—Part C06: Colour Fastness to Domestic and Commercial Laundering. International Organization for Standardization: Geneva, Switzerland, 2010.
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef] [PubMed]
- M26-A; Methods for Determining Bactericidal Activity for Antimicrobial Agents. Clinical and Laboratory Standards Institute; National Committee for Clinical Laboratory Standards: Wayne, PA, USA, 1999.
- Padrão, J.; Nicolau, T.; Felgueiras, H.P.; Calçada, C.; Veiga, M.I.; Osório, N.S.; Martins, M.S.; Dourado, N.; Taveira-Gomes, A.; Ferreira, F.; et al. Development of an Ultraviolet-C Irradiation Room in a Public Portuguese Hospital for Safe Re-Utilization of Personal Protective Respirators. Int. J. Environ. Res. Public Health 2022, 19, 4854. [Google Scholar] [CrossRef]
- Nicolau, T.; Gomes Filho, N.; Padrão, J.; Zille, A. A Comprehensive Analysis of the UVC LEDs’ Applications and Decontamination Capability. Materials 2022, 15, 2854. [Google Scholar] [CrossRef]
- Owaid, M.N.; Rabeea, M.A.; Abdul Aziz, A.; Jameel, M.S.; Dheyab, M.A. Mushroom-assisted synthesis of triangle gold nanoparticles using the aqueous extract of fresh Lentinula edodes (shiitake), Omphalotaceae. Environ. Nanotechnol. Monit. Manag. 2019, 12, 100270. [Google Scholar] [CrossRef]
- Xia, L.; Wang, A.; Wang, Y.; Zhang, C.; Wang, Y.; Zhou, S.; Fu, Z.; Zhao, H.; Ding, C.; Xu, W. Eco-friendly dyeing of raw cotton fibres in an ethanol–water mixture without scouring and bleaching pretreatments. Green Chem. 2021, 23, 796–807. [Google Scholar] [CrossRef]
- Barbosa, S.G.; Peixoto, L.; Meulman, B.; Alves, M.M.; Pereira, M.A. A design of experiments to assess phosphorous removal and crystal properties in struvite precipitation of source separated urine using different Mg sources. Chem. Eng. J. 2016, 298, 146–153. [Google Scholar] [CrossRef]
- Kowalczyk, D.; Kaminska, I. Effect of pH and surfactants on the electrokinetic properties of nanoparticles dispersions and their application to the PET fibres modification. J. Mol. Liq. 2020, 320, 320. [Google Scholar] [CrossRef]
- Ordóñez, F.; Chejne, F.; Pabón, E.; Cacua, K. Synthesis of ZrO2 nanoparticles and effect of surfactant on dispersion and stability. Ceram. Int. 2020, 46, 11970–11977. [Google Scholar] [CrossRef]
- Cortés, H.; Hernández-Parra, H.; Bernal-Chávez, S.A.; Prado-Audelo, M.L.D.; Caballero-Florán, I.H.; Borbolla-Jiménez, F.V.; González-Torres, M.; Magaña, J.J.; Leyva-Gómez, G. Non-Ionic Surfactants for Stabilization of Polymeric Nanoparticles for Biomedical Uses. Materials 2021, 14, 3197. [Google Scholar] [CrossRef]
- Sandanuwan, T.; Hendeniya, N.; Amarasinghe, D.A.S.; Attygalle, D.; Weragoda, S. The effect of atmospheric pressure plasma treatment on wetting and absorbance properties of cotton fabric. Mater. Today Proc. 2021, 45, 5065–5068. [Google Scholar] [CrossRef]
- Bian, X.; Fan, S.; Xia, G.; Chae, Y.; Yu, H.; Xin, J.H. Sustainable ultrasonic dyeing of unscoured and unbleached cotton sliver using low liquor ratio. J. Clean. Prod. 2022, 379, 134853. [Google Scholar] [CrossRef]
- Muhammed, N.; Govindan, N. Chemical Modification of Cotton Cellulose by Carbamation with Urea and Its Dyeability with Reactive Dyes without the Use of Electrolyte. J. Nat. Fibers 2020, 19, 1402–1418. [Google Scholar] [CrossRef]
- Song, X.; Cvelbar, U.; Strazar, P.; Vossebein, L.; Zille, A. Antimicrobial Efficiency and Surface Interactions of Quaternary Ammonium Compound Absorbed on Dielectric Barrier Discharge (DBD) Plasma Treated Fiber-Based Wiping Materials. ACS Appl. Mater. Interfaces 2019, 12, 298–311. [Google Scholar] [CrossRef]
- Zille, A.; Oliveira, F.R.; Souto, A.P. Plasma Treatment in Textile Industry. Plasma Process. Polym. 2015, 12, 98–131. [Google Scholar] [CrossRef]
- Ghaheh, F.S.; Khoddami, A.; Alihosseini, F.; Jing, S.; Ribeiro, A.; Cavaco-Paulo, A.; Silva, C. Antioxidant cosmetotextiles: Cotton coating with nanoparticles containing vitamin E. Process Biochem. 2017, 59, 46–51. [Google Scholar] [CrossRef]
- Siddiqua, U.H.; Ali, S.; Iqbal, M.; Hussain, T. Relationship between structure and dyeing properties of reactive dyes for cotton dyeing. J. Mol. Liq. 2017, 241, 839–844. [Google Scholar] [CrossRef]
- Cay, A.; Tarakçıoğlu, I.; Hepbasli, A. Assessment of finishing processes by exhaustion principle for textile fabrics: An exergetic approach. Appl. Therm. Eng. 2009, 29, 2554–2561. [Google Scholar] [CrossRef]
- Silva, I.O.; Ladchumananandasivam, R.; Nascimento, J.H.O.; Silva, K.K.O.; Oliveira, F.R.; Souto, A.P.; Felgueiras, H.P.; Zille, A. Multifunctional Chitosan/Gold Nanoparticles Coatings for Biomedical Textiles. Nanomaterials 2019, 9, 1064. [Google Scholar] [CrossRef]
- Ivanovska, A.; Lađarević, J.; Asanović, K.; Barać, N.; Mihajlovski, K.; Kostić, M.; Mangovska, B. Quality of Cotton and cotton/elastane Single Jersey Knitted Fabrics before and after Softening and in Situ Synthesis of Cu-based Nanoparticles. J. Nat. Fibers 2022, 19, 15139–15150. [Google Scholar] [CrossRef]
- Ma, Z.; Yin, M.; Qi, Z.; Ren, X. Preparation of Durable Antibacterial Cellulose with AgCl Nanoparticles. Fibers Polym. 2018, 19, 2097–2102. [Google Scholar] [CrossRef]




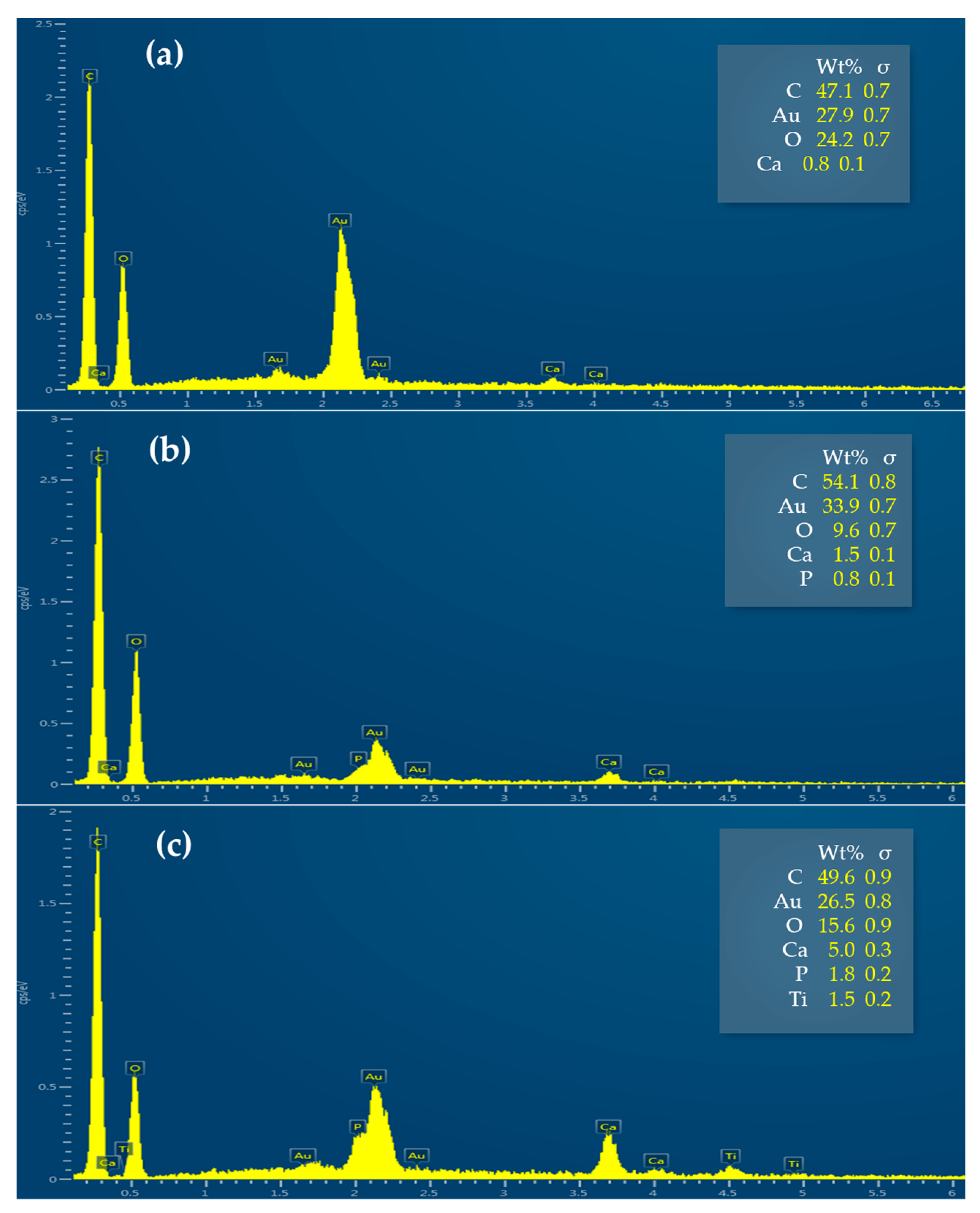

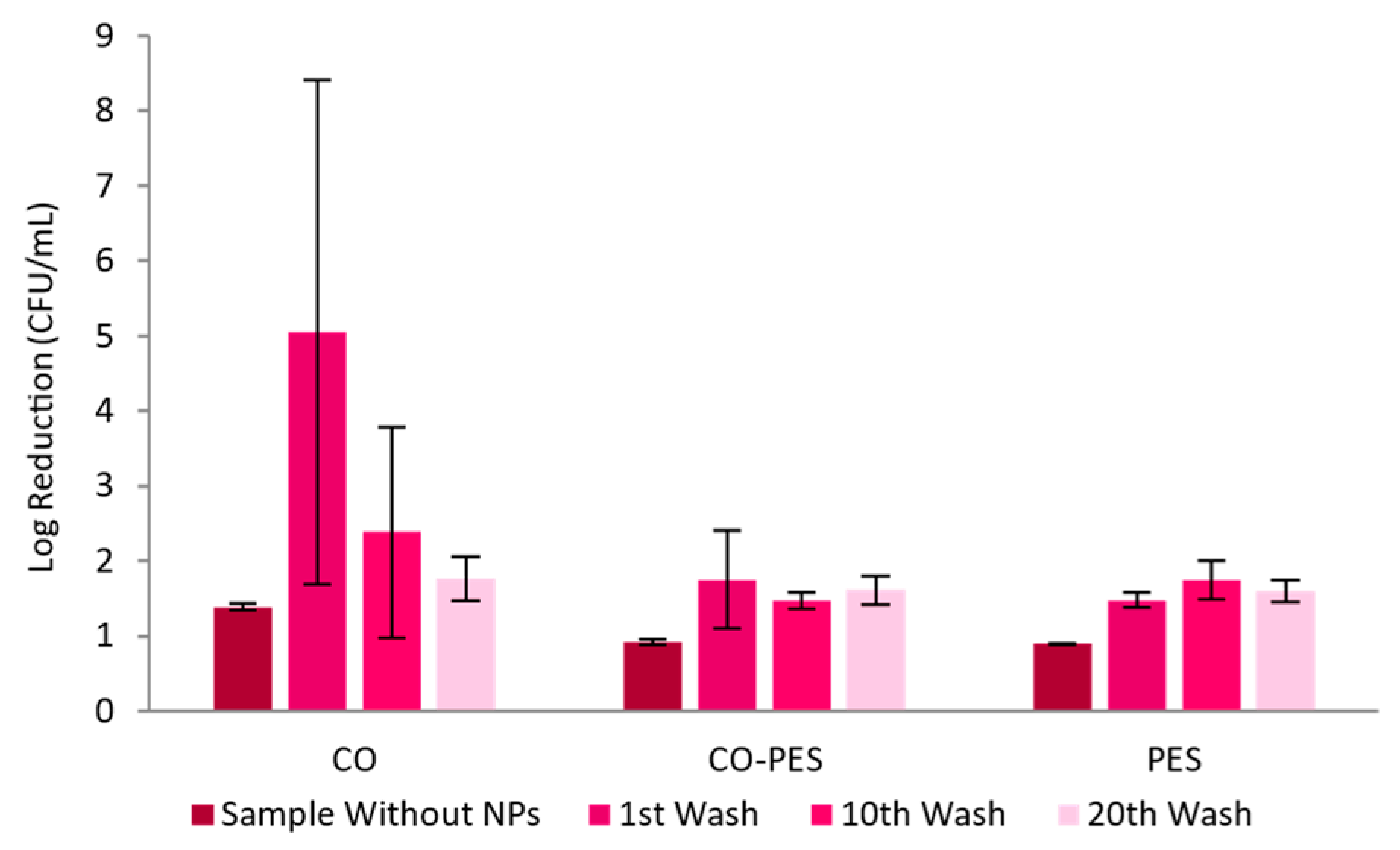



| Process | AuNPs-HAp Concentration (mg/mL) | pH | Temperature (°C) | Time (min) | Bath Ratio | Additives |
|---|---|---|---|---|---|---|
| Padding | 0.1; 0.3 | 6; 9 | Room temperature | - | - | Na2CO3 (pH adjustment). Surfactants A (4 g/L), B (0.5 g/L), C (3 g/L), and D (5 g/L) |
| Exhaustion | 0.1; 0.3 | 6; 9 | 20–80 | 10–60 | 7:1–20:1 |
| Viability Log10 Reduction | Viability Reduction (%) | Classification |
|---|---|---|
| Log10 < 1 | <90 | No activity |
| 1 ≤ Log10 < 2 | 90 ≤ R < 99 | Weak decontaminant |
| 2 ≤ Log10 < 3 | 99 ≤ R < 99.9 | Strong decontaminant |
| 3 ≤ Log10 < 4 | 99.9 ≤ R < 99.99 | Weak disinfectant |
| 4 ≤ Log10 < 5 | 99.99 ≤ R < 99.999 | Moderate disinfectant |
| 5 ≤ Log10 < 6 | 99.999 ≤ R < 99.9999 | Strong disinfectant |
| 6 ≤ Log10 | 99.9999 ≤ R | Sterilizing |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 22.82 | 3 | 7.61 | 38.62 | 0.0253 | significant |
| Time | 0.93 | 1 | 0.93 | 4.74 | 0.1613 | |
| Temperature | 19.21 | 1 | 19.21 | 97.56 | 0.0101 | significant |
| Time and Temperature | 2.67 | 1 | 2.67 | 13.55 | 0.0665 | |
| Curvature | 3.24 | 1 | 3.24 | 16.46 | 0.0557 | |
| Pure Error | 0.3939 | 2 | 0.1969 | |||
| Cor Total | 26.45 | 6 |
| Sample | Au after 1 Washing Cycle (mg/L) | Au after 20 Washing Cycles (mg/L) |
|---|---|---|
| CO | 10.5 | 8.0 |
| CO–PES | 7.5 | 3.8 |
| PES | 8.5 | 2.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vieira, B.; Padrão, J.; Alves, C.; Silva, C.J.; Vilaça, H.; Zille, A. Enhancing Functionalization of Health Care Textiles with Gold Nanoparticle-Loaded Hydroxyapatite Composites. Nanomaterials 2023, 13, 1752. https://doi.org/10.3390/nano13111752
Vieira B, Padrão J, Alves C, Silva CJ, Vilaça H, Zille A. Enhancing Functionalization of Health Care Textiles with Gold Nanoparticle-Loaded Hydroxyapatite Composites. Nanomaterials. 2023; 13(11):1752. https://doi.org/10.3390/nano13111752
Chicago/Turabian StyleVieira, Bárbara, Jorge Padrão, Cátia Alves, Carla Joana Silva, Helena Vilaça, and Andrea Zille. 2023. "Enhancing Functionalization of Health Care Textiles with Gold Nanoparticle-Loaded Hydroxyapatite Composites" Nanomaterials 13, no. 11: 1752. https://doi.org/10.3390/nano13111752
APA StyleVieira, B., Padrão, J., Alves, C., Silva, C. J., Vilaça, H., & Zille, A. (2023). Enhancing Functionalization of Health Care Textiles with Gold Nanoparticle-Loaded Hydroxyapatite Composites. Nanomaterials, 13(11), 1752. https://doi.org/10.3390/nano13111752







