Ionized Jet Deposition of Calcium Phosphates-Based Nanocoatings: Tuning Coating Properties and Cell Behavior by Target Composition and Substrate Heating
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Target Preparation and Characterization
2.3. IJD Deposition
2.4. Film Characterization
2.5. Stability Profile
2.6. Biological Evaluation
3. Results and Discussion
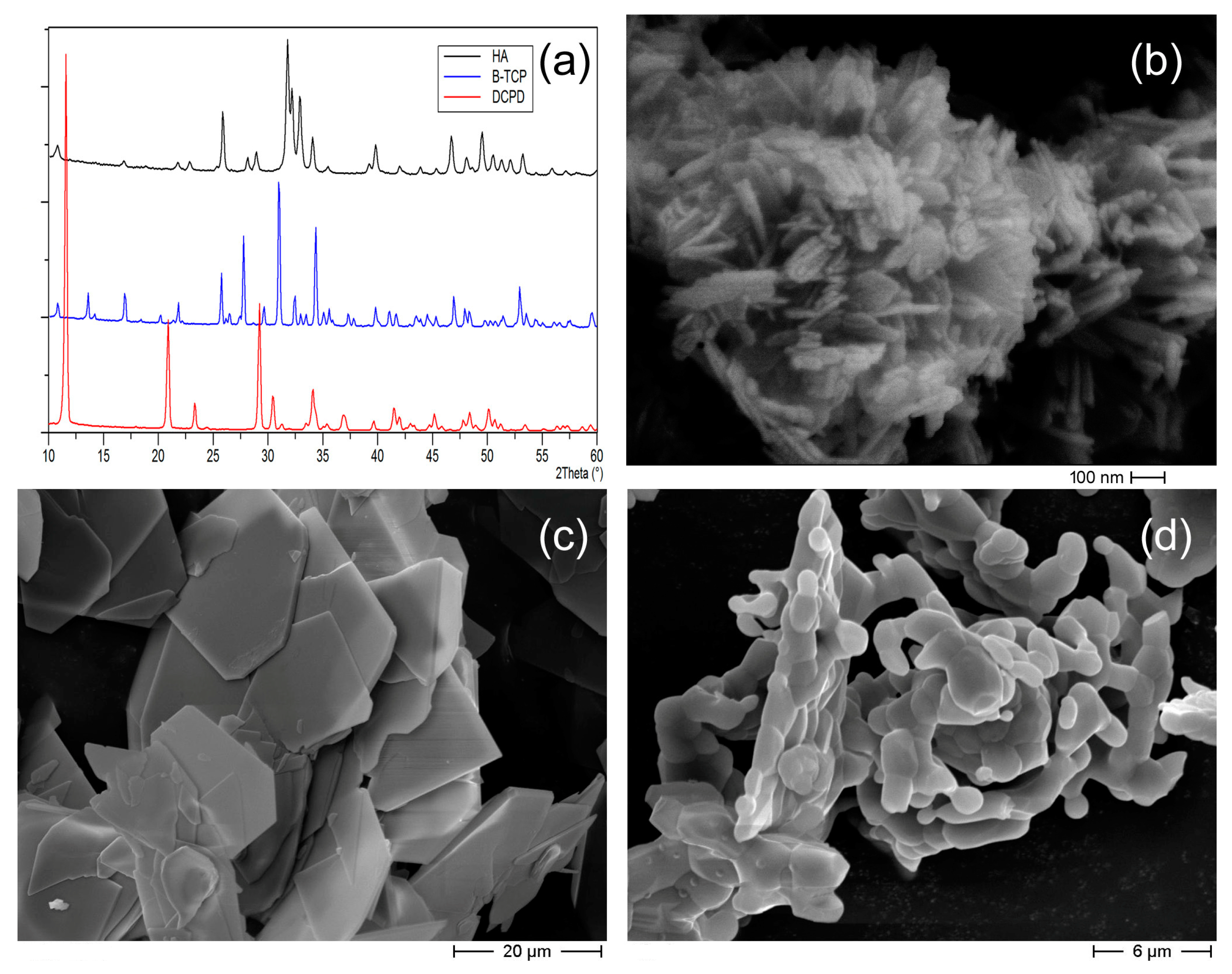
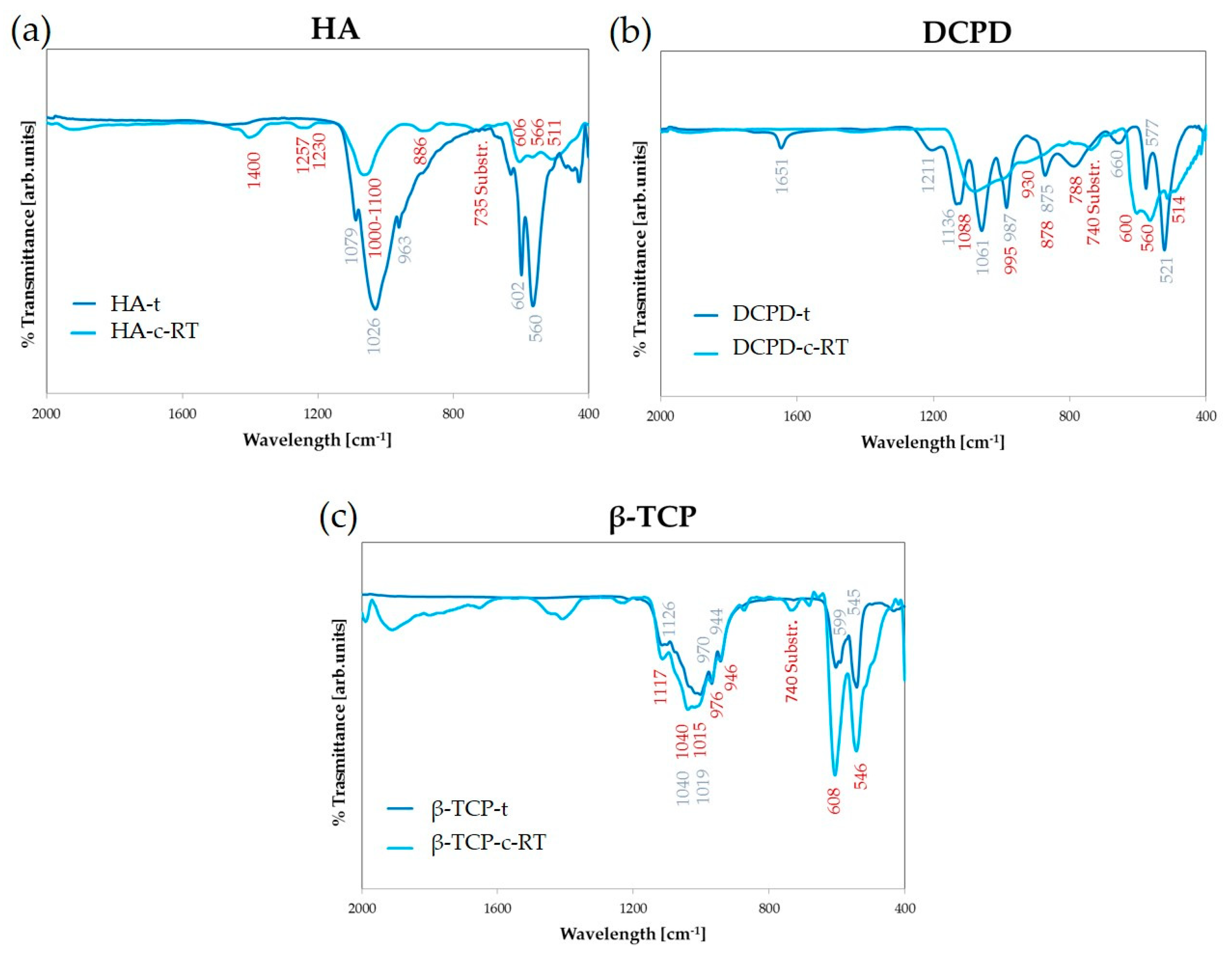
3.1. Targets Characterization
3.2. Coating Characterization
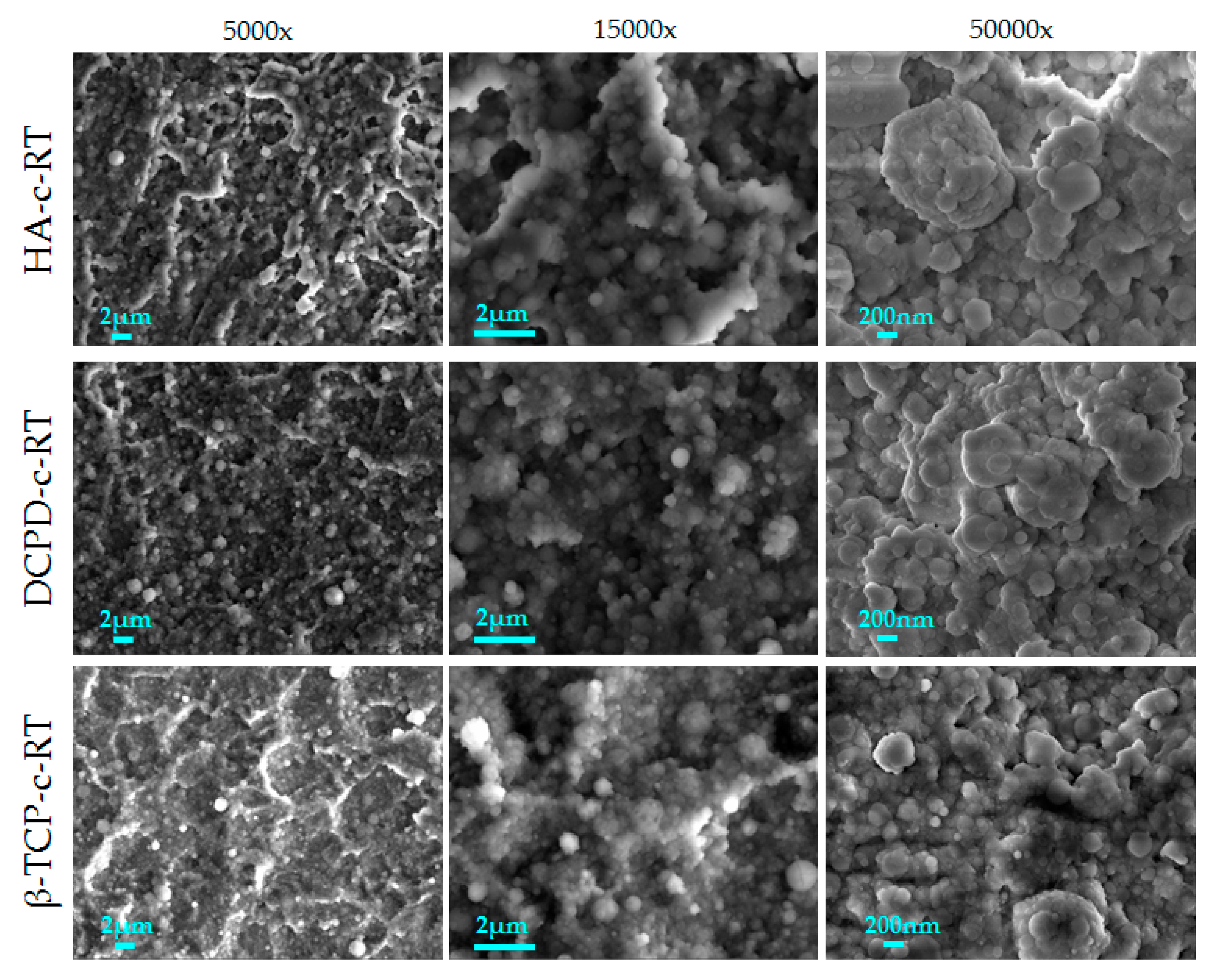
3.2.1. Deposition of Different Calcium Phosphates
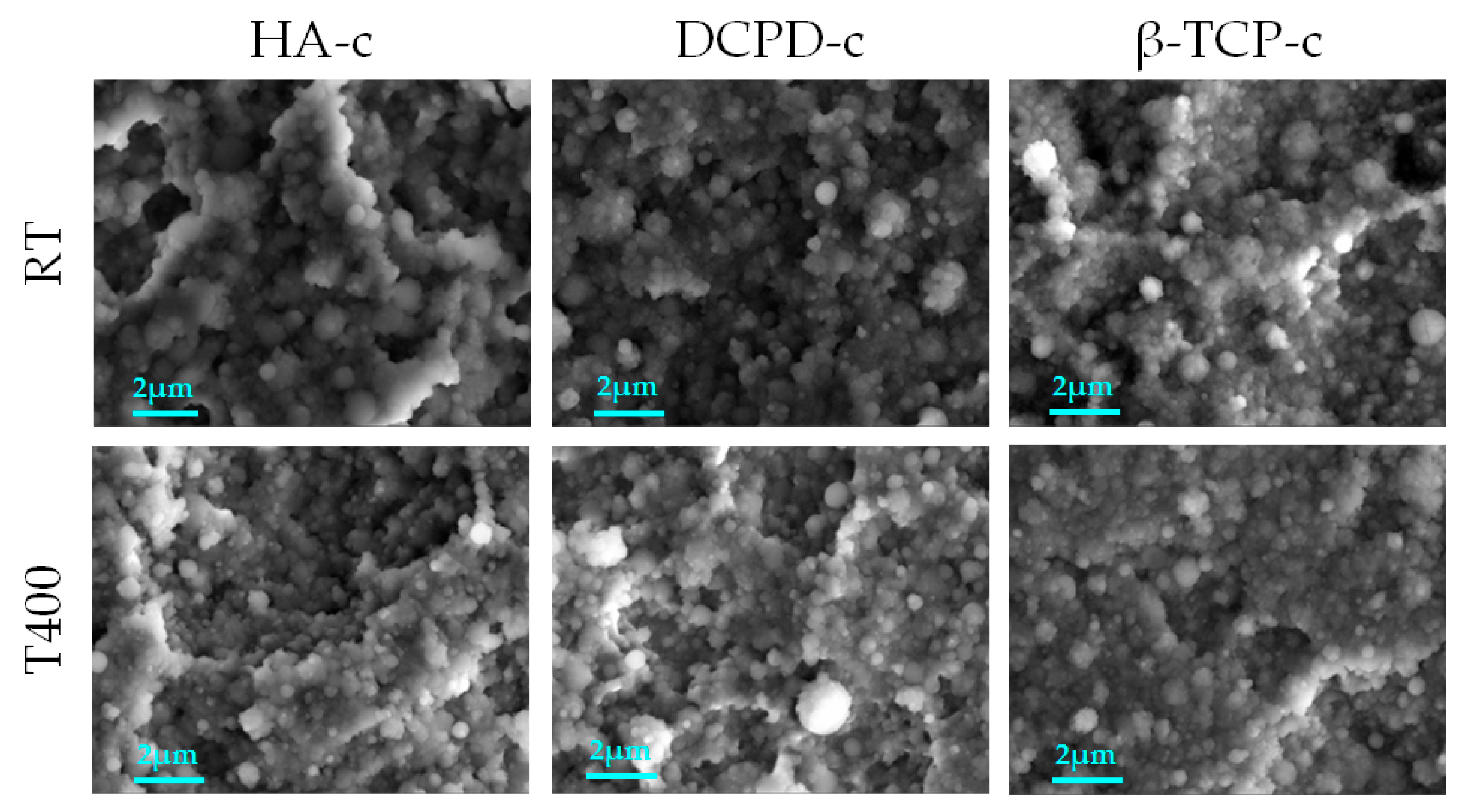
3.2.2. Deposition at High Temperature
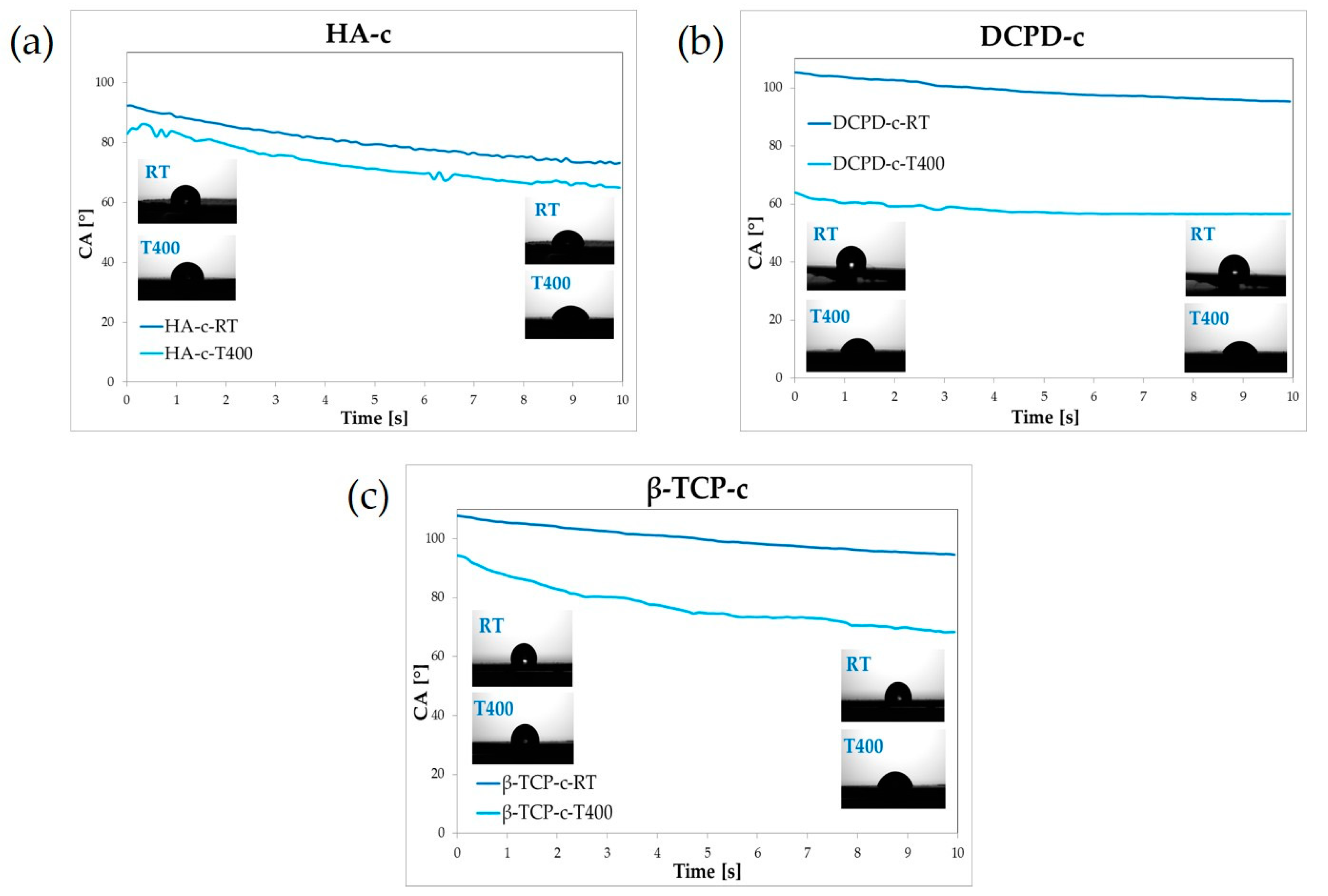
3.3. Stability Profile
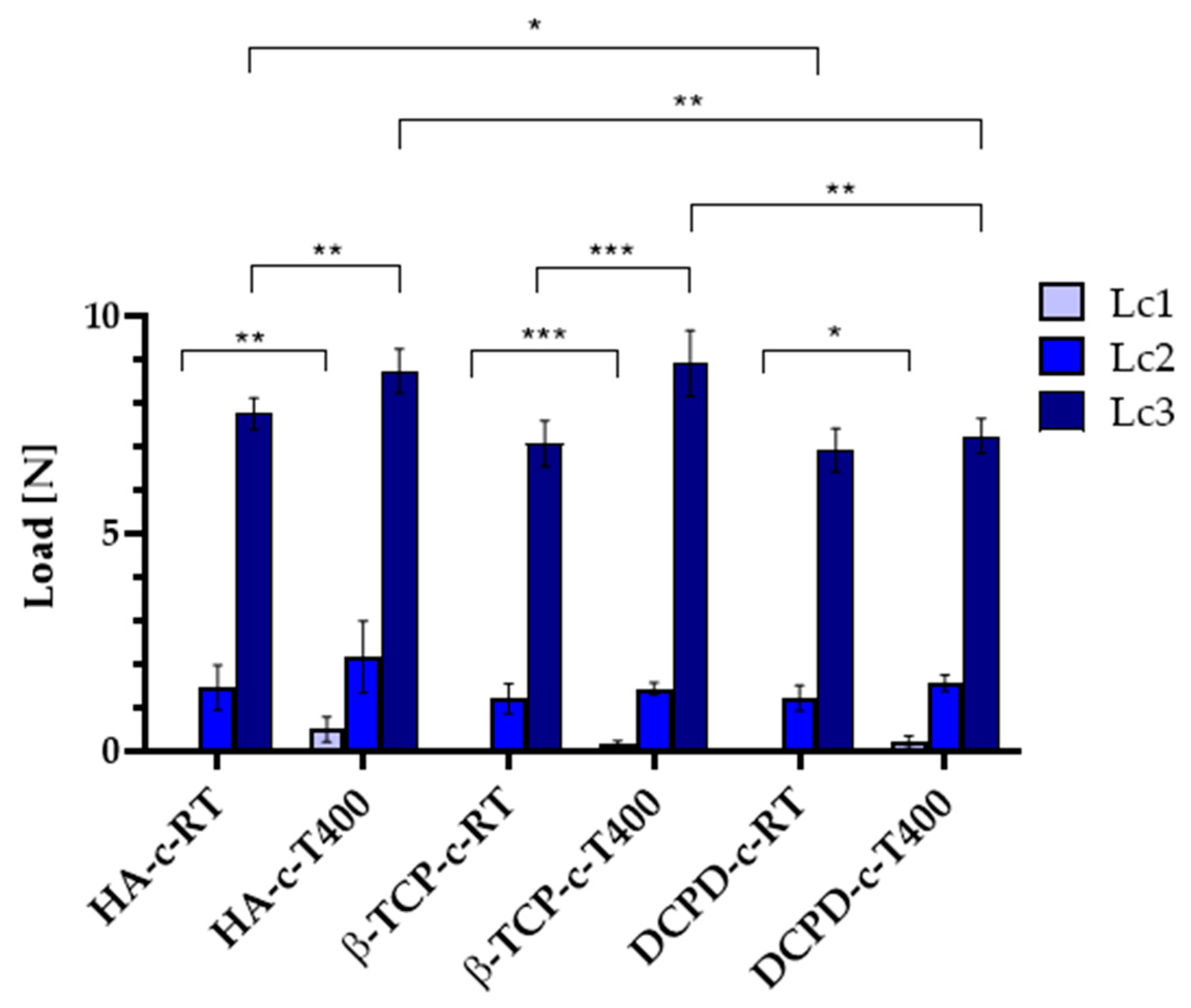
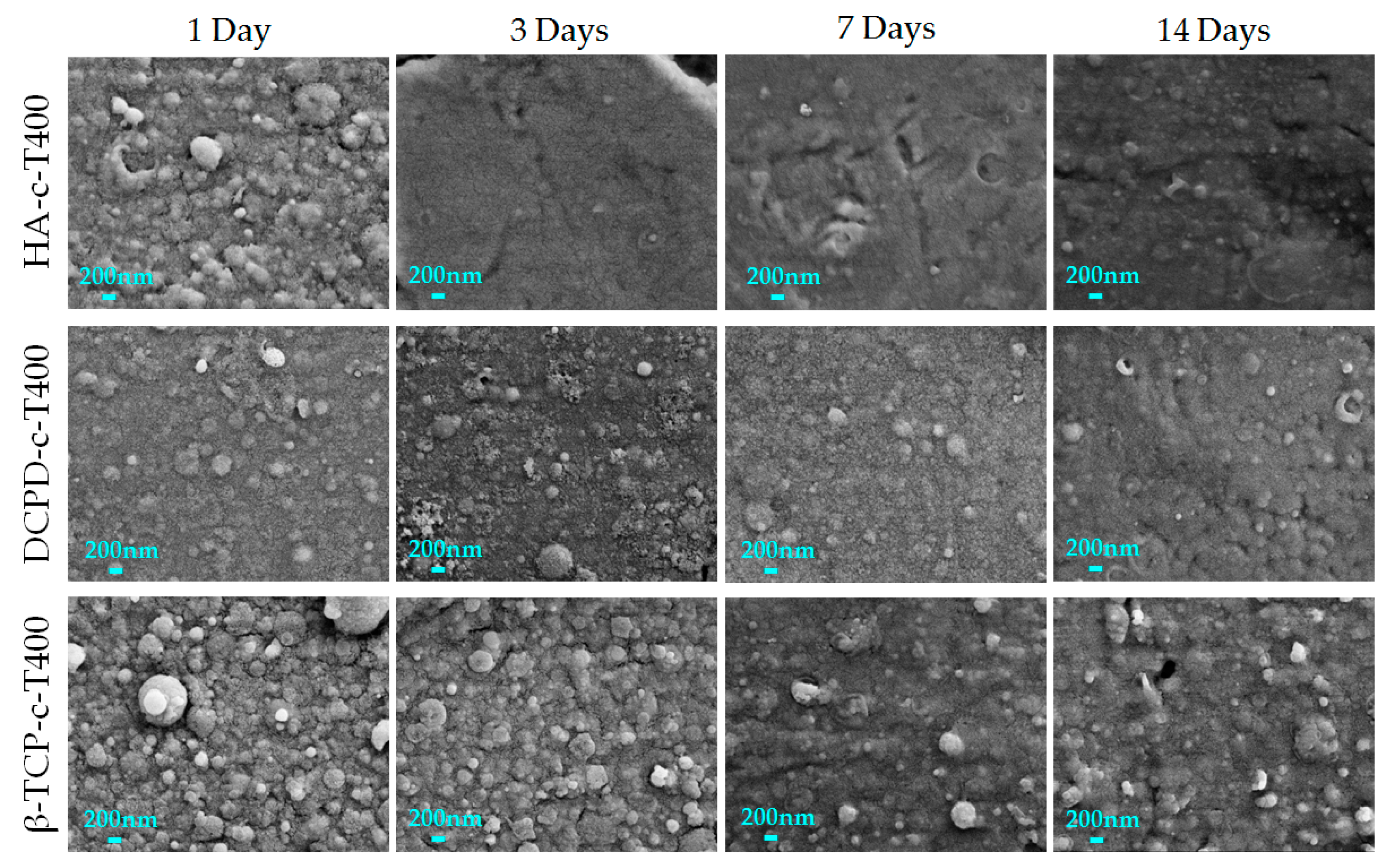
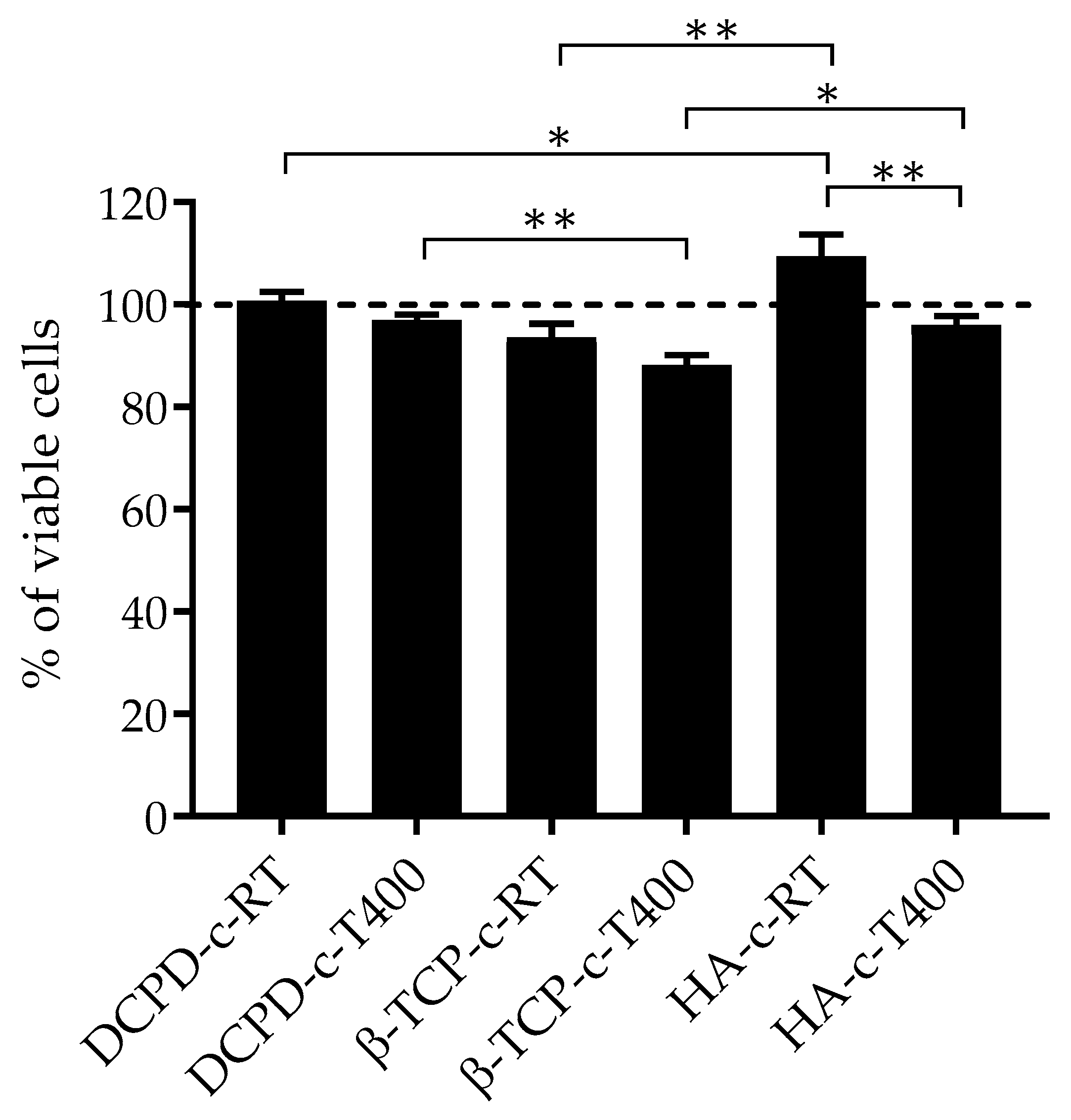
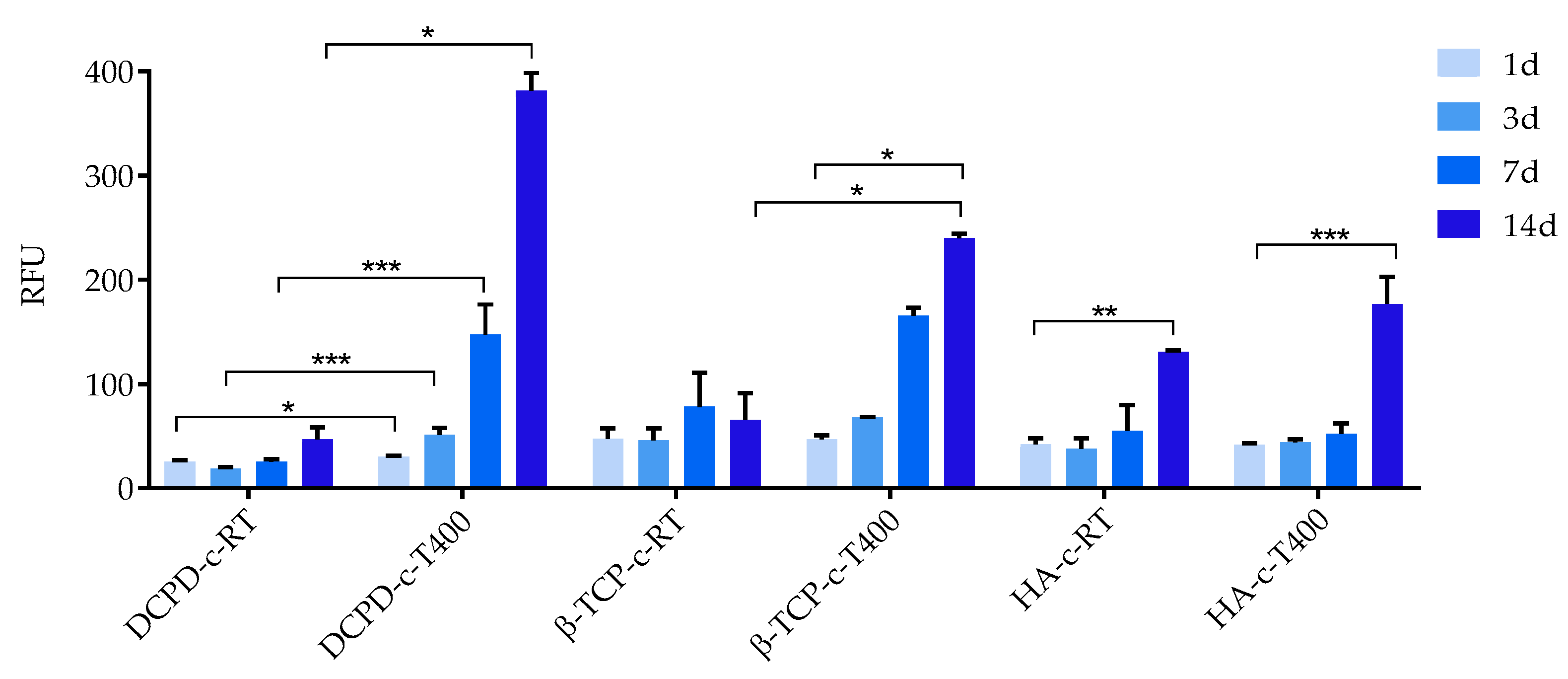
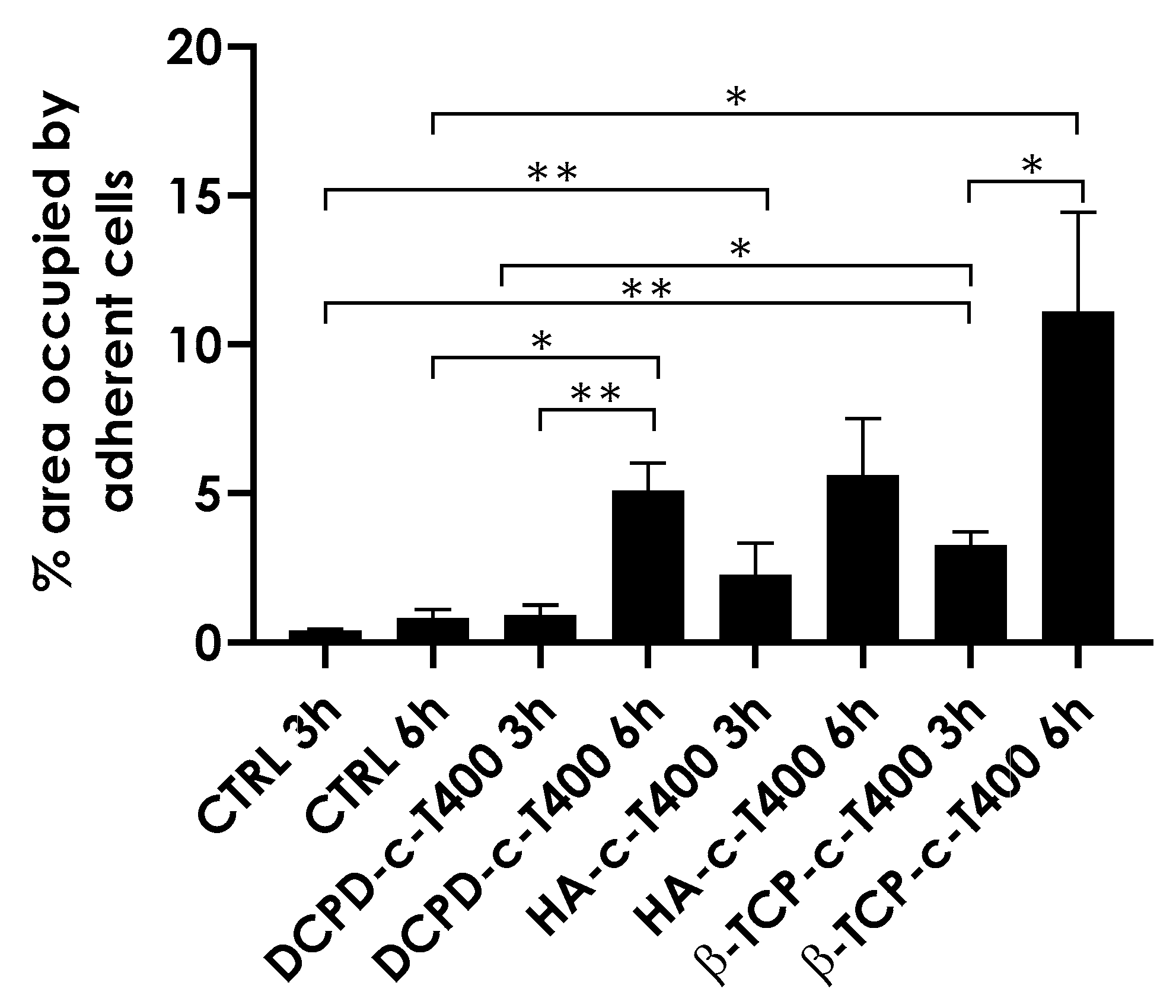
3.4. Biological Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Eliaz, N.; Metoki, N. Calcium Phosphate Bioceramics: A Review of Their History, Structure, Properties, Coating Technologies and Biomedical Applications. Materials 2017, 10, 334. [Google Scholar] [CrossRef] [PubMed]
- Bigi, A.; Boanini, E. Functionalized Biomimetic Calcium Phosphates for Bone Tissue Repair. J. Appl. Biomater. Funct. Mater. 2017, 15, e313–e325. [Google Scholar] [CrossRef] [PubMed]
- Surmenev, R.A. A review of plasma-assisted methods for calcium phosphate-based coatings fabrication. Surf. Coat. Technol. 2012, 206, 2035–2056. [Google Scholar] [CrossRef]
- Sun, L.; Berndt, C.C.; Gross, K.A.; Kucuk, A. Material fundamentals and clinical performance of plasma-sprayed hydroxyapatite coatings: A review. J. Biomed. Mater. Res. 2001, 58, 570–592. [Google Scholar] [CrossRef]
- Mocanu, A.; Cadar, O.; Frangopol, P.T.; Petean, I.; Tomoaia, G.; Paltinean, G.-A.; Racz, C.P.; Horovitz, O.; Tomoaia-Cotisel, M. Ion release from hydroxyapatite and substituted hydroxyapatites in different immersion liquids: In vitro experiments and theoretical modelling study. R. Soc. Open Sci. 2021, 8, 201785. [Google Scholar] [CrossRef]
- Harun, W.S.W.; Asri, R.I.M.; Sulong, A.B.; Ghani, S.A.C.; Ghazalli, Z. Hydroxyapatite-Based Coating on Biomedical Implant. In Hydroxyapatite—Advances in Composite Nanomaterials, Biomedical Applications and Its Technological Facets; InTechOpen Ltd.: London, UK, 2018; Volume 11, p. 13. ISBN 0000957720. [Google Scholar]
- Boanini, E.; Silingardi, F.; Gazzano, M.; Bigi, A. Synthesis and Hydrolysis of Brushite (DCPD): The Role of Ionic Substitution. Cryst. Growth Des. 2021, 21, 1689–1697. [Google Scholar] [CrossRef]
- Rubini, K.; Boanini, E.; Bigi, A. Role of Aspartic and Polyaspartic Acid on the Synthesis and Hydrolysis of Brushite. J. Funct. Biomater. 2019, 10, 11. [Google Scholar] [CrossRef] [PubMed]
- Bolbasov, E.N.; Lapin, I.N.; Svetlichnyi, V.A.; Lenivtseva, Y.D.; Malashicheva, A.; Malashichev, Y.; Golovkin, A.S.; Anissimov, Y.G.; Tverdokhlebov, S.I. The formation of calcium phosphate coatings by pulse laser deposition on the surface of polymeric ferroelectric. Appl. Surf. Sci. 2015, 349, 420–429. [Google Scholar] [CrossRef]
- Liu, X.; Li, M.; Zhu, Y.; Yeung, K.W.K.; Chu, P.K.; Wu, S. The modulation of stem cell behaviors by functionalized nanoceramic coatings on Ti-based implants. Bioact. Mater. 2016, 1, 65–76. [Google Scholar] [CrossRef]
- Sartori, M.; Graziani, G.; Sassoni, E.; Pagani, S.; Boi, M.; Maltarello, M.C.; Baldini, N.; Fini, M. Nanostructure and biomimetics orchestrate mesenchymal stromal cell differentiation: An in vitro bioactivity study on new coatings for orthopedic applications. Mater. Sci. Eng. C 2021, 123, 112031. [Google Scholar] [CrossRef]
- Von Erlach, T.C.; Bertazzo, S.; Wozniak, M.A.; Horejs, C.-M.; Maynard, S.A.; Attwood, S.; Robinson, B.K.; Autefage, H.; Kallepitis, C.; del Río Hernández, A.; et al. Cell-geometry-dependent changes in plasma membrane order direct stem cell signalling and fate. Nat. Mater. 2018, 17, 237–242. [Google Scholar] [CrossRef]
- Bellucci, D.; Bianchi, M.; Graziani, G.; Gambardella, A.; Berni, M.; Russo, A.; Cannillo, V. Pulsed Electron Deposition of nanostructured bioactive glass coatings for biomedical applications. Ceram. Int. 2017, 43, 15862–15867. [Google Scholar] [CrossRef]
- Bianchi, M.; Gambardella, A.; Graziani, G.; Liscio, F.; Cristina Maltarello, M.; Boi, M.; Berni, M.; Bellucci, D.; Marchiori, G.; Valle, F.; et al. Plasma-assisted deposition of bone apatite-like thin films from natural apatite. Mater. Lett. 2017, 199, 32–36. [Google Scholar] [CrossRef]
- Graziani, G.; Barbaro, K.; Fadeeva, I.V.; Ghezzi, D.; Fosca, M.; Sassoni, E.; Vadalà, G.; Cappelletti, M.; Valle, F.; Baldini, N.; et al. Ionized jet deposition of antimicrobial and stem cell friendly silver-substituted tricalcium phosphate nanocoatings on titanium alloy. Bioact. Mater. 2021, 6, 2629–2642. [Google Scholar] [CrossRef]
- Dorozhkin, S. V Calcium orthophosphate coatings, films and layers. Prog. Biomater. 2012, 1, 1. [Google Scholar] [CrossRef] [PubMed]
- Graziani, G.; Berni, M.; Gambardella, A.; De Carolis, M.; Maltarello, M.C.; Boi, M.; Carnevale, G.; Bianchi, M. Fabrication and characterization of biomimetic hydroxyapatite thin films for bone implants by direct ablation of a biogenic source. Mater. Sci. Eng. C 2019, 99, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Pagnotta, G.; Graziani, G.; Baldini, N.; Maso, A.; Focarete, M.L.; Berni, M.; Biscarini, F.; Bianchi, M.; Gualandi, C. Nanodecoration of electrospun polymeric fibers with nanostructured silver coatings by ionized jet deposition for antibacterial tissues. Mater. Sci. Eng. C 2020, 113, 110998. [Google Scholar] [CrossRef] [PubMed]
- Di Pompo, G.; Liguori, A.; Carlini, M.; Avnet, S.; Boi, M.; Baldini, N.; Focarete, M.L.; Bianchi, M.; Gualandi, C.; Graziani, G. Electrospun fibers coated with nanostructured biomimetic hydroxyapatite: A new platform for regeneration at the bone interfaces. Biomater. Adv. 2023, 144, 213231. [Google Scholar] [CrossRef]
- Choi, S.; Murphy, W.L. A screening approach reveals the influence of mineral coating morphology on human mesenchymal stem cell differentiation. Biotechnol. J. 2013, 8, 496–501. [Google Scholar] [CrossRef]
- Skočdopole, J.; Kalvoda, L.; Nozar, P.; Netopilík, M. Preparation of polymeric coatings by ionized jet deposition method. Chem. Pap. 2018, 72, 1735–1739. [Google Scholar] [CrossRef]
- Binitha, M.P.; Pradyumnan, P.P. Dielectric Property Studies of Biologically Compatible Brushite Single Crystals Used as Bone Graft Substitute. J. Biomater. Nanobiotechnol. 2013, 4, 119–122. [Google Scholar] [CrossRef]
- Lenis, J.A.; Hurtado, F.M.; Gómez, M.A.; Bolívar, F.J. Effect of thermal treatment on structure, phase and mechanical properties of hydroxyapatite thin films grown by RF magnetron sputtering. Thin Solid Films 2019, 669, 571–578. [Google Scholar] [CrossRef]
- Graziani, G.; Bianchi, M.; Sassoni, E.; Russo, A.; Marcacci, M. Ion-substituted calcium phosphate coatings deposited by plasma-assisted techniques: A review. Mater. Sci. Eng. C 2017, 74, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Dalby, M.J.; McCloy, D.; Robertson, M.; Wilkinson, C.D.W.; Oreffo, R.O.C. Osteoprogenitor response to defined topographies with nanoscale depths. Biomaterials 2006, 27, 1306–1315. [Google Scholar] [CrossRef]
- Curtis, A.; Wilkinson, C. Nantotechniques and approaches in biotechnology. Trends Biotechnol. 2001, 19, 97–101. [Google Scholar] [CrossRef]
- Eriksson, C.; Nygren, H.; Ohlson, K. Implantation of hydrophilic and hydrophobic titanium discs in rat tibia: Cellular reactions on the surfaces during the first 3 weeks in bone. Biomaterials 2004, 25, 4759–4766. [Google Scholar] [CrossRef]
- Junker, R.; Dimakis, A.; Thoneick, M.; Jansen, J.A. Effects of implant surface coatings and composition on bone integration: A systematic review. Clin. Oral Implant. Res. 2009, 20, 185–206. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.; Haluska, M.; Narayan, R.J.; Snyder, R.L. In situ annealing of hydroxyapatite thin films. Mater. Sci. Eng. C 2006, 26, 1312–1316. [Google Scholar] [CrossRef]
- Fernández-Pradas, J.; García-Cuenca, M.; Clèries, L.; Sardin, G.; Morenza, J. Influence of the interface layer on the adhesion of pulsed laser deposited hydroxyapatite coatings on titanium alloy. Appl. Surf. Sci. 2002, 195, 31–37. [Google Scholar] [CrossRef]
- Graziani, G.; Boi, M.; Bianchi, M. A Review on Ionic Substitutions in Hydroxyapatite Thin Films: Towards Complete Biomimetism. Coatings 2018, 8, 269. [Google Scholar] [CrossRef]
- Bianchi, M.; Pisciotta, A.; Bertoni, L.; Berni, M.; Gambardella, A.; Visani, A.; Russo, A.; De Pol, A.; Carnevale, G. Osteogenic differentiation of hDPSCs on biogenic bone apatite thin films. Stem Cells Int. 2017, 2017, 3579283. [Google Scholar] [CrossRef] [PubMed]


| Dm | dm | da | Size Distribution (% of Aggregates for Each Range) | |||||
|---|---|---|---|---|---|---|---|---|
| (nm) | (nm) | (nm) | d < 100 nm | 100 nm ≤ d < 250 nm | 250 nm ≤ d < 500 nm | 500 nm ≤ d < 1000 nm | d ≥ 1000 | |
| HA-c-RT | 1372 ± 221 | 79 ± 25 | 304 ± 209 | 1 | 53 | 35 | 9 | 3 |
| HA-c-T400 | 1045 ± 161 | 106 ± 12 | 296 ± 174 | 1 | 53 | 36 | 10 | 1 |
| DCPD-c-RT | 1293 ± 684 | 105 ± 3 | 307 ± 221 | 0 | 49 | 41 | 8 | 1 |
| DCPD-c-T400 | 1550 ± 379 | 106 ± 13 | 296 ± 194 | 1 | 48 | 43 | 6 | 2 |
| β-TCP-c-RT | 1526 ± 203 | 118 ± 9 | 278 ± 212 | 0 | 63 | 31 | 4 | 2 |
| β-TCP-c-T400 | 1530 ± 345 | 105 ± 9 | 262 ± 208 | 1 | 67 | 25 | 5 | 2 |
| RMS (nm) | Maximum Height (nm) | |||
|---|---|---|---|---|
| RT | T400 | RT | T400 | |
| HA-c | 107 | 62 | 505 | 423 |
| DCPD-c | 98 | 130 | 537 | 661 |
| β-TCP-c | 26 | 96 | 238 | 507 |
| Ca/P | HA | DCPD | β-TCP |
|---|---|---|---|
| Stoichiometric (target) | 1.67 | 1 | 1.50 |
| Coating | 1.91 | 0.83 | 1.42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montesissa, M.; Borciani, G.; Rubini, K.; Valle, F.; Boi, M.; Baldini, N.; Boanini, E.; Graziani, G. Ionized Jet Deposition of Calcium Phosphates-Based Nanocoatings: Tuning Coating Properties and Cell Behavior by Target Composition and Substrate Heating. Nanomaterials 2023, 13, 1758. https://doi.org/10.3390/nano13111758
Montesissa M, Borciani G, Rubini K, Valle F, Boi M, Baldini N, Boanini E, Graziani G. Ionized Jet Deposition of Calcium Phosphates-Based Nanocoatings: Tuning Coating Properties and Cell Behavior by Target Composition and Substrate Heating. Nanomaterials. 2023; 13(11):1758. https://doi.org/10.3390/nano13111758
Chicago/Turabian StyleMontesissa, Matteo, Giorgia Borciani, Katia Rubini, Francesco Valle, Marco Boi, Nicola Baldini, Elisa Boanini, and Gabriela Graziani. 2023. "Ionized Jet Deposition of Calcium Phosphates-Based Nanocoatings: Tuning Coating Properties and Cell Behavior by Target Composition and Substrate Heating" Nanomaterials 13, no. 11: 1758. https://doi.org/10.3390/nano13111758







