High-Performance Perovskite Solar Cells and Modules Fabricated by Slot-Die Coating with Nontoxic Solvents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Solution Preparations
2.3. Device Fabrication
2.4. Device Characterization
3. Results
3.1. Slot-Die Coating of NiOX HTL
3.2. Slot-Die of Perovskite Films with Nontoxic Solvents
3.3. Slot-Die Coating of PC61BM and WF Layer Using Nontoxic Solvent
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miyata, A.; Mitioglu, A.; Plochocka, P.; Portugall, O.; Wang, J.T.-W.; Stranks, S.D.; Snaith, H.J.; Nicholas, R.J. Direct measurement of the exciton binding energy and effective masses for charge carriers in organic–inorganic tri-halide perovskites. Nat. Phys. 2015, 11, 582–587. [Google Scholar] [CrossRef]
- Snaith, H.J. Perovskites: The emergence of a new era for low-cost, high-efficiency solar cells. J. Phys. Chem. Lett. 2013, 4, 3623–3630. [Google Scholar] [CrossRef]
- Agbaoye, R.O.; Akinlami, J.O.; Afolabi, T.A.; Adebayo, G.A. Unraveling the stable phase, high absorption coefficient, optical and mechanical properties of hybrid perovskite CH3NH3PbXMg1−XI3: Density functional approach. J. Inorg. Organomet. Polym. Mater. 2020, 30, 299–309. [Google Scholar] [CrossRef]
- Oga, H.; Saeki, A.; Ogomi, Y.; Hayase, S.; Seki, S. Improved understanding of the electronic and energetic landscapes of perovskite solar cells: High local charge carrier mobility, reduced recombination, and extremely shallow traps. J. Am. Chem. Soc. 2014, 136, 13818–13825. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Jeong, J.; Lu, H.; Lee, T.K.; Eickemeyer, F.T.; Liu, Y.; Choi, I.W.; Choi, S.J.; Jo, Y.; Kim, H.-B.; et al. Conformal quantum dot–SnO2 layers as electron transporters for efficient perovskite solar cells. Science 2022, 375, 302–306. [Google Scholar] [CrossRef] [PubMed]
- Rajagopal, A.; Yao, K.; Jen, A.K. Toward perovskite solar cell commercialization: A perspective and research roadmap based on interfacial engineering. Adv. Mater. 2018, 30, 1800455. [Google Scholar] [CrossRef]
- Yang, Y.; Xue, Z.; Chen, L.; Lau, C.F.J.; Wang, Z. Large-area perovskite films for PV applications: A perspective from nucleation and crystallization. J. Energy Chem. 2021, 59, 626–641. [Google Scholar] [CrossRef]
- Wei, Z.; Chen, H.; Yan, K.; Yang, S. Inkjet printing and instant chemical transformation of a CH3NH3PbI3/nanocarbon electrode and interface for planar perovskite solar cells. Angew. Chem. Int. Ed. Engl. 2014, 53, 13239–13243. [Google Scholar] [CrossRef]
- Yang, J.; Lim, E.L.; Tan, L.; Wei, Z. Ink engineering in blade-coating large-area perovskite solar cells. Adv. Energy Mater. 2022, 12, 2200975. [Google Scholar] [CrossRef]
- Huang, Y.-C.; Li, C.-F.; Huang, Z.-H.; Liu, P.-H.; Tsao, C.-S. Rapid and sheet-to-sheet slot-die coating manufacture of highly efficient perovskite solar cells processed under ambient air. Sol. Energy 2019, 177, 255–261. [Google Scholar] [CrossRef]
- Huang, S.H.; Guan, C.K.; Lee, P.H.; Huang, H.C.; Li, C.F.; Huang, Y.C.; Su, W.F. Toward All slot-die fabricated high efficiency large area perovskite solar cell using rapid near infrared heating in ambient air. Adv. Energy Mater. 2020, 10, 2001567. [Google Scholar] [CrossRef]
- Li, J.; Dagar, J.; Shargaieva, O.; Flatken, M.A.; Köbler, H.; Fenske, M.; Schultz, C.; Stegemann, B.; Just, J.; Többens, D.M.; et al. 20.8% slot-die coated mapbi3 perovskite solar cells by optimal dmso-content and age of 2-me based precursor inks. Adv. Energy Mater. 2021, 11, 2003460. [Google Scholar] [CrossRef]
- Lan, D.H.; Hong, S.H.; Chou, L.H.; Wang, X.F.; Liu, C.L. High throughput two-step ultrasonic spray deposited CH3NH3PbI3 thin film layer for solar cell application. J. Power Sources 2018, 390, 270–277. [Google Scholar] [CrossRef]
- Bishop, J.E.; Read, C.D.; Smith, J.A.; Routledge, T.J.; Lidzey, D.G. Fully spray-coated triple-cation perovskite solar cells. Sci. Rep. 2020, 10, 6610. [Google Scholar] [CrossRef] [PubMed]
- Rana, P.J.S.; Febriansyah, B.; Koh, T.M.; Muhammad, B.T.; Salim, T.; Hooper, T.J.N.; Kanwat, A.; Ghosh, B.; Kajal, P.; Lew, J.H.; et al. Alkali additives enable efficient large area (>55 cm2) slot-die coated perovskite solar modules. Adv. Funct. Mater. 2022, 32, 2113026. [Google Scholar] [CrossRef]
- Lee, H.-J.; Na, S.-I. Performance improvement of slot-die-deposition based perovskite solar cells using diglycolic acid additives. Mater. Lett. 2022, 312, 131651. [Google Scholar] [CrossRef]
- Gao, L.; Huang, K.; Long, C.; Zeng, F.; Liu, B.; Yang, J. Fully slot-die-coated perovskite solar cells in ambient condition. Appl. Phys. A 2020, 126, 452. [Google Scholar] [CrossRef]
- Le, T.S.; Saranin, D.; Gostishchev, P.; Ermanova, I.; Komaricheva, T.; Luchnikov, L.; Muratov, D.; Uvarov, A.; Vyacheslavova, E.; Mukhin, I. All-slot-die-coated inverted perovskite solar cells in ambient conditions with chlorine additives. Sol. RRL 2022, 6, 2100807. [Google Scholar] [CrossRef]
- Wang, S.; Wang, B.; He, S.; Wang, Y.; Cheng, J.; Li, Y. Enhancing the photovoltaic performance of planar heterojunction perovskite solar cells via introducing binary-mixed organic electron transport layers. New J. Chem. 2023, 47, 5048–5055. [Google Scholar] [CrossRef]
- Wei, Y.; Chen, C.; Tan, C.; He, L.; Ren, Z.; Zhang, C.; Peng, S.; Han, J.; Zhou, H.; Wang, J. High-performance visible to near-infrared broadband Bi2O2Se nanoribbon photodetectors. Adv. Opt. Mater. 2022, 10, 2201396. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, J.; Huang, C.; Bi, Z.; Xu, X.; Yu, H. SnO2/2D-Bi2O2Se new hybrid electron transporting layer for efficient and stable perovskite solar cells. Chem. Eng. J. 2021, 410, 128436. [Google Scholar] [CrossRef]
- Zhu, L.; Lu, Q.; Li, C.; Wang, Y.; Deng, Z. Graded interface engineering of 3D/2D halide perovskite solar cells through ultrathin (PEA)2PbI4 nanosheets. Chin. Chem. Lett. 2021, 32, 2259–2262. [Google Scholar] [CrossRef]
- Kranthiraja, K.; Parashar, M.; Mehta, R.K.; Aryal, S.; Temsal, M.; Kaul, A.B. Stability and degradation in triple cation and methyl ammonium lead iodide perovskite solar cells mediated via Au and Ag electrodes. Sci. Rep. 2022, 12, 18574. [Google Scholar] [CrossRef] [PubMed]
- Rivkin, B.; Fassl, P.; Sun, Q.; Taylor, A.D.; Chen, Z.; Vaynzof, Y. Effect of ion migration-induced electrode degradation on the operational stability of perovskite solar cells. ACS Omega 2018, 3, 10042–10047. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, Y.; Zhang, F.; Lee, C.S. A novel aluminum–graphite dual-ion battery. Adv. Energy Mater. 2016, 6, 1502588. [Google Scholar] [CrossRef]
- Wang, M.; Jiang, C.; Zhang, S.; Song, X.; Tang, Y.; Cheng, H.-M. Reversible calcium alloying enables a practical room-temperature rechargeable calcium-ion battery with a high discharge voltage. Nat. Chem. 2018, 10, 667–672. [Google Scholar] [CrossRef]
- Huang, Z.; Luo, P.; Zheng, H.; Lyu, Z.; Ma, X. Novel one-dimensional V3S4@ NC nanofibers for sodium-ion batteries. J. Phys. Chem. Solids 2023, 172, 111081. [Google Scholar] [CrossRef]
- Lee, J.-W.; Kim, H.-S.; Park, N.-G. Lewis acid–base adduct approach for high efficiency perovskite solar cells. Acc. Chem. Res. 2016, 49, 311–319. [Google Scholar] [CrossRef]
- Lv, M.; Dong, X.; Fang, X.; Lin, B.; Zhang, S.; Ding, J.; Yuan, N. A promising alternative solvent of perovskite to induce rapid crystallization for high-efficiency photovoltaic devices. Rsc Adv. 2015, 5, 20521–20529. [Google Scholar] [CrossRef]
- Gardner, K.L.; Tait, J.G.; Merckx, T.; Qiu, W.; Paetzold, U.W.; Kootstra, L.; Jaysankar, M.; Gehlhaar, R.; Cheyns, D.; Heremans, P. Nonhazardous solvent systems for processing perovskite photovoltaics. Adv. Energy Mater. 2016, 6, 1600386. [Google Scholar] [CrossRef]
- Ahn, N.; Son, D.-Y.; Jang, I.-H.; Kang, S.M.; Choi, M.; Park, N.-G. Highly reproducible perovskite solar cells with average efficiency of 18.3% and best efficiency of 19.7% fabricated via Lewis base adduct of lead (II) iodide. J. Am. Chem. Soc. 2015, 137, 8696–8699. [Google Scholar] [CrossRef]
- Henderson, R.K.; Jiménez-González, C.; Constable, D.J.; Alston, S.R.; Inglis, G.G.; Fisher, G.; Sherwood, J.; Binks, S.P.; Curzons, A.D. Expanding GSK’s solvent selection guide–embedding sustainability into solvent selection starting at medicinal chemistry. Green Chem. 2011, 13, 854–862. [Google Scholar] [CrossRef]
- Wu, Y.; Islam, A.; Yang, X.; Qin, C.; Liu, J.; Zhang, K.; Peng, W.; Han, L. Retarding the crystallization of PbI 2 for highly reproducible planar-structured perovskite solar cells via sequential deposition. Energy Environ. Sci. 2014, 7, 2934–2938. [Google Scholar] [CrossRef]
- Noel, N.K.; Habisreutinger, S.N.; Wenger, B.; Klug, M.T.; Hörantner, M.T.; Johnston, M.B.; Nicholas, R.J.; Moore, D.T.; Snaith, H.J. A low viscosity, low boiling point, clean solvent system for the rapid crystallisation of highly specular perovskite films. Energy Environ. Sci. 2017, 10, 145–152. [Google Scholar] [CrossRef]
- Galagan, Y.; Di Giacomo, F.; Gorter, H.; Kirchner, G.; de Vries, I.; Andriessen, R.; Groen, P. Roll-to-roll slot die coated perovskite for efficient flexible solar cells. Adv. Energy Mater. 2018, 8, 1801935. [Google Scholar] [CrossRef]
- Wang, J.; Di Giacomo, F.; Brüls, J.; Gorter, H.; Katsouras, I.; Groen, P.; Janssen, R.A.J.; Andriessen, R.; Galagan, Y. Highly efficient perovskite solar cells using non-toxic industry compatible solvent system. Sol. RRL 2017, 1, 1700091. [Google Scholar] [CrossRef]
- Dong, W.; Qiao, W.; Xiong, S.; Yang, J.; Wang, X.; Ding, L.; Yao, Y.; Bao, Q. Surface passivation and energetic modification suppress nonradiative recombination in perovskite solar cells. Nano-Micro Lett. 2022, 14, 108. [Google Scholar] [CrossRef]
- Wang, K.; Liu, C.; Du, P.; Chen, L.; Zhu, J.; Karim, A.; Gong, X. Efficiencies of perovskite hybrid solar cells influenced by film thickness and morphology of CH3NH3PbI3−xClx layer. Org. Electron. 2015, 21, 19–26. [Google Scholar] [CrossRef]
- Liang, Q.; Liu, K.; Sun, M.; Ren, Z.; Fong, P.W.K.; Huang, J.; Qin, M.; Wu, Z.; Shen, D.; Lee, C.-S.; et al. Manipulating crystallization kinetics in high-performance blade-coated perovskite solar cells via cosolvent-assisted phase transition. Adv. Mater. 2022, 34, 2200276. [Google Scholar] [CrossRef]
- Xia, F.; Wu, Q.; Zhou, P.; Li, Y.; Chen, X.; Liu, Q.; Zhu, J.; Dai, S.; Lu, Y.; Yang, S. Efficiency enhancement of inverted structure perovskite solar cells via oleamide doping of pcbm electron transport layer. ACS Appl. Mater. Interfaces 2015, 7, 13659–13665. [Google Scholar] [CrossRef]
- Cho, S.; Pandey, P.; Park, J.; Lee, T.-W.; Kang, D.-W. Mixed solvent engineering for morphology optimization of the electron transport layer in perovskite photovoltaics. ACS Appl. Energ. Mater. 2022, 5, 387–396. [Google Scholar] [CrossRef]
- Seo, J.; Park, S.; Kim, Y.C.; Jeon, N.J.; Noh, J.H.; Yoon, S.C.; Seok, S.I. Benefits of very thin PCBM and LiF layers for solution-processed p–i–n perovskite solar cells. Energy Environ. Sci. 2014, 7, 2642–2646. [Google Scholar] [CrossRef]
- Marinova, N.; Tress, W.; Humphry-Baker, R.; Dar, M.I.; Bojinov, V.; Zakeeruddin, S.M.; Nazeeruddin, M.K.; Grätzel, M. Light harvesting and charge recombination in CH3NH3PbI3 perovskite solar cells studied by hole transport layer thickness variation. ACS Nano 2015, 9, 4200–4209. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.-H.; Wu, T.-T.; Tian, K.-Y.; Li, C.-F.; Hou, C.-H.; Shyue, J.-J.; Lu, C.-F.; Huang, Y.-C.; Su, W.-F. Work-function-tunable electron transport layer of molecule-capped metal oxide for a high-efficiency and stable p–i–n perovskite solar cell. ACS Appl. Mater. Interfaces 2020, 12, 45936–45949. [Google Scholar] [CrossRef] [PubMed]

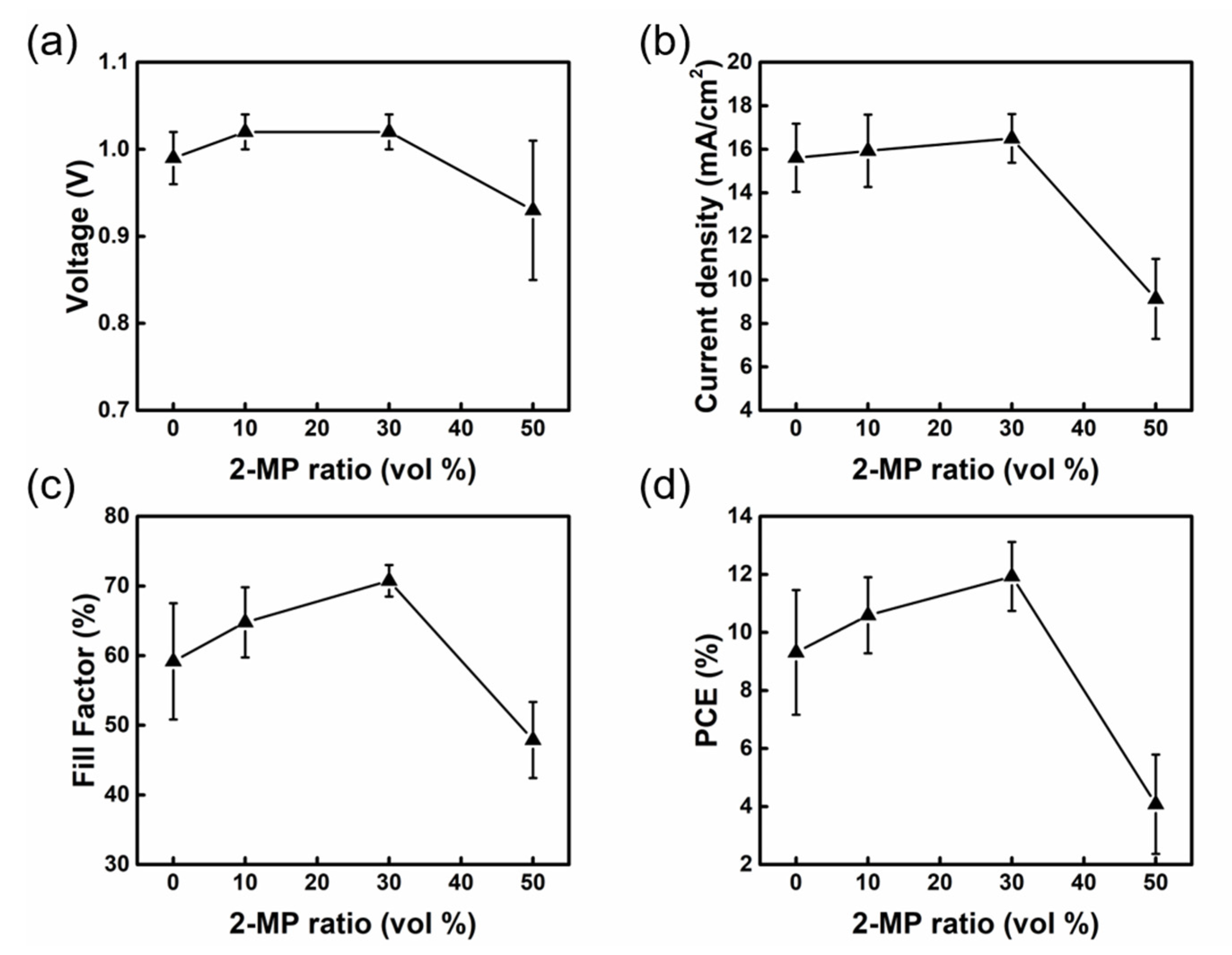

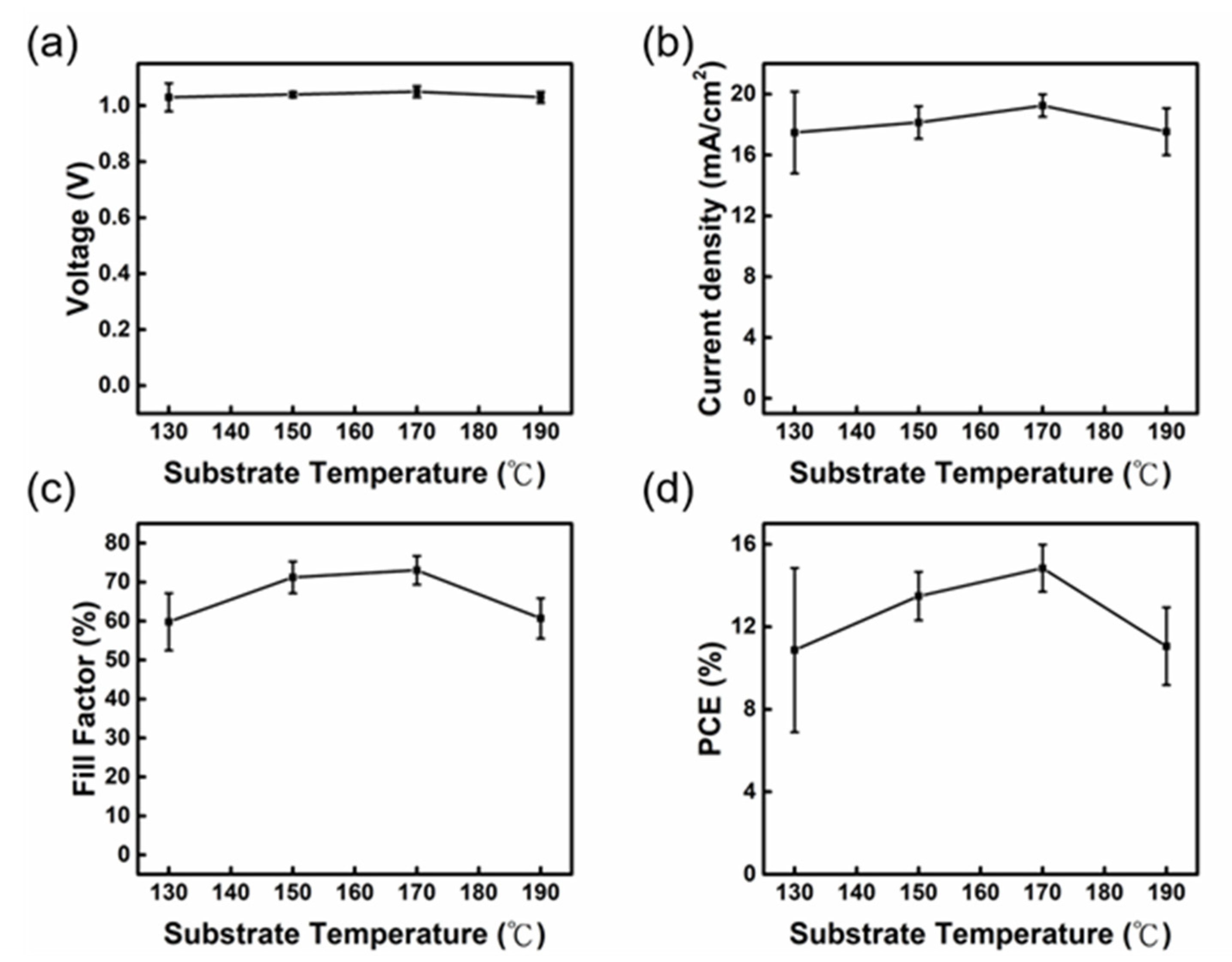
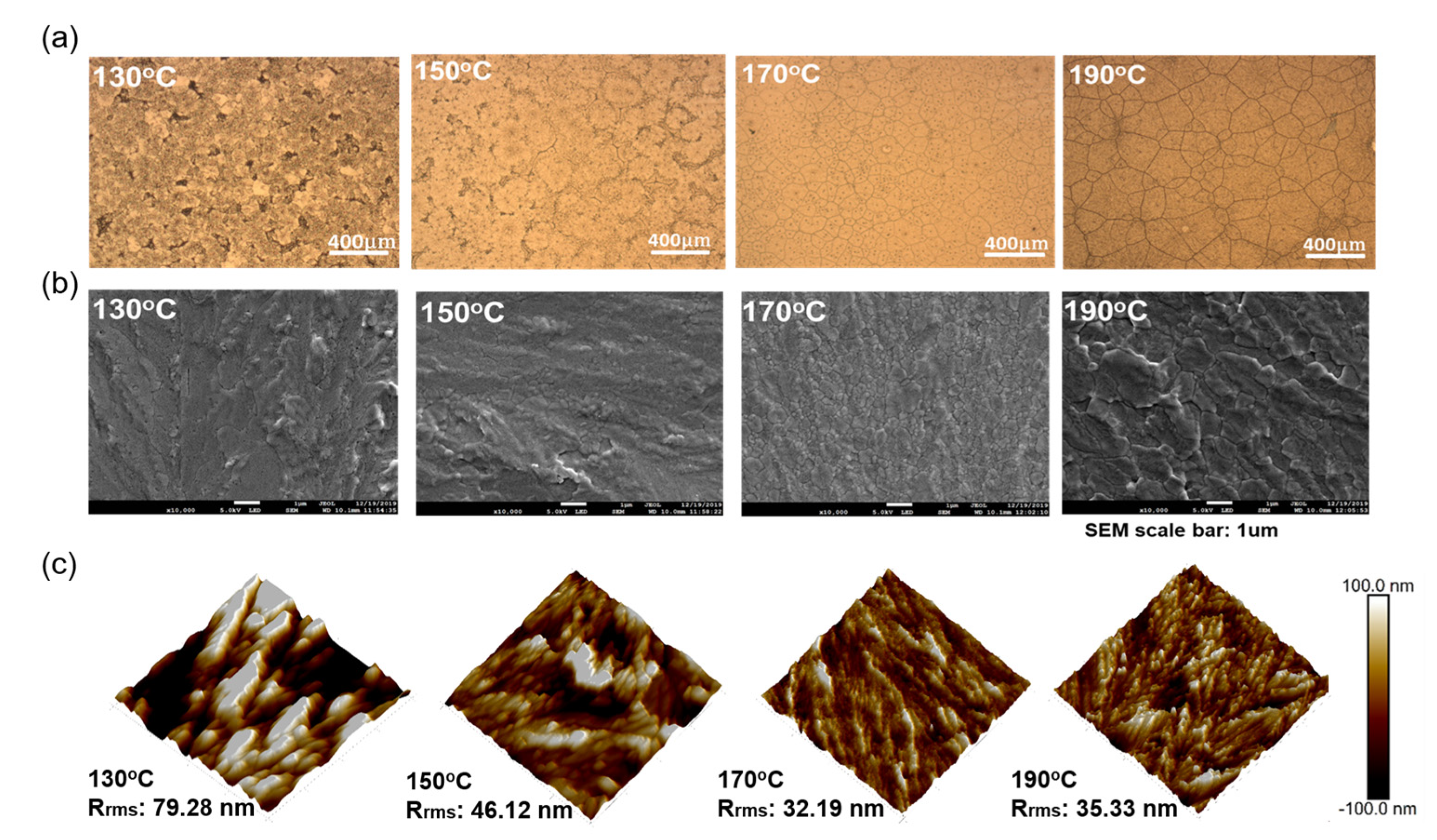
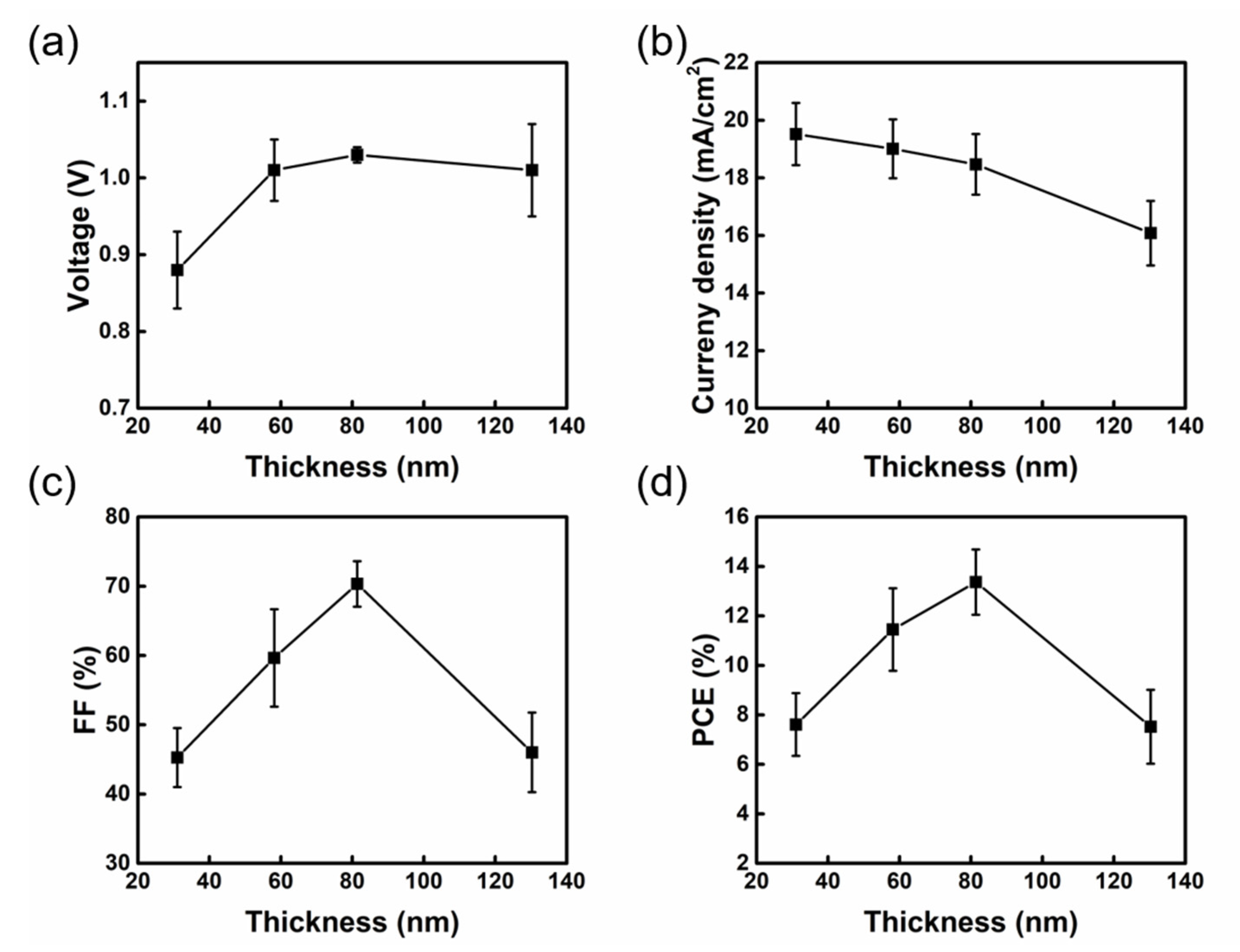

| 2-MP. (Vol%) | VOC (V) | JSC (mA/cm2) | FF (%) | PCE (%) |
|---|---|---|---|---|
| 0 | 0.99 ± 0.03 | 15.61 ± 1.57 | 59.18 ± 8.36 | 9.31 ± 2.15 |
| 10 | 1.02 ± 0.02 | 15.93 ± 1.66 | 64.79 ± 5.04 | 10.59 ± 1.31 |
| 30 | 1.02 ± 0.02 | 16.50 ± 1.12 | 70.75 ± 2.27 | 11.93 ± 1.19 |
| 50 | 0.93 ± 0.08 | 9.13 ± 1.84 | 47.89 ± 5.45 | 4.08 ± 1.71 |
| Process | Moving Rate (m/min) | Thickness (nm) | R (10−1 ohm) | VOC (V) | JSC (mA/cm2) | FF (%) | PCE (%) |
|---|---|---|---|---|---|---|---|
| Slot−die (SD) | 1.5 | 31.03 ± 7.61 | 9.30 | 0.88 ± 0.05 (0.94) | 19.52 ± 1.08 (20.98) | 45.27 ± 4.25 (49.81) | 7.61 ± 1.27 (9.88) |
| 2.5 | 58.14 ± 7.52 | 12.58 | 1.01 ± 0.04 (1.03) | 19.01 ± 1.02 (18.76) | 59.65 ± 7.04 (68.99) | 11.45 ± 1.67 (13.46) | |
| 3.5 | 81.38 ± 8.09 | 18.58 | 1.03 ± 0.01 (1.07) | 18.47 ± 1.05 (19.18) | 70.33 ± 3.28 (77.17) | 13.37 ± 1.32 (15.95) | |
| 4.5 | 130.28 ± 29.06 | 29.47 | 1.01 ± 0.06 (1.04) | 16.08 ± 1.12 (18.29) | 46.02 ± 5.75 (57.30) | 7.52 ± 1.49 (10.98) | |
| Spin (SP) | - | 80.44 ± 5.01 | 18.33 | 1.03 ± 0.02 (1.06) | 18.44 ± 0.80 (19.34) | 71.52 ± 3.71 (74.72) | 13.68 ± 1.09 (15.35) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.-F.; Huang, H.-C.; Huang, S.-H.; Hsiao, Y.-H.; Chaudhary, P.; Chang, C.-Y.; Tsai, F.-Y.; Su, W.-F.; Huang, Y.-C. High-Performance Perovskite Solar Cells and Modules Fabricated by Slot-Die Coating with Nontoxic Solvents. Nanomaterials 2023, 13, 1760. https://doi.org/10.3390/nano13111760
Li C-F, Huang H-C, Huang S-H, Hsiao Y-H, Chaudhary P, Chang C-Y, Tsai F-Y, Su W-F, Huang Y-C. High-Performance Perovskite Solar Cells and Modules Fabricated by Slot-Die Coating with Nontoxic Solvents. Nanomaterials. 2023; 13(11):1760. https://doi.org/10.3390/nano13111760
Chicago/Turabian StyleLi, Chia-Feng, Hung-Che Huang, Shih-Han Huang, Yu-Hung Hsiao, Priyanka Chaudhary, Chun-Yu Chang, Feng-Yu Tsai, Wei-Fang Su, and Yu-Ching Huang. 2023. "High-Performance Perovskite Solar Cells and Modules Fabricated by Slot-Die Coating with Nontoxic Solvents" Nanomaterials 13, no. 11: 1760. https://doi.org/10.3390/nano13111760
APA StyleLi, C.-F., Huang, H.-C., Huang, S.-H., Hsiao, Y.-H., Chaudhary, P., Chang, C.-Y., Tsai, F.-Y., Su, W.-F., & Huang, Y.-C. (2023). High-Performance Perovskite Solar Cells and Modules Fabricated by Slot-Die Coating with Nontoxic Solvents. Nanomaterials, 13(11), 1760. https://doi.org/10.3390/nano13111760







