Advancement of Physical and Photoelectrochemical Properties of Nanostructured CdS Thin Films toward Optoelectronic Applications
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Structural Analysis
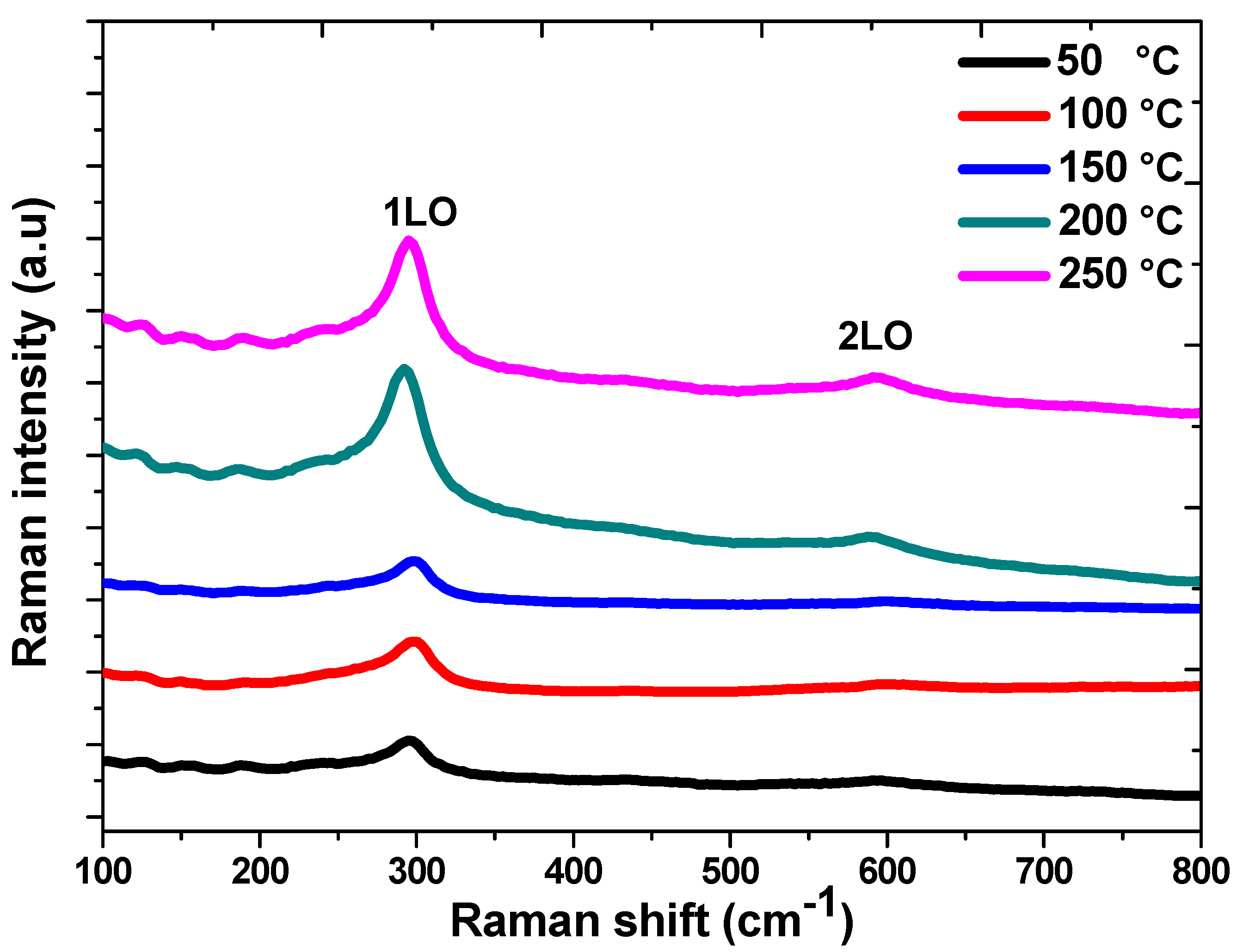
3.2. Raman Spectroscopy Analysis
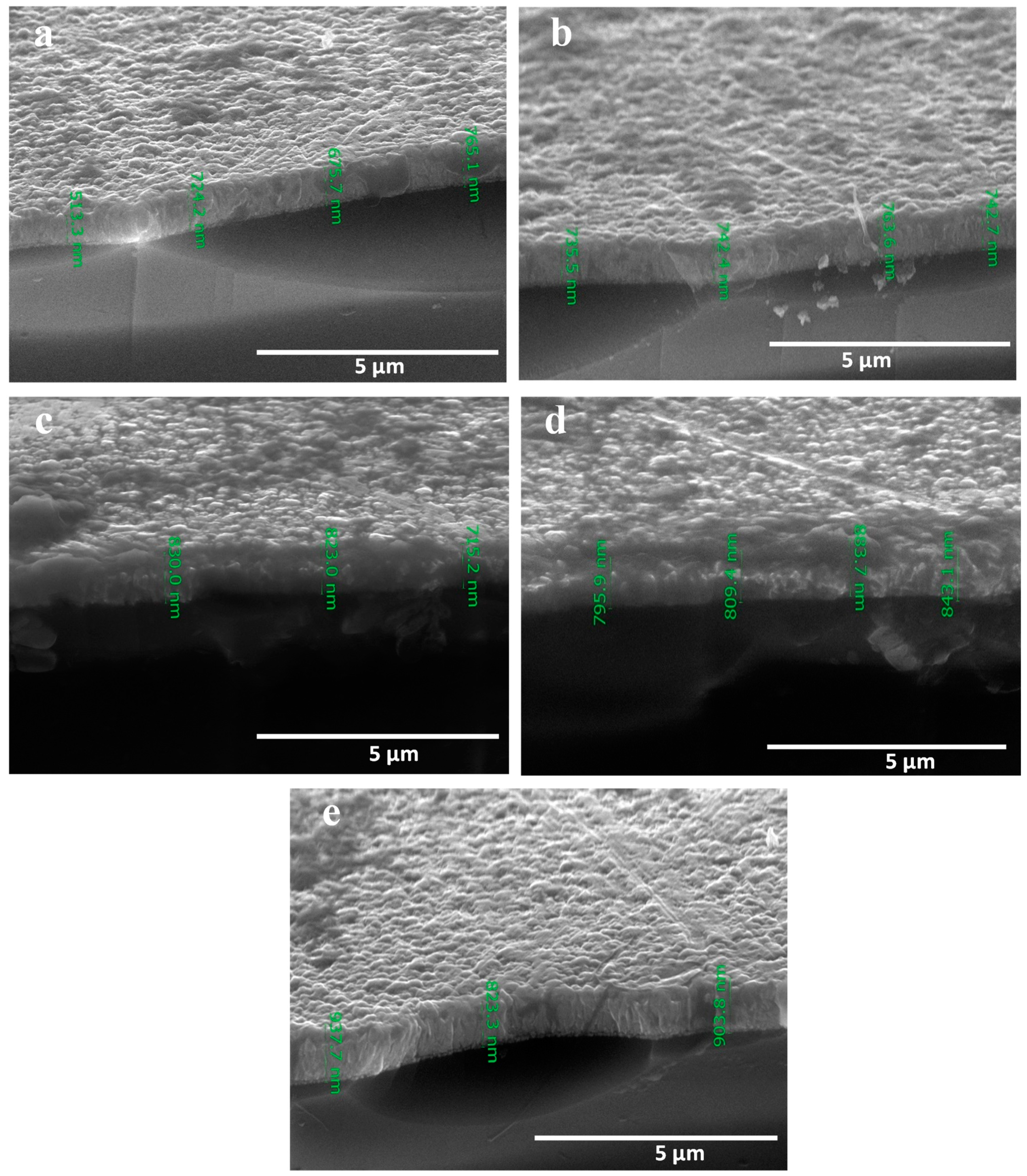
3.3. Surface Morphology Analysis
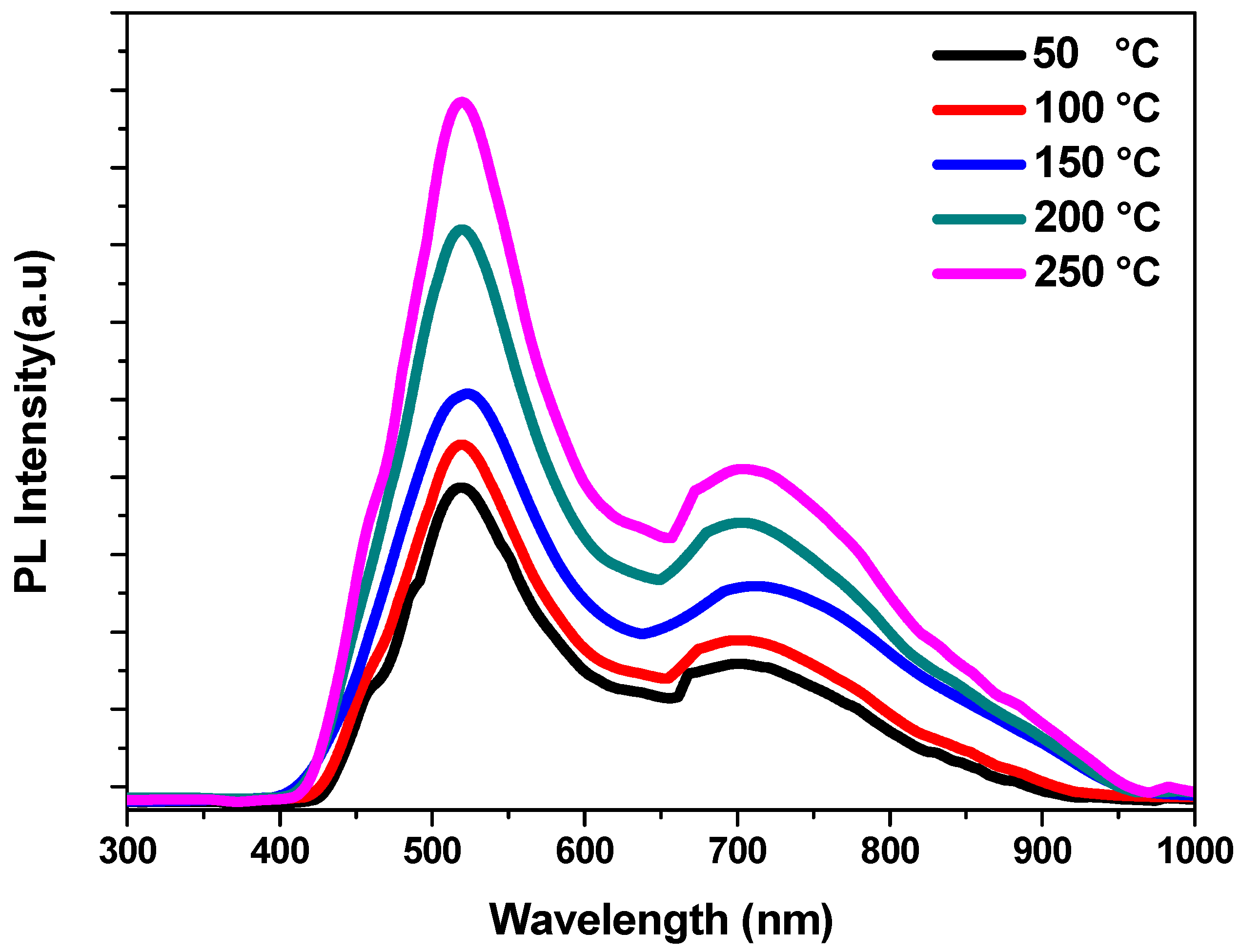
3.4. Photoluminescence (PL) Analysis
3.5. Optical Analysis
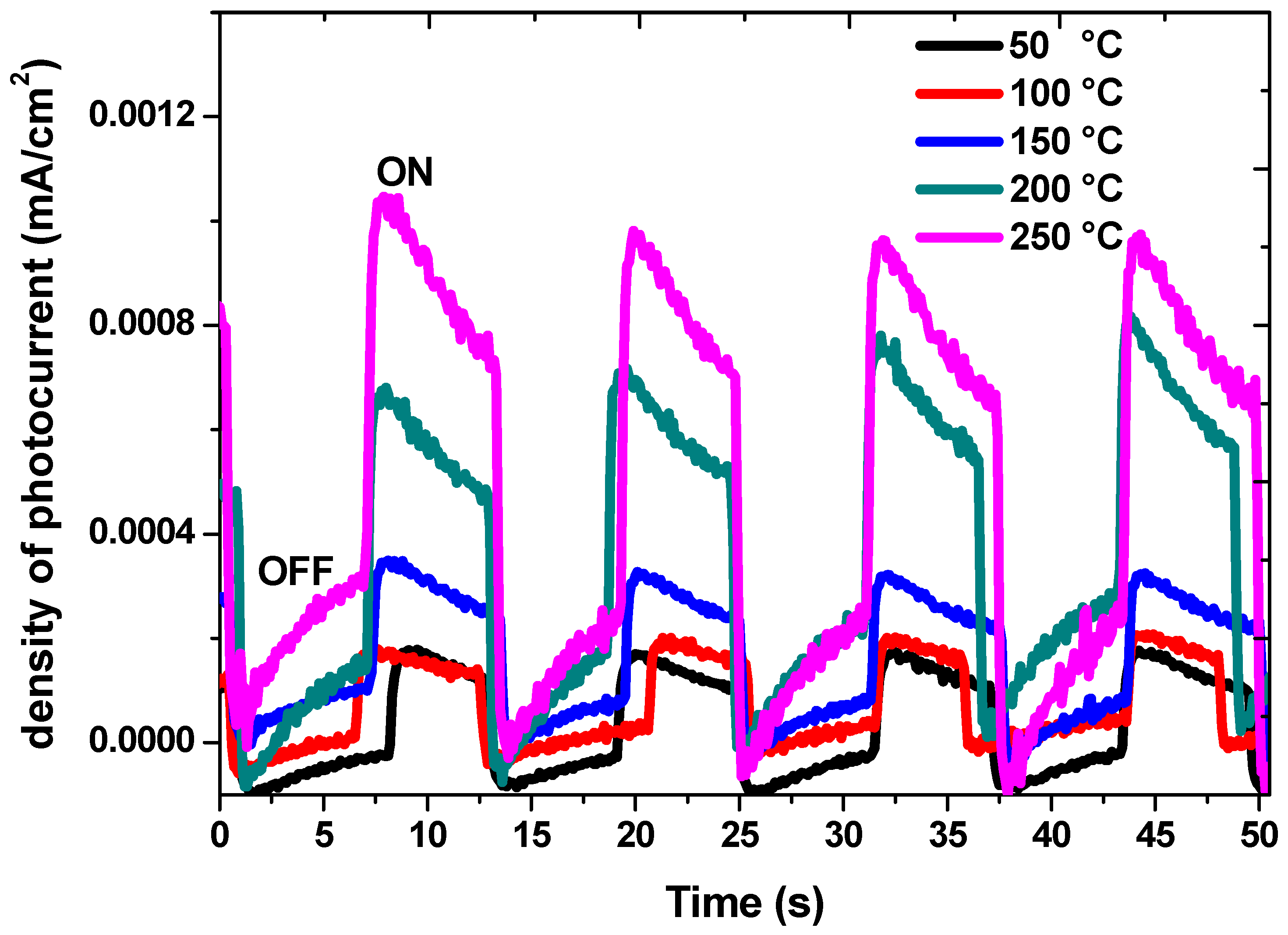
3.6. Photocurrent Measurements
3.7. Mott–Schottky and Electrochemical Impedance Spectroscopy (EIS) Analysis
3.7.1. EIS Measurements
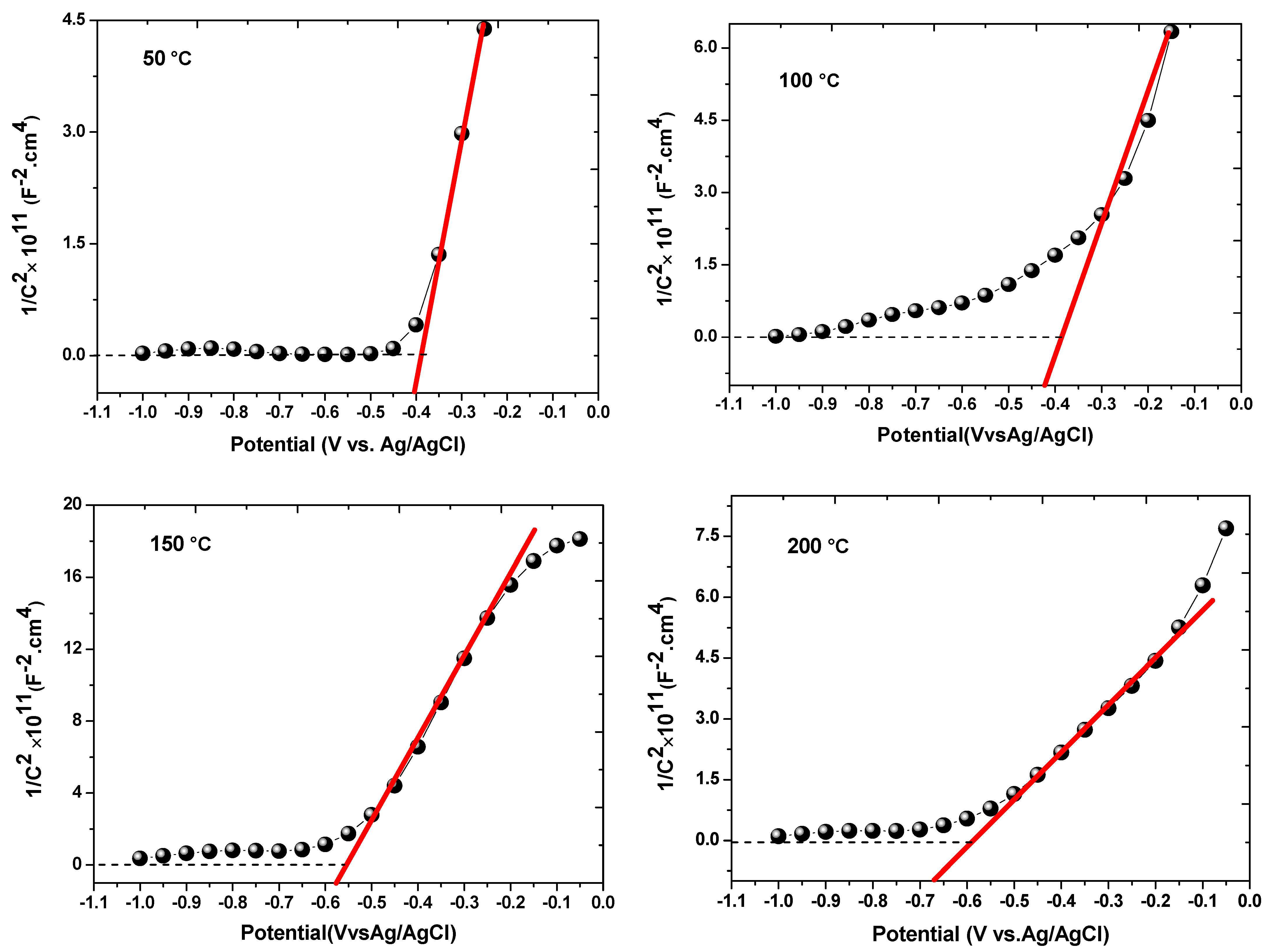
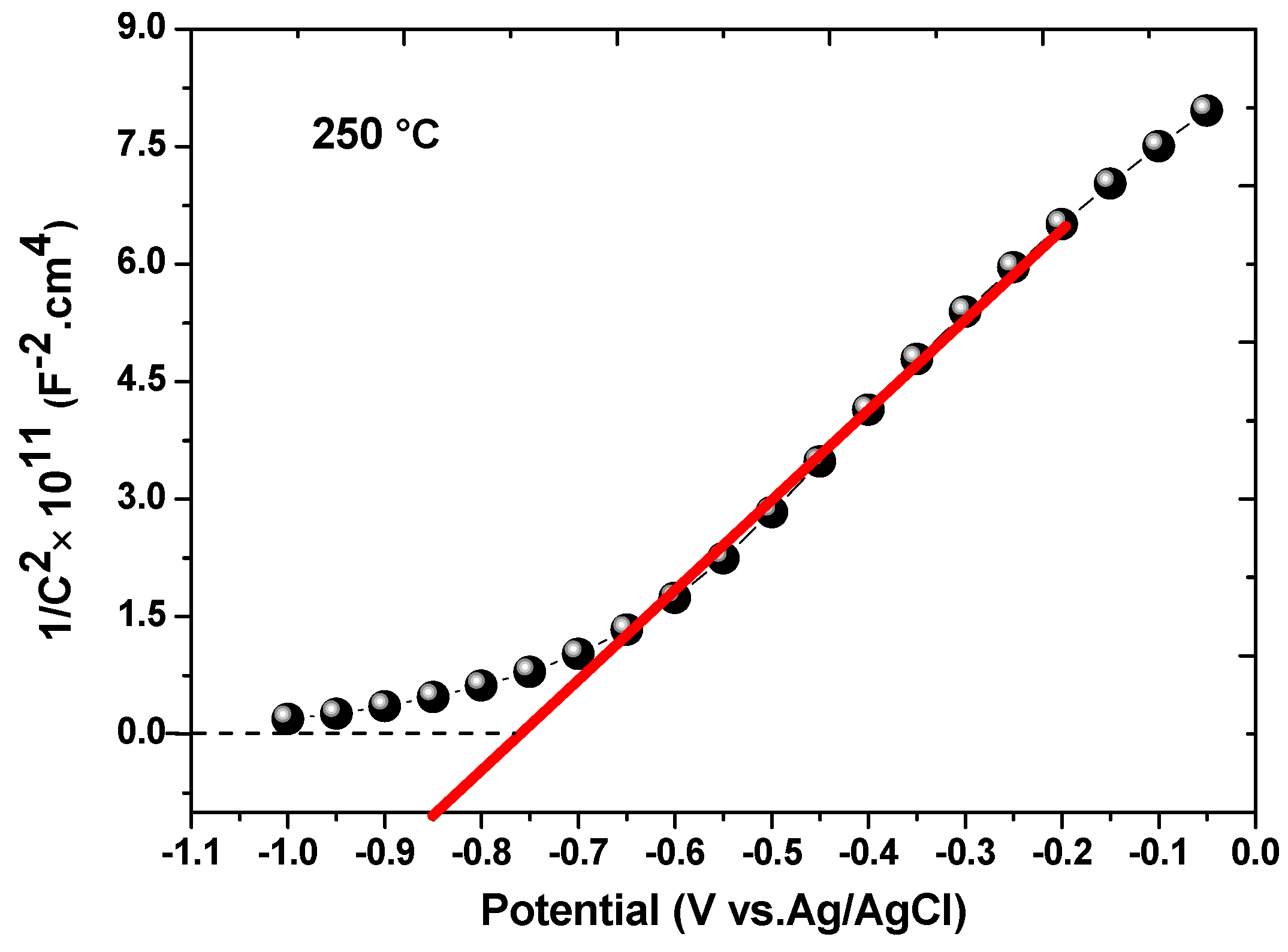
3.7.2. Mott–Schottky Measurements
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhai, T.; Fang, X.; Li, L.; Bando, Y.; Golberg, D. One-dimensional CdS nanostructures: Synthesis, properties, and applications. Nanoscale 2010, 2, 168–187. [Google Scholar] [CrossRef] [PubMed]
- Sivaraman, T.; Narasimman, V.; Nagarethinam, V.; Balu, A. Effect of chlorine doping on the structural, morphological, optical and electrical properties of spray deposited CdS thin films. Prog. Nat. Sci. Mater. Int. 2015, 25, 392–398. [Google Scholar] [CrossRef]
- Yuvaraj, S.; Fernandez, A.C.; Sundararajan, M.; Dash, C.S.; Sakthivel, P. Hydrothermal synthesis of ZnO–CdS nanocomposites: Structural, optical and electrical behavior. Ceram. Int. 2020, 46, 391–402. [Google Scholar] [CrossRef]
- Murugesan, R.; Sivakumar, S.; Karthik, K.; Anandan, P.; Haris, M. Structural, optical and magnetic behaviors of Fe/Mn-doped and co-doped CdS thin films prepared by spray pyrolysis method. Appl. Phys. A 2019, 125, 281. [Google Scholar] [CrossRef]
- Göde, F.; Ünlü, S. Synthesis and characterization of CdS window layers for PbS thin film solar cells. Mater. Sci. Semicond. Process. 2019, 90, 92–100. [Google Scholar] [CrossRef]
- Shkir, M.; Ashraf, I.; Khan, A.; Khan, M.T.; El-Toni, A.M.; AlFaify, S. A facile spray pyrolysis fabrication of Sm: CdS thin films for high-performance photodetector applications. Sens. Actuators A Phys. 2020, 306, 111952. [Google Scholar] [CrossRef]
- Wondmagegn, W.; Mejia, I.; Salas-Villasenor, A.; Stiegler, H.; Quevedo-Lopez, M.; Pieper, R.; Gnade, B. CdS thin film transistor for inverter and operational amplifier circuit applications. Microelectron. Eng. 2016, 157, 64–70. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, F.; Wang, H.; Wang, L.; Wang, F.; Lin, Q.; Shen, H.; Li, L.S. High-efficiency CdSe/CdS nanorod–based red light–emitting diodes. Opt. Express 2019, 27, 7935–7944. [Google Scholar] [CrossRef]
- Khan, M.S.; Aziz, A.; Rahman, S.A.; Khan, Z.R. Spectroscopic studies of sol–gel grown CdS nanocrystalline thin films for optoelectronic devices. Mater. Sci. Semicond. Process. 2013, 16, 1894–1898. [Google Scholar]
- Bairy, R.; Jayarama, A.; Shivakumar, G.; Kulkarni, S.D.; Maidur, S.R.; Patil, P.S. Effect of Aluminium doping on photoluminescence and third-order nonlinear optical properties of nanostructured CdS thin films for photonic device applications. Phys. B Condens. Matter 2019, 555, 145–151. [Google Scholar] [CrossRef]
- Isik, M.; Gullu, H.; Delice, S.; Parlak, M.; Gasanly, N.M. Structural and temperature-dependent optical properties of thermally evaporated CdS thin films. Mater. Sci. Semicond. Process. 2019, 93, 148–152. [Google Scholar] [CrossRef]
- El-Shaer, A.; Ezzat, S.; Habib, M.A.; Alduaij, O.K.; Meaz, T.M.; El-Attar, S.A. Influence of Deposition Time on Structural, Morphological, and Optical Properties of CdS Thin Films Grown by Low-Cost Chemical Bath Deposition. Crystals 2023, 13, 788. [Google Scholar] [CrossRef]
- Shkir, M.; Ashraf, I.; AlFaify, S.; El-Toni, A.M.; Ahmed, M.; Khan, A. A noticeable effect of Pr doping on key optoelectrical properties of CdS thin films prepared using spray pyrolysis technique for high-performance photodetector applications. Ceram. Int. 2020, 46, 4652–4663. [Google Scholar] [CrossRef]
- Petrus, R.Y.; Ilchuk, H.; Kashuba, A.; Semkiv, I.; Zmiiovska, E. Optical-energy properties of CdS thin films obtained by the method of high-frequency magnetron sputtering. Opt. Spectrosc. 2019, 126, 220–225. [Google Scholar] [CrossRef]
- Chol, K.H.; Ho, C.H.; Jo, K.Y.; Il, S.G. Effect of the reactant concentration, bath temperature and deposition time on the properties of CdS thin film prepared by the chemical bath deposition method. Opt. Mater. 2021, 112, 110790. [Google Scholar] [CrossRef]
- Dey, M.; Das, N.; Gupta, A.S.; Dey, M.; Hossain, M.; Matin, M.; Amin, N. Deposition of CdS thin film by thermal evaporation. In Proceedings of the 2019 International Conference on Electrical, Computer and Communication Engineering (ECCE), Cox’s Bazar, Bangladesh, 7–9 February 2019; pp. 1–5. [Google Scholar]
- Liu, X.; Cao, L.; Guo, Z.; Li, Y.; Gao, W.; Zhou, L. A review of perovskite photovoltaic materials’ synthesis and applications via chemical vapor deposition method. Materials 2019, 12, 3304. [Google Scholar] [CrossRef]
- Wu, H.; Zheng, Z.; Tang, Y.; Huang, N.M.; Amal, R.; Lim, H.N.; Ng, Y.H. Pulsed electrodeposition of CdS on ZnO nanorods for highly sensitive photoelectrochemical sensing of copper (II) ions. Sustain. Mater. Technol. 2018, 18, e00075. [Google Scholar] [CrossRef]
- Khan, Z.R.; Shkir, M.; Alshammari, A.S.; Ganesh, V.; AlFaify, S.; Gandouzi, M. Structural, linear and third order nonlinear optical properties of sol-gel grown Ag-CdS nanocrystalline thin films. J. Electron. Mater. 2019, 48, 1122–1132. [Google Scholar] [CrossRef]
- Nivetha, A.; Mangala Devi, S.; Prabha, I. Fascinating physic-chemical properties and resourceful applications of selected cadmium nanomaterials. J. Inorg. Organomet. Polym. Mater. 2019, 29, 1423–1438. [Google Scholar] [CrossRef]
- Dhaygude, H.; Shinde, S.; Takale, M.; Dubal, D.; Lohar, G.; Fulari, V.J. Electrodeposited nanosphere like CdxZn1−x S electrodes for photoelectrochemical cell. J. Mater. Sci. Mater. Electron. 2016, 27, 5145–5152. [Google Scholar] [CrossRef]
- Judran, H.K.; Yousif, N.A.; AL-Jawad, S.M. Preparation and characterization of CdS prepared by hydrothermal method. J. Sol-Gel Sci. Technol. 2021, 97, 48–62. [Google Scholar] [CrossRef]
- Oliva, A.; Castro-Rodrıguez, R.; Ceh, O.; Bartolo-Perez, P.; Caballero-Briones, F.; Sosa, V. First stages of growth of CdS films on different substrates. Appl. Surf. Sci. 1999, 148, 42–49. [Google Scholar] [CrossRef]
- Kozhevnikova, N.S.; Vorokh, A.S.; Uritskaya, A.A. Cadmium sulfide nanoparticles prepared by chemical bath deposition. Russ. Chem. Rev. 2015, 84, 225. [Google Scholar] [CrossRef]
- Najm, A.S.; Naeem, H.S.; Alwarid, D.A.R.M.; Aljuhani, A.; Hasbullah, S.A.; Hasan, H.A.; Sopian, K.; Bais, B.; Al-Iessa, H.J.; Majdi, H.S.; et al. Mechanism of Chemical Bath Deposition of CdS Thin Films: Influence of Sulphur Precursor Concentration on Microstructural and Optoelectronic Characterizations. Coatings 2022, 12, 1400. [Google Scholar] [CrossRef]
- Peng, S.; Zhu, P.; Thavasi, V.; Mhaisalkar, S.G.; Ramakrishna, S. Facile solution deposition of ZnIn2S4 nanosheet films on FTO substrates for photoelectric application. Nanoscale 2011, 3, 2602–2608. [Google Scholar] [CrossRef]
- Ali, A.; Chiang, Y.W.; Santos, R.M. X-ray diffraction techniques for mineral characterization: A review for engineers of the fundamentals, applications, and research directions. Minerals 2022, 12, 205. [Google Scholar] [CrossRef]
- Das, N.; Chakrabartty, J.; Farhad, S.; Gupta, A.S.; Ahamed, E.I.; Rahman, K.; Wafi, A.; Alkahtani, A.; Matin, M.; Amin, N. Effect of substrate temperature on the properties of RF sputtered CdS thin films for solar cell applications. Results Phys. 2020, 17, 103132. [Google Scholar] [CrossRef]
- Islam, M.; Hossain, M.; Aliyu, M.; Chelvanathan, P.; Huda, Q.; Karim, M.; Sopian, K.; Amin, N. Comparison of structural and optical properties of CdS thin films grown by CSVT, CBD and sputtering techniques. Energy Procedia 2013, 33, 203–213. [Google Scholar] [CrossRef]
- Begum, A.; Hussain, A.; Rahman, A. Effect of deposition temperature on the structural and optical properties of chemically prepared nanocrystalline lead selenide thin films. Beilstein J. Nanotechnol. 2012, 3, 438–443. [Google Scholar] [CrossRef]
- Hone, F.G.; Ampong, F.K. Effect of deposition temperature on the structural, morphological and optical band gap of lead selenide thin films synthesized by chemical bath deposition method. Mater. Chem. Phys. 2016, 183, 320–325. [Google Scholar] [CrossRef]
- Lu, X.; Liu, Z.; Zhao, X.; Xu, W.; Hou, H.; Qian, J. CdS Nanoparticles Decorated 1D CeO2 Nanorods for Enhanced Photocatalytic Desulfurization Performance. Catalysts 2022, 12, 1478. [Google Scholar] [CrossRef]
- Diwate, K.; Pawbake, A.; Rondiya, S.; Kulkarni, R.; Waykar, R.; Jadhavar, A.; Rokade, A.; Funde, A.; Mohite, K.; Shinde, M. Substrate temperature dependent studies on properties of chemical spray pyrolysis deposited CdS thin films for solar cell applications. J. Semicond. 2017, 38, 23001. [Google Scholar] [CrossRef]
- Saleem, M.F.; Zhang, H.; Deng, Y.; Wang, D. Resonant Raman scattering in nanocrystalline thin CdS film. J. Nanosci. Nanotechnol. 2017, 48, 224–229. [Google Scholar] [CrossRef]
- Rengaraj, S.; Jee, S.H.; Venkataraj, S.; Kim, Y.; Vijayalakshmi, S.; Repo, E.; Koistinen, A.; Sillanpää, M. CdS microspheres composed of nanocrystals and their photocatalytic activity. J. Nanosci. Nanotechnol. 2011, 11, 2090–2099. [Google Scholar] [CrossRef] [PubMed]
- Habte, A.G.; Hone, F.G.; Dejene, F. The influence of malonic acid on the structural, morphological and optical properties of CdSe thin films prepared by chemical bath deposition method. Inorg. Chem. Commun. 2019, 103, 107–112. [Google Scholar] [CrossRef]
- Khimani, A.J.; Chaki, S.H.; Malek, T.J.; Tailor, J.P.; Chauhan, S.M.; Deshpande, M. Cadmium sulphide (CdS) thin films deposited by chemical bath deposition (CBD) and dip coating techniques—A comparative study. Mater. Res. Express 2018, 5, 36406. [Google Scholar] [CrossRef]
- Wilson, K.; Ahamed, M.B. Influence of bath temperature on surface modification and optoelectronic properties of chemical bath deposited CdS thin film nanostructures. Mater. Sci. Eng. B 2019, 251, 114444. [Google Scholar] [CrossRef]
- Kalsi, T.; Godara, S.K.; Medwal, R.; Kumar, P. Dopant mediated augmentation of nanotwinning and anomalous emission behaviour. J. Lumin. 2023, 255, 119544. [Google Scholar] [CrossRef]
- Saxena, N.; Kalsi, T.; Uttam, P.; Kumar, P. Morphological evolution in nanocrystalline CdS thin films from flowers to salt rock like structures. Opt. Mater. 2018, 84, 625–630. [Google Scholar] [CrossRef]
- Devi, M.D.; Juliet, A.V.; Hari Prasad, K.; Alshahrani, T.; Alshehri, A.; Shkir, M.; AIFaify, S. An effect of precursor concentrations on the photodetection capabilities of CdS thin films for high-efficiency visible-light photodetector applications. Appl. Phys. A 2020, 126, 960. [Google Scholar] [CrossRef]
- Kumar, P.; Saxena, N.; Chandra, R.; Gupta, V.; Agarwal, A.; Kanjilal, D. Nanotwinning and structural phase transition in CdS quantum dots. Nanoscale Res. Lett. 2012, 7, 584. [Google Scholar] [CrossRef] [PubMed]
- Shkir, M.; Khan, Z.; Alshammari, A.S.; Gandouzi, M.; Ashraf, I.; AlFaify, S. A comprehensive experimental investigation of La@ CdS nanostructured thin films: Structural, opto-nonlinear and photodetection properties. Surf. Interfaces 2021, 24, 101063. [Google Scholar] [CrossRef]
- Shkir, M.; Anis, M.; Shafik, S.; Manthrammel, M.A.; Sayeed, M.; Hamdy, M.S.; AlFaify, S. An effect of Zn content doping on opto-third order nonlinear characteristics of nanostructured CdS thin films fabricated through spray pyrolysis for optoelectronics. Phys. E Low-Dimens. Syst. Nanostruct. 2020, 118, 113955. [Google Scholar] [CrossRef]
- Toma, O.; Ion, L.; Iftimie, S.; Radu, A.; Antohe, S. Structural, morphological and optical properties of rf–Sputtered CdS thin films. Mater. Des. 2016, 100, 198–203. [Google Scholar] [CrossRef]
- Bouachri, M.; Oubakalla, M.; El Farri, H.; Díaz-Guerra, C.; Mhalla, J.; Zimou, J.; El-Habib, A.; Beraich, M.; Nouneh, K.; Fahoume, M. Substrate temperature effects on the structural, morphological and optical properties of Bi2S3 thin films deposited by spray pyrolysis: An experimental and first-principles study. Opt. Mater. 2023, 135, 113215. [Google Scholar] [CrossRef]
- Wang, P.; Wu, H.; Tang, Y.; Amal, R.; Ng, Y.H. Electrodeposited Cu2O as Photoelectrodes with Controllable Conductivity Type for Solar Energy Conversion. J. Phys. Chem. C 2015, 119, 26275–26282. [Google Scholar] [CrossRef]
- Sakai, Y.; Okimura, H. Properties of photoconductive CdS evaporated films. Jpn. J. Appl. Phys. 1964, 3, 144. [Google Scholar] [CrossRef]
- Yu, X.; Du, R.; Li, B.; Zhang, Y.; Liu, H.; Qu, J.; An, X. Biomolecule-assisted self-assembly of CdS/MoS2/graphene hollow spheres as high-efficiency photocatalysts for hydrogen evolution without noble metals. Appl. Catal. B Environ. 2016, 182, 504–512. [Google Scholar] [CrossRef]
- Xu, H.; Liu, S.; Guo, L.; Li, Y.; Shen, K.; Guan, C.; Wang, W. Comparing Electrochemical Behaviour of Amorphous Ni−P Powders and Film. Int. J. Electrochem. Sci. 2015, 10, 4985–5000. [Google Scholar]
- Abdelfatah, M.; Darwesh, N.; Habib, M.A.; Alduaij, O.K.; El-Shaer, A.; Ismail, W. Enhancement of Structural, Optical and Photoelectrochemical Properties of n−Cu2O Thin Films with K Ions Doping toward Biosensor and Solar Cell Applications. Nanomaterials 2023, 13, 1272. [Google Scholar] [CrossRef]
- Bouznit, Y.; Henni, A. Characterization of Sb doped SnO2 films prepared by spray technique and their application to photocurrent generation. Mater. Chem. Phys. 2019, 233, 242–248. [Google Scholar] [CrossRef]
- Nong, J.; Lan, G.; Jin, W.; Luo, P.; Guo, C.; Tang, X.; Zang, Z.; Wei, W. Eco-friendly and high-performance photoelectrochemical anode based on AgInS 2 quantum dots embedded in 3D graphene nanowalls. J. Mater. Chem. C 2019, 7, 9830–9839. [Google Scholar] [CrossRef]
- Gonçalves, B.S.; Palhares, H.G.; Souza, T.C.C.d.; Castro, V.G.d.; Silva, G.G.; Silva, B.C.; Krambrock, K.; Soares, R.B.; Lins, V.F.C.; Houmard, M.; et al. Effect of the carbon loading on the structural and photocatalytic properties of reduced graphene oxide-TiO2 nanocomposites prepared by hydrothermal synthesis. J. Mater. Res. Technol. 2019, 8, 6262–6274. [Google Scholar] [CrossRef]
- Tiwari, S.; Jhamb, N.; Kumar, S.; Ganguli, A.K. Synthesis of Photocorrosion-Resistant VS4-MoS2-rGO Based Nanocomposite with Efficient Photoelectrochemical Water-Splitting Activity. ChemNanoMat 2022, 8, e202100429. [Google Scholar] [CrossRef]
- Gajanayake, G.; De Silva, D.; Atapattu, H. Altering NH4OH concentration in producing chemical bath deposited CdS to steadily support electrodeposited CdTe. Mater. Sci. Eng. B 2021, 265, 114952. [Google Scholar] [CrossRef]
- El-rouby, M.; Aliyev, A.S. Electrical, electrochemical and photo-electrochemical studies on the electrodeposited n-type semiconductor hexagonal crystalline CdS thin film on nickel substrate. J. Mater. Sci. Mater. Electron. 2014, 25, 5618–5629. [Google Scholar] [CrossRef]
- Han, Z.; He, C.; Lian, J.; Zhao, Y.; Chen, X. Effects of temperature on corrosion behaviour of 2205 duplex stainless steel in carbon dioxide containing environments. Int. J. Electrochem. Sci. 2020, 73, 3627–3645. [Google Scholar] [CrossRef]
- Li, Q.; Qiao, X.-Q.; Jia, Y.; Hou, D.; Li, D.-S. Noble-metal-free amorphous CoMoSx modified CdS core-shell nanowires for dramatically enhanced photocatalytic hydrogen evolution under visible light irradiation. Appl. Surf. Sci. 2019, 498, 143863. [Google Scholar] [CrossRef]
- Barma, S.V.; Rondiya, S.R.; Jadhav, Y.A.; Jathar, S.B.; Rahane, G.K.; Rokade, A.; Cross, R.W.; Nasane, M.P.; Jadkar, V.; Dzade, N.Y.; et al. Structural, optoelectronic, and photoelectrochemical investigation of CdSe NC’s prepared by hot injection method. ES Mater. Manuf. 2020, 11, 50–56. [Google Scholar] [CrossRef]
- Sivula, K. Mott–Schottky Analysis of Photoelectrodes: Sanity Checks Are Needed. ACS Energy Lett. 2021, 6, 2549–2551. [Google Scholar] [CrossRef]
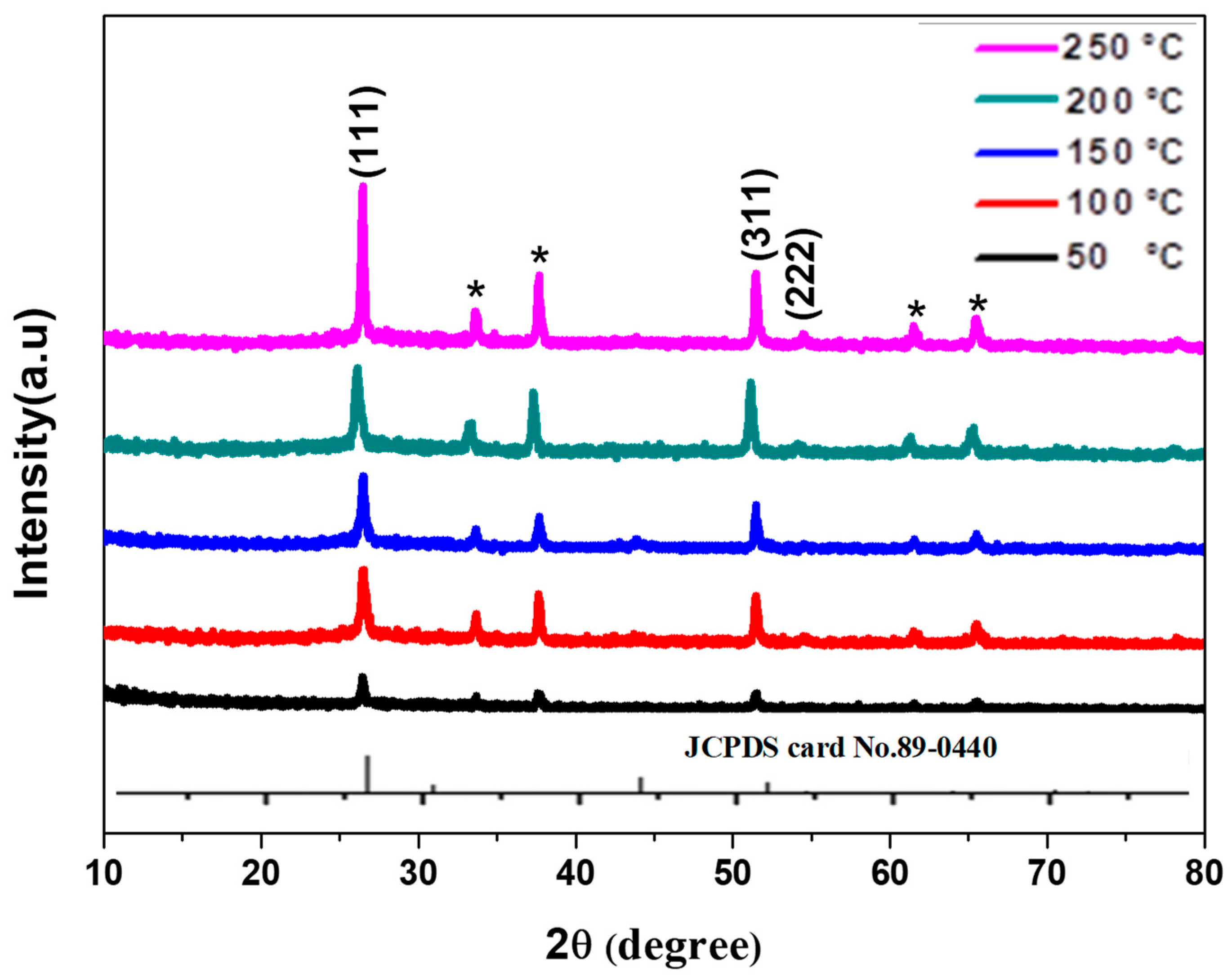
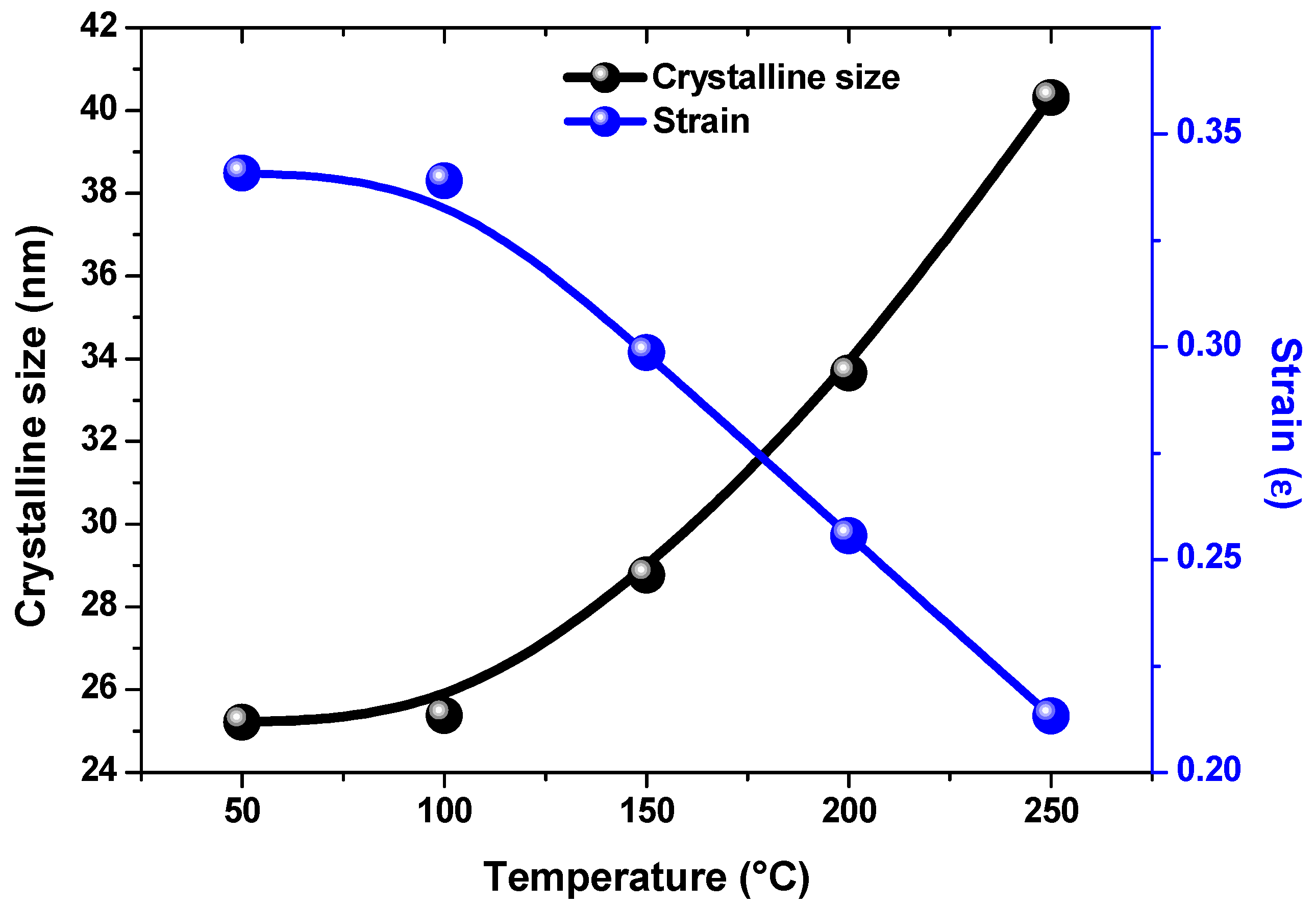





| Temperature (°C) | 2θ(Degree) | FWHM (Degree) | Crystallite Size (D) (nm) | Micro Strain (ε) Lines−2 m4 | Dislocation Density (δ) ×10−4 | Energy Band Gap E(g) (eV) |
|---|---|---|---|---|---|---|
| 50 | 26.48 | 0.32 | 25.21 | 0.34 | 15.73 | 2.50 |
| 100 | 26.45 | 0.31 | 25.37 | 0.34 | 15.53 | 2.49 |
| 150 | 26.48 | 0.28 | 29.77 | 0.30 | 12.08 | 2.45 |
| 200 | 26.45 | 0.24 | 33.64 | 0.26 | 8.83 | 2.40 |
| 250 | 26.46 | 0.20 | 40.31 | 0.21 | 6.15 | 2.39 |
| Temperature (°C) | Donner Density (ND) (cm−3) | Vfb (V vs. Ag/AgCl) |
|---|---|---|
| 50 | 4.41 × 1018 | −0.39 |
| 100 | 6.34 × 1018 | −0.40 |
| 150 | 7.18 × 1018 | −0.55 |
| 200 | 13.7 × 1018 | −0.59 |
| 250 | 15.8 × 1018 | −0.76 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ismail, W.; Ibrahim, G.; Habib, M.A.; Alduaij, O.K.; Abdelfatah, M.; El-Shaer, A. Advancement of Physical and Photoelectrochemical Properties of Nanostructured CdS Thin Films toward Optoelectronic Applications. Nanomaterials 2023, 13, 1764. https://doi.org/10.3390/nano13111764
Ismail W, Ibrahim G, Habib MA, Alduaij OK, Abdelfatah M, El-Shaer A. Advancement of Physical and Photoelectrochemical Properties of Nanostructured CdS Thin Films toward Optoelectronic Applications. Nanomaterials. 2023; 13(11):1764. https://doi.org/10.3390/nano13111764
Chicago/Turabian StyleIsmail, Walid, Ghada Ibrahim, Mohamed A. Habib, Omar K. Alduaij, Mahmoud Abdelfatah, and Abdelhamid El-Shaer. 2023. "Advancement of Physical and Photoelectrochemical Properties of Nanostructured CdS Thin Films toward Optoelectronic Applications" Nanomaterials 13, no. 11: 1764. https://doi.org/10.3390/nano13111764





