Influence of Thermal and Flash-Lamp Annealing on the Thermoelectrical Properties of Cu2ZnSnS4 Nanocrystals Obtained by “Green” Colloidal Synthesis
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
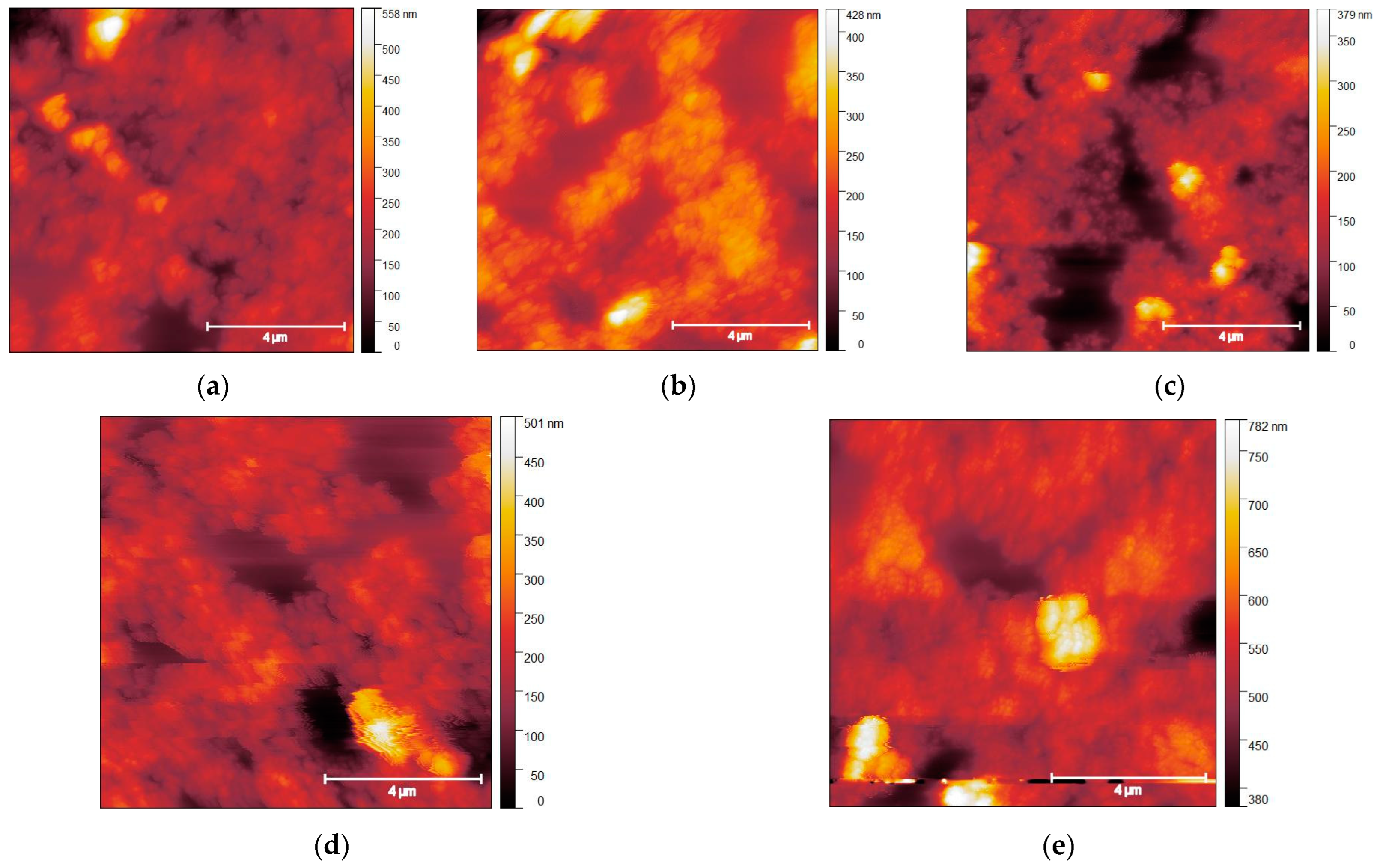
3.1. Surface Morphology
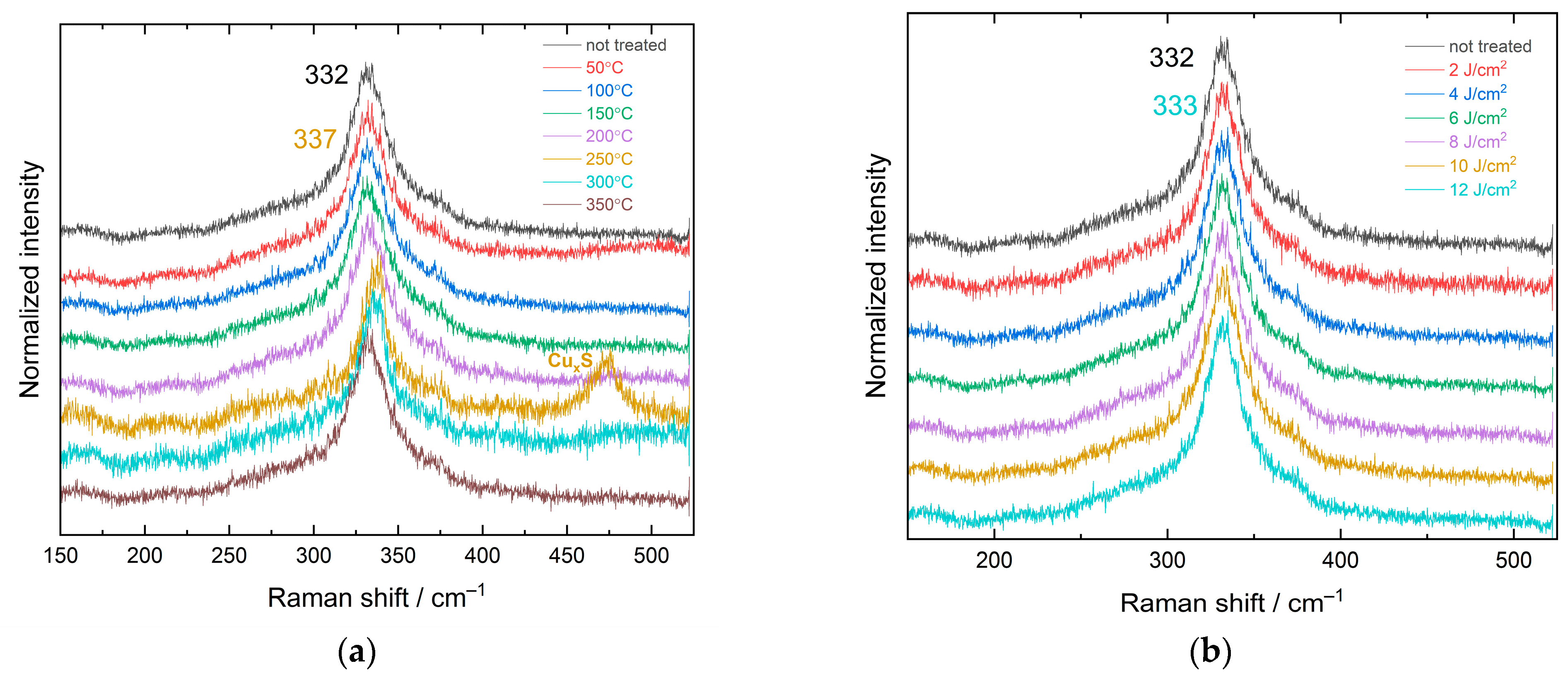
3.2. Raman Study
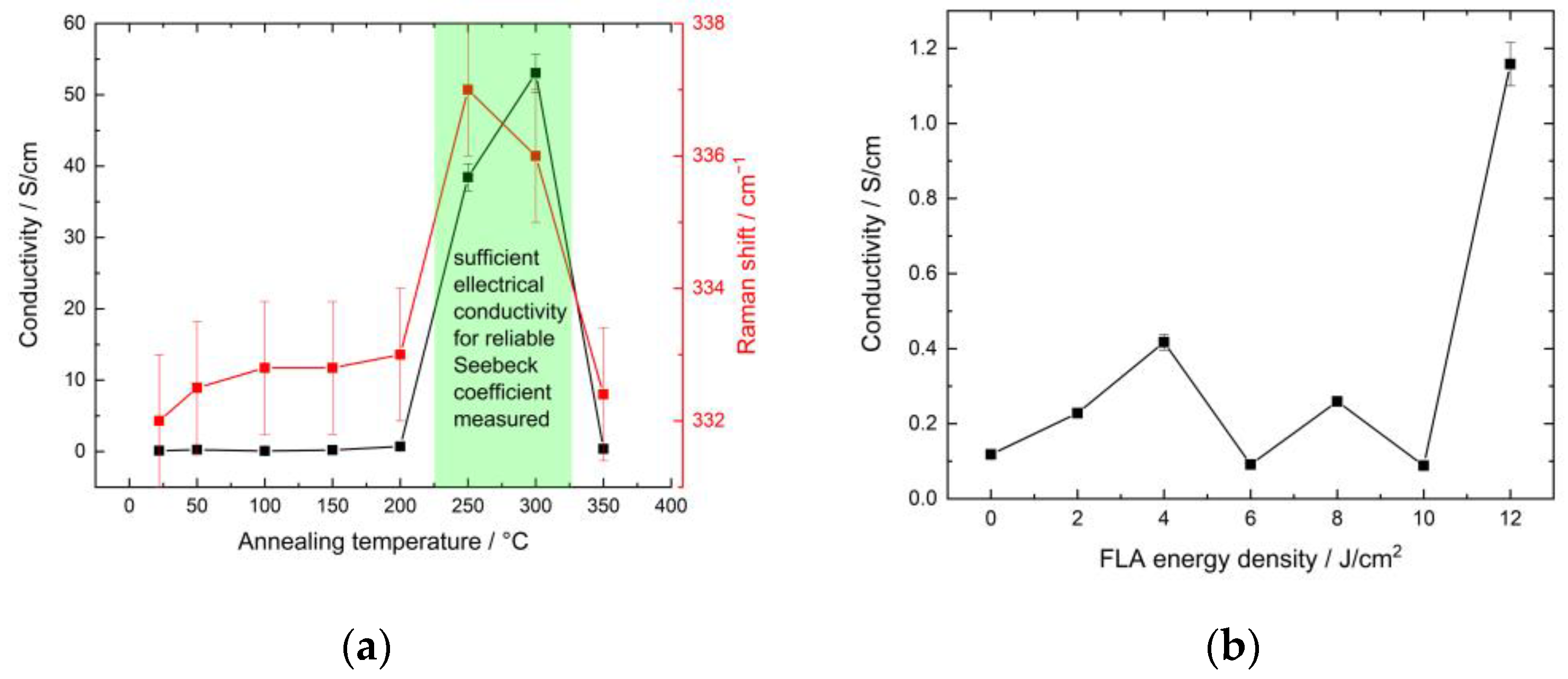
3.3. Thermoelectrical Characterization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, F.; Wang, Z.; Zhu, H.; Pellet, N.; Luo, J.; Yi, C.; Liu, X.; Liu, H.; Wang, S.; Li, X.; et al. Over 20% PCE Perovskite Solar Cells with Superior Stability Achieved by Novel and Low-Cost Hole-Transporting Materials. Nano Energy 2017, 41, 469–475. [Google Scholar] [CrossRef]
- Unold, T. Accelerating Research on Novel Photovoltaic Materials. Faraday Discuss. 2022, 239, 235–249. [Google Scholar] [CrossRef]
- Hashemi, S.A.; Ramakrishna, S.; Aberle, A.G. Recent Progress in Flexible–Wearable Solar Cells for Self-Powered Electronic Devices. Energy Environ. Sci. 2020, 13, 685–743. [Google Scholar] [CrossRef]
- Husain, A.A.F.; Hasan, W.Z.W.; Shafie, S.; Hamidon, M.N.; Pandey, S.S. A Review of Transparent Solar Photovoltaic Technologies. Renew. Sustain. Energy Rev. 2018, 94, 779–791. [Google Scholar] [CrossRef]
- Simon, M.; Meyer, E.L. Detection and Analysis of Hot-Spot Formation in Solar Cells. Sol. Energy Mater. Sol. Cells 2010, 94, 106–113. [Google Scholar] [CrossRef]
- Chow, T.T. A Review on Photovoltaic/Thermal Hybrid Solar Technology. Appl. Energy 2010, 87, 365–379. [Google Scholar] [CrossRef]
- Tripanagnostopoulos, Y.; Nousia, T.H.; Souliotis, M.; Yianoulis, P. Hybrid Photovoltaic/Thermal Solar Systems. Sol. Energy 2002, 72, 217–234. [Google Scholar] [CrossRef]
- Ashok Kumar, L.; Indragandhi, V.; Teekaraman, Y.; Kuppusamy, R.; Radhakrishnan, A. Design and Implementation of Automatic Water Spraying System for Solar Photovoltaic Module. Math. Probl. Eng. 2022, 2022, 1–11. [Google Scholar] [CrossRef]
- Sahin, A.Z.; Ismaila, K.G.; Yilbas, B.S.; Al-Sharafi, A. A Review on the Performance of Photovoltaic/Thermoelectric Hybrid Generators. Int. J. Energy Res. 2020, 44, 3365–3394. [Google Scholar] [CrossRef]
- Deng, Y.; Zhu, W.; Wang, Y.; Shi, Y. Enhanced Performance of Solar-Driven Photovoltaic-Thermoelectric Hybrid System in an Integrated Design. Sol. Energy 2013, 88, 182–191. [Google Scholar] [CrossRef]
- Wang, N.; Han, L.; He, H.; Park, N.H.; Koumoto, K. A Novel High-Performance Photovoltaic-Thermoelectric Hybrid Device. Energy Environ. Sci. 2011, 4, 3676–3679. [Google Scholar] [CrossRef]
- Xu, L.; Xiong, Y.; Mei, A.; Hu, Y.; Rong, Y.; Zhou, Y.; Hu, B.; Han, H. Efficient Perovskite Photovoltaic-Thermoelectric Hybrid Device. Adv. Energy Mater. 2018, 8, 1702937. [Google Scholar] [CrossRef]
- Kraemer, D.; Hu, L.; Muto, A.; Chen, X.; Chen, G.; Chiesa, M. Photovoltaic-Thermoelectric Hybrid Systems: A General Optimization Methodology. Appl. Phys. Lett. 2008, 92, 243503. [Google Scholar] [CrossRef]
- Wei, J.; Yang, L.; Ma, Z.; Song, P.; Zhang, M.; Ma, J.; Yang, F.; Wang, X. Review of Current High-ZT Thermoelectric Materials. J. Mater. Sci. 2020, 55, 12642–12704. [Google Scholar] [CrossRef]
- Stroyuk, O.; Raevskaya, A.; Gaponik, N. Solar Light Harvesting with Multinary Metal Chalcogenide Nanocrystals. Chem. Soc. Rev. 2018, 47, 5354–5422. [Google Scholar] [CrossRef] [PubMed]
- Giraldo, S.; Jehl, Z.; Placidi, M.; Izquierdo-Roca, V.; Pérez-Rodríguez, A.; Saucedo, E. Progress and Perspectives of Thin Film Kesterite Photovoltaic Technology: A Critical Review. Adv. Mater. 2019, 31, 1806692. [Google Scholar] [CrossRef]
- Akcay, N.; Zaretskaya, E.P.; Ozcelik, S. Development of a CZTS Solar Cell with CdS Buffer Layer Deposited by RF Magnetron Sputtering. J. Alloys Compd. 2019, 772, 782–792. [Google Scholar] [CrossRef]
- Akcay, N.; Baskose, U.C.; Ozcelik, S.; Gremenok, V.F.; Zaretskaya, E.P. Na Incorporation into Cu2ZnSnS4 Thin Film Absorbers from RF-Sputtered NaF Precursors. Sol. Energy 2021, 217, 280–291. [Google Scholar] [CrossRef]
- Islam, M.F.; Yatim, N.M.; Hashim, M.A. A Review of CZTS Thin Film Solar Cell Technology. J. Adv. Res. Fluid Mech. Therm. Sci. 2021, 81, 73–87. [Google Scholar] [CrossRef]
- Akhavan, V.A.; Goodfellow, B.W.; Panthani, M.G.; Steinhagen, C.; Harvey, T.B.; Stolle, C.J.; Korgel, B.A. Colloidal CIGS and CZTS Nanocrystals: A Precursor Route to Printed Photovoltaics. J. Solid State Chem. 2012, 189, 2–12. [Google Scholar] [CrossRef]
- Isotta, E.; Pugno, N.M.; Scardi, P. Nanostructured Kesterite (Cu2ZnSnS4) for Applications in Thermoelectric Devices. Powder Diffr. 2019, 34, S42–S47. [Google Scholar] [CrossRef]
- Ali, A.; Jacob, J.; Arshad, M.I.; Ajaz un Nabi, M.; Ashfaq, A.; Mahmood, K.; Amin, N.; Ikram, S.; Rehman, U.; Hussain, S.; et al. Enhancement of Thermoelectric Properties of Sulphurized CZTS Nano-Crystals by the Engineering of Secondary Phases. Solid State Sci. 2020, 103, 106198. [Google Scholar] [CrossRef]
- Ali, A.; Jacob, J.; Ashfaq, A.; Mahmood, K.; Ahmad, S.; Rehman, U.; Ahmad, W.; Amin, N.; Ikram, S.; Hussain, S.; et al. Effect of Tin Concentration on the Structural, Optical and Thermoelectric Properties of CZTS Thin Films Gown by Chemical Solution Method. Ceram. Int. 2019, 45, 22513–22516. [Google Scholar] [CrossRef]
- Kumar, S.; Ansari, M.Z.; Khare, N. Influence of Compactness and Formation of Metallic Secondary Phase on the Thermoelectric Properties of Cu2ZnSnS4 Thin Films. Thin Solid Films 2018, 645, 300–304. [Google Scholar] [CrossRef]
- Khottummee, N.; Sumphao, T.; Samapisut, S.; Seetawan, T. Seebeck Coefficient of Cu2ZnSnS4 Film Synthesized by Sol-Gel Process. Mater. Today Proc. 2018, 5, 14135–14139. [Google Scholar] [CrossRef]
- Nagaoka, A.; Masuda, T.; Yasui, S.; Taniyama, T.; Nose, Y. The Single-Crystal Multinary Compound Cu2ZnSnS4 as an Environmentally Friendly High-Performance Thermoelectric Material. Appl. Phys. Express 2018, 11, 051203. [Google Scholar] [CrossRef]
- Ashfaq, A.; Jacon, J.; Ali, A.; Mehboob, K.; Mahmood, K.; Rehman, U.; Ahmad, W.; Ikram, S.; Amin, N.; Tahir, S.; et al. Designing of Seebeck Coefficient and Electrical Conductivity in CZTS Thin Films for Giant Power Factor. Ceram. Int. 2020, 46, 9646–9655. [Google Scholar] [CrossRef]
- Sharma, S.D.; Khasimsaheb, B.; Chen, Y.Y.; Neeleshwar, S. Enhanced Thermoelectric Performance of Cu2ZnSnS4 (CZTS) by Incorporating Ag Nanoparticles. Ceram. Int. 2019, 45, 2060–2068. [Google Scholar] [CrossRef]
- Yang, H.; Jauregui, L.A.; Zhang, G.; Chen, Y.P.; Wu, Y. Nontoxic and Abundant Copper Zinc Tin Sulfide Nanocrystals for Potential High-Temperature Thermoelectric Energy Harvesting. Nano Lett. 2012, 12, 540–545. [Google Scholar] [CrossRef]
- Isotta, E.; Andrade-Arvizu, J.; Syafiq, U.; Jiménez-Arguijo, A.; Navarro-Güell, A.; Guc, M.; Saucedo, E.; Scardi, P. Towards Low Cost and Sustainable Thin Film Thermoelectric Devices Based on Quaternary Chalcogenides. Adv. Funct. Mater. 2022, 32, 2202157. [Google Scholar] [CrossRef]
- Isotta, E.; Syafiq, U.; Ataollahi, N.; Chiappini, A.; Malerba, C.; Luong, S.; Trifiletti, V.; Fenwick, O.; Pugno, N.M.; Scardi, P. Thermoelectric Properties of CZTS Thin Films: Effect of Cu-Zn Disorder. Phys. Chem. Chem. Phys. 2021, 23, 13148–13158. [Google Scholar] [CrossRef] [PubMed]
- Isotta, E.; Fanciulli, C.; Pugno, N.M.; Scardi, P. Effect of the Order-Disorder Transition on the Seebeck Coefficient of Nanostructured Thermoelectric Cu2ZnSnS4. Nanomaterials 2019, 9, 762. [Google Scholar] [CrossRef] [PubMed]
- Isotta, E.; Mukherjee, B.; Fanciulli, C.; Ataollahi, N.; Sergueev, I.; Stankov, S.; Edla, R.; Pugno, N.M.; Scardi, P. Origin of a Simultaneous Suppression of Thermal Conductivity and Increase of Electrical Conductivity and Seebeck Coefficient in Disordered Cubic Cu2ZnSnS4. Phys. Rev. Appl. 2020, 14, 064073. [Google Scholar] [CrossRef]
- Scragg, J.J.S.; Choubrac, L.; Lafond, A.; Ericson, T.; Platzer-Björkman, C. A Low-Temperature Order-Disorder Transition in Cu2ZnSnS4 Thin Films. Appl. Phys. Lett. 2014, 104, 041911. [Google Scholar] [CrossRef]
- Dzhagan, V.; Selyshchev, O.; Kondratenko, S.; Mazur, N.; Havryliuk, Y.; Raievska, O.; Stroyuk, O.; Zahn, D.R.T. Copper-Content Dependent Structural and Electrical Properties of CZTS Films Formed by “Green” Colloidal Nanocrystals. Electron. Mater. 2022, 3, 136–153. [Google Scholar] [CrossRef]
- Druffel, T.; Dharmadasa, R.; Lavery, B.W.; Ankireddy, K. Intense Pulsed Light Processing for Photovoltaic Manufacturing. Sol. Energy Mater. Sol. Cells 2018, 174, 359–369. [Google Scholar] [CrossRef]
- Singh, M.; Rana, S.; Agarwal, S. Light Induced Morphological Reforms in Thin Film of Advanced Nano-Materials for Energy Generation: A Review. Opt. Laser Technol. 2020, 129, 106284. [Google Scholar] [CrossRef]
- Williams, B.A.; Smeaton, M.A.; Holgate, C.S.; Trejo, N.D.; Francis, L.F.; Aydil, E.S. Intense Pulsed Light Annealing of Copper Zinc Tin Sulfide Nanocrystal Coatings. J. Vac. Sci. Technol. A Vac. Surf. Film. 2016, 34, 051204. [Google Scholar] [CrossRef]
- McMahon, R.A.; Smith, M.P.; Seffen, K.A.; Voelskow, M.; Anwand, W.; Skorupa, W. Flash-Lamp Annealing of Semiconductor Materials—Applications and Process Models. Vacuum 2007, 81, 1301–1305. [Google Scholar] [CrossRef]
- Cao, V.M.H.; Bae, J.; Shim, J.; Hong, B.; Jee, H.; Lee, J. Fabrication of the Cu2ZnSnS4 Thin Film Solar Cell via a Photo-Sintering Technique. Appl. Sci. 2021, 12, 38. [Google Scholar] [CrossRef]
- Havryliuk, Y.; Selyshchev, O.; Valakh, M.; Raevskaya, A.; Stroyuk, O.; Schmidt, C.; Dzhagan, V.; Zahn, D.R.T. Raman Study of Flash-Lamp Annealed Aqueous Cu2ZnSnS4 Nanocrystals. Beilstein J. Nanotechnol. 2019, 10, 222–227. [Google Scholar] [CrossRef]
- Dimitrievska, M.; Fairbrother, A.; Fontané, X.; Jawhari, T.; Izquierdo-Roca, V.; Saucedo, E.; Pérez-Rodríguez, A. Multiwavelength Excitation Raman Scattering Study of Polycrystalline Kesterite Cu2ZnSnS4 Thin Films. Appl. Phys. Lett. 2014, 104, 021901. [Google Scholar] [CrossRef]
- Dimitrievska, M.; Boero, F.; Litvinchuk, A.P.; Delsante, S.; Borzone, G.; Perez-Rodriguez, A.; Izquierdo-Roca, V. Structural Polymorphism in “Kesterite” Cu2ZnSnS4: Raman Spectroscopy and First-Principles Calculations Analysis. Inorg. Chem. 2017, 56, 3467–3474. [Google Scholar] [CrossRef] [PubMed]
- Dumcenco, D.; Huang, Y.S. The Vibrational Properties Study of Kesterite Cu2ZnSnS4 Single Crystals by Using Polarization Dependent Raman Spectroscopy. Opt. Mater. 2013, 35, 419–425. [Google Scholar] [CrossRef]
- Guc, M.; Levcenko, S.; Bodnar, I.V.; Izquierdo-Roca, V.; Fontane, X.; Volkova, L.V.; Arushanov, E.; Pérez-Rodríguez, A. Polarized Raman Scattering Study of Kesterite Type Cu2ZnSnS4 Single Crystals. Sci. Rep. 2016, 6, 19414. [Google Scholar] [CrossRef]
- Fan, F.J.; Wu, L.; Yu, S.H. Energetic I-III-VI2 and I2-II-IV-VI4 Nanocrystals: Synthesis, Photovoltaic and Thermoelectric Applications. Energy Environ. Sci. 2014, 7, 190–208. [Google Scholar] [CrossRef]
- Havryliuk, Y.; Dzhagan, V.; Karnaukhov, A.; Selyshchev, O.; Hann, J.; Zahn, D.R.T. Raman Spectroscopy and Thermoelectric Characterization of Composite Thin Films of Cu2ZnSnS4 Nanocrystals Embedded in a Conductive Polymer PEDOT:PSS. Nanomaterials 2022, 13, 41. [Google Scholar] [CrossRef]
- Stroyuk, O.; Raevskaya, A.; Selyshchev, O.; Dzhagan, V.; Gaponik, N.; Zahn, D.R.T.; Eychmüller, A. “Green” Aqueous Synthesis and Advanced Spectral Characterization of Size-Selected Cu2ZnSnS4 Nanocrystal Inks. Sci. Rep. 2018, 8, 13677. [Google Scholar] [CrossRef]
- Selyshchev, O.; Havryliuk, Y.; Valakh, M.Y.; Yukhymchuk, V.O.; Raievska, O.; Stroyuk, O.L.; Dzhagan, V.; Zahn, D.R.T. Raman and X-ray Photoemission Identification of Colloidal Metal Sulfides as Potential Secondary Phases in Nanocrystalline Cu2ZnSnS4 Photovoltaic Absorbers. ACS Appl. Nano Mater. 2020, 3, 5706–5717. [Google Scholar] [CrossRef]
- Ashfaq, A.; Jacob, J.; Bano, N.; Nabi, M.A.U.; Ali, A.; Ahmad, W.; Mahmood, K.; Arshad, M.I.; Ikram, S.; Rehman, U.; et al. A Two Step Technique to Remove the Secondary Phases in CZTS Thin Films Grown by Sol-Gel Method. Ceram. Int. 2019, 45, 10876–10881. [Google Scholar] [CrossRef]
- Fernandes, P.A.; Salomé, P.M.P.; da Cunha, A.F. Study of Polycrystalline Cu2ZnSnS4 Films by Raman Scattering. J. Alloys Compd. 2011, 509, 7600–7606. [Google Scholar] [CrossRef]
- Furuta, K.; Sakai, N.; Kato, T.; Sugimoto, H.; Kurokawa, Y.; Yamada, A. Improvement of Cu2ZnSn(S,Se)4 Solar Cell Efficiency by Surface Treatment. Phys. Status Solidi (C) Curr. Top. Solid State Phys. 2015, 12, 704–707. [Google Scholar] [CrossRef]
- Lee, K.D.; Seo, S.W.; Lee, D.-K.; Kim, H.; Ko, M.J.; Kim, B.; Kim, D.H.; Kim, J.Y. The Effect of a Heating Rate on the Properties of Cu2ZnSnS4 Thin Films Solar Cells. ECS Trans. 2013, 50, 101–106. [Google Scholar] [CrossRef]
- Havryliuk, Y.; Valakh, M.Y.; Dzhagan, V.; Greshchuk, O.; Yukhymchuk, V.; Raevskaya, A.; Stroyuk, O.; Selyshchev, O.; Gaponik, N.; Zahn, D.R.T. Raman Characterization of Cu2ZnSnS4 Nanocrystals: Phonon Confinement Effect and Formation of CuxS Phases. RSC Adv. 2018, 8, 30736–30746. [Google Scholar] [CrossRef] [PubMed]
- Caballero, R.; Garcia-Llamas, E.; Merino, J.M.; León, M.; Babichuk, I.; Dzhagan, V.; Strelchuk, V.; Valakh, M. Non-Stoichiometry Effect and Disorder in Cu2ZnSnS4 Thin Films Obtained by Flash Evaporation: Raman Scattering Investigation. Acta Mater. 2014, 65, 412–417. [Google Scholar] [CrossRef]
- Camara, S.M.; Wang, L.; Zhang, X. Easy Hydrothermal Preparation of Cu2ZnSnS4 (CZTS) Nanoparticles for Solar Cell Application. Nanotechnology 2013, 24, 495401. [Google Scholar] [CrossRef]
- Ji, S.; Shi, T.; Qiu, X.; Zhang, J.; Xu, G.; Chen, C.; Jiang, Z.; Ye, C. A Route to Phase Controllable Cu2ZnSn(S1−xSex)4 Nanocrystals with Tunable Energy Bands. Sci. Rep. 2013, 3, 2733. [Google Scholar] [CrossRef]
- Valakh, M.Y.; Kolomys, O.F.; Ponomaryov, S.S.; Yukhymchuk, V.O.; Babichuk, I.S.; Izquierdo-Roca, V.; Saucedo, E.; Perez-Rodriguez, A.; Morante, J.R.; Schorr, S.; et al. Raman Scattering and Disorder Effect in Cu2ZnSnS4. Phys. Status Solidi-Rapid Res. Lett. 2013, 7, 258–261. [Google Scholar] [CrossRef]
- Valakh, M.Y.; Dzhagan, V.M.; Babichuk, I.S.; Fontane, X.; Perez-Rodriquez, A.; Schorr, S. Optically Induced Structural Transformation in Disordered Kesterite Cu2ZnSnS4. JETP Lett. 2013, 98, 255–258. [Google Scholar] [CrossRef]
- Dimitrievska, M.; Oliva, F.; Guc, M.; Giraldo, S.; Saucedo, E.; Pérez-Rodríguez, A.; Izquierdo-Roca, V. Defect Characterisation in Cu2ZnSnSe4 Kesterites: Via Resonance Raman Spectroscopy and the Impact on Optoelectronic Solar Cell Properties. J. Mater. Chem. A Mater. 2019, 7, 13293–13304. [Google Scholar] [CrossRef]
- Munce, C.G.; Parker, G.K.; Holt, S.A.; Hope, G.A. A Raman Spectroelectrochemical Investigation of Chemical Bath Deposited CuxS Thin Films and Their Modification. Colloids Surf. A Physicochem. Eng. Asp. 2007, 295, 152–158. [Google Scholar] [CrossRef]
- Jiang, Q.; Yan, H.; Khaliq, J.; Shen, Y.; Simpson, K.; Reece, M.J. Enhancement of Thermoelectric Properties by Atomic-Scale Percolation in Digenite CuxS. J. Mater. Chem. A Mater. 2014, 2, 9486–9489. [Google Scholar] [CrossRef]
- Tarachand; Hussain, S.; Lalla, N.P.; Kuo, Y.K.; Lakhani, A.; Sathe, V.G.; Deshpande, U.; Okram, G.S. Thermoelectric Properties of Ag-Doped CuS Nanocomposites Synthesized by a Facile Polyol Method. Phys. Chem. Chem. Phys. 2018, 20, 5926–5935. [Google Scholar] [CrossRef]
- Nagaoka, A.; Yoshino, K.; Masuda, T.; Sparks, T.D.; Scarpulla, M.A.; Nishioka, K. Environmentally Friendly Thermoelectric Sulphide Cu2ZnSnS4 Single Crystals Achieving a 1.6 Dimensionless Figure of Merit ZT. J. Mater. Chem. A Mater. 2021, 9, 15595–15604. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Havryliuk, Y.; Dzhagan, V.; Karnaukhov, A.; Selyshchev, O.; Hann, J.; Zahn, D.R.T. Influence of Thermal and Flash-Lamp Annealing on the Thermoelectrical Properties of Cu2ZnSnS4 Nanocrystals Obtained by “Green” Colloidal Synthesis. Nanomaterials 2023, 13, 1775. https://doi.org/10.3390/nano13111775
Havryliuk Y, Dzhagan V, Karnaukhov A, Selyshchev O, Hann J, Zahn DRT. Influence of Thermal and Flash-Lamp Annealing on the Thermoelectrical Properties of Cu2ZnSnS4 Nanocrystals Obtained by “Green” Colloidal Synthesis. Nanomaterials. 2023; 13(11):1775. https://doi.org/10.3390/nano13111775
Chicago/Turabian StyleHavryliuk, Yevhenii, Volodymyr Dzhagan, Anatolii Karnaukhov, Oleksandr Selyshchev, Julia Hann, and Dietrich R. T. Zahn. 2023. "Influence of Thermal and Flash-Lamp Annealing on the Thermoelectrical Properties of Cu2ZnSnS4 Nanocrystals Obtained by “Green” Colloidal Synthesis" Nanomaterials 13, no. 11: 1775. https://doi.org/10.3390/nano13111775




