Comparative Study of the U(VI) Adsorption by Hybrid Silica-Hyperbranched Poly(ethylene imine) Nanoparticles and Xerogels
Abstract
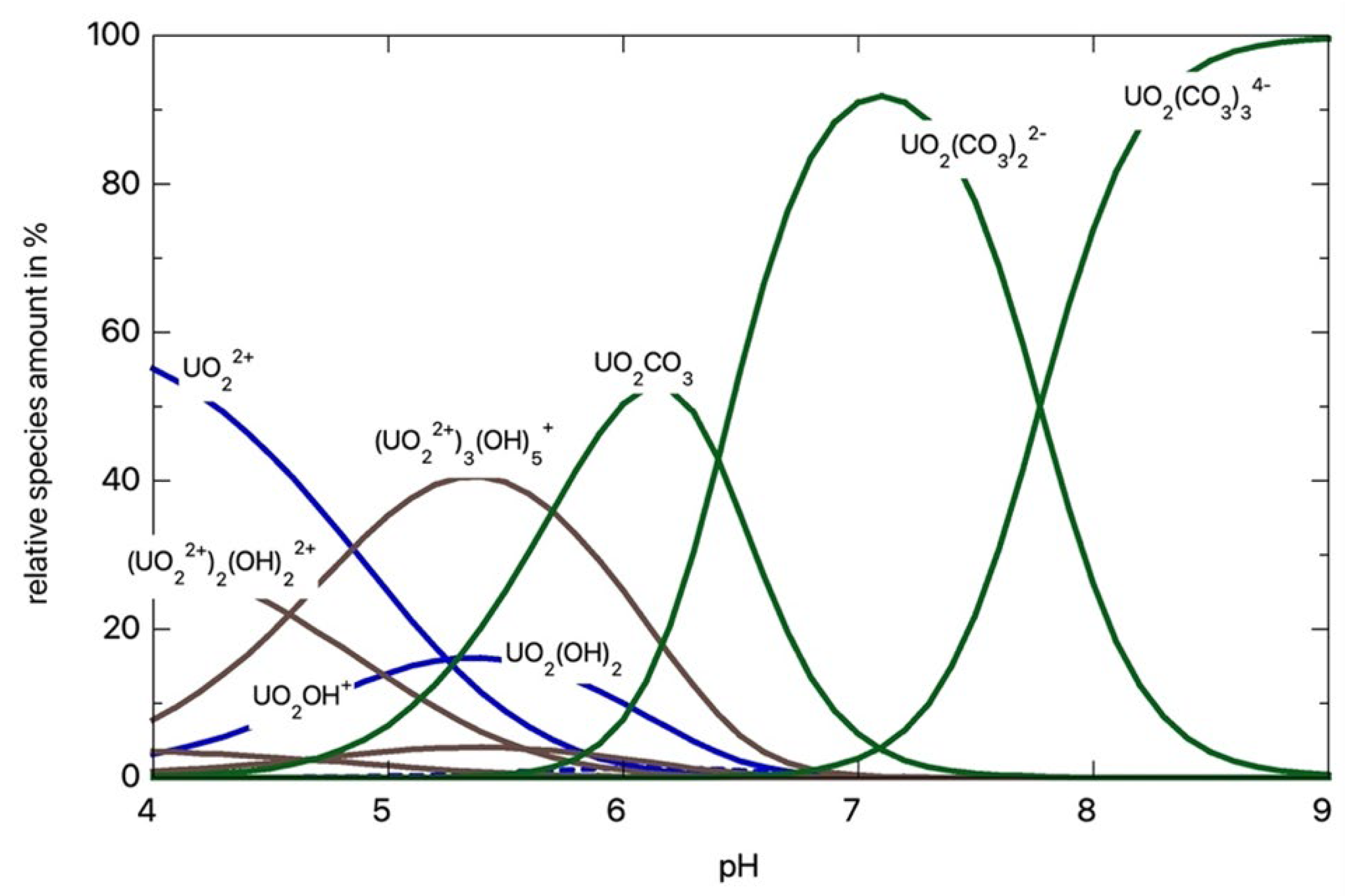
:1. Introduction
2. Materials and Methods
2.1. Preparation of Hybrid Silica-PEI Nanoparticles
2.2. Preparation of Silica-PEI Xerogels
2.3. Determination of Sorption Kinetics
2.4. Determination of Sorption Isotherms
3. Results and Discussion
3.1. Xerogel and Nanoparticle Characterization
3.1.1. Thermogravimetry
3.1.2. IR Spectroscopy of Nanoparticles and Xerogels
3.1.3. LN2 Porosimetry
3.1.4. Size (DLS) and Charge (ζ-Potential)
3.2. Adsorption Kinetics
3.3. Adsorption Isotherms
Effects of Temperature and Calcination
3.4. Adsorbent Characterization Posterior to the Uranyl Adsorption
3.4.1. Scanning Electron Microscopy (SEM)
3.4.2. Size (DLS) and Charge (ζ-Potential)
3.4.3. IR Spectroscopy
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Markich, S.J. Uranium Speciation and Bioavailability in Aquatic Systems: An Overview. Sci. World J. 2002, 2, 707–729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mühr-Ebert, E.L.; Wagner, F.; Walther, C. Speciation of Uranium: Compilation of a Thermodynamic Database and Its Experimental Evaluation Using Different Analytical Techniques. Appl. Geochem. 2019, 100, 213–222. [Google Scholar] [CrossRef]
- Fanghänel, T.; Neck, V. Aquatic Chemistry and Solubility Phenomena of Actinide Oxides/Hydroxides. Pure Appl. Chem. 2002, 74, 1895–1907. [Google Scholar] [CrossRef]
- Azzaz, A.A.; Jellali, S.; Akrout, H.; Assadi, A.A.; Bousselmi, L. Dynamic investigations on cationic dye desorption from chemically modified lignocellulosic material using a low-cost eluent: Dye recovery and anodic oxidation efficiencies of the desorbed solutions. J. Clean. Prod. 2018, 201, 28–38. [Google Scholar] [CrossRef]
- Zhou, G.; Li, S.; Niu, C.; Wang, Q.; Zhang, X.; Meng, Q.; Li, L. Fir sawdust as a low-cost and easily recyclable adsorbent: Efficient removal of Pb (II), Cu (II), and Zn (II) contaminants from wastewater. Environ. Sci. Pollut. Res. 2023, 2023, 39169–39183. [Google Scholar] [CrossRef] [PubMed]
- Philippou, K.; Savva, I.; Pashalidis, I. Uranium(VI) Binding by Pine Needles Prior and after Chemical Modification. J. Radioanal. Nucl. Chem. 2018, 318, 2205–2211. [Google Scholar] [CrossRef]
- Liatsou, I.; Michail, G.; Demetriou, M.; Pashalidis, I. Uranium Binding by Biochar Fibres Derived from Luffa Cylindrica after Controlled Surface Oxidation. J. Radioanal. Nucl. Chem. 2017, 311, 871–875. [Google Scholar] [CrossRef]
- Hadjittofi, L.; Pashalidis, I. Uranium Sorption from Aqueous Solutions by Activated Biochar Fibres Investigated by FTIR Spectroscopy and Batch Experiments. J. Radioanal. Nucl. Chem. 2015, 304, 897–904. [Google Scholar] [CrossRef]
- Stasi, C.; Georgiou, E.; Ioannidis, I.; Pashalidis, I. Uranium Removal from Laboratory and Environmental Waters by Oxidised Biochar Prepared from Palm Tree Fibres. J. Radioanal. Nucl. Chem. 2022, 331, 375–381. [Google Scholar] [CrossRef]
- Bhalara, P.D.; Punetha, D.; Balasubramanian, K. A Review of Potential Remediation Techniques for Uranium(VI) Ion Retrieval from Contaminated Aqueous Environment. J. Environ. Chem. Eng. 2014, 2, 1621–1634. [Google Scholar] [CrossRef]
- Guo, H.; Mei, P.; Xiao, J.; Huang, X.; Ishag, A.; Sun, Y. Carbon Materials for Extraction of Uranium from Seawater. Chemosphere 2021, 278, 130411. [Google Scholar] [CrossRef] [PubMed]
- Philippou, K.; Anastopoulos, I.; Dosche, C.; Pashalidis, I. Synthesis and Characterization of a Novel Fe3O4-Loaded Oxidized Biochar from Pine Needles and Its Application for Uranium Removal. Kinetic, Thermodynamic, and Mechanistic Analysis. J. Environ. Manag. 2019, 252, 109677. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Song, X.; Zhang, L.; Chen, W.; Chu, D.; Tan, L. Recovery of Uranium (VI) from Aqueous Solutions by the Polyethyleneimine-Functionalized Reduced Graphene Oxide/Molybdenum Disulfide Composition Aerogels. J. Taiwan Inst. Chem. Eng. 2020, 106, 198–205. [Google Scholar] [CrossRef]
- Huang, Z.; Li, Z.; Zheng, L.; Zhou, L.; Chai, Z.; Wang, X.; Shi, W. Interaction Mechanism of Uranium(VI) with Three-Dimensional Graphene Oxide-Chitosan Composite: Insights from Batch Experiments, IR, XPS, and EXAFS Spectroscopy. Chem. Eng. J. 2017, 328, 1066–1074. [Google Scholar] [CrossRef]
- Ioannou, K.; Hadjiyiannis, P.; Liatsou, I.; Pashalidis, I. U(VI) Adsorption by Biochar Fiber–MnO2 Composites. J. Radioanal. Nucl. Chem. 2019, 320, 425–432. [Google Scholar] [CrossRef]
- Philippou, K.; Christou, C.N.; Socoliuc, V.; Vekas, L.; Tanasă, E.; Miclau, M.; Pashalidis, I.; Krasia-Christoforou, T. Superparamagnetic Polyvinylpyrrolidone/Chitosan/Fe3O4 Electrospun Nanofibers as Effective U(VI) Adsorbents. J. Appl. Polym. Sci. 2021, 138, 50212. [Google Scholar] [CrossRef]
- Panagiotou, N.; Liatsou, I.; Pournara, A.; Angeli, G.K.; Giappa, R.M.; Tylianakis, E.; Manos, M.J.; Froudakis, G.E.; Trikalitis, P.N.; Pashalidis, I.; et al. Water-STable 2-D Zr MOFs with Exceptional UO22+ Sorption Capability. J. Mater. Chem. A 2020, 8, 1849–1857. [Google Scholar] [CrossRef]
- Liu, H.; Fu, T.; Mao, Y. Metal–Organic Framework-Based Materials for Adsorption and Detection of Uranium(VI) from Aqueous Solution. ACS Omega 2022, 7, 14430–14456. [Google Scholar] [CrossRef]
- Koppula, S.; Manabolu Surya, S.; Katari, N.K.; Dhami, P.S.; Sivasankaran Nair, R.K. Mesoporous MOF Composite for Efficient Removal of Uranium, Methyl Orange, Methylene Blue, and Congo Red Dyes from Aqueous Solutions. Appl. Organomet. Chem. 2022, 36, e6554. [Google Scholar] [CrossRef]
- Li, N.; Yang, L.; Wang, D.; Tang, C.; Deng, W.; Wang, Z. High-Capacity Amidoxime-Functionalized β-Cyclodextrin/Graphene Aerogel for Selective Uranium Capture. Environ. Sci. Technol. 2021, 55, 9181–9188. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, L.; Chen, F.; Wang, H.; Wang, Q.; Liang, K. Efficiency and Mechanism of Adsorption of Low-Concentration Uranium from Water by a New Chitosan/Aluminum Sludge Composite Aerogel. Dalton Trans. 2020, 49, 3209–3221. [Google Scholar] [CrossRef] [PubMed]
- Ioannidis, I.; Kinigopoulou, V.; Giannakoudakis, D.A.; Arkas, M.; Anastopoulos, I.; Triantafyllidis, K.S.; Pashalidis, I. Microplastics and disposable face masks as “Trojan Horse” for radionuclides pollution in water bodies—A review with emphasis on the involved interactions. Sustain. Chem. Environ. 2023, 2023, 100005. [Google Scholar] [CrossRef]
- Anastopoulos, I.; Milojković, J.V.; Tsigkou, K.; Zafiri, C.; Lopičić, Z.R.; Kornaros, M.; Pashalidis, I. A Nappies Management By-Product for the Treatment of Uranium-Contaminated Waters. J. Hazard. Mater. 2021, 404, 124147. [Google Scholar] [CrossRef] [PubMed]
- Liatsou, I.; Savva, I.; Vasile, E.; Vekas, L.; Marinica, O.; Mpekris, F.; Pashalidis, I.; Krasia-Christoforou, T. Magnetoresponsive Polymer Networks as Adsorbents for the Removal of U(VI) Ions from Aqueous Media. Eur. Polym. J. 2017, 97, 138–146. [Google Scholar]
- Yin, J.; Yang, S.; He, W.; Zhao, T.; Li, C.; Hua, D. Biogene-Derived Aerogels for Simultaneously Selective Adsorption of Uranium(VI) and Strontium(II) by Co-Imprinting Method. Sep. Purif. Technol. 2021, 271, 118849. [Google Scholar] [CrossRef]
- Yu, J.; Wang, J.; Jiang, Y. Removal of Uranium from Aqueous Solution by Alginate Beads. Nucl. Eng. Technol. 2017, 49, 534–540. [Google Scholar] [CrossRef] [Green Version]
- Ilaiyaraja, P.; Deb, A.S.; Ponraju, D.; Ali, S.M.; Venkatraman, B. Surface engineering of PAMAM-SDB chelating resin with diglycolamic acid (DGA) functional group for efficient sorption of U (VI) and Th (IV) from aqueous medium. J. Hazard. Mater. 2017, 328, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Douloudi, M.; Nikoli, E.; Katsika, T.; Arkas, M. Dendritic polymers for water resources remediation. In Novel Materials for Environmental Remediation Applications; Elsevier: Amsterdam, The Netherlands, 2023; pp. 435–490. [Google Scholar]
- Jikei, M.; Kakimoto, M.A. Hyperbranched polymers: A promising new class of materials. Prog. Polym. Sci. 2001, 26, 1233–1285. [Google Scholar] [CrossRef]
- Kim, Y.H. Hyperbranched polymers 10 years after. J. Polym. Sci. Part A Polym. Chem. 1998, 36, 1685–1698. [Google Scholar] [CrossRef]
- Malmström, E.; Hult, A. Hyperbranched polymers. J. Macromol. Sci. Part C Polym. Rev. 1997, 37, 555–579. [Google Scholar] [CrossRef]
- Sunder, A.; Heinemann, J.; Frey, H. Controlling the growth of polymer trees: Concepts and perspectives for hyperbranched polymers. Chem. A Eur. J. 2000, 6, 2499–2506. [Google Scholar] [CrossRef]
- Voit, B.I. Hyperbranched polymers: A chance and a challenge. Comptes Rendus Chim. 2003, 6, 821–832. [Google Scholar] [CrossRef]
- Yates, C.R.; Hayes, W. Synthesis and applications of hyperbranched polymers. Eur. Polym. J. 2004, 40, 1257–1281. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, S.; Weng, Z.; Gao, C. Hyperbranched polymers: Advances from synthesis to applications. Chem. Soc. Rev. 2015, 44, 4091–4130. [Google Scholar] [CrossRef] [PubMed]
- Ardoin, N.; Astruc, D. Molecular trees: From syntheses towards applications. Bull. Société Chim. Fr. 1995, 9, 875–909. [Google Scholar]
- Bosman, D.A.; Janssen, H.M.; Meijer, E.W. About dendrimers: Structure, physical properties, applications. Chem. Rev. 1999, 99, 1665–1688. [Google Scholar] [CrossRef]
- Dvornic, P.R.; Tomalia, D.A. November. Starburst® dendrimers: A conceptual approach to nanoscopic chemistry and architecture. In Macromolecular Symposia; Springer: Heidelberg/Berlin, Germany, 1994; Volume 88, pp. 123–148. [Google Scholar] [CrossRef]
- Tully, D.C.; Fréchet, J.M. Dendrimers at surfaces and interfaces: Chemistry and applications. Chem. Commun. 2001, 14, 1229–1239. [Google Scholar] [CrossRef]
- Zeng, F.; Zimmerman, S.C. Dendrimers in supramolecular chemistry: From molecular recognition to self-assembly. Chem. Rev. 1997, 97, 1681–1712. [Google Scholar] [CrossRef]
- Grayson, S.M.; Frechet, J.M. Convergent dendrons and dendrimers: From synthesis to applications. Chem. Rev. 2001, 101, 3819–3868. [Google Scholar] [CrossRef]
- Rosen, B.M.; Wilson, C.J.; Wilson, D.A.; Peterca, M.; Imam, M.R.; Percec, V. Dendron-mediated self-assembly, disassembly, and self-organization of complex systems. Chem. Rev. 2009, 109, 6275–6540. [Google Scholar] [CrossRef]
- Teertstra, S.J.; Gauthier, M. Dendrigraft polymers: Macromolecular engineering on a mesoscopic scale. Prog. Polym. Sci. 2004, 29, 277–327. [Google Scholar] [CrossRef]
- Afang, Z. Synthesis, characterization and applications of dendronized polymers. Prog. Chem. 2005, 17, 157–171. [Google Scholar] [CrossRef]
- Chen, Y.; Xiong, X. Tailoring dendronized polymers. Chem. Commun. 2010, 46, 5049–5060. [Google Scholar] [CrossRef]
- Frauenrath, H. Dendronized polymers—Building a new bridge from molecules to nanoscopic objects. Prog. Polym. Sci. 2005, 30, 325–384. [Google Scholar] [CrossRef]
- Schlüter, A.D.; Rabe, J.P. Dendronized polymers: Synthesis, characterization, assembly at interfaces, and manipulation. Angew. Chem. Int. Ed. 2000, 39, 864–883. [Google Scholar] [CrossRef]
- Buhleier, E.; Wehner, W.; Vogtle, F. “Cascade”- and “nonskid-chain-like” syntheses of molecular cavity topologies. Synthesis 1978, 1978, 155–158. [Google Scholar] [CrossRef]
- de Gennes, P.G.; Hervet, H. Statistics of «starburst» polymers. J. Phys. Lett. 1983, 44, 351–360. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Fréchet, J.M. Discovery of dendrimers and dendritic polymers: A brief historical perspective. J. Polym. Sci. Part A: Polym. Chem. 2002, 40, 2719–2728. [Google Scholar] [CrossRef]
- Vögtle, F.; Gestermann, S.; Hesse, R.; Schwierz, H.; Windisch, B. Functional dendrimers. Prog. Polym. Sci. 2000, 25, 987–1041. [Google Scholar] [CrossRef]
- Caminade, A.M.; Turrin, C.O.; Laurent, R.; Ouali, A.; Delavaux-Nicot, B. (Eds.) Dendrimers: Towards Catalytic, Material, and Biomedical Uses; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Marcos, M.; Martín-Rapún, R.; Omenat, A.; Serrano, J.L. Highly congested liquid crystal structures: Dendrimers, dendrons, dendronized and hyperbranched polymers. Chem. Soc. Rev. 2007, 36, 1889–1901. [Google Scholar] [CrossRef]
- Tsiourvas, D.; Arkas, M. Columnar and smectic self-assembly deriving from non-ionic amphiphilic hyperbranched polyethylene imine polymers and induced by hydrogen bonding and segregation into polar and non-polar parts. Polymer 2013, 54, 1114–1122. [Google Scholar] [CrossRef]
- Arkas, M.; Kitsou, I.; Gkouma, A.; Papageorgiou, M. The role of hydrogen bonds in the mesomorphic behaviour of supramolecular assemblies organized in dendritic architectures. Liq. Cryst. Rev. 2019, 7, 60–105. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Hosseini, S.H.; Alizadeh, M.; Bennett, C. Magnetic pH-responsive nanocarrier with long spacer length and high colloidal stability for controlled delivery of doxorubicin. Colloids Surf. B Biointerfaces 2014, 116, 49–54. [Google Scholar] [CrossRef]
- Yetisgin, A.A.; Cetinel, S.; Zuvin, M.; Kosar, A.; Kutlu, O. Therapeutic nanoparticles and their targeted delivery applications. Molecules 2020, 25, 2193. [Google Scholar] [CrossRef] [PubMed]
- Le, N.T.T.; Nguyen, T.N.Q.; Cao, V.D.; Hoang, D.T.; Ngo, V.C.; Hoang Thi, T.T. Recent progress and advances of multi-stimuli-responsive dendrimers in drug delivery for cancer treatment. Pharmaceutics 2019, 11, 591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paleos, C.M.; Tsiourvas, D.; Sideratou, Z.; Tziveleka, L.A. Drug delivery using multifunctional dendrimers and hyperbranched polymers. Expert Opin. Drug Deliv. 2010, 7, 1387–1398. [Google Scholar] [CrossRef] [PubMed]
- Arkas, M.; Vardavoulias, M.; Kythreoti, G.; Giannakoudakis, D.A. Dendritic Polymers in Tissue Engineering: Contributions of PAMAM, PPI PEG and PEI to Injury Restoration and Bioactive Scaffold Evolution. Pharmaceutics 2023, 15, 524. [Google Scholar] [CrossRef] [PubMed]
- Calabretta, M.K.; Kumar, A.; McDermott, A.M.; Cai, C. Antibacterial activities of poly (amidoamine) dendrimers terminated with amino and poly (ethylene glycol) groups. Biomacromolecules 2007, 8, 1807–1811. [Google Scholar] [CrossRef] [Green Version]
- Stach, M.; Siriwardena, T.N.; Köhler, T.; Van Delden, C.; Darbre, T.; Reymond, J.L. Combining Topology and Sequence Design for the Discovery of Potent Antimicrobial Peptide Dendrimers against Multidrug-Resistant Pseudomonas aeruginosa. Angew. Chem. 2014, 126, 13041–13045. [Google Scholar] [CrossRef]
- Pérez-Anes, A.; Spataro, G.; Coppel, Y.; Moog, C.; Blanzat, M.; Turrin, C.O.; Caminade, A.M.; Rico-Lattes, I.; Majoral, J.P. Phosphonate terminated PPH dendrimers: Influence of pendant alkyl chains on the in vitro anti-HIV-1 properties. Org. Biomol. Chem. 2009, 7, 3491–3498. [Google Scholar] [CrossRef]
- AWang, S.K.; Liang, P.H.; Astronomo, R.D.; Hsu, T.L.; Hsieh, S.L.; Burton, D.R.; Wong, C.H. Targeting the carbo-hydrates on HIV-1: Interaction of oligomannose dendrons with human monoclonal antibody 2G12 and DC-SIGN. Proc. Natl. Acad. Sci. USA 2008, 105, 3690–3695. [Google Scholar] [CrossRef] [Green Version]
- Meyers, S.R.; Juhn, F.S.; Griset, A.P.; Luman, N.R.; Grinstaff, M.W. Anionic amphiphilic dendrimers as antibacterial agents. J. Am. Chem. Soc. 2008, 130, 14444–14445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.Z.; Beck-Tan, N.C.; Dhurjati, P.; van Dyk, T.K.; LaRossa, R.A.; Cooper, S.L. Quaternary ammonium functionalized poly (propylene imine) dendrimers as effective antimicrobials: Structure-activity studies. Biomacromolecules 2000, 1, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Arkas, M.; Kythreoti, G.; Favvas, E.P.; Giannakopoulos, K.; Mouti, N.; Arvanitopoulou, M.; Athanasiou, A.; Douloudi, M.; Nikoli, E.; Vardavoulias, M.; et al. Hydrophilic Antimicrobial Coatings for Medical Leathers from Silica-Dendritic Polymer-Silver Nanoparticle Composite Xerogels. Textiles 2022, 2, 464–485. [Google Scholar] [CrossRef]
- Shi, X.; Wang, S.H.; Van Antwerp, M.E.; Chen, X.; Baker Jr, J.R. Targeting and detecting cancer cells using spontaneously formed multifunctional dendrimer-stabilized gold nanoparticles. Analyst 2009, 134, 1373–1379. [Google Scholar] [CrossRef] [Green Version]
- JLi, H.; Sun, J.; Zhu, H.; Wu, H.; Zhang, H.; Gu, Z.; Luo, K. Recent advances in development of dendritic polymer-based nanomedicines for cancer diagnosis. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2021, 13, e1670. [Google Scholar] [CrossRef]
- Wiener, E.; Brechbiel, M.W.; Brothers, H.; Magin, R.L.; Gansow, O.A.; Tomalia, D.A.; Lauterbur, P.C. Dendrimer-based metal chelates: A new class of magnetic resonance imaging contrast agents. Magn. Reson. Med. 1994, 31, 1–8. [Google Scholar] [CrossRef]
- Nwe, K.; Bryant, L.H., Jr.; Brechbiel, M.W. Poly (amidoamine) dendrimer based MRI contrast agents exhibiting enhanced relaxivities derived via metal preligation techniques. Bioconjug. Chem. 2010, 21, 1014–1017. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Mou, Q.; Wang, D.; Zhu, X.; Yan, D. Dendritic polymers for theranostics. Theranostics 2016, 6, 930. [Google Scholar] [CrossRef]
- Korake, S.; Shaikh, A.; Salve, R.; Gajbhiye, K.R.; Gajbhiye, V.; Pawar, A. Biodegradable dendritic Boltorn™ nano-constructs: A promising avenue for cancer theranostics. Int. J. Pharm. 2021, 594, 120177. [Google Scholar] [CrossRef]
- Haensler, J.; Szoka, F.C., Jr. Polyamidoamine cascade polymers mediate efficient transfection of cells in culture. Bioconjug. Chem. 1993, 4, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Kabanov, V.A.; Sergeyev, V.G.; Pyshkina, O.A.; Zinchenko, A.A.; Zezin, A.B.; Joosten, J.G.H.; Brackman, J.; Yoshikawa, K. Interpolyelectrolyte complexes formed by DNA and astramol poly (propylene imine) dendrimers. Macromolecules 2000, 33, 9587–9593. [Google Scholar] [CrossRef]
- Patil, M.L.; Zhang, M.; Taratula, O.; Garbuzenko, O.B.; He, H.; Minko, T. Internally cationic polyamidoamine PAMAM-OH dendrimers for siRNA delivery: Effect of the degree of quaternization and cancer targeting. Biomacromolecules 2009, 10, 258–266. [Google Scholar] [CrossRef] [Green Version]
- German, N.; Popov, A.; Ramanavicius, A.; Ramanaviciene, A. Development and practical application of glucose biosensor based on dendritic gold nanostructures modified by conducting polymers. Biosensors 2022, 12, 641. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, A.; Armada, M.P.G.; Losada, J.; Villena, C.; Alonso, B.; Casado, C.M. Amperometric biosensors for NADH based on hyperbranched dendritic ferrocene polymers and Pt nanoparticles. Sens. Actuators B Chem. 2014, 190, 111–119. [Google Scholar] [CrossRef]
- Shukla, R.; Singh, A.; Pardhi, V.; Sunil Dubey, P.K. Dendrimer (polyamidoamine, polypropylene imine, poly-L-lysine, carbosilane dendrimers, triazine dendrimers) as promising tool for anticancer therapeutics. In Polymeric Nanoparticles as a Promising Tool for Anti-Cancer Therapeutics; Wolff, A.G., Ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2019; pp. 233–255. ISBN 9780128169636. [Google Scholar]
- Wang, B.Y.; Liao, M.L.; Hong, G.C.; Chang, W.W.; Chu, C.C. Near-infrared-triggered photodynamic therapy toward breast cancer cells using dendrimer-functionalized upconversion nanoparticles. Nanomaterials 2017, 7, 269. [Google Scholar] [CrossRef] [Green Version]
- Dhanikula, R.S.; Argaw, A.; Bouchard, J.F.; Hildgen, P. Methotrexate loaded polyether-copolyester dendrimers for the treatment of gliomas: Enhanced efficacy and intratumoral transport capability. Mol. Pharm. 2008, 5, 105–116. [Google Scholar] [CrossRef]
- Klajnert, B.; Cangiotti, M.; Calici, S.; Majoral, J.P.; Caminade, A.M.; Cladera, J.; Bryszewska, M.; Ottaviani, M.F. EPR study of the interactions between dendrimers and peptides involved in Alzheimer’s and prion diseases. Macromol. Biosci. 2007, 7, 1065–1074. [Google Scholar] [CrossRef]
- Fruchon, S.; Poupot, M.; Martinet, L.; Turrin, C.O.; Majoral, J.P.; Fournié, J.J.; Caminade, A.M.; Poupot, R. Anti-inflammatory and immunosuppressive activation of human monocytes by a bioactive dendrimer. J. Leukoc. Biol. 2009, 85, 553–562. [Google Scholar] [CrossRef]
- Chandrasekar, D.; Sistla, R.; Ahmad, F.J.; Khar, R.K.; Diwan, P.V. Folate-coupled poly (ethyleneglycol) conjugates of anionic poly (amidoamine) dendrimer for inflammatory tissue-specific drug delivery. J. Biomed. Mater. Res. Part A 2007, 82, 92–103. [Google Scholar] [CrossRef]
- Douloudi, M.; Nikoli, E.; Katsika, T.; Vardavoulias, M.; Arkas, M. Dendritic polymers as promising additives for the manufacturing of hybrid organoceramic nanocomposites with ameliorated properties suitable for an extensive diversity of applications. Nanomaterials 2020, 11, 19. [Google Scholar] [CrossRef] [PubMed]
- Kitsou, I.; Panagopoulos, P.; Maggos, T.; Arkas, M.; Tsetsekou, A. Development of SiO2@TiO2 core-shell nanospheres for catalytic applications. Appl. Surf. Sci. 2018, 441, 223–231. [Google Scholar] [CrossRef]
- Tsiourvas, D.; Papavasiliou, A.; Deze, E.G.; Papageorgiou, S.K.; Katsaros, F.K.; Romanos, G.E.; Poulakis, E.; Philippopoulos, C.J.; Xin, Q.; Cool, P. A green route to copper loaded silica nanoparticles using hyperbranched poly(Ethylene imine) as a biomimetic template: Application in heterogeneous catalysis. Catalysts 2017, 7, 390. [Google Scholar] [CrossRef] [Green Version]
- Kitsou, I.; Arkas, M.; Tsetsekou, A. Synthesis and characterization of ceria-coated silica nanospheres: Their application in heterogeneous catalysis of organic pollutants. SN Appl. Sci. 2019, 1, 1557. [Google Scholar] [CrossRef] [Green Version]
- Jensen, L.K.; Jensen, H.E.; Blirup-Plum, S.A.; Bue, M.; Hanberg, P.; Kvich, L.; Aalbæk, B.; López, Y.; Soto, S.M.; Douloudi, M.; et al. Coating of bone implants with silica, hyperbranched polyethyleneimine, and gentamicin prevents development of osteomyelitis in a porcine model. Materialia 2022, 24, 101473. [Google Scholar] [CrossRef]
- Pang, Y.; Zeng, G.; Tang, L.; Zhang, Y.; Liu, Y.; Lei, X.; Li, Z.; Zhang, J.; Xie, G. PEI-grafted magnetic porous powder for highly effective adsorption of heavy metal ions. Desalination 2011, 281, 278–284. [Google Scholar] [CrossRef]
- Tsetsekou, A.; Arkas, M.; Kritikaki, A.; Simonetis, S.; Tsiourvas, D. Optimization of hybrid hyperbranched polymer/ceramic filters for the efficient absorption of polyaromatic hydrocarbons from water. J. Membr. Sci. 2008, 311, 128–135. [Google Scholar] [CrossRef]
- Arkas, M.; Tsiourvas, D.; Paleos, C.M. Organosilicon dendritic networks in porous ceramics for water purification. Chem. Mater. 2005, 17, 3439–3444. [Google Scholar] [CrossRef]
- Arkas, M.; Tsiourvas, D. Organic/inorganic hybrid nanospheres based on hyperbranched poly (ethylene imine) encapsulated into silica for the sorption of toxic metal ions and polycyclic aromatic hydrocarbons from water. J. Hazard. Mater. 2009, 170, 35–42. [Google Scholar] [CrossRef]
- Knecht, M.R.; Wright, D.W. Amine-terminated dendrimers as biomimetic templates for silica nanosphere formation. Langmuir 2004, 20, 4728–4732. [Google Scholar] [CrossRef]
- Knecht, M.R.; Sewell, S.L.; Wright, D.W. Size control of dendrimer-templated silica. Langmuir 2005, 21, 2058–2061. [Google Scholar] [CrossRef] [PubMed]
- Giannakoudakis, D.A.; Anastopoulos, I.; Barczak, M.; Antoniou, Ε.; Terpiłowski, K.; Mohammadi, E.; Shams, M.; Coy, E.; Bakandritsos, A.; Katsoyiannis, I.A.; et al. Enhanced uranium removal from acidic wastewater by phosphonate-functionalized ordered mesoporous silica: Surface chemistry matters the most. J. Hazard. Mater. 2021, 413, 125279. [Google Scholar] [CrossRef] [PubMed]
- Arkas, M.; Douloudi, M.; Nikoli, E.; Karountzou, G.; Kitsou, I.; Kavetsou, E.; Korres, D.; Vouyiouka, S.; Tsetsekou, A.; Giannakopoulos, K.; et al. Investigation of two bioinspired reaction mechanisms for the optimization of nano catalysts generated from hyperbranched polymer matrices. React. Funct. Polym. 2022, 174, 105238. [Google Scholar] [CrossRef]
- Tsiourvas, D.; Tsetsekou, A.; Arkas, M.; Diplas, S.; Mastrogianni, E. Covalent attachment of a bioactive hyperbranched polymeric layer to titanium surface for the biomimetic growth of calcium phosphates. J. Mater. Sci. Mater. Med. 2011, 22, 85–96. [Google Scholar] [CrossRef] [Green Version]
- Kristensen, J.B.; Meyer, R.L.; Poulsen, C.H.; Kragh, K.M.; Besenbacher, F.; Laursen, B.S. Biomimetic Silica Encapsulation of Enzymes for Replacement of Biocides in Antifouling Coatings. Green Chem. 2010, 12, 387–394. [Google Scholar] [CrossRef]
- Knecht, M.R.; Wright, D.W. Functional Analysis of the Biomimetic Silica Precipitating Activity of the R5 Peptide from Cylindrotheca Fusiformis. Chem. Commun. 2003, 24, 3038–3039. [Google Scholar] [CrossRef]
- Kroger, N.; Deutzmann, R.; Sumper, M. Polycationic Peptides from Diatom Biosilica That Direct Silica Nanosphere Formation. Science 1999, 286, 1129–1132. [Google Scholar] [CrossRef] [Green Version]
- Kroger, N.; Deutzmann, R.; Bergsdorf, C.; Sumper, M. Species-Specific Polyamines from Diatoms Control Silica Morphology. Proc. Natl. Acad. Sci. USA 2000, 97, 14133–14138. [Google Scholar] [CrossRef] [Green Version]
- Kröger, N.; Lorenz, S.; Brunner, E.; Sumper, M. Self-Assembly of Highly Phosphorylated Silaffins and Their Function in Biosilica Morphogenesis. Science 2002, 298, 548–586. [Google Scholar] [CrossRef]
- Dillon, R.E.A.; Shriver, D.F. Ion transport and vibrational spectra of branched polymer and dendrimer electrolytes. Chem. Mater. 2001, 13, 1369–1373. [Google Scholar] [CrossRef]
- Hu, H.; Ortíz-Aguilar, B.E.; Hechavarría, L.E. Effect of pH value of poly(ethylenimine)–H2SO4 electrolyte on electrochromic response of polyaniline thin films. Opt. Mater. 2007, 29, 579–584. [Google Scholar] [CrossRef]
- Bellamy, L.J. The Infrared Spectra of Complex Molecules, 3rd ed.; Chapman and Hall: London, UK, 1975; pp. 16–17. [Google Scholar]
- Kłonkowski, A.M.; Grobelna, B.; Widernik, T.; Jankowska-Frydel, A.; Mozgawa, W. Coordination state of copper(II) complexes anchored and grafted onto the surface of organically modified silicates. Langmuir 1999, 15, 5814–5819. [Google Scholar] [CrossRef]
- Shimizu, I.; Okabayashi, H.; Taga, K.; Nishio, E.; O’Connor, C.J. Diffuse reflectance infrared Fourier transform spectral study of the thermal and adsorbed-water effects of a 3-aminopropyltriethoxysilane layer modified onto the surface of silica gel. Vib. Spectrosc. 1997, 14, 113–123. [Google Scholar] [CrossRef]
- Yoshino, H.; Kamiya, K.; Nasu, H. IR Study on the Structural Evolution of Sol-Gel Derived SiO2 Gels in the Early Stage of Conversion to Glasses. J. Non. Cryst. Solids 1990, 126, 68–78. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Amer. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Li, K.; Jiang, J.; Tian, S.; Yan, F.; Chen, X. Polyethyleneimine–nano silica composites: A low-cost and promising adsorbent for CO2 capture. J. Mater. Chem. A 2015, 3, 2166–2175. [Google Scholar] [CrossRef]
- Bchellaoui, N.; Hayat, Z.; Mami, M.; Dorbez-Sridi, R.; El Abed, A.I. Microfluidic-assisted formation of highly mono-disperse and mesoporous silica soft microcapsules. Sci. Rep. 2017, 7, 16326. [Google Scholar] [CrossRef] [Green Version]
- Dahliyanti, A.; Yunitama, D.A.; Rofiqoh, I.M.; Mustapha, M. Synthesis and characterization of silica xerogel from corn husk waste as cationic dyes adsorbent. F1000Research 2022, 11, 305. [Google Scholar] [CrossRef] [PubMed]
- Arenas, L.T.; Simm, C.W.; Gushikem, Y.; Dias, S.L.; Moro, C.C.; Costa, T.M.; Benvenutti, E.V. Synthesis of silica xerogels with high surface area using acetic acid as catalyst. J. Braz. Chem. Soc. 2007, 18, 886–890. [Google Scholar] [CrossRef]
- Ding, W.; Wang, X.; Chen, D.; Li, T.; Shen, J. Cast-In-Situ, Large-Sized Monolithic Silica Xerogel Prepared in Aqueous System. Molecules 2018, 23, 1178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Rasmussen, C.J.; Vishnyakov, A.; Thommes, M.; Smarsly, B.M.; Kleitz, F.; Neimark, A.V. Cavitation in metastable liquid nitrogen confined to nanoscale pores. Langmuir 2010, 26, 10147–10157. [Google Scholar] [CrossRef] [PubMed]
- Paleos, C.M.; Arkas, M.; Skoulios, A. Mesomorphic character of quaternary ammonium salts affected by secondary hydrogen bonding interactions. Mol. Cryst. Liq. Cryst. Sci. Technol. Sect. A. Mol. Cryst. Liq. Cryst. 1998, 309, 237–250. [Google Scholar] [CrossRef]
- Nikokavoura, A.; Tsiourvas, D.; Arkas, M.; Sideratou, Z.; Paleos, C.M. Thermotropic liquid crystalline behaviour of piperazinium and homopiperazinium alkylsulphates. Liq. Cryst. 2002, 29, 1547–1553. [Google Scholar] [CrossRef]
- Lantenois, S.; Prélot, B.; Douillard, J.-M.; Szczodrowski, K.; Charbonnel, M.-C. Flow microcalorimetry: Experimental development and application to adsorption of heavy metal cations on silica. Appl. Surf. Sci. 2007, 253, 5807–5813. [Google Scholar] [CrossRef]
- da Silva, L.C.C.; Santos, L.B.O.D.; Abate, G.; Cosentino, I.C.; Fantini, M.C.A.; Masini, J.C.; Matos, J.R. Adsorption of Pb2+, Cu2+ and Cd2+ in FDU-1 silica and FDU-1 silica modified with humic acid. Micropor. Mesopor. Mater. 2008, 110, 250–259. [Google Scholar] [CrossRef]
- Georgiou, E.; Raptopoulos, G.; Anastopoulos, I.; Giannakoudakis, D.A.; Arkas, M.; Paraskevopoulou, P.; Pashalidis, I. Uranium Removal from Aqueous Solutions by Aerogel-Based Adsorbents—A Critical Review. Nanomaterials 2023, 13, 363. [Google Scholar] [CrossRef]
- Wang, F.; Liao, Y.; Xia, L. Poly (amidoamine) dendrimer decorated dendritic fibrous nano-silica for efficient removal of uranium (VI). J. Solid State Chem. 2021, 303, 122511. [Google Scholar] [CrossRef]
- Xiong, T.; Li, Q.; Liao, J.; Zhang, Y.; Zhu, W. Highly Enhanced Adsorption Performance to Uranium(VI) by Facile Synthesized Hydroxyapatite Aerogel. J. Hazard. Mater. 2022, 423, 127184. [Google Scholar] [CrossRef]
- Xiong, T.; Li, Q.; Liao, J.; Zhang, Y.; Zhu, W. Design of Hydroxyapatite Aerogel with Excellent Adsorption Performance to Uranium. J. Environ. Chem. Eng. 2021, 9, 106364. [Google Scholar] [CrossRef]
- Georgiou, E.; Raptopoulos, G.; Papastergiou, M.; Paraskevopoulou, P.; Pashalidis, I. Extremely Efficient Uranium Removal from Aqueous Environments with Polyurea-Cross-Linked Alginate Aerogel Beads. ACS Appl. Polym. Mater. 2022, 4, 920–928. [Google Scholar] [CrossRef]
- Zhao, M.; Tesfay Reda, A.; Zhang, D. Reduced Graphene Oxide/ZIF-67 Aerogel Composite Material for Uranium Adsorption in Aqueous Solutions. ACS Omega 2020, 5, 8012–8022. [Google Scholar] [CrossRef] [Green Version]
- Lei, Y.; Li, K.; Liao, J.; Zhang, Y.; Zhang, L.; Zhu, W. Design of 3D Alumina-Doped Magnesium Oxide Aerogels with a High-Efficiency Removal of Uranium(VI) from Wastewater. Inorg. Chem. Front. 2021, 8, 2561–2574. [Google Scholar] [CrossRef]
- Chen, M.; Liu, T.; Zhang, X.; Zhang, R.; Tang, S.; Yuan, Y.; Xie, Z.; Liu, Y.; Wang, H.; Fedorovich, K.V.; et al. Photoinduced Enhancement of Uranium Extraction from Seawater by MOF/Black Phosphorus Quantum Dots Heterojunction Anchored on Cellulose Nanofiber Aerogel. Adv. Funct. Mater. 2021, 31, 2100106. [Google Scholar] [CrossRef]
- Arkas, M.; Douloudi, M.; Nikoli, E.; Karountzou, G.; Kitsou, I.; Kavetsou, E.; Korres, D.; Vouyiouka, S.; Tsetsekou, A.; Giannakopoulos, K.; et al. Additional data on the investigation of the reaction mechanisms for the production of silica hyperbranched polyethylene imine silver nanoparticle composites. Data Brief 2022, 43, 108374. [Google Scholar] [CrossRef]
- Ioannidis, I.; Anastopoulos, I.; Giannakopoulos, K.; Arkas, M.; Dosche, C.; Pashalidis, I. A comprehensive investigation on the sorption of U (VI) and Eu (III) by polyamide microplastics: Surface-assisted microparticle formation. J. Mol. Liq. 2022, 368, 120757. [Google Scholar] [CrossRef]
- Groenewold, G.S.; Gianotto, A.K.; McIlwain, M.E.; Van Stipdonk, M.J.; Kullman, M.; Moore, D.T.; Polfer, N.; Oomens, J.; Infante, I.; Visscher, L.; et al. Infrared Spectroscopy of Discrete Uranyl Anion Complexes. J. Phys. Chem. A 2008, 112, 508–521. [Google Scholar] [CrossRef] [Green Version]
- Gan, F.; Wu, K.; Ma, F.; Du, C. In Situ Determination of Nitrate in Water Using Fourier Transform Mid-Infrared Attenuated Total Reflectance Spectroscopy Coupled with Deconvolution Algorithm. Molecules 2020, 25, 5838. [Google Scholar] [CrossRef]
- Allabashi, R.; Arkas, M.; Hörmann, G.; Tsiourvas, D. Removal of some organic pollutants in water employing ceramic membranes impregnated with cross-linked silylated dendritic and cyclodextrin polymers. Water Res. 2007, 41, 476–486. [Google Scholar] [CrossRef]
- Arkas, M.; Tsiourvas, D.; Paleos, C.M. Functional dendrimeric “nanosponges” for the removal of polycyclic aromatic hydrocarbons from water. Chem. Mater. 2003, 15, 2844–2847. [Google Scholar] [CrossRef]
- Arkas, M.; Eleades, L.; Paleos, C.M.; Tsiourvas, D. Alkylated hyperbranched polymers as molecular nanosponges for the purification of water from polycyclic aromatic hydrocarbons. J. Appl. Polym. Sci. 2005, 97, 2299–2305. [Google Scholar] [CrossRef]
- Arkas, M.; Tsiourvas, D.; Paleos, C.M. Functional dendritic polymers for the development of hybrid materials for water purification. Macromol. Mater. Eng. 2010, 295, 883–898. [Google Scholar] [CrossRef]
- Arkas, M.; Kithreoti, G.; Boukos, N.; Kitsou, I.; Petrakli, F.; Panagiotaki, K. Two completely different biomimetic reactions mediated by the same matrix producing inorganic/organic/inorganic hybrid nanoparticles. Nano-Struct. Nano-Objects 2018, 14, 138–148. [Google Scholar] [CrossRef]
- Arkas, M.; Anastopoulos, I.; Giannakoudakis, D.A.; Pashalidis, I.; Katsika, T.; Nikoli, E.; Panagiotopoulos, R.; Fotopoulou, A.; Vardavoulias, M.; Douloudi, M. Catalytic Neutralization of Water Pollutants Mediated by Dendritic Polymers. Nanomaterials 2022, 12, 445. [Google Scholar] [CrossRef] [PubMed]










| Adsorbent | Weight Loss 170 °C (Water/Ethanol) (%) | Total Weight Loss 3 h 700 °C (%) | Organic Content (%) |
|---|---|---|---|
| Xerogel 2000 | 6.99 | 15.97 | 8.98 |
| Xerogel 5000 | 4.92 | 14.89 | 9.91 |
| Xerogel 25,000 | 7.15 | 17.28 | 10.13 |
| Xerogel 750,000 | 7.05 | 16.01 | 8.96 |
| Nanoparticles 2000 | 8.28 | 31.08 | 22.80 |
| Nanoparticles 5000 | 7.40 | 28.90 | 21.50 |
| Nanoparticles 25,000 | 7.80 | 32.23 | 24.43 |
| Nanoparticles 750,000 | 6.53 | 28.84 | 22.31 |
| Band Assignment 1 | PEI25000 | Xerogels | Nanoparticles |
|---|---|---|---|
| νs SiO-H free | - | 3750 (vw) | - |
| νs SiO-H Hydrogen bonded | - | 3450 (w/b) | 3450 (w/b) |
| νas NH (primary, secondary) | 3350 (m) | 3400 (w) | 3370 (sh) |
| νs NH (primary, secondary) | 3276 (m) | op | 3200 (w) |
| νas CH2 | 2935 (m) | 2981 (vw) | 2962 (vw) |
| νs CH2 | 2810 (s) | 2820 (vw) | 2853 (vw) |
| δ NH, NH2 | 1585 (m) | 1640 (vw) | - |
| δas NH2+, NH3+ | - | 1530 (vw) | 1524 (vw) |
| δs NH2+, NH3+ | - | - | 1473 (vw) |
| νas C-N | 1105 (m) | sh | sh |
| νs C-N | 1045 (m) | op | op |
| ν Si-O-Si | - | 1074 (s) | 1042 (s) |
| ν Si-OH, Si-O- | - | 960 (m) | 964 (m) |
| δ Si-O-Si | - | 788 (m) | 785 (m) |
| ρ CH2 | 760 (s) | op | op |
| δ Si-OH | - | 540 (m) | 540 (m) |
| Sample | TPV 1 | SBET | dmean 2 | dBJH 3 | dDFT 4 |
|---|---|---|---|---|---|
| (mL/g) | (m2/g) | (nm) | (nm) | (nm) | |
| Nanoparticles 750,000 | 0.640 | 139.0 | 18.4 | 32.0 | 28.4 |
| Nanoparticles 25,000 | 0.326 | 77.9 | 16.7 | 53.5 | 55.8 |
| Nanoparticles 5000 | 0.880 | 148.3 | 23.7 | 31.0 | 28.4 |
| Nanoparticles 2000 | 0.508 | 98.5 | 20.6 | 53.6 | 28.4 |
| Xerogel 750,000 | 0.461 | 271.4 | 6.8 | 3.8 | 5.1 |
| Xerogel 25,000 | 0.829 | 714.1 | 4.6 | 3.8 | 5.9 |
| Xerogel 5000 | 0.390 | 220.3 | 7.1 | 4.7 | 6.1 |
| Xerogel 2000 | 0.297 | 194.3 | 6.1 | 5.4 | 7.0 |
| Sample | SBET (m2/g) | Reference |
|---|---|---|
| Nanoparticles 5000 | 148.3 | This Work |
| Xerogel 25,000 | 714.1 | This Work |
| Nano-silica composites- PEI 30% | 104 | [111] |
| Nano-silica composites- PEI 60% | 0.65 | [111] |
| Mesoporous silica microcapsules | 514 | [112] |
| Silica xerogels | 363.72 | [113] |
| Silica xerogels | 335–850 | [114] |
| PEG-silica xerogels | 19–127 | [115] |
| Sample | qmax (mol kg−1) | Reference |
|---|---|---|
| Nanoparticles 750,000 | 11.5 | This Work |
| Nanoparticles 25,000 | 9.5 | This Work |
| Nanoparticles 5000 | 10.6 | This Work |
| Nanoparticles 2000 | 8.1 | This Work |
| Xerogel 750,000 | 3.7 | This Work |
| Xerogel 25,000 | 1.5 | This Work |
| Xerogel 5000 | 1.7 | This Work |
| Xerogel 2000 | 2.3 | This Work |
| Nano-silica with hyperbranched PAMAM | 0.91 | [124] |
| Hydroxyapatite with konjac gum | 9.0 | [125,126] |
| Polyurea-crosslinked alginate | 8.72 | [127] |
| Reduced graphene oxide/ZIF-67 | 8.14 | [128] |
| Al2O3/MgO | 4.51 | [129] |
| MOF/black phosphorus quantum dots on cellulose | 3.7 | [130] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arkas, M.; Giannakopoulos, K.; Favvas, E.P.; Papageorgiou, S.; Theodorakopoulos, G.V.; Giannoulatou, A.; Vardavoulias, M.; Giannakoudakis, D.A.; Triantafyllidis, K.S.; Georgiou, E.; et al. Comparative Study of the U(VI) Adsorption by Hybrid Silica-Hyperbranched Poly(ethylene imine) Nanoparticles and Xerogels. Nanomaterials 2023, 13, 1794. https://doi.org/10.3390/nano13111794
Arkas M, Giannakopoulos K, Favvas EP, Papageorgiou S, Theodorakopoulos GV, Giannoulatou A, Vardavoulias M, Giannakoudakis DA, Triantafyllidis KS, Georgiou E, et al. Comparative Study of the U(VI) Adsorption by Hybrid Silica-Hyperbranched Poly(ethylene imine) Nanoparticles and Xerogels. Nanomaterials. 2023; 13(11):1794. https://doi.org/10.3390/nano13111794
Chicago/Turabian StyleArkas, Michael, Konstantinos Giannakopoulos, Evangelos P. Favvas, Sergios Papageorgiou, George V. Theodorakopoulos, Artemis Giannoulatou, Michail Vardavoulias, Dimitrios A. Giannakoudakis, Konstantinos S. Triantafyllidis, Efthalia Georgiou, and et al. 2023. "Comparative Study of the U(VI) Adsorption by Hybrid Silica-Hyperbranched Poly(ethylene imine) Nanoparticles and Xerogels" Nanomaterials 13, no. 11: 1794. https://doi.org/10.3390/nano13111794
APA StyleArkas, M., Giannakopoulos, K., Favvas, E. P., Papageorgiou, S., Theodorakopoulos, G. V., Giannoulatou, A., Vardavoulias, M., Giannakoudakis, D. A., Triantafyllidis, K. S., Georgiou, E., & Pashalidis, I. (2023). Comparative Study of the U(VI) Adsorption by Hybrid Silica-Hyperbranched Poly(ethylene imine) Nanoparticles and Xerogels. Nanomaterials, 13(11), 1794. https://doi.org/10.3390/nano13111794












