Dextran-Coated Iron Oxide Nanoparticles Loaded with 5-Fluorouracil for Drug-Delivery Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis and Characterization of the Drug-Delivery System
2.1.1. Materials
2.1.2. Dextran-Coated IONPs Loaded with 5-FU
2.1.3. Physiochemical Characterization
2.1.4. Cell Culture and Treatment
2.1.5. MTT Assay
2.1.6. Microscopic Imaging of Cell Morphology
2.1.7. Detection of Lactate Dehydrogenase (LDH) Leakage
2.1.8. Quantification of Total Intracellular Iron
2.1.9. Measurement of H2O2 Production
2.1.10. Immunoblotting
2.1.11. Drug-Loading and Drug-Release Studies
2.1.12. Statistical Analysis
3. Results
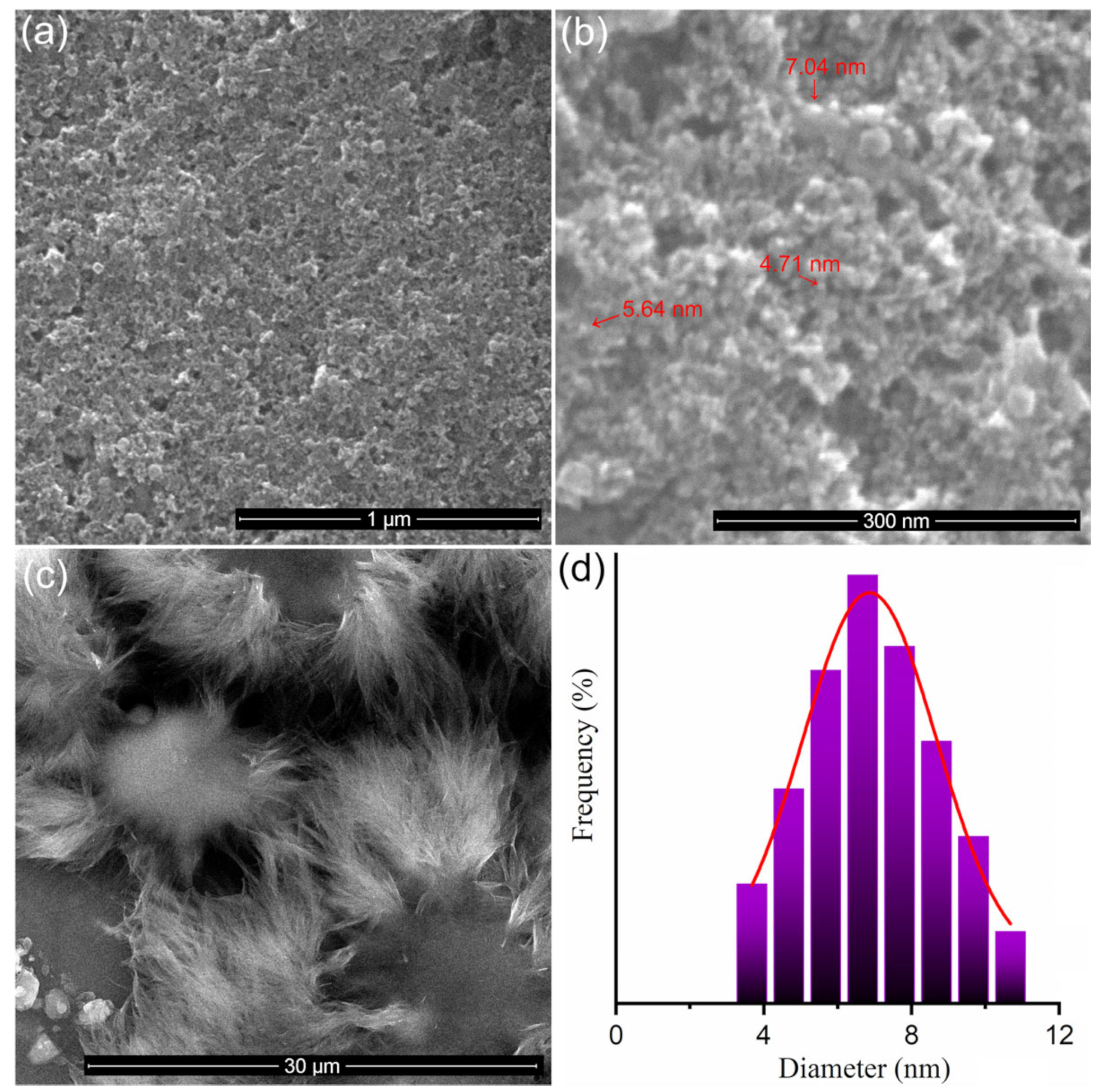
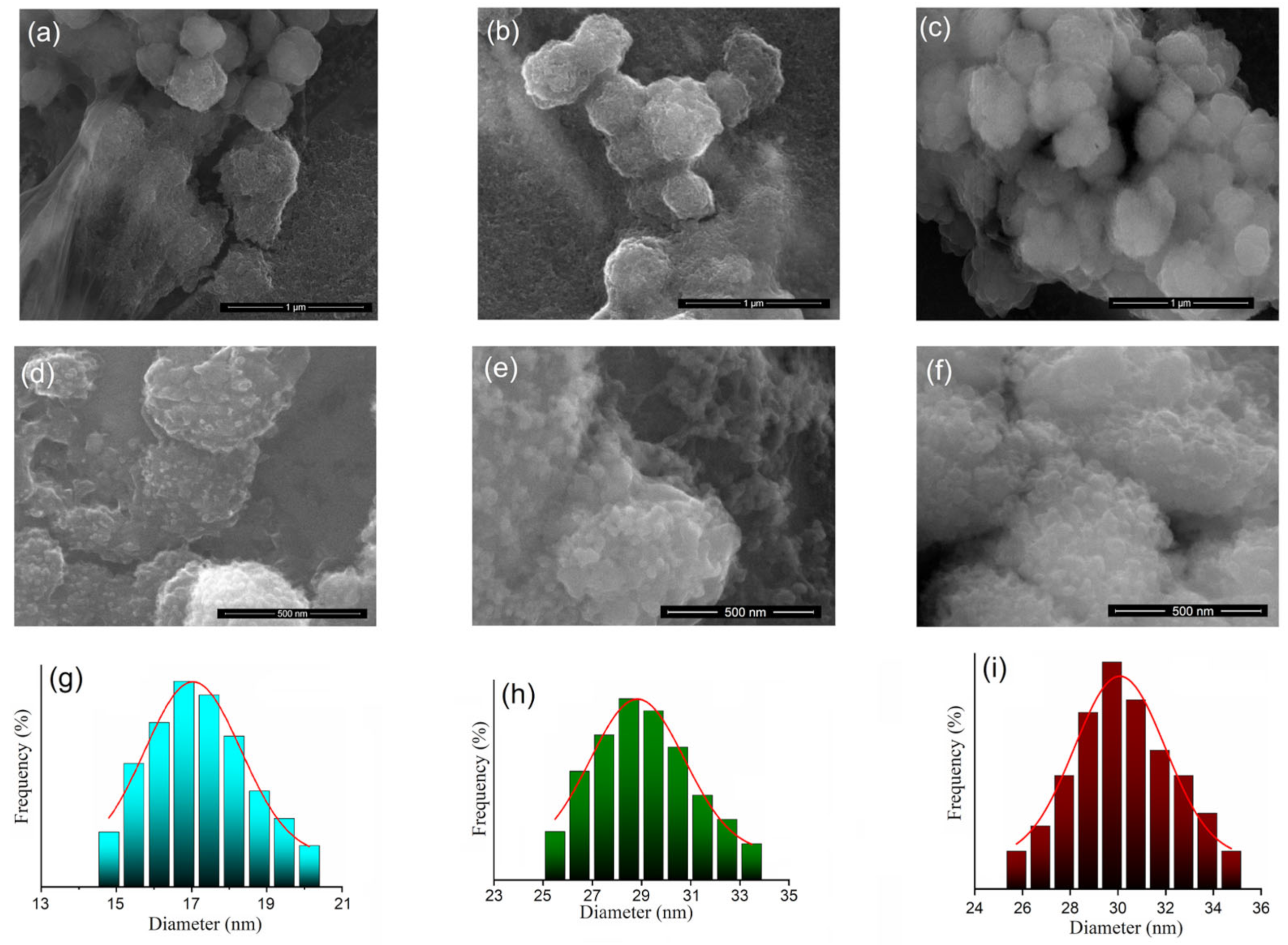
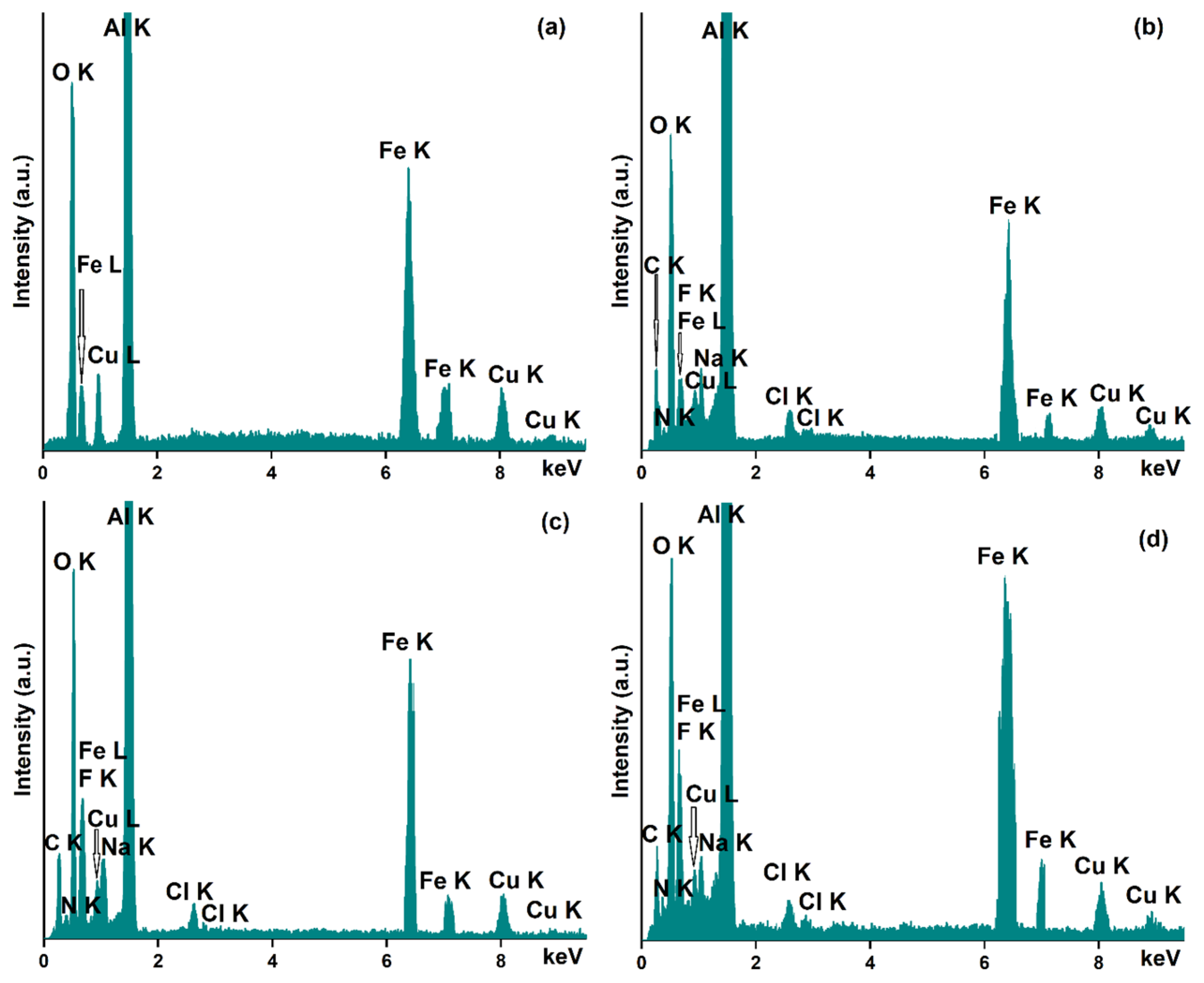
3.1. Characterization of Dextran-Coated IONP Loaded with 5-FU
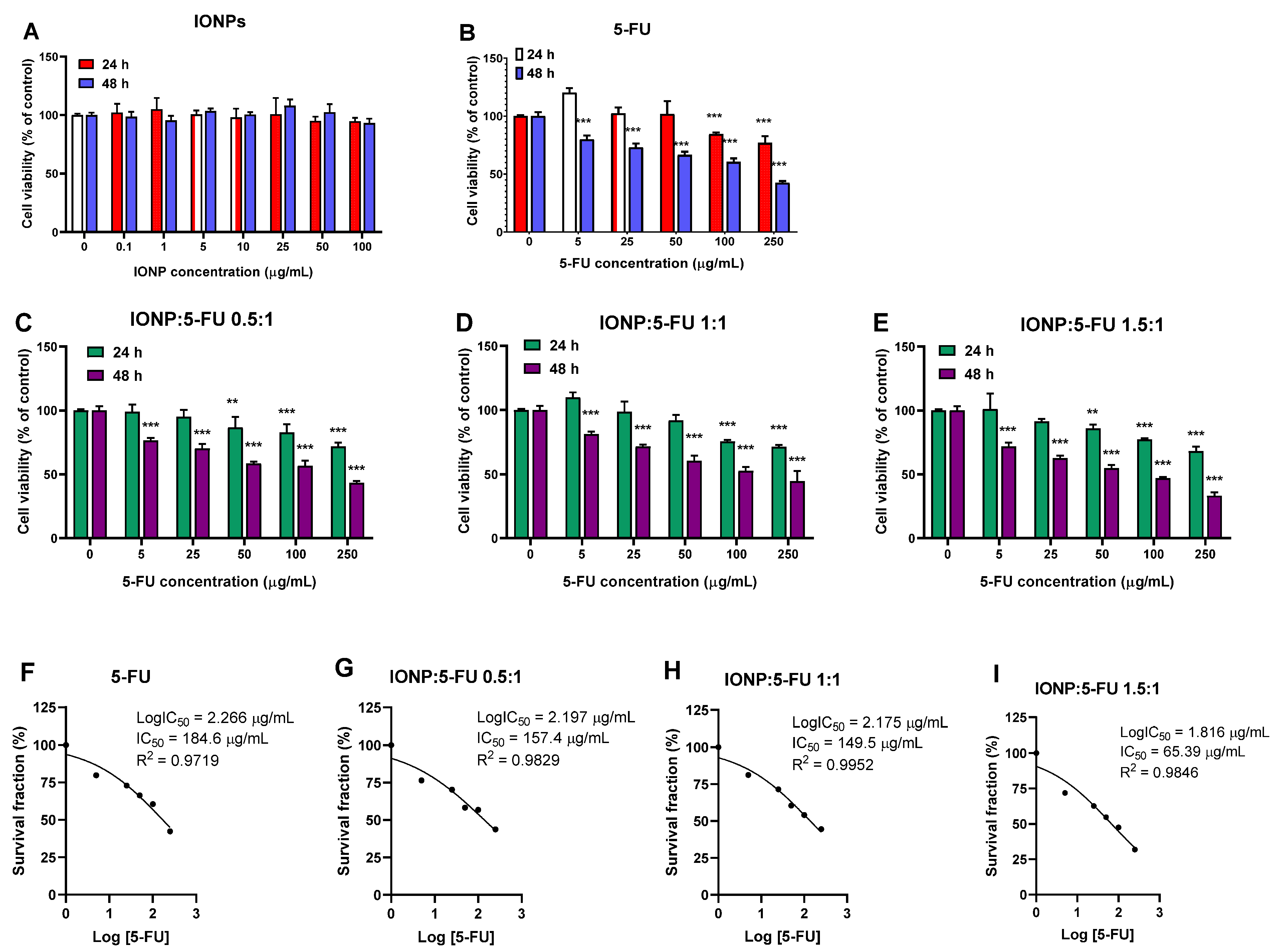
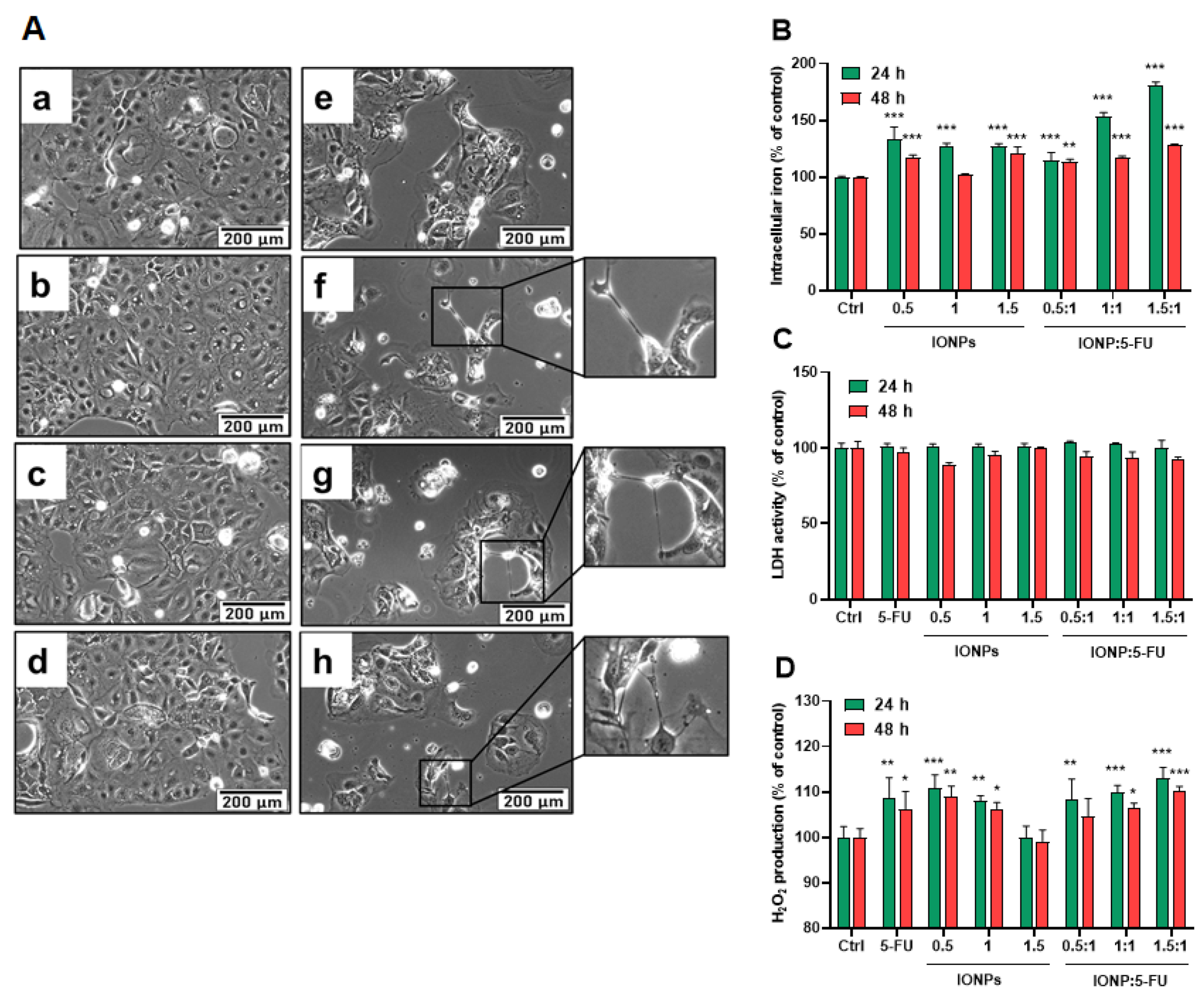
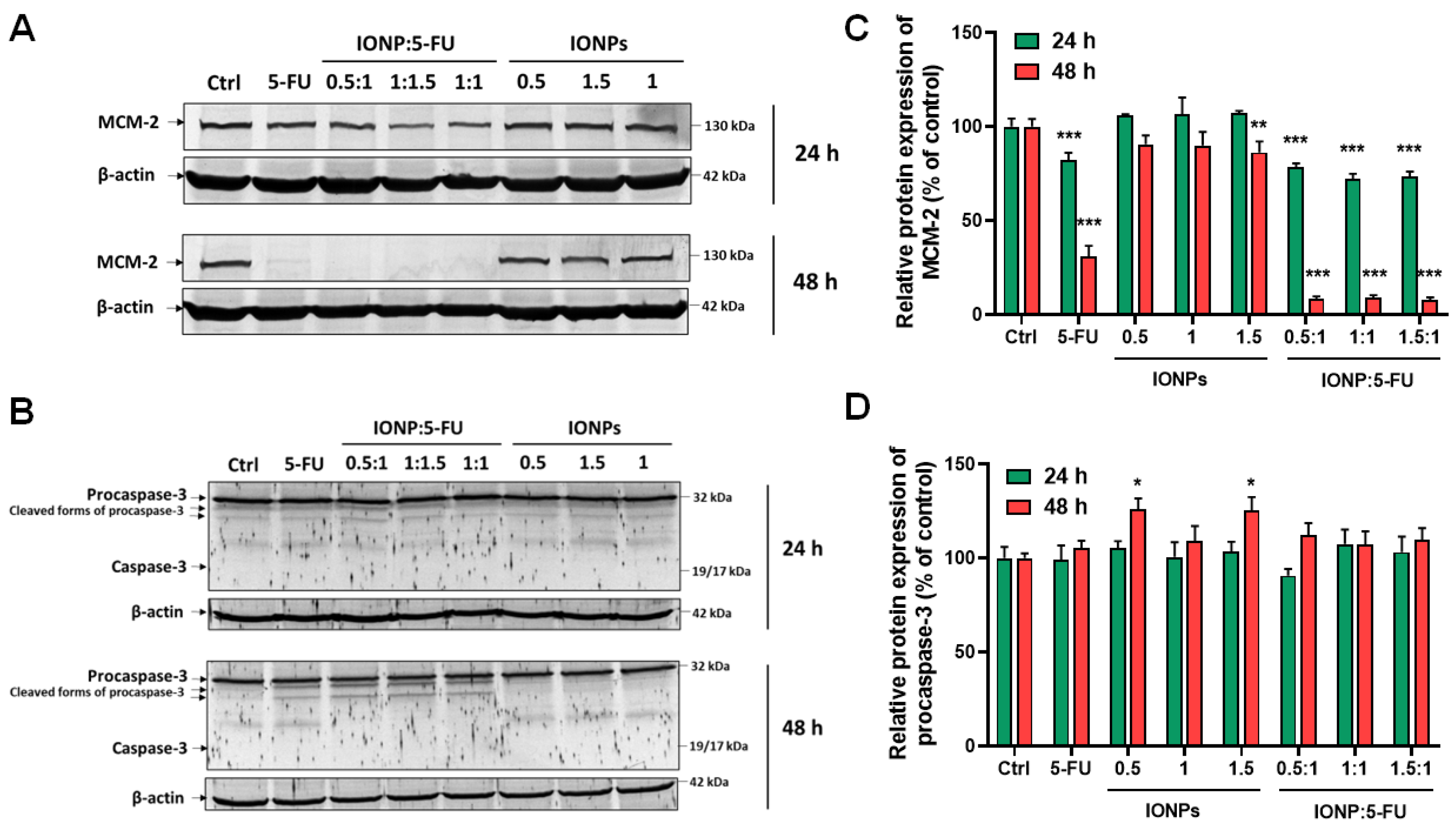
3.2. Evaluation of the Anti-Tumoral Activity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Wagle, N.S.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- White, A.; Ironmonger, L.; Steele, R.J.C.; Ormiston-Smith, N.; Crawford, C.; Seims, A. A review of sex related differences in colorectal cancer incidence, screening uptake, routes to diagnosis, cancer stage and survival in the UK. BMC Cancer 2018, 18, 906. [Google Scholar] [CrossRef] [PubMed]
- Jasperson, K.W.; Tuohy, T.M.; Neklason, D.W.; Burt, R.W. Hereditary and Familial Colon cancer. Gastroenterology 2010, 138, 2044–2058. [Google Scholar] [CrossRef]
- Ishimaru, K.; Tominaga, T.; Nonaka, T.; Fukuda, A.; Moriyama, M.; Oyama, S.; Ishii, M.; Sawai, T.; Nagayasu, T. Colorectal cancer in Crohn’s disease-a series of 6 cases. Surg. Case Rep. 2021, 7, 152. [Google Scholar] [CrossRef] [PubMed]
- Ye, P.; Xi, Y.; Huang, Z.; Xu, P. Linking Obesity with Colorectal Cancer: Epidemiology and Mechanistic Insights. Cancers 2020, 12, 1408. [Google Scholar] [CrossRef]
- Oruç, Z.; Kaplan, M.A. Effect of exercise on colorectal cancer prevention and treatment. World J. Gastrointest. Oncol. 2019, 11, 348–366. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Wei, B.; Zhai, Z.; Zheng, Y.; Yao, J.; Wang, S.; Xiang, D.; Hu, J.; Ye, X.; Yang, S.; et al. Dietary-Related Colorectal Cancer Burden: Estimates From 1990 to 2019. Front. Nutr. 2021, 8, 690663. [Google Scholar] [CrossRef]
- Gran, I.T.; Park, S.-Y.; Wilkens, L.R.; Haiman, C.A.; Le Marchand, L. Smoking-Related Risks of Colorectal Cancer by Anatomical Subsite and Sex. Am. J. Epidemiol. 2020, 189, 543–553. [Google Scholar] [CrossRef]
- Rossi, M.; Anwar, M.J.; Usman, A.; Keshavaezian, A.; Bishehsar, F. Colorectal Cancer and Alcohol Consumption-Populations to Molecules. Cancers 2018, 10, 38. [Google Scholar] [CrossRef]
- Xi, Y.; Xu, P. Global colorectal cancer burden in 2020 and projections to 2024. Translational. Oncol. 2021, 14, 101174. [Google Scholar] [CrossRef]
- Kotani, D.; Oki, E.; Nakamura, Y.; Yukami, H.; Mishima, S.; Bando, H.; Shirasu, H.; Yamazaki, K.; Watanabe, J.; Kotaka, M.; et al. Molecular residual disease and efficacy of adjuvant chemotherapy in patients with colorectal cancer. Nat. Med. 2023, 29, 127–134. [Google Scholar] [CrossRef]
- Vodenkova, S.; Buchler, T.; Cervena, K.; Veskrnova, V.; Vodicka, P.; Vymetalkova, V. 5-fluorouracil and other fluoropyrimidines in colorectal cancer: Past, present and future. Pharm. Ther. 2020, 206, 107447. [Google Scholar] [CrossRef] [PubMed]
- Okumura, K.; Shiomi, H.; Mekata, E.; Kaizuka, M.; Endo, Y.; Kurumi, Y.; Tani, T. Correlation between chemosensitivity and mRNA expression of 5-fluorouracil-related metabolic enzymes during liver metastasis of colorectal cancer. Oncol. Rep. 2006, 15, 875–882. [Google Scholar] [CrossRef]
- Sara, J.D.; Kaur, J.; Khodadadi, R.; Rehman, M.; Lobo, R.; Chakrabarti, S.; Herrmann, J.; Lerman, A.; Grothey, A. 5-fluorouracil and cardiotoxicity: A review. Ther. Adv. Med. Oncol. 2018, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Anaka, M.; Abdel-Rahman, O. Managing 5FU Cardiotoxicology in Colorectal Cancer Treatment. Cancer Manag. Res. 2022, 14, 273–285. [Google Scholar] [CrossRef]
- Krishmaiah, Y.; Satyanarayana, V.; Kumar, B.D.; Karthikeyan, R.; Bhaskar, P. In vivo pharmacokinetics in human volunteers: Oral administered guar gum-based colon-targeted 5-fluorouracil tablets. Eur. J. Pharm. Sci. 2003, 19, 355–362. [Google Scholar] [CrossRef]
- Entezar-Almahdi, E.; Mohammadi-Samani, S.; Tayebi, L.; Farjadian, F. Recent Advances in Designing 5-Fluorouracil Delivery Systems: A Stepping Stone in the Safe Treatment of Colorectal Cancer. Int. J. Nanomed. 2020, 15, 5445–5458. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Le, V.; Lang, M.; Liu, J. Preparation of polysaccharide derivates chitosan-graft-poly(ε caprolactone) amphilic copolymer micelles for 5-fluorouracil drug delivery. Colloids Surf. B 2014, 116, 745–750. [Google Scholar] [CrossRef]
- Zheng, Y.; Yang, W.; Wang, C.; Hu, J.; Fu, S.; Dong, L.; Wu, L.; Shen, X. Nanoparticles based on the complex of chitosan and polyaspartic acid sodium salt: Preparation, characterization, and the use for 5-fluorouracil delivery. Eur. J. Pharm. Biopharm. 2007, 67, 621–631. [Google Scholar] [CrossRef]
- Maghsoudi, A.; Shojaosadati, S.A.; Farahni, E.V. 5-fluorouracil-loaded BSA nanoparticles: Formulation, optimization and in vitro release study. AAPS PharmSciTech 2008, 9, 1092–1096. [Google Scholar] [CrossRef]
- Balas, M.; Constanda, S.; Duma-Voiculet, A.; Prodana, M.; Hermenean, A.; Pop, S.; Demetrescu, I.; Dinischiotu, A. Fabrication and toxicity characterization of a hybrid material based on oxidized and aminated MWCNT loaded with carboplatin. Toxicol. Vitr. 2016, 37, 189–200. [Google Scholar] [CrossRef] [PubMed]
- González-Lavado, E.; Valdivia, L.; García-Castaño, A.; González, F.; Pesquera, C.; Valiente, R.; Fanarraga, M.L. Multi-walled carbon nanotubes complement the anti-tumoral effect of 5-fluorouracil. Oncotarget 2019, 10, 2022–2029. [Google Scholar] [CrossRef] [PubMed]
- Hiremath, C.; Kariduraganavar, M.Y.; Hiremath, M.B. Synergistic delivery of 5-fluorouracil and curcumin using human serum albumin-coated iron oxide nanoparticles by folic acid targeting. Prog. Biomater. 2018, 7, 297–306. [Google Scholar] [CrossRef]
- Kiamohammadi, L.; Asadi, L.; Shivalilou, S.; Khoei, S.; Khoee, S.; Soleimani, M.; Minaei, S.E. Physical and Biological Properties of 5-Fluorouracil Polymer-Coated Magnetite Nanographene Oxide as a New Thermosensitizer for Alternative Magnetic hyperthermia and a Magnetic resonance Imaging Contrast Agent: In Vitro and In Vivo study. ACS Omega 2021, 6, 20192–20204. [Google Scholar] [CrossRef] [PubMed]
- Tavares Luiz, M.; Santos Rosa Viegas, J.; Palma Abriata, J.; Viegas, F.; Testa Moura de Carvalho Vicentini, F.; Lopes Badra Bentley, M.V.; Chorilli, M.; Maldonado Marchetti, J.; Tapia-Blácido, D.R. Design of experiments (DoE) to develop and to optimize nanoparticles as drug delivery systems. Eur. J. Pharm. Biopharm. 2021, 165, 127–148. [Google Scholar] [CrossRef]
- Lal, R.; Kumar Marwaha, R.; Pandita, D.; Dureja, H. Formulation and Optimization of 5-Fluorouracil Loaded Chitosan Nanoparticles Employing Central Composite Design. Drug Deliv. Lett. 2012, 2, 281–289. [Google Scholar] [CrossRef]
- Massart, R.; Dubois, E.; Cabuil, V.; Hasmonay, E. Preparation and properties of monodisperse magnetic fluids. J. Magn. Magn. Mater. 1995, 149, 1–5. [Google Scholar] [CrossRef]
- Predoi, S.-A.; Iconaru, S.L.; Predoi, D. In Vitro and In Vivo Biological Assays of Dextran Coated Iron Oxide Aqueous Magnetic Fluids. Pharmaceutics 2023, 15, 177. [Google Scholar] [CrossRef]
- Prodan, A.M.; Iconaru, S.L.; Ciobanu, C.S.; Chifiriuc, M.C.; Stoicea, M.; Predoi, D. Iron Oxide Magnetic Nanoparticles: Characterization and Toxicity Evaluation by In Vitro and In Vivo Assays. J. Nanomat. 2013, 2013, 587021. [Google Scholar] [CrossRef]
- Prodan, A.M.; Iconaru, S.L.; Chifiriuc, C.M.; Bleotu, C.; Ciobanu, C.S.; Motelica-Heino, M.; Sizaret, S.; Predoi, D. Magnetic Properties and Biological Activity Evaluation of Iron Oxide Nanoparticles. J. Nanomat. 2013, 2013, 893970. [Google Scholar] [CrossRef]
- Ciobanu, C.S.; Iconaru, S.L.; Gyorgy, E.; Radu, M.; Costache, M.; Dinischiotu, A.; Le Coustumer, P.; Lafdi, K.; Predoi, D. Biomedical properties and preparation of iron oxide-dextran nanostructures by MAPLE technique. Chem. Cent. J. 2012, 6, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Iconaru, S.L.; Ciobanu, C.S.; Le Coustumer, P.; Predoi, D. Structural Characterization and Magnetic Properties of Iron Oxides Biological Polymers. J. Supercond. Nov. Magn. 2013, 26, 851–855. [Google Scholar] [CrossRef]
- Balas, M.; Dumitrache, F.; Badea, M.A.; Fleaca, C.; Badoi, A.; Tanasa, E.; Dinischiotu, A. Coating Dependent In Vitro Biocompatibility of New Fe-Si Nanoparticles. Nanomaterials 2018, 8, 495. [Google Scholar] [CrossRef] [PubMed]
- Amini-Fazl, M.S.; Mohammadi, R.; Kheiri, K. 5-Fluorouracil loaded chitosan/polyacrylic acid/Fe3O4 magnetic nanocomposite hydrogel as a potential anticancer drug delivery system. Int. J. Biol. Macromol. 2019, 132, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Ayyanaar, S.; Bhaskar, R.; Esthar, S.; Vadivel, M.; Rajesh, J.; Rajagopal, G. Design and development of 5-fluorouracil loaded biodegradable magnetic microspheres as site-specific drug delivery vehicle for cancer therapy. J. Magn. Magn. 2022, 546, 168853. [Google Scholar] [CrossRef]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Vander Elst, L.; Muller, R.N. Magnetic iron oxide nanoparticles: Synthesis, stabilization, vectorization, physicochemical Characterizations, and Biological Applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef]
- Honary, S.; Zahir, F. Effect of zeta potential on the properties of nano-drug delivery systems—A review (Part 2). Trop. J. Pharm. Res. 2013, 12, 265–273. [Google Scholar]
- Samimi, S.; Maghsoudnia, N.; Eftekhari, R.B.; Dorkoosh, F. Lipid-Based Nanoparticles for Drug Delivery Systems; Elsevier Inc.: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Gumustas, M.; Sengel-Turk, C.T.; Gumustas, A.; Ozkan, S.A.; Uslu, B. Effect of Polymer-Based Nanoparticles on the Assay of Antimicrobial Drug Delivery Systems; Elsevier Inc.: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Ebadi, M.; Saifullah, B.; Buskaran, K.; Hussein, M.Z.; Fakurazi, S. Synthesis and properties of magnetic nan-otheranostics coated with polyethylene glycol/5-fluorouracil/layered double hydroxid. Int. J. Nanomed. 2019, 14, 6661–6678. [Google Scholar] [CrossRef]
- Cortajarena, A.L.; Ortega, D.; Ocampo, S.M.; Gonzalez-García, A.; Couleaud, P.; Miranda, R.; Belda-Iniesta, C.; Ayuso-Sacido, A. Engineering Iron Oxide Nanoparticles for Clinical Settings. Nanobiomedicine 2014, 1, 2. [Google Scholar] [CrossRef]
- Arbab, A.S.; Wilson, L.B.; Ashari, P.; Jordan, E.K.; Lewis, B.K.; Frank, J.A. A model of Lysosomal metabolism of dextran coated supermagnetic iron oxide (SPIO)nanoparticles: Implications for cellular magnetic resonance imaging. NMR Biomed. 2005, 18, 383–389. [Google Scholar] [CrossRef]
- Cromer Berman, S.M.; Wang, C.J.; Orukari, I.; Levchenko, A.; Bulte, J.W.; Walczak, P. Cell motility of neural stem cells is reduced after SPIO-labeling, which is mitigated after exocytosis. Magn. Reason. Med. 2013, 69, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Oh, N.; Park, J.H. Endocytosis and exocytosis of nanoparticles in mammalian cells. Int. J. Nanomed. 2014, 9, 51–63. [Google Scholar] [CrossRef]
- Bruschi, M.L.; de Toledo, L.d.A.S. Pharmaceutical Applications of Iron-Oxide Magnetic Nanoparticles. Magnetochemistry 2019, 5, 50. [Google Scholar] [CrossRef]
- Aranda, A.; Sequedo, L.; Tolosa, L.; Quintas, G.; Burello, E.; Castell, J.V.; Gombau, L. Dichloro-dihydro-fluorescein diacetate (DCFH-DA) assay: A quantitative method for oxida-tive stress assessment of nanoparticle-treated cells. Toxicol. Vitr. 2013, 27, 954–963. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: Oxidative eustress. Redox Biol. 2017, 11, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Cheng, Z.; Liu, S. MCM2 in human cancer: Functions, mechanisms, and clinical significance. Mol. Med. 2022, 28, 128. [Google Scholar] [CrossRef] [PubMed]
- Mhaidat, N.M.; Bouklihacene, M.; Thorne, R.F. 5-Fluorouracil-induced apoptosis in colorectal cancer cells is caspase-9-dependent and mediated by activation of protein kinase C-δ. Oncol. Lett. 2014, 8, 699–704. [Google Scholar] [CrossRef]
- Flanagan, L.; Meyer, M.; Fay, J.; Curry, S.; Bacon, O.; Duessmann, H.; John, K.; Boland, K.C.; McNamara, D.A.; Kay, E.W.; et al. Low levels of Caspase-3 predict favourable response to 5FU-based chemotherapy in advanced colorectal cancer: Caspase-3 inhibition as a therapeutic approach. Cell Death Dis. 2016, 7, e2087. [Google Scholar] [CrossRef]
- Yang, J.W.; Zhang, Q.H.; Liu, T. Autophagy facilitates anticancer effect of 5-fluorouracil in HCT-116 cells. J. Cancer Res. Ther. 2018, 14, S1141–S1147. [Google Scholar] [CrossRef]
- Zhou, M.; Liu, X.; Li, Z.; Huang, Q.; Li, F.; Li, C.Y. Caspase-3 regulates the migration, invasion and metastasis of colon cancer cells. Int. J. Cancer 2018, 143, 921–930. [Google Scholar] [CrossRef]
- Akay, S.; Kayan, B.; Yang, Y. Solubility and Chromatographic Separation of 5-Fluorouracil under Subcritical Water Conditions. J. Chem. Eng. Data 2017, 62, 1538–1543. [Google Scholar] [CrossRef]
- Poller, J.M.; Zaloga, J.; Schreiber, E.; Unterweger, H.; Janko, C.; Radon, P.; Eberbeck, D.; Trahms, L.; Alexiou, C.; Friedrich, R.P. Selection of potential iron oxide nanoparticles for breast cancer treatment based on in vitro cytotoxicity and cellular uptake. Int. J. Nanomed. 2017, 12, 3207. [Google Scholar] [CrossRef] [PubMed]
- Sahay, G.; Alakhova, D.Y.; Kabanov, A.V. Endocytosis of nanomedicines. J. Control. Release 2010, 145, 182–195. [Google Scholar] [CrossRef] [PubMed]
- Miralinaghi, P.; Kashani, P.; Moniri, E.; Miralinaghi, M. Non-linear kinetic, equilibrium, and thermodynamic studies of 5-fluorouracil adsorption onto chitosan–functionalized graphene oxide. Mater. Res. Express 2019, 6, 065305. [Google Scholar] [CrossRef]

| Time (Days) | 1 | 7 | 14 | 28 | |
|---|---|---|---|---|---|
| Dh | IONP:5-FU 0.5:1 | 38.6 | 42.4 | 45.8 | 46.7 |
| IONP:5-FU 1:1 | 44.8 | 48.6 | 55.5 | 56.8 | |
| IONP:5-FU 1.5:1 | 57.6 | 63.9 | 66.2 | 69.4 | |
| Time (Days) | 1 | 7 | 14 | 28 | |
|---|---|---|---|---|---|
| ζ | IONP:5-FU 0.5:1 | −39.6 | −38.9 | −37.9 | −36.8 |
| IONP:5-FU 1:1 | −40.5 | −39.9 | −38.3 | −37.7 | |
| IONP:5-FU 1.5:1 | −41.9 | −41 | −40.6 | −39.9 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Predoi, D.; Balas, M.; Badea, M.A.; Ciobanu, S.C.; Buton, N.; Dinischiotu, A. Dextran-Coated Iron Oxide Nanoparticles Loaded with 5-Fluorouracil for Drug-Delivery Applications. Nanomaterials 2023, 13, 1811. https://doi.org/10.3390/nano13121811
Predoi D, Balas M, Badea MA, Ciobanu SC, Buton N, Dinischiotu A. Dextran-Coated Iron Oxide Nanoparticles Loaded with 5-Fluorouracil for Drug-Delivery Applications. Nanomaterials. 2023; 13(12):1811. https://doi.org/10.3390/nano13121811
Chicago/Turabian StylePredoi, Daniela, Mihaela Balas, Madalina Andreea Badea, Steluta Carmen Ciobanu, Nicolas Buton, and Anca Dinischiotu. 2023. "Dextran-Coated Iron Oxide Nanoparticles Loaded with 5-Fluorouracil for Drug-Delivery Applications" Nanomaterials 13, no. 12: 1811. https://doi.org/10.3390/nano13121811
APA StylePredoi, D., Balas, M., Badea, M. A., Ciobanu, S. C., Buton, N., & Dinischiotu, A. (2023). Dextran-Coated Iron Oxide Nanoparticles Loaded with 5-Fluorouracil for Drug-Delivery Applications. Nanomaterials, 13(12), 1811. https://doi.org/10.3390/nano13121811











