An Overview of the Use of Nanoparticles in Vaccine Development
Abstract
1. Introduction
2. Conventional Vaccines
3. Nanotechnology in Vaccines
3.1. New Vaccine Technologies
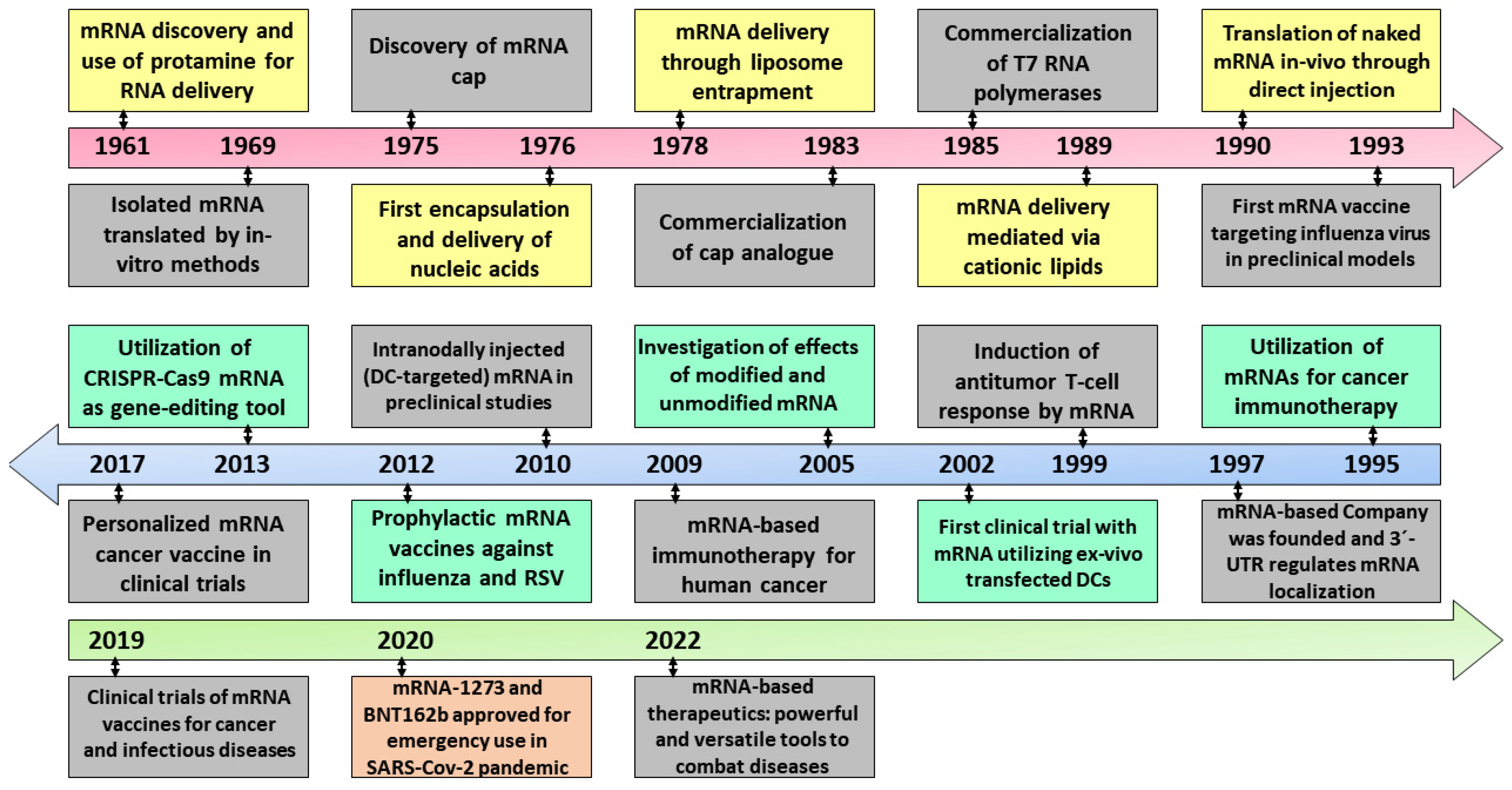
3.1.1. mRNA Technology
3.1.2. mRNA Delivery Platforms
Lipid Nanoparticles
Human Cells
Extracellular Vesicles
Biomimetic Nanoparticles
3.1.3. DNA Technology
3.2. Nanoparticles in Biomedicine
3.3. Nanoparticles Employed in Vaccines Technologies
3.3.1. Polymeric Nanoparticles
3.3.2. Virus-Like Nanoparticles
3.3.3. Immune Stimulating Complexes
3.3.4. Inorganic Nanoparticles
3.3.5. Liposomes and Lipid Nanoparticles
3.4. Nanoparticle Platforms as Vaccine Adjuvants
3.5. Interaction with the Body
4. Success Case of Vaccines Based on Nanoparticles to Fight SARS-CoV-2
4.1. Jonhson & Johnson/Jansen and Oxford/Astra Zeneca Vaccines
4.2. Moderna and Pfizer Vaccines
5. Conclusions and Final Remarks
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Amanna, I.J.; Slifka, M.K. Successful Vaccines BT—Vaccination Strategies Against Highly Variable Pathogens; Hangartner, L., Burton, D.R., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–30. [Google Scholar] [CrossRef]
- Greenwood, B. The Contribution of Vaccination to Global Health: Past, Present and Future. Philos. Trans. R. Soc. London. Ser. B Biol. Sci. 2014, 369, 20130433. [Google Scholar] [CrossRef] [PubMed]
- Plotkin, S.A.; Plotkin, S.L. The Development of Vaccines: How the Past Led to the Future. Nat. Rev. Microbiol. 2011, 9, 889–893. [Google Scholar] [CrossRef] [PubMed]
- Crimmins, E.M. Lifespan and Healthspan: Past, Present, and Promise. Gerontologist 2015, 55, 901–911. [Google Scholar] [CrossRef]
- Cox, R.J.; Brokstad, K.A.; Ogra, P. Influenza Virus: Immunity and Vaccination Strategies. Comparison of the Immune Response to Inactivated and Live, Attenuated Influenza Vaccines. Scand. J. Immunol. 2004, 59, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Delany, I.; Rappuoli, R.; De Gregorio, E. Vaccines for the 21st Century. EMBO Mol. Med. 2014, 6, 708–720. [Google Scholar] [CrossRef] [PubMed]
- Plotkin, S.A. Vaccines: Past, Present and Future. Nat. Med. 2005, 11, S5–S11. [Google Scholar] [CrossRef]
- Heng, W.T.; Yew, J.S.; Poh, C.L. Nanovaccines against Viral Infectious Diseases. Pharmaceutics 2022, 14, 2554. [Google Scholar] [CrossRef]
- Chauhan, G.; Madou, M.J.; Kalra, S.; Chopra, V.; Ghosh, D.; Martinez-Chapa, S.O. Nanotechnology for COVID-19: Therapeutics and Vaccine Research. ACS Nano 2020, 14, 7760–7782. [Google Scholar] [CrossRef]
- Kirtane, A.R.; Verma, M.; Karandikar, P.; Furin, J.; Langer, R.; Traverso, G. Nanotechnology Approaches for Global Infectious Diseases. Nat. Nanotechnol. 2021, 16, 369–384. [Google Scholar] [CrossRef] [PubMed]
- Gregory, A.E.; Titball, R.; Williamson, D. Vaccine Delivery Using Nanoparticles. Front. Cell Infect. Microbiol. 2013, 3, 13. [Google Scholar] [CrossRef]
- Pollard, A.J.; Bijker, E.M. A Guide to Vaccinology: From Basic Principles to New Developments. Nat. Rev. Immunol. 2021, 21, 83–100. [Google Scholar] [CrossRef] [PubMed]
- Riedel, S. Edward Jenner and the History of Smallpox and Vaccination. In Baylor University Medical Center Proceedings; Taylor & Francis: Oxfordshire, UK, 2005; Volume 18, pp. 21–25. [Google Scholar] [CrossRef]
- Hajj Hussein, I.; Chams, N.; Chams, S.; El Sayegh, S.; Badran, R.; Raad, M.; Gerges-Geagea, A.; Leone, A.; Jurjus, A. Vaccines Through Centuries: Major Cornerstones of Global Health. Front. Public Health 2015, 3, 269. [Google Scholar] [CrossRef] [PubMed]
- Khan, Y.H.; Saifullah, A.; Mallhi, T.H. Bacterial Vaccines. In Encyclopedia of Infection and Immunity; Rezaei, N., Ed.; Elsevier: Oxford, UK, 2022; pp. 530–544. [Google Scholar] [CrossRef]
- Nascimento, I.P.; Leite, L.C.C. Recombinant Vaccines and the Development of New Vaccine Strategies. Braz. J. Med. Biol. Res. 2012, 45, 1102–1111. [Google Scholar] [CrossRef]
- Coler, R.N.; Reed, S.G. Second-Generation Vaccines against Leishmaniasis. Trends Parasitol. 2005, 21, 244–249. [Google Scholar] [CrossRef]
- Martins, R.; Carlos, A.R.; Braza, F.; Thompson, J.A.; Bastos-Amador, P.; Ramos, S.; Soares, M.P. Disease Tolerance as an Inherent Component of Immunity. Annu. Rev. Immunol. 2019, 37, 405–437. [Google Scholar] [CrossRef] [PubMed]
- Boraschi, D.; Italiani, P. From Antigen Delivery System to Adjuvanticy: The Board Application of Nanoparticles in Vaccinology. Vaccines 2015, 3, 930–939. [Google Scholar] [CrossRef]
- Gurunathan, S.; Klinman, D.M.; Seder, R.A. DNA Vaccines: Immunology, Application, and Optimization. Annu. Rev. Immunol. 2000, 18, 927–974. [Google Scholar] [CrossRef]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. MRNA Vaccines—A New Era in Vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef] [PubMed]
- Hilleman, M.R. Strategies and Mechanisms for Host and Pathogen Survival in Acute and Persistent Viral Infections. Proc. Natl. Acad. Sci. USA 2004, 101 (Suppl. S2), 14560–14566. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.Y.S.; Rutka, J.T.; Chan, W.C.W. Nanomedicine. N. Engl. J. Med. 2010, 363, 2434–2443. [Google Scholar] [CrossRef]
- Webster, T.J. Nanomedicine: What’s in a Definition? Int. J. Nanomed. 2006, 1, 115–116. [Google Scholar] [CrossRef] [PubMed]
- Pelaz, B.; Alexiou, C.; Alvarez-Puebla, R.A.; Alves, F.; Andrews, A.M.; Ashraf, S.; Balogh, L.P.; Ballerini, L.; Bestetti, A.; Brendel, C.; et al. Diverse Applications of Nanomedicine. ACS Nano 2017, 11, 2313–2381. [Google Scholar] [CrossRef] [PubMed]
- Lammers, T.; Ferrari, M. The Success of Nanomedicine. Nano Today 2020, 31, 100853. [Google Scholar] [CrossRef]
- He, Q.; Mao, Q.; Peng, X.; He, Z.; Lu, S.; Zhang, J.; Gao, F.; Bian, L.; An, C.; Yu, W.; et al. Immunogenicity and Protective Efficacy of a Recombinant Protein Subunit Vaccine and an Inactivated Vaccine against SARS-CoV-2 Variants in Non-Human Primates. Signal Transduct. Target. Ther. 2022, 7, 69. [Google Scholar] [CrossRef]
- Ghattas, M.; Dwivedi, G.; Lavertu, M.; Alameh, M.-G. Vaccine Technologies and Platforms for Infectious Diseases: Current Progress, Challenges, and Opportunities. Vaccines 2021, 9, 1490. [Google Scholar] [CrossRef]
- Chavda, V.P.; Soni, S.; Vora, L.K.; Soni, S.; Khadela, A.; Ajabiya, J. MRNA-Based Vaccines and Therapeutics for COVID-19 and Future Pandemics. Vaccines 2022, 10, 2150. [Google Scholar] [CrossRef]
- Sahin, U.; Kariko, K.; Tureci, O. MRNA-Based Therapeutics—Developing a New Class of Drugs. Nat. Rev. Drug Discov. 2014, 13, 759–780. [Google Scholar] [CrossRef]
- Kulkarni, J.A.; Witzigmann, D.; Thomson, S.B.; Chen, S.; Leavitt, B.R.; Cullis, P.R.; van der Meel, R. The Current Landscape of Nucleic Acid Therapeutics. Nat. Nanotechnol. 2021, 16, 630–643. [Google Scholar] [CrossRef]
- Vishweshwaraiah, Y.L.; Dokholyan, N.V. MRNA Vaccines for Cancer Immunotherapy. Front. Immunol. 2022, 13, 1029069. [Google Scholar] [CrossRef]
- Schlake, T.; Thess, A.; Fotin-Mleczek, M.; Kallen, K.-J. Developing MRNA-Vaccine Technologies. RNA Biol. 2012, 9, 1319–1330. [Google Scholar] [CrossRef]
- Rohner, E.; Yang, R.; Foo, K.S.; Goedel, A.; Chien, K.R. Unlocking the Promise of MRNA Therapeutics. Nat. Biotechnol. 2022, 40, 1586–1600. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhu, L.; Wang, X.; Jin, H. RNA-Based Therapeutics: An Overview and Prospectus. Cell Death Dis. 2022, 13, 644. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Troilo, P.J.; Wang, X.; Griffiths, T.G.; Pacchione, S.J.; Barnum, A.B.; Harper, L.B.; Pauley, C.J.; Niu, Z.; Denisova, L.; et al. Detection of Integration of Plasmid DNA into Host Genomic DNA Following Intramuscular Injection and Electroporation. Gene Ther. 2004, 11, 711–721. [Google Scholar] [CrossRef]
- Zheng, C.; Baum, B.J.; Iadarola, M.J.; O’Connell, B.C. Genomic Integration and Gene Expression by a Modified Adenoviral Vector. Nat. Biotechnol. 2000, 18, 176–180. [Google Scholar] [CrossRef]
- Aldén, M.; Olofsson Falla, F.; Yang, D.; Barghouth, M.; Luan, C.; Rasmussen, M.; De Marinis, Y. Intracellular Reverse Transcription of Pfizer BioNTech COVID-19 MRNA Vaccine BNT162b2 In Vitro in Human Liver Cell Line. Curr. Issues Mol. Biol. 2022, 44, 1115–1126. [Google Scholar] [CrossRef]
- Yang, L.; Gong, L.; Wang, P.; Zhao, X.; Zhao, F.; Zhang, Z.; Li, Y.; Huang, W. Recent Advances in Lipid Nanoparticles for Delivery of MRNA. Pharmaceutics 2022, 14, 2682. [Google Scholar] [CrossRef]
- Khurana, A.; Allawadhi, P.; Khurana, I.; Allwadhi, S.; Weiskirchen, R.; Banothu, A.K.; Chhabra, D.; Joshi, K.; Bharani, K.K. Role of Nanotechnology behind the Success of MRNA Vaccines for COVID-19. Nano Today 2021, 38, 101142. [Google Scholar] [CrossRef]
- Swetha, K.; Kotla, N.G.; Tunki, L.; Jayaraj, A.; Bhargava, S.K.; Hu, H.; Bonam, S.R.; Kurapati, R. Recent Advances in the Lipid Nanoparticle-Mediated Delivery of MRNA Vaccines. Vaccines 2023, 11, 658. [Google Scholar] [CrossRef]
- Pilkington, E.H.; Suys, E.J.A.; Trevaskis, N.L.; Wheatley, A.K.; Zukancic, D.; Algarni, A.; Al-Wassiti, H.; Davis, T.P.; Pouton, C.W.; Kent, S.J.; et al. From Influenza to COVID-19: Lipid Nanoparticle MRNA Vaccines at the Frontiers of Infectious Diseases. Acta Biomater. 2021, 131, 16–40. [Google Scholar] [CrossRef]
- Zeng, C.; Zhang, C.; Walker, P.G.; Dong, Y. Formulation and Delivery Technologies for MRNA Vaccines. Curr. Top. Microbiol. Immunol. 2022, 440, 71–110. [Google Scholar] [CrossRef]
- Hou, X.C.; Zaks, T.; Langer, R.; Dong, Y.Z. Lipid Nanoparticles for MRNA Delivery. Nat. Rev. Mater. 2021, 6, 1078–1094. [Google Scholar] [CrossRef]
- Liu, P.; Chen, G.; Zhang, J. A Review of Liposomes as a Drug Delivery System: Current Status of Approved Products, Regulatory Environments, and Future Perspectives. Molecules 2022, 27, 1372. [Google Scholar] [CrossRef]
- Herrmann, I.K.; Wood, M.J.A.; Fuhrmann, G. Extracellular Vesicles as a Next-Generation Drug Delivery Platform. Nat. Nanotechnol. 2021, 16, 748–759. [Google Scholar] [CrossRef]
- Qin, S.; Tang, X.; Chen, Y.; Chen, K.; Fan, N.; Xiao, W.; Zheng, Q.; Li, G.; Teng, Y.; Wu, M.; et al. MRNA-Based Therapeutics: Powerful and Versatile Tools to Combat Diseases. Signal Transduct. Target. Ther. 2022, 7, 166. [Google Scholar] [CrossRef]
- Lorenzen, N.; LaPatra, S.E. DNA Vaccines for Aquacultured Fish. Rev. Sci. Tech. 2005, 24, 201–213. [Google Scholar] [CrossRef]
- Pardi, N.; Hogan, M.J.; Naradikian, M.S.; Parkhouse, K.; Cain, D.W.; Jones, L.; Moody, M.A.; Verkerke, H.P.; Myles, A.; Willis, E.; et al. Nucleoside-Modified MRNA Vaccines Induce Potent T Follicular Helper and Germinal Center B Cell Responses. J. Exp. Med. 2018, 215, 1571–1588. [Google Scholar] [CrossRef]
- pCI-Neo Mammalian Expression Vector. Available online: https://www.promega.com/products/vectors/protein-expression-vectors/pci_neo-mammalian-expression-vector/?catNum=E1841 (accessed on 6 June 2023).
- Alcolea, P.J.; Alonso, A.; Larraga, V. The Antibiotic Resistance-Free Mammalian Expression Plasmid Vector PPAL for Development of Third Generation Vaccines. Plasmid 2019, 101, 35–42. [Google Scholar] [CrossRef]
- Alcolea, P.J.; Larraga, J.; Rodríguez-Martín, D.; Alonso, A.; Loayza, F.J.; Rojas, J.M.; Ruiz-García, S.; Louloudes-Lázaro, A.; Carlón, A.B.; Sánchez-Cordón, P.J.; et al. Non-Replicative Antibiotic Resistance-Free DNA Vaccine Encoding S and N Proteins Induces Full Protection in Mice against SARS-CoV-2. Front. Immunol. 2022, 13, 1023255. [Google Scholar] [CrossRef]
- Liu, M.A. DNA Vaccines: An Historical Perspective and View to the Future. Immunol. Rev. 2011, 239, 62–84. [Google Scholar] [CrossRef]
- Yang, B.; Jeang, J.; Yang, A.; Wu, T.C.; Hung, C.-F. DNA Vaccine for Cancer Immunotherapy. Hum. Vaccin. Immunother. 2014, 10, 3153–3164. [Google Scholar] [CrossRef]
- Lan, T.; Kandimalla, E.R.; Yu, D.; Bhagat, L.; Li, Y.; Wang, D.; Zhu, F.; Tang, J.X.; Putta, M.R.; Cong, Y.; et al. Stabilized Immune Modulatory RNA Compounds as Agonists of Toll-like Receptors 7 and 8. Proc. Natl. Acad. Sci. USA 2007, 104, 13750–13755. [Google Scholar] [CrossRef]
- Khobragade, A.; Bhate, S.; Ramaiah, V.; Deshpande, S.; Giri, K.; Phophle, H.; Supe, P.; Godara, I.; Revanna, R.; Nagarkar, R.; et al. Efficacy, Safety, and Immunogenicity of the DNA SARS-CoV-2 Vaccine (ZyCoV-D): The Interim Efficacy Results of a Phase 3, Randomised, Double-Blind, Placebo-Controlled Study in India. Lancet 2022, 399, 1313–1321. [Google Scholar] [CrossRef]
- Hobernik, D.; Bros, M. DNA Vaccines—How Far From Clinical Use? Int. J. Mol. Sci. 2018, 19, 3605. [Google Scholar] [CrossRef]
- Batty, C.J.; Bachelder, E.M.; Ainslie, K.M. Historical Perspective of Clinical Nano and Microparticle Formulations for Delivery of Therapeutics. Trends Mol. Med. 2021, 27, 516–519. [Google Scholar] [CrossRef]
- Sainz, V.; Conniot, J.; Matos, A.I.; Peres, C.; Zupančič, E.; Moura, L.; Silva, L.C.; Florindo, H.F.; Gaspar, R.S. Regulatory Aspects on Nanomedicines. Biochem. Biophys. Res. Commun. 2015, 468, 504–510. [Google Scholar] [CrossRef]
- Etheridge, M.L.; Campbell, S.A.; Erdman, A.G.; Haynes, C.L.; Wolf, S.M.; McCullough, J. The Big Picture on Nanomedicine: The State of Investigational and Approved Nanomedicine Products. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 1–14. [Google Scholar] [CrossRef]
- Duncan, R.; Gaspar, R. Nanomedicine(s) under the Microscope. Mol. Pharm. 2011, 8, 2101–2141. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.d.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano Based Drug Delivery Systems: Recent Developments and Future Prospects. J. Nanobiotechnology 2018, 16, 71. [Google Scholar] [CrossRef]
- Vallabani, N.V.S.; Singh, S. Recent Advances and Future Prospects of Iron Oxide Nanoparticles in Biomedicine and Diagnostics. 3 Biotech 2018, 8, 279. [Google Scholar] [CrossRef]
- Fenske, D.B.; Cullis, P.R. Liposomal Nanomedicines. Expert Opin. Drug Deliv. 2008, 5, 25–44. [Google Scholar] [CrossRef]
- Rezvantalab, S.; Drude, N.I.; Moraveji, M.K.; Guvener, N.; Koons, E.K.; Shi, Y.; Lammers, T.; Kiessling, F. PLGA-Based Nanoparticles in Cancer Treatment. Front. Pharmacol. 2018, 9, 1260. [Google Scholar] [CrossRef]
- Soppimath, K.S.; Aminabhavi, T.M.; Kulkarni, A.R.; Rudzinski, W.E. Biodegradable Polymeric Nanoparticles as Drug Delivery Devices. J. Control. Release 2001, 70, 1–20. [Google Scholar] [CrossRef]
- Scott, A.M.; Wolchok, J.D.; Old, L.J. Antibody Therapy of Cancer. Nat. Rev. Cancer 2012, 12, 278–287. [Google Scholar] [CrossRef]
- Zhao, L.; Seth, A.; Wibowo, N.; Zhao, C.X.; Mitter, N.; Yu, C.Z.; Middelberg, A.P.J. Nanoparticle Vaccines. Vaccine 2014, 32, 327–337. [Google Scholar] [CrossRef]
- Peek, L.J.; Middaugh, C.R.; Berkland, C. Nanotechnology in Vaccine Delivery. Adv. Drug Deliv. Rev. 2008, 60, 915–928. [Google Scholar] [CrossRef]
- Diaz-Arévalo, D.; Zeng, M. Nanoparticle-Based Vaccines: Opportunities and Limitations. In Nanopharmaceuticals; Elsevier: Amsterdam, The Netherlands, 2020; pp. 135–150. [Google Scholar] [CrossRef]
- Pati, R.; Shevtsov, M.; Sonawane, A. Nanoparticle Vaccines Against Infectious Diseases. Front. Immunol. 2018, 9, 2224. [Google Scholar] [CrossRef]
- Dobrovolskaia, M.A.; Aggarwal, P.; Hall, J.B.; McNeil, S.E. Preclinical Studies To Understand Nanoparticle Interaction with the Immune System and Its Potential Effects on Nanoparticle Biodistribution. Mol. Pharm. 2008, 5, 487–495. [Google Scholar] [CrossRef]
- Zolnik, B.S.; González-Fernández, A.; Sadrieh, N.; Dobrovolskaia, M.A. Nanoparticles and the Immune System. Endocrinology 2010, 151, 458–465. [Google Scholar] [CrossRef]
- Mottram, P.L.; Leong, D.; Crimeen-Irwin, B.; Gloster, S.; Xiang, S.D.; Meanger, J.; Ghildyal, R.; Vardaxis, N.; Plebanski, M. Type 1 and 2 Immunity Following Vaccination Is Influenced by Nanoparticle Size: Formulation of a Model Vaccine for Respiratory Syncytial Virus. Mol. Pharm. 2007, 4, 73–84. [Google Scholar] [CrossRef]
- Sato, Y.; Yokoyama, A.; Shibata, K.; Akimoto, Y.; Ogino, S.; Nodasaka, Y.; Kohgo, T.; Tamura, K.; Akasaka, T.; Uo, M. Influence of Length on Cytotoxicity of Multi-Walled Carbon Nanotubes against Human Acute Monocytic Leukemia Cell Line THP-1 In Vitro and Subcutaneous Tissue of Rats In Vivo. Mol. Biosyst. 2005, 1, 176–182. [Google Scholar] [CrossRef]
- Oh, N.; Park, J.-H. Endocytosis and Exocytosis of Nanoparticles in Mammalian Cells. Int. J. Nanomed. 2014, 9 (Suppl. S1), 51–63. [Google Scholar] [CrossRef]
- Kim, S.; Oh, W.-K.; Jeong, Y.S.; Hong, J.-Y.; Cho, B.-R.; Hahn, J.-S.; Jang, J. Cytotoxicity of, and Innate Immune Response to, Size-Controlled Polypyrrole Nanoparticles in Mammalian Cells. Biomaterials 2011, 32, 2342–2350. [Google Scholar] [CrossRef]
- Son, Y.J.; Kim, H.; Leong, K.W.; Yoo, H.S. Multifunctional Nanorods Serving as Nanobridges to Modulate T Cell-Mediated Immunity. ACS Nano 2013, 7, 9771–9779. [Google Scholar] [CrossRef]
- Foged, C.; Brodin, B.; Frokjaer, S.; Sundblad, A. Particle Size and Surface Charge Affect Particle Uptake by Human Dendritic Cells in an In Vitro Model. Int. J. Pharm. 2005, 298, 315–322. [Google Scholar] [CrossRef]
- Bezbaruah, R.; Chavda, V.P.; Nongrang, L.; Alom, S.; Deka, K.; Kalita, T.; Ali, F.; Bhattacharjee, B.; Vora, L. Nanoparticle-Based Delivery Systems for Vaccines. Vaccines 2022, 10, 1946. [Google Scholar] [CrossRef]
- Hassett, K.J.; Higgins, J.; Woods, A.; Levy, B.; Xia, Y.; Hsiao, C.J.; Acosta, E.; Almarsson, Ö.; Moore, M.J.; Brito, L.A. Impact of Lipid Nanoparticle Size on MRNA Vaccine Immunogenicity. J. Control. Release 2021, 335, 237–246. [Google Scholar] [CrossRef]
- Daramola, O.O.; Adara, P.; Adewuyi, B.O.; Sadiku, E.R.; Kupolati, W.K. 3-Polymer Nanoparticles (Nanomedicine) for Therapeutic Applications. In Polymeric Biomaterials for Healthcare Applications; Varaprasad, K., Ed.; Woodhead Publishing Series in Biomaterials; Woodhead Publishing: Sawston, UK, 2022; pp. 71–123. [Google Scholar] [CrossRef]
- Perotti, M.; Perez, L. Virus-Like Particles and Nanoparticles for Vaccine Development against HCMV. Viruses 2019, 12, 35. [Google Scholar] [CrossRef]
- Yadav, R.; Zhai, L.; Tumban, E. Virus-like Particle-Based L2 Vaccines against HPVs: Where Are We Today? Viruses 2020, 12, 18. [Google Scholar] [CrossRef]
- Irvine, D.J.; Aung, A.; Silva, M. Controlling Timing and Location in Vaccines. Adv. Drug Deliv. Rev. 2020, 158, 91–115. [Google Scholar] [CrossRef]
- Poon, C.; Patel, A.A. Organic and Inorganic Nanoparticle Vaccines for Prevention of Infectious Diseases. Nano Express 2020, 1, 12001. [Google Scholar] [CrossRef]
- Fries, C.N.; Curvino, E.J.; Chen, J.-L.; Permar, S.R.; Fouda, G.G.; Collier, J.H. Advances in Nanomaterial Vaccine Strategies to Address Infectious Diseases Impacting Global Health. Nat. Nanotechnol. 2021, 16, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Tenchov, R.; Bird, R.; Curtze, A.E.; Zhou, Q. Lipid Nanoparticles—From Liposomes to MRNA Vaccine Delivery, a Landscape of Research Diversity and Advancement. ACS Nano 2021, 15, 16982–17015. [Google Scholar] [CrossRef]
- Jia, F.; Gao, Y.; Wang, H. Recent Advances in Drug Delivery System Fabricated by Microfluidics for Disease Therapy. Bioengineering 2022, 9, 625. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Chen, Z.; Wang, Y.; Chen, C. Design and Application of Nanoparticles as Vaccine Adjuvants against Human Corona Virus Infection. J. Inorg. Biochem. 2021, 219, 111454. [Google Scholar] [CrossRef] [PubMed]
- Hassanzadeh, P. Nanotheranostics against COVID-19: From Multivalent to Immune-Targeted Materials. J. Control. Release Off. J. Control. Release Soc. 2020, 328, 112–126. [Google Scholar] [CrossRef]
- Meng, L.; Teng, Z.; Yang, S.; Wang, N.; Guan, Y.; Chen, X.; Liu, Y. Biomimetic Nanoparticles for DC Vaccination: A Versatile Approach to Boost Cancer Immunotherapy. Nanoscale 2023, 15, 6432–6455. [Google Scholar] [CrossRef]
- Momin, T.; Kansagra, K.; Patel, H.; Sharma, S.; Sharma, B.; Patel, J.; Mittal, R.; Sanmukhani, J.; Maithal, K.; Dey, A.; et al. Safety and Immunogenicity of a DNA SARS-CoV-2 Vaccine (ZyCoV-D): Results of an Open-Label, Non-Randomized Phase I Part of Phase I/II Clinical Study by Intradermal Route in Healthy Subjects in India. eClinicalMedicine 2021, 38, 101020. [Google Scholar] [CrossRef]
- Curley, S.M.; Putnam, D. Biological Nanoparticles in Vaccine Development. Front. Bioeng. Biotechnol. 2022, 10, 867119. [Google Scholar] [CrossRef]
- Collier, A.-R.Y.; Brown, C.M.; McMahan, K.A.; Yu, J.; Liu, J.; Jacob-Dolan, C.; Chandrashekar, A.; Tierney, D.; Ansel, J.L.; Rowe, M.; et al. Characterization of Immune Responses in Fully Vaccinated Individuals after Breakthrough Infection with the SARS-CoV-2 Delta Variant. Sci. Transl. Med. 2022, 14, eabn6150. [Google Scholar] [CrossRef]
- Fiolet, T.; Kherabi, Y.; MacDonald, C.-J.; Ghosn, J.; Peiffer-Smadja, N. Comparing COVID-19 Vaccines for Their Characteristics, Efficacy and Effectiveness against SARS-CoV-2 and Variants of Concern: A Narrative Review. Clin. Microbiol. Infect. 2022, 28, 202–221. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the MRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2020, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- El Sahly, H.M.; Baden, L.R.; Essink, B.; Doblecki-Lewis, S.; Martin, J.M.; Anderson, E.J.; Campbell, T.B.; Clark, J.; Jackson, L.A.; Fichtenbaum, C.J.; et al. Efficacy of the MRNA-1273 SARS-CoV-2 Vaccine at Completion of Blinded Phase. N. Engl. J. Med. 2021, 385, 1774–1785. [Google Scholar] [CrossRef] [PubMed]
- Frenck, R.W., Jr.; Dormitzer, P.R.; Gurtman, A. BNT162b2 COVID-19 Vaccine in Adolescents. N. Engl. J. Med. 2021, 385, 1342–1343. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 MRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.J.; Perez, J.L.; Lockhart, S.P.; Hariharan, S.; Kitchin, N.; Bailey, R.; Liau, K.; Lagkadinou, E.; Türeci, Ö.; Şahin, U.; et al. Efficacy and Safety of the BNT162b2 MRNA COVID-19 Vaccine in Participants with a History of Cancer: Subgroup Analysis of a Global Phase 3 Randomized Clinical Trial. Vaccine 2022, 40, 1483–1492. [Google Scholar] [CrossRef]
- Mannar, D.; Saville, J.W.; Zhu, X.; Srivastava, S.S.; Berezuk, A.M.; Tuttle, K.S.; Marquez, A.C.; Sekirov, I.; Subramaniam, S. SARS-CoV-2 Omicron Variant: Antibody Evasion and Cryo-EM Structure of Spike Protein-ACE2 Complex. Science 2022, 375, 760–764. [Google Scholar] [CrossRef]
- Waltz, E. Omicron-Targeted Vaccines Do No Better than Original Jabs in Early Tests. Nature 2022, 10, e139024. [Google Scholar] [CrossRef]
- Ahmed, A.; Nezami, M.; Alkattan, A. Pitfalls at Chemistry of Adenoviral Vector Vaccine against COVID-19 and How to Circumvent It. Adv. Pharm. Bull. 2022, 12, 217–218. [Google Scholar] [CrossRef]
- Reynolds, C.J.; Gibbons, J.M.; Pade, C.; Lin, K.-M.; Sandoval, D.M.; Pieper, F.; Butler, D.K.; Liu, S.; Otter, A.D.; Joy, G.; et al. Heterologous Infection and Vaccination Shapes Immunity against SARS-CoV-2 Variants. Science 2022, 375, 183–192. [Google Scholar] [CrossRef]
- Toh, Z.Q.; Anderson, J.; Mazarakis, N.; Neeland, M.; Higgins, R.A.; Rautenbacher, K.; Dohle, K.; Nguyen, J.; Overmars, I.; Donato, C.; et al. Comparison of Seroconversion in Children and Adults With Mild COVID-19. JAMA Netw. Open 2022, 5, e221313. [Google Scholar] [CrossRef]
- Choi, S.J.; Kim, D.-U.; Noh, J.Y.; Kim, S.; Park, S.-H.; Jeong, H.W.; Shin, E.-C. T Cell Epitopes in SARS-CoV-2 Proteins Are Substantially Conserved in the Omicron Variant. Cell. Mol. Immunol. 2022, 19, 447–448. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Aid, M.; Chandrashekar, A.; Yu, J.; McMahan, K.; Wegmann, F.; Jacob-Dolan, C.; Maron, J.S.; Atyeo, C.; Wan, H.; et al. A Homologous or Variant Booster Vaccine after Ad26.COV2.S Immunization Enhances SARS-CoV-2-Specific Immune Responses in Rhesus Macaques. Sci. Transl. Med. 2022, 14, eabm4996. [Google Scholar] [CrossRef] [PubMed]
- Altmann, D.M.; Boyton, R.J. COVID-19 Vaccination: The Road Ahead. Science 2022, 375, 1127–1132. [Google Scholar] [CrossRef] [PubMed]
- Tebas, P.; Kraynyak, K.A.; Patel, A.; Maslow, J.N.; Morrow, M.P.; Sylvester, A.J.; Knoblock, D.; Gillespie, E.; Amante, D.; Racine, T.; et al. Intradermal SynCon® Ebola GP DNA Vaccine Is Temperature Stable and Safely Demonstrates Cellular and Humoral Immunogenicity Advantages in Healthy Volunteers. J. Infect. Dis. 2019, 220, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Liu, J.; Jiang, W.; Yue, S.; Liu, H.; Wei, S. IL-6 and CD8+ T Cell Counts Combined Are an Early Predictor of in-Hospital Mortality of Patients with COVID-19. JCI Insight 2020, 5, e139024. [Google Scholar] [CrossRef] [PubMed]
- Diao, B.; Wang, C.; Tan, Y.; Chen, X.; Liu, Y.; Ning, L.; Chen, L.; Li, M.; Liu, Y.; Wang, G.; et al. Reduction and Functional Exhaustion of T Cells in Patients With Coronavirus Disease 2019 (COVID-19). Front. Immunol. 2020, 11, 827. [Google Scholar] [CrossRef]
- Hellerstein, M. What Are the Roles of Antibodies versus a Durable, High Quality T-Cell Response in Protective Immunity against SARS-CoV-2? Vaccine X 2020, 6, 100076. [Google Scholar] [CrossRef]
- Mayr, L.M.; Su, B.; Moog, C. Non-Neutralizing Antibodies Directed against HIV and Their Functions. Front. Immunol. 2017, 8, 1590. [Google Scholar] [CrossRef]
- Bootz, A.; Karbach, A.; Spindler, J.; Kropff, B.; Reuter, N.; Sticht, H.; Winkler, T.H.; Britt, W.J.; Mach, M. Protective Capacity of Neutralizing and Non-Neutralizing Antibodies against Glycoprotein B of Cytomegalovirus. PLoS Pathog. 2017, 13, e1006601. [Google Scholar] [CrossRef]
- Carragher, D.M.; Kaminski, D.A.; Moquin, A.; Hartson, L.; Randall, T.D. A Novel Role for Non-Neutralizing Antibodies against Nucleoprotein in Facilitating Resistance to Influenza Virus. J. Immunol. 2008, 181, 4168–4176. [Google Scholar] [CrossRef]
- Gussow, A.B.; Auslander, N.; Faure, G.; Wolf, Y.I.; Zhang, F.; Koonin, E.V. Genomic Determinants of Pathogenicity in SARS-CoV-2 and Other Human Coronaviruses. bioRxiv, 2020. [Google Scholar] [CrossRef]
- Voysey, M.; Costa Clemens, S.A.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Single-Dose Administration and the Influence of the Timing of the Booster Dose on Immunogenicity and Efficacy of ChAdOx1 NCoV-19 (AZD1222) Vaccine: A Pooled Analysis of Four Randomised Trials. Lancet 2021, 397, 881–891. [Google Scholar] [CrossRef] [PubMed]
- Wiersinga, W.J.; Rhodes, A.; Cheng, A.C.; Peacock, S.J.; Prescott, H.C. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19) A Review. JAMA—J. Am. Med. Assoc. 2020, 324, 782–793. [Google Scholar] [CrossRef] [PubMed]
- Horejs, C. From Lipids to Lipid Nanoparticles to MRNA Vaccines. Nat. Rev. Mater. 2021, 6, 1075–1076. [Google Scholar] [CrossRef] [PubMed]
- Dolgin, E. The Tangled History of MRNA Vaccines. Nature 2021, 597, 318–324. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lozano, D.; Larraga, V.; Vallet-Regí, M.; Manzano, M. An Overview of the Use of Nanoparticles in Vaccine Development. Nanomaterials 2023, 13, 1828. https://doi.org/10.3390/nano13121828
Lozano D, Larraga V, Vallet-Regí M, Manzano M. An Overview of the Use of Nanoparticles in Vaccine Development. Nanomaterials. 2023; 13(12):1828. https://doi.org/10.3390/nano13121828
Chicago/Turabian StyleLozano, Daniel, Vicente Larraga, María Vallet-Regí, and Miguel Manzano. 2023. "An Overview of the Use of Nanoparticles in Vaccine Development" Nanomaterials 13, no. 12: 1828. https://doi.org/10.3390/nano13121828
APA StyleLozano, D., Larraga, V., Vallet-Regí, M., & Manzano, M. (2023). An Overview of the Use of Nanoparticles in Vaccine Development. Nanomaterials, 13(12), 1828. https://doi.org/10.3390/nano13121828







