Highly Selective NH3 Sensor Based on MoS2/WS2 Heterojunction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
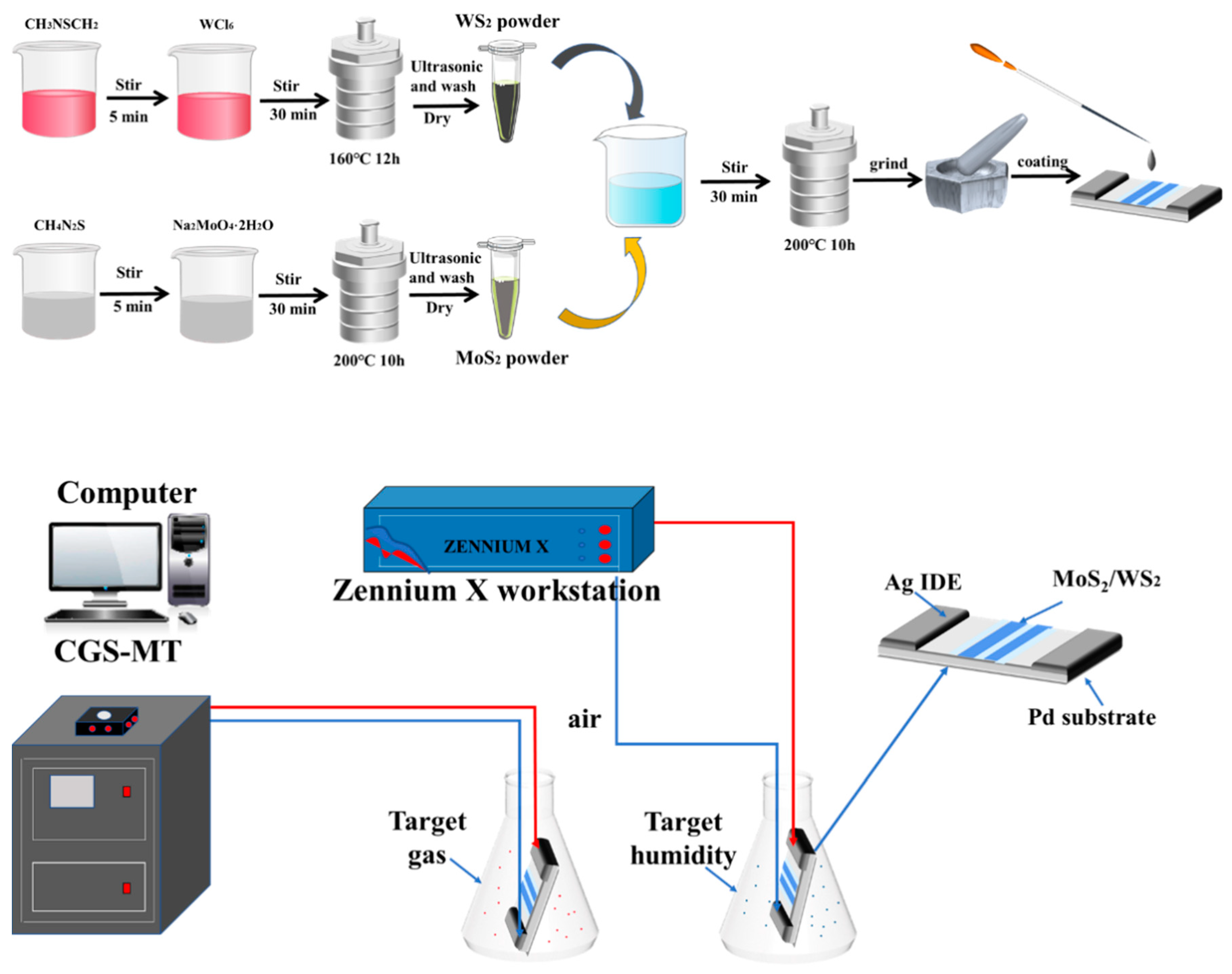
2.2. Preparation of WS2
2.3. Preparation of MoS2
2.4. Preparation of MoS2/WS2 Composites
2.5. Characterization
2.6. Gas Detection
2.7. Moisture Resistance Measurement
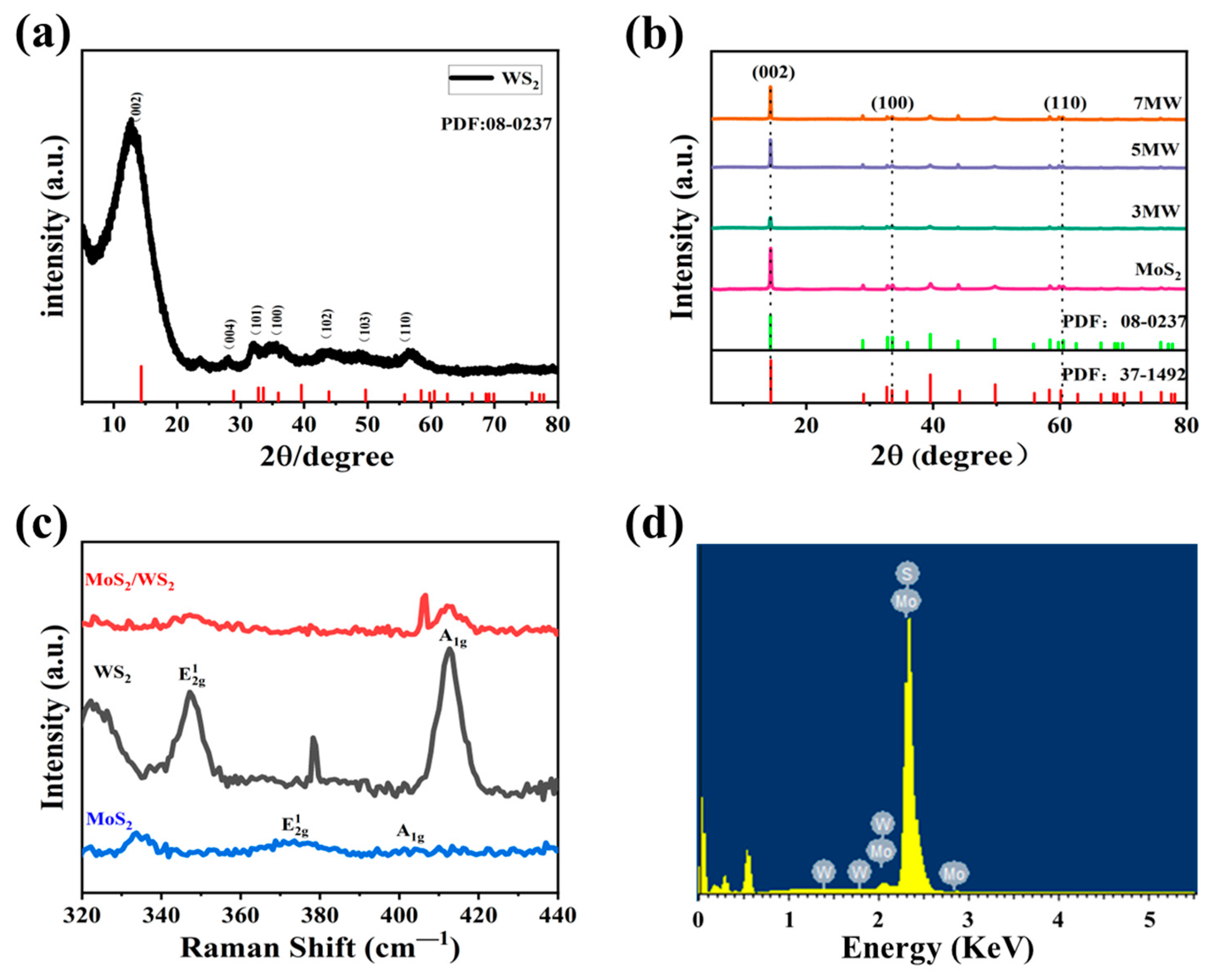
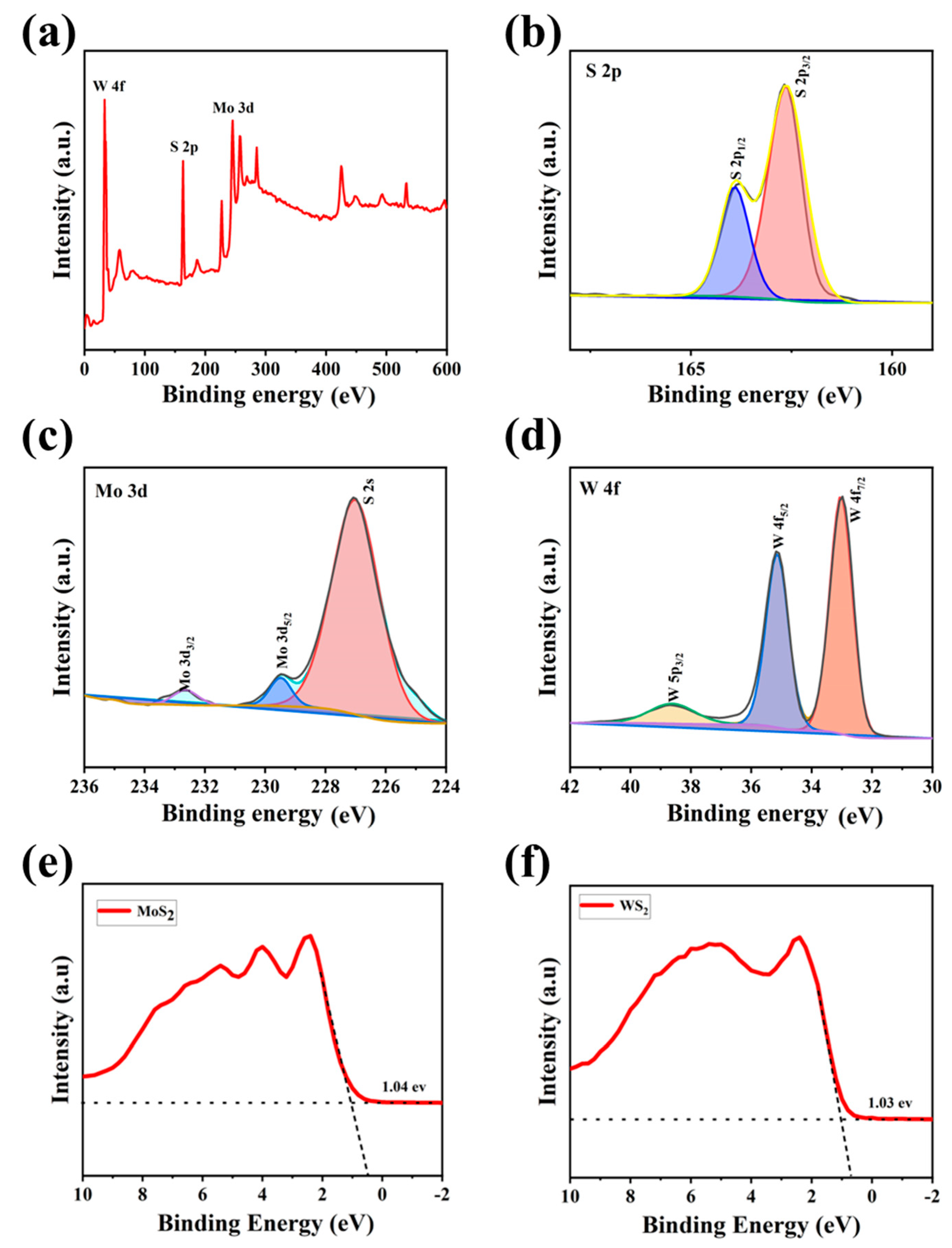
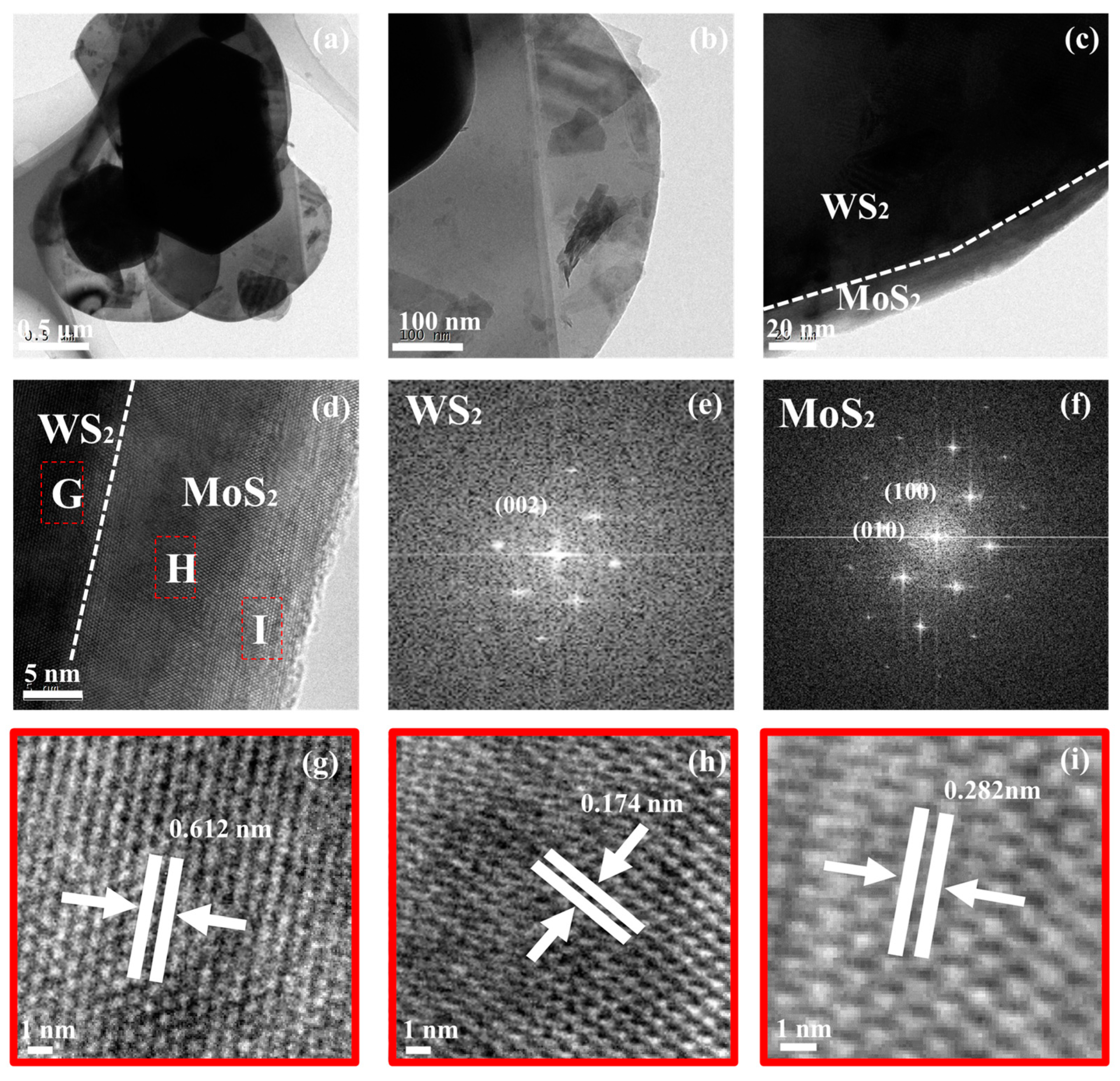
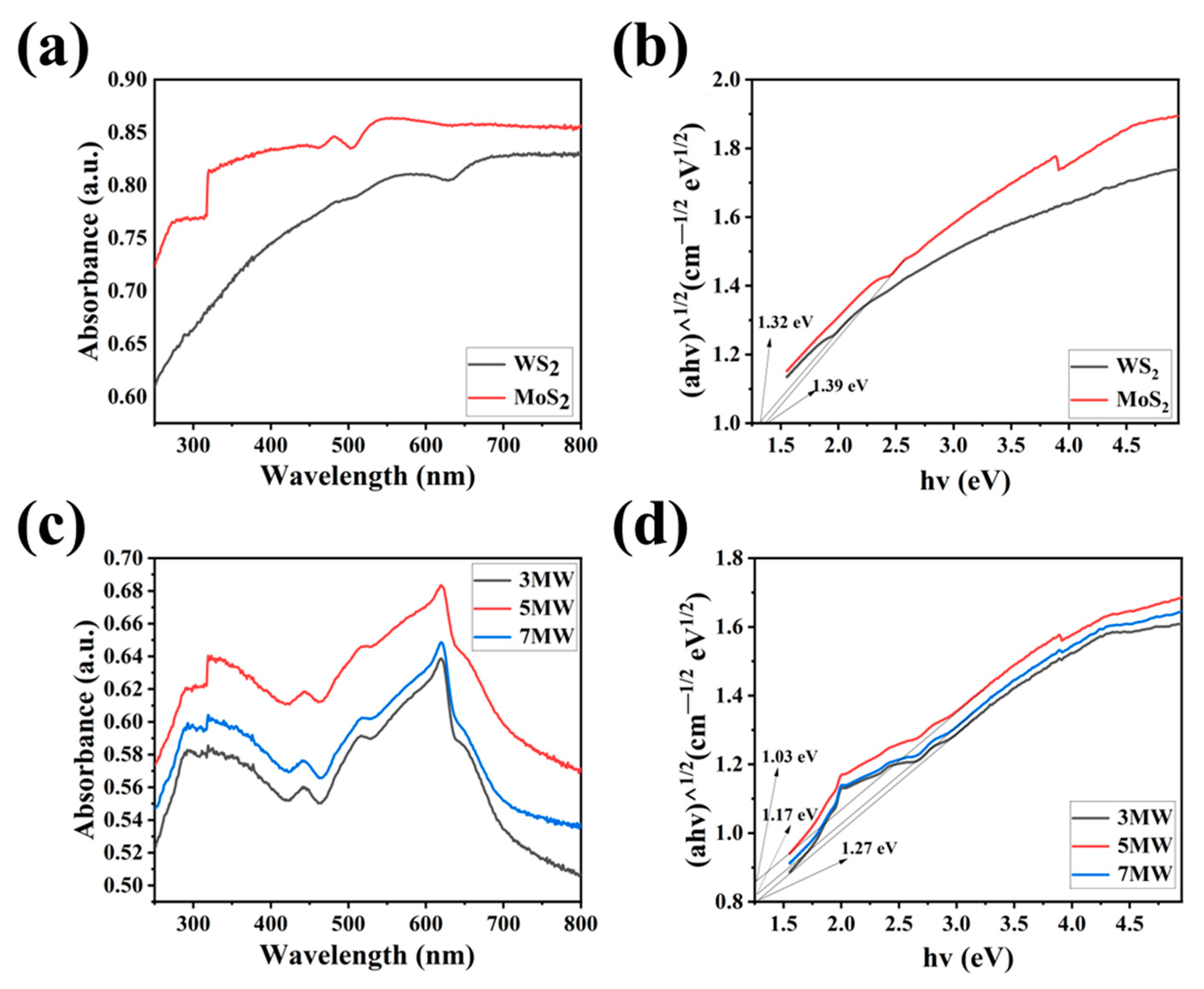
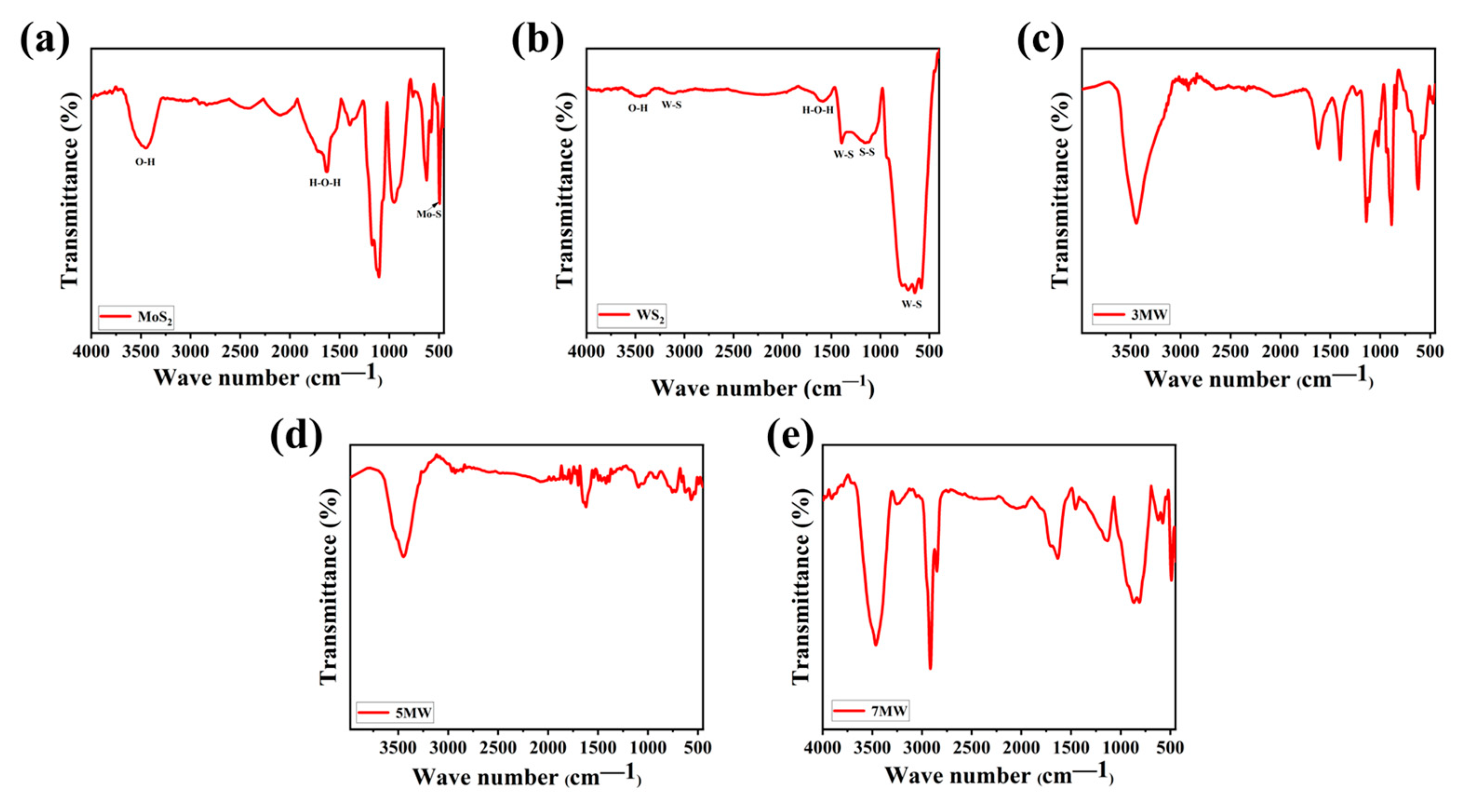
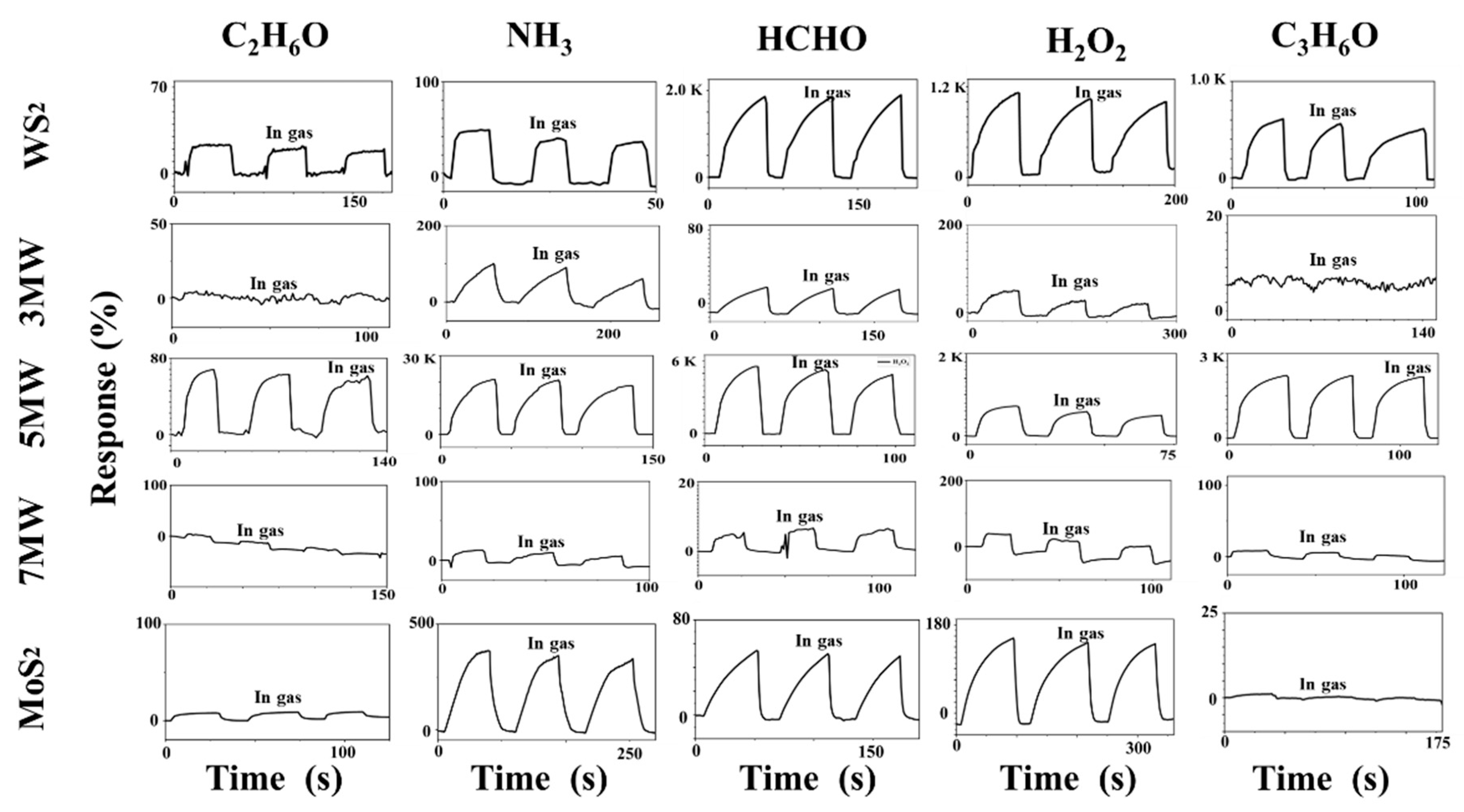
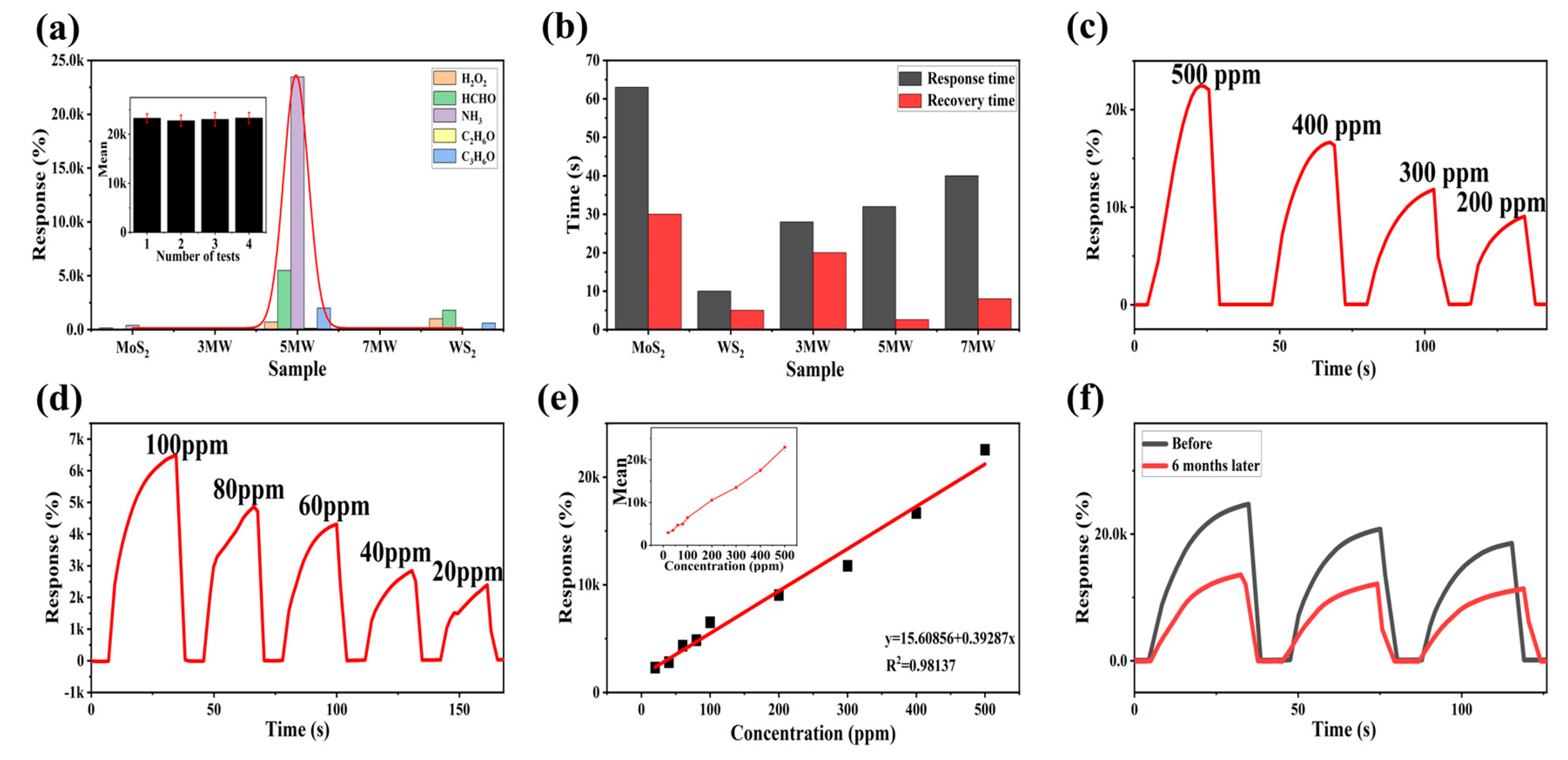
3. Results
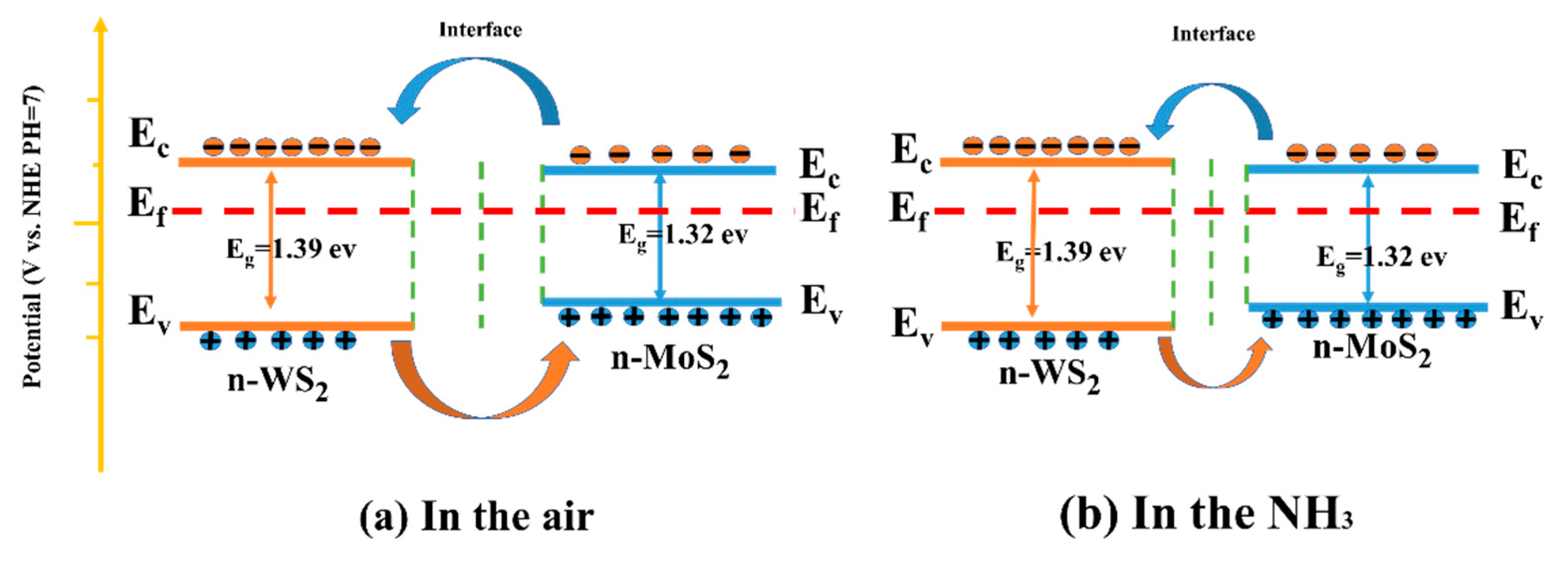
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Krupa, S.V. Effects of atmospheric ammonia (NH3) on terrestrial vegetation: A review. Environ. Pollut. 2003, 124, 179–221. [Google Scholar] [CrossRef] [PubMed]
- Boron, W.F.; De Weer, P. Intracellular pH transients in squid giant axons caused by CO2, NH3, and metabolic inhibitors. J. Gen. Physiol. 1976, 67, 91–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Chang, H.; Ma, L.; Hao, J.; Yang, R.T. Low-temperature selective catalytic reduction of NOx with NH3 over metal oxide and zeolite catalysts—A review. Catal. Today 2011, 175, 147–156. [Google Scholar] [CrossRef]
- Sufriadi, E.; Meilina, H.; Munawar, A.A.; Muhammad, S.; Idroes, R. Identification of β-Caryophyllene (BCP) in Aceh Patchouli Essential Oil (PEO) using Gas Chromatography-Mass Pectrophotometry (GC-MS), IOP Conference Series: Earth and Environmental Science, 2021; IOP Publishing: Bristol, UK, 2021; p. 12032. [Google Scholar]
- Bartle, K.D.; Myers, P. History of gas chromatography. TrAC Trends Anal. Chem. 2002, 21, 547–557. [Google Scholar] [CrossRef]
- Bakker, E.; Telting-Diaz, M. Electrochemical sensors. Anal. Chem. 2002, 74, 2781–2800. [Google Scholar] [CrossRef]
- Fine, G.F.; Cavanagh, L.M.; Afonja, A.; Binions, R. Metal oxide semi-conductor gas sensors in environmental monitoring. Sensors-Basel 2010, 10, 5469–5502. [Google Scholar] [CrossRef] [Green Version]
- Nikolic, M.V.; Milovanovic, V.; Vasiljevic, Z.Z.; Stamenkovic, Z. Semiconductor gas sensors: Materials, technology, design, and application. Sensors 2020, 20, 6694. [Google Scholar] [CrossRef]
- Gu, J.; Chakraborty, B.; Khatoniar, M.; Menon, V.M. A room-temperature polariton light-emitting diode based on monolayer WS2. Nat. Nanotechnol. 2019, 14, 1024–1028. [Google Scholar] [CrossRef]
- Kim, J.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Realization of Au-decorated WS2 nanosheets as low power-consumption and selective gas sensors. Sens. Actuators B Chem. 2019, 296, 126659. [Google Scholar] [CrossRef]
- Sharma, S.; Saini, R.; Gupta, G.; Late, D.J. Room-temperature highly sensitive and selective NH3 gas sensor using vertically aligned WS2 nanosheets. Nanotechnology 2022, 34, 45704. [Google Scholar] [CrossRef]
- Xu, P.; Cheng, Z.; Pan, Q.; Xu, J.; Xiang, Q.; Yu, W.; Chu, Y. High aspect ratio In2O3 nanowires: Synthesis, mechanism and NO2 gas-sensing properties. Sens. Actuators B Chem. 2008, 130, 802–808. [Google Scholar] [CrossRef]
- Qin, Z.; Song, X.; Wang, J.; Li, X.; Wu, C.; Wang, X.; Yin, X.; Zeng, D. Development of flexible paper substrate sensor based on 2D WS2 with S defects for room-temperature NH3 gas sensing. Appl. Surf. Sci. 2022, 573, 151535. [Google Scholar] [CrossRef]
- Yu, S.M.; Kim, J.H.; Yoon, K.R.; Jung, J.W.; Oh, J.H.; Kim, I.D. Single Layers of WS2 Nanoplates Anchored to Hollow N-Doped Carbon Nanofibers as Efficient Electrocatalysts for Hydrogen Evolution, 2015 Fall MRS, 2015; Materials Research Society: Warrendale, PA, USA, 2015. [Google Scholar]
- Zhao, F.; Li, Z.; Fu, Y.; Wang, Q. Gas-Sensitive Characteristics of Graphene Composite Tungsten Disulfide to Ammonia. Sensors 2022, 22, 8672. [Google Scholar] [CrossRef] [PubMed]
- Järvinen, T.; Lorite, G.S.; Peräntie, J.; Toth, G.; Saarakkala, S.; Virtanen, V.K.; Kordas, K. WS2 and MoS2 thin film gas sensors with high response to NH3 in air at low temperature. Nanotechnology 2019, 30, 405501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Late, D.J.; Kanawade, R.V.; Kannan, P.K.; Rout, C.S. Atomically thin WS2 nanosheets based gas sensor. Sens. Lett. 2016, 14, 1249–1254. [Google Scholar] [CrossRef]
- Perrozzi, F.; Emamjomeh, S.M.; Paolucci, V.; Taglieri, G.; Ottaviano, L.; Cantalini, C. Thermal stability of WS2 flakes and gas sensing properties of WS2/WO3 composite to H2, NH3 and NO2. Sens. Actuators B Chem. 2017, 243, 812–822. [Google Scholar] [CrossRef]
- Luo, H.; Shi, J.; Liu, C.; Chen, X.; Lv, W.; Zhou, Y.; Zeng, M.; Yang, J.; Wei, H.; Zhou, Z. Design of p–p heterojunctions based on CuO decorated WS2 nanosheets for sensitive NH3 gas sensing at room temperature. Nanotechnology 2021, 32, 445502. [Google Scholar] [CrossRef]
- Ouyang, C.; Chen, Y.; Qin, Z.; Zeng, D.; Zhang, J.; Wang, H.; Xie, C. Two-dimensional WS2-based nanosheets modified by Pt quantum dots for enhanced room-temperature NH3 sensing properties. Appl. Surf. Sci. 2018, 455, 45–52. [Google Scholar] [CrossRef]
- Singh, S.; Saggu, I.S.; Chen, K.; Xuan, Z.; Swihart, M.T.; Sharma, S. Humidity-tolerant room-temperature selective dual sensing and discrimination of NH3 and no using a WS2/MWCNT Composite. ACS Appl. Mater. Inter. 2022, 14, 40382–40395. [Google Scholar] [CrossRef]
- Paolucci, V.; Emamjomeh, S.M.; Ottaviano, L.; Cantalini, C. Near room temperature light-activated WS2-decorated rGO as NO2 gas sensor. Sensors 2019, 19, 2617. [Google Scholar] [CrossRef] [Green Version]
- Yadav, A.A.; Hunge, Y.M.; Kang, S. Visible Light-Responsive CeO2/MoS2 Composite for Photocatalytic Hydrogen Production. Catalysts 2022, 12, 1185. [Google Scholar] [CrossRef]
- Hunge, Y.M.; Yadav, A.A.; Kang, S.; Lim, S.J.; Kim, H. Visible light activated MoS2/ZnO composites for photocatalytic degradation of ciprofloxacin antibiotic and hydrogen production. J. Photochem. Photobiol. A Chem. 2023, 434, 114250. [Google Scholar] [CrossRef]
- Hunge, Y.M.; Yadav, A.A.; Kang, S. Photocatalytic Degradation of Eriochrome Black-T Using BaWO4/MoS2 Composite. Catalysts 2022, 12, 1290. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, J.; Chen, P.; Sun, Y.; Wu, S.; Jia, Z.; Lu, X.; Yu, H.; Chen, W.; Zhu, J. Observation of strong interlayer coupling in MoS2/WS2 heterostructures. Adv. Mater. 2016, 28, 1950–1956. [Google Scholar] [CrossRef] [PubMed]
- Vikraman, D.; Hussain, S.; Akbar, K.; Truong, L.; Kathalingam, A.; Chun, S.; Jung, J.; Park, H.J.; Kim, H. Improved hydrogen evolution reaction performance using MoS2–WS2 heterostructures by physicochemical process. ACS Sustain. Chem. Eng. 2018, 6, 8400–8409. [Google Scholar] [CrossRef]
- Yu, Y.; Hu, S.; Su, L.; Huang, L.; Liu, Y.; Jin, Z.; Purezky, A.A.; Geohegan, D.B.; Kim, K.W.; Zhang, Y. Equally efficient interlayer exciton relaxation and improved absorption in epitaxial and nonepitaxial MoS2/WS2 heterostructures. Nano Lett. 2015, 15, 486–491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Wen, X.; Zhang, J.; Wu, T.; Gong, Y.; Zhang, X.; Yuan, J.; Yi, C.; Lou, J.; Ajayan, P.M. Ultrafast formation of interlayer hot excitons in atomically thin MoS2/WS2 heterostructures. Nat. Commun. 2016, 7, 12512. [Google Scholar] [CrossRef]
- Chen, S.; Pan, Y. Enhancing catalytic properties of noble metal@ MoS2/WS2 heterojunction for the hydrogen evolution reaction. Appl. Surf. Sci. 2022, 591, 153168. [Google Scholar] [CrossRef]
- Deilmann, T.; Thygesen, K.S. Interlayer trions in the MoS2/WS2 van der Waals heterostructure. Nano Lett. 2018, 18, 1460–1465. [Google Scholar] [CrossRef] [Green Version]
- Kaushik, V.; Ahmad, M.; Agarwal, K.; Varandani, D.; Belle, B.D.; Das, P.; Mehta, B.R. Charge transport in 2D MoS2, WS2, and MoS2–WS2 heterojunction-based field-effect transistors: Role of ambipolarity. J. Phys. Chem. C 2020, 124, 23368–23379. [Google Scholar] [CrossRef]
- Chiappe, D.; Asselberghs, I.; Sutar, S.; Iacovo, S.; Afanas’ Ev, V.; Stesmans, A.; Balaji, Y.; Peters, L.; Heyne, M.; Mannarino, M. Controlled sulfurization process for the synthesis of large area MoS2 films and MoS2/WS2 heterostructures. Adv. Mater. Interfaces 2016, 3, 1500635. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.; Mahlouji, R.; Wu, L.; Verheijen, M.A.; Vandalon, V.; Balasubramanyam, S.; Hofmann, J.P.; Kessels, W.E.; Bol, A.A. Large area, patterned growth of 2D MoS2 and lateral MoS2–WS2 heterostructures for nano-and opto-electronic applications. Nanotechnology 2020, 31, 255603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, X.; Kim, J.; Shi, S.; Zhang, Y.; Jin, C.; Sun, Y.; Tongay, S.; Wu, J.; Zhang, Y.; Wang, F. Ultrafast charge transfer in atomically thin MoS2/WS2 heterostructures. Nat Nanotechnol 2014, 9, 682–686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, Y.F.; Chen, J.H.; Lu, Y.Q.; Xu, F. Broadband optical-fiber-compatible photodetector based on a graphene-MoS2-WS2 heterostructure with a synergetic photogenerating mechanism. Adv. Electron. Mater. 2019, 5, 1800562. [Google Scholar] [CrossRef] [Green Version]
- Tang, M.; Zhang, D.; Wang, D.; Deng, J.; Kong, D.; Zhang, H. Performance prediction of 2D vertically stacked MoS2-WS2 heterostructures base on first-principles theory and Pearson correlation coefficient. Appl. Surf. Sci. 2022, 596, 153498. [Google Scholar] [CrossRef]
- Zhao, X.; Tang, G.; Li, Y.; Zhang, M.; Nie, Y. Biaxial strain improving the thermoelectric performance of a two-dimensional MoS2/WS2 heterostructure. ACS Appl. Electron. Ma 2021, 3, 2995–3004. [Google Scholar] [CrossRef]
- Zhang, X.; Huangfu, L.; Gu, Z.; Xiao, S.; Zhou, J.; Nan, H.; Gu, X.; Ostrikov, K. Controllable Epitaxial Growth of Large-Area MoS2/WS2 Vertical Heterostructures by Confined-Space Chemical Vapor Deposition. Small 2021, 17, 2007312. [Google Scholar] [CrossRef]
- Choudhary, N.; Park, J.; Hwang, J.Y.; Chung, H.; Dumas, K.H.; Khondaker, S.I.; Choi, W.; Jung, Y. Centimeter scale patterned growth of vertically stacked few layer only 2D MoS2/WS2 van der Waals heterostructure. Sci. Rep. 2016, 6, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Pesci, F.M.; Sokolikova, M.S.; Grotta, C.; Sherrell, P.C.; Reale, F.; Sharda, K.; Ni, N.; Palczynski, P.; Mattevi, C. MoS2/WS2 heterojunction for photoelectrochemical water oxidation. ACS Catal. 2017, 7, 4990–4998. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Tahir, M.; Chen, H.; Sambur, J.B. Probing charge carrier transport and recombination pathways in monolayer MoS2/WS2 heterojunction photoelectrodes. Nano Lett. 2019, 19, 9084–9094. [Google Scholar] [CrossRef]
- Lin, Z.; Luo, P.; Zeng, W.; Lai, H.; Xie, W.; Deng, W.; Luo, Z. Improvement of photoelectric properties of MoS2/WS2 heterostructure photodetector with interlayer of Au nanoparticles. Opt. Mater. 2020, 108, 110191. [Google Scholar] [CrossRef]
- Zhang, X.; Xiao, S.; Nan, H.; Mo, H.; Wan, X.; Gu, X.; Ostrikov, K.K. Controllable one-step growth of bilayer MoS2–WS2/WS2 heterostructures by chemical vapor deposition. Nanotechnology 2018, 29, 455707. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Sahin, H.; Peeters, F.M. Tuning carrier confinement in the MoS2/WS2 lateral heterostructure. J. Phys. Chem. C 2015, 119, 9580–9586. [Google Scholar] [CrossRef]
- Xue, Y.; Zhang, Y.; Liu, Y.; Liu, H.; Song, J.; Sophia, J.; Liu, J.; Xu, Z.; Xu, Q.; Wang, Z. Scalable production of a few-layer MoS2/WS2 vertical heterojunction array and its application for photodetectors. ACS Nano 2016, 10, 573–580. [Google Scholar] [CrossRef]
- Shan, J.; Li, J.; Chu, X.; Xu, M.; Jin, F.; Fang, X.; Wei, Z.; Wang, X. Enhanced photoresponse characteristics of transistors using CVD-grown MoS2/WS2 heterostructures. Appl. Surf. Sci. 2018, 443, 31–38. [Google Scholar] [CrossRef]
- Zeng, Y.; Dai, W.; Ma, R.; Li, Z.; Ou, Z.; Wang, C.; Yu, Y.; Zhu, T.; Liu, X.; Wang, T. Distinguishing Ultrafast Energy Transfer in Atomically Thin MoS2/WS2 Heterostructures. Small 2022, 18, 2204317. [Google Scholar] [CrossRef]
- Wang, F.; Wang, J.; Guo, S.; Zhang, J.; Hu, Z.; Chu, J. Tuning coupling behavior of stacked heterostructures based on MoS2, WS2, and WSe2. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Li, Z.; Zhang, S.; Yan, S.; Cao, B.; Wang, Z.; Fu, Y. Enhanced NH3 gas-sensing performance of silica modified CeO2 nanostructure based sensors. Sens. Actuators B Chem. 2018, 255, 862–870. [Google Scholar] [CrossRef]
- Zhao, J.; Li, N.; Yu, H.; Wei, Z.; Liao, M.; Chen, P.; Wang, S.; Shi, D.; Sun, Q.; Zhang, G. Highly sensitive MoS2 humidity sensors array for noncontact sensation. Adv. Mater. 2017, 29, 1702076. [Google Scholar] [CrossRef]
- Kim, T.; Lee, T.H.; Park, S.Y.; Eom, T.H.; Cho, I.; Kim, Y.; Kim, C.; Lee, S.A.; Choi, M.; Suh, J.M. Drastic Gas Sensing Selectivity in 2-Dimensional MoS2 Nanoflakes by Noble Metal Decoration. Acs Nano 2023, 17, 4404–4413. [Google Scholar] [CrossRef]
- Lee, E.; VahidMohammadi, A.; Prorok, B.C.; Yoon, Y.S.; Beidaghi, M.; Kim, D. Room temperature gas sensing of two-dimensional titanium carbide (MXene). ACS Appl. Mater. Inter. 2017, 9, 37184–37190. [Google Scholar] [CrossRef] [PubMed]
- Rabchinskii, M.K.; Sysoev, V.V.; Glukhova, O.E.; Brzhezinskaya, M.; Stolyarova, D.Y.; Varezhnikov, A.S.; Solomatin, M.A.; Barkov, P.V.; Kirilenko, D.A.; Pavlov, S.I. Guiding Graphene Derivatization for the On-Chip Multisensor Arrays: From the Synthesis to the Theoretical Background. Adv. Mater. Technol. 2022, 7, 2101250. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, L.; Zou, Y.; Li, Y.; Xuan, J.; Wang, X.; Jia, F.; Yin, G.; Sun, M. Enhanced ammonia sensing response based on Pt-decorated Ti3C2Tx/TiO2 composite at room temperature. Nanotechnology 2023, 34, 205501. [Google Scholar] [CrossRef]
- Liu, Z.; Han, D.; Liu, L.; Li, D.; Han, X.; Chen, Y.; Liu, X.; Zhuo, K.; Cheng, Y.; Sang, S. Ultrasensitive ammonia gas sensor based on Ti3C2Tx/Ti3AlC2 planar composite at room temperature. Sens. Actuators B Chem. 2023, 378, 133149. [Google Scholar] [CrossRef]
- Wang, L.; Meng, W.; He, Z.; Meng, W.; Li, Y.; Dai, L. Enhanced selective performance of mixed potential ammonia gas sensor by Au nanoparticles decorated CeVO4 sensing electrode. Sens. Actuators B Chem. 2018, 272, 219–228. [Google Scholar] [CrossRef]
- Rigosi, A.F.; Hill, H.M.; Li, Y.; Chernikov, A.; Heinz, T.F. Probing interlayer interactions in transition metal dichalcogenide heterostructures by optical spectroscopy: MoS2/WS2 and MoSe2/WSe2. Nano Lett. 2015, 15, 5033–5038. [Google Scholar] [CrossRef] [PubMed]
- Bogaert, K.; Liu, S.; Chesin, J.; Titow, D.; Gradecak, S.; Garaj, S. Diffusion-mediated synthesis of MoS2/WS2 lateral heterostructures. Nano Lett. 2016, 16, 5129–5134. [Google Scholar] [CrossRef]
- He, X.; Li, H.; Zhu, Z.; Dai, Z.; Yang, Y.; Yang, P.; Zhang, Q.; Li, P.; Schwingenschlogl, U.; Zhang, X. Strain engineering in monolayer WS2, MoS2, and the WS2/MoS2 heterostructure. Appl. Phys. Lett. 2016, 109, 173105. [Google Scholar] [CrossRef] [Green Version]


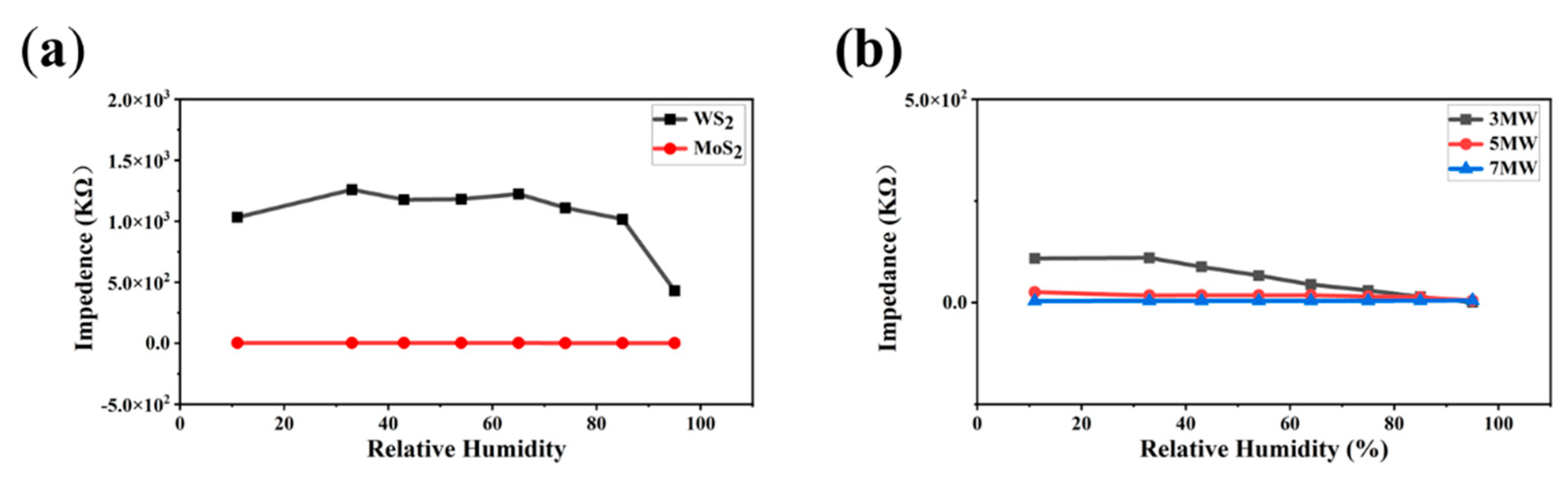
| Materials | T (°C) | Concentration (ppm) | Response/Recovery Time (s) | Limit of Detection (ppm) | Gas Type | Response | Ref. |
|---|---|---|---|---|---|---|---|
| P-MoS2 | 150 °C | 50 | 1300/1250 | 10 | NH3 | 651.53% | [52] |
| Ti3C2Tx | RT | 100 | - | 9.27 | NH3 | 21% | [53] |
| C-xy graphene | RT | 500 | - | - | NH3 | 4200% | [54] |
| Pt-Ti3C2Tx/TiO2 | RT | 100 | 23/24 | 10 | NH3 | 45.5% | [55] |
| Ti3C2Tx/Ti3AlC2 | RT | 0.5 | 90/75 | 0.05 | NH3 | 1.2% | [56] |
| MoS2/WS2 | RT | 500 | 30/2.6 | 20 | NH3 | 23643% | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Zhang, J. Highly Selective NH3 Sensor Based on MoS2/WS2 Heterojunction. Nanomaterials 2023, 13, 1835. https://doi.org/10.3390/nano13121835
Zhang M, Zhang J. Highly Selective NH3 Sensor Based on MoS2/WS2 Heterojunction. Nanomaterials. 2023; 13(12):1835. https://doi.org/10.3390/nano13121835
Chicago/Turabian StyleZhang, Min, and Jinzhu Zhang. 2023. "Highly Selective NH3 Sensor Based on MoS2/WS2 Heterojunction" Nanomaterials 13, no. 12: 1835. https://doi.org/10.3390/nano13121835
APA StyleZhang, M., & Zhang, J. (2023). Highly Selective NH3 Sensor Based on MoS2/WS2 Heterojunction. Nanomaterials, 13(12), 1835. https://doi.org/10.3390/nano13121835






