A Study on the Impermeability of Nanodispersible Modified Bentonite Based on Colloidal Osmotic Pressure Mechanisms and the Adsorption of Harmful Substances
Abstract
:1. Introduction
2. Experimental Methods
2.1. Materials
2.2. Preparation of Polymer-Modified Bentonite
2.3. Material Characterization
2.4. Free Swell Index
2.5. Hydraulic Conductivity
2.6. Colloidal Osmotic Pressure
2.7. Adsorption Experiments
2.7.1. Phenol Adsorption Experiments
2.7.2. Methylene Blue Adsorption Experiments
2.7.3. Heavy Metal Ion Adsorption Experiments
3. Results and Discussions
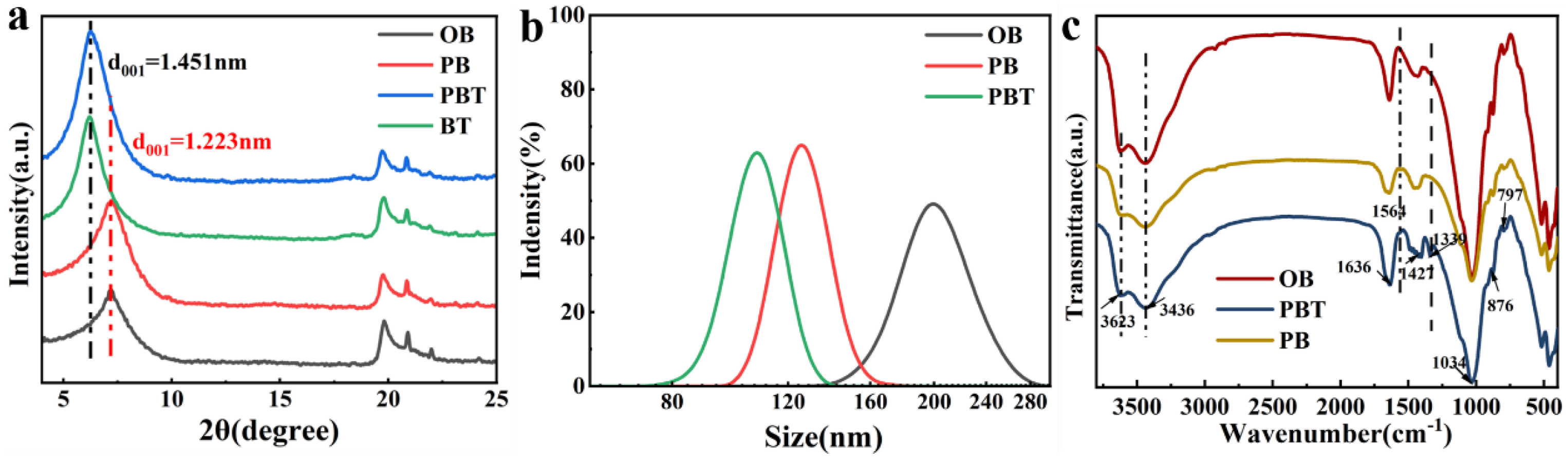
3.1. Characterization
3.2. Free Swell Index
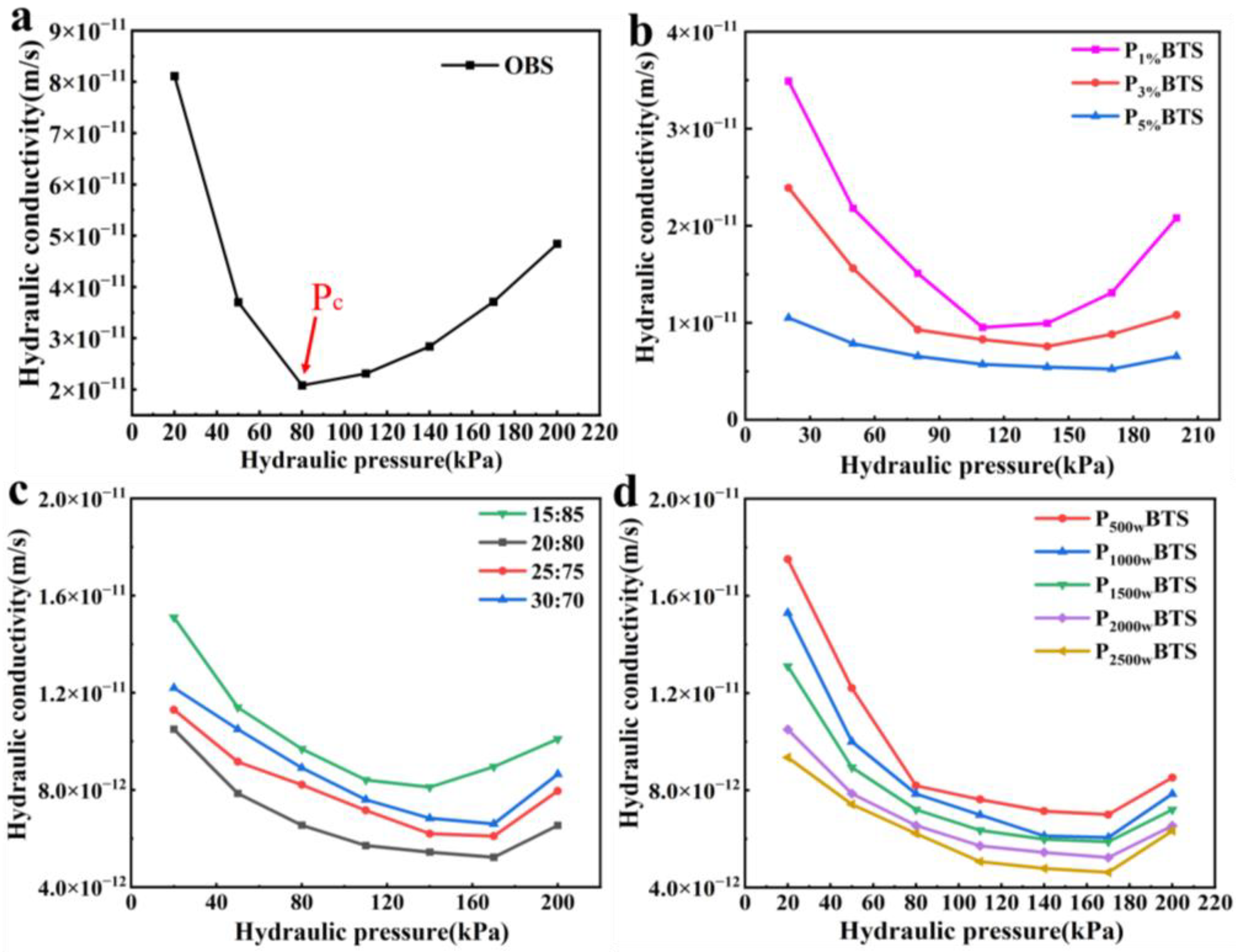
3.3. Hydraulic Conductivity
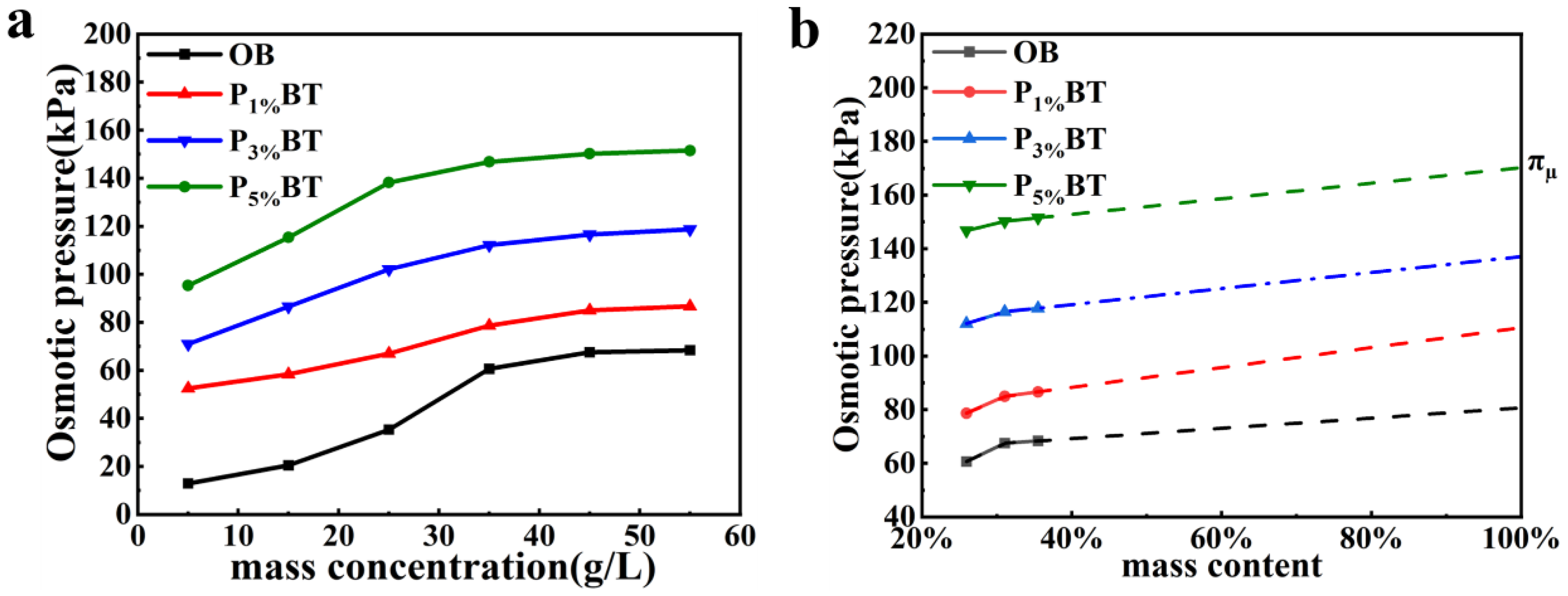
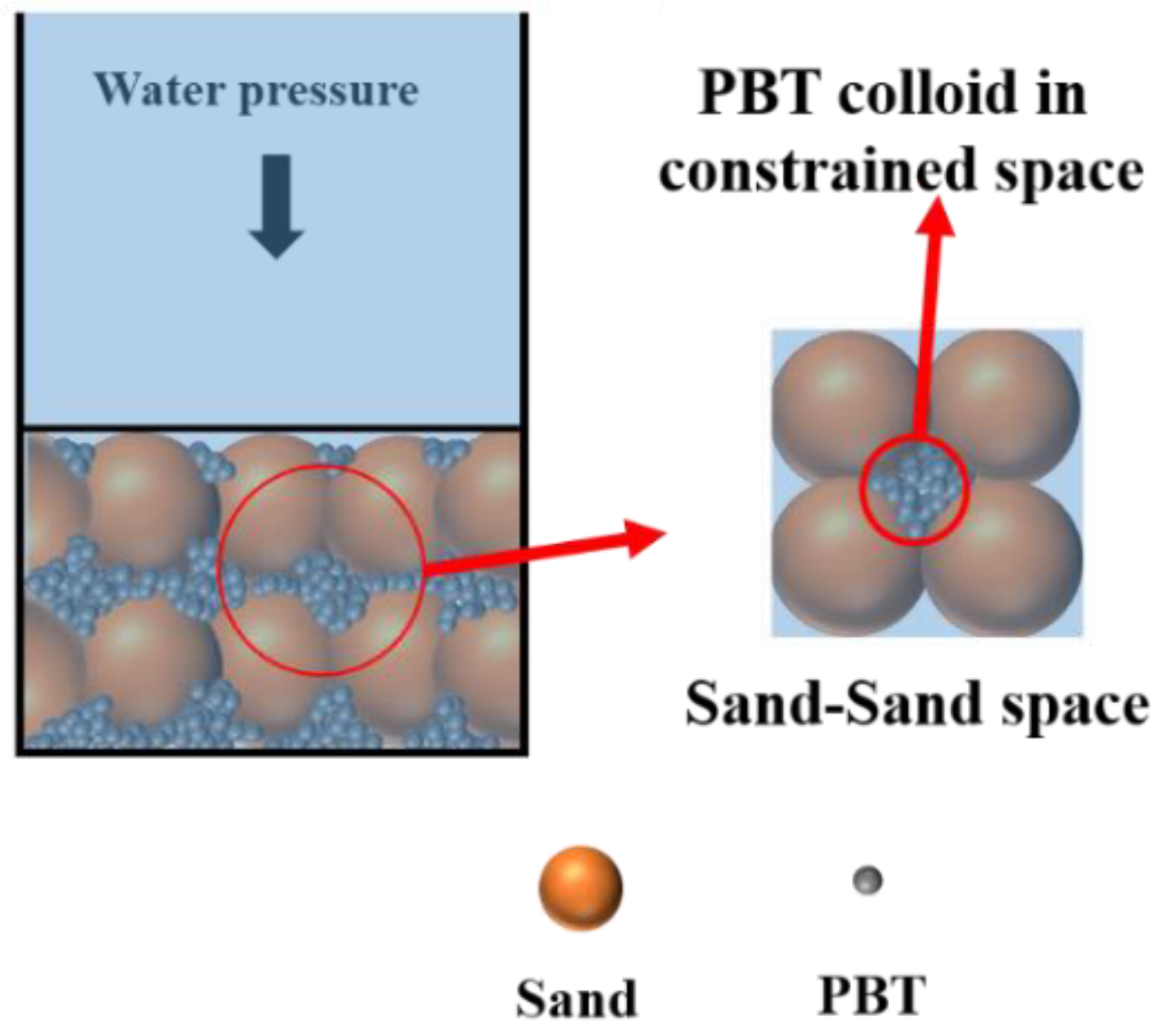
3.4. Colloidal Osmotic Pressure Mechanism
3.5. Adsorption Properties
3.5.1. Phenol Adsorption
3.5.2. Methylene Blue Adsorption
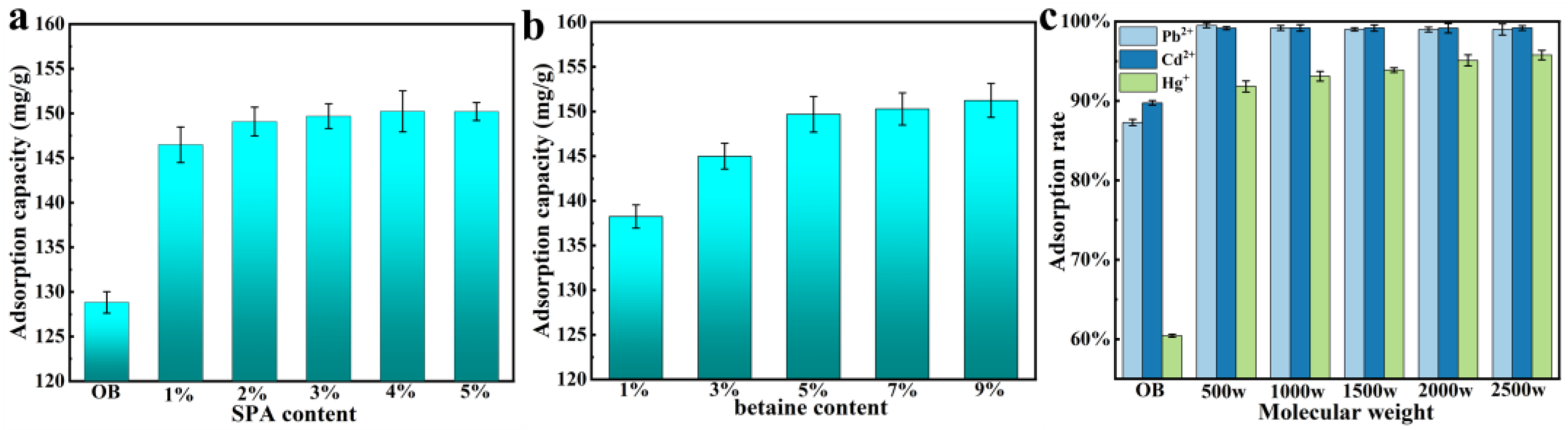
3.5.3. Heavy Metal Ion Adsorption
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wang, S.; Lu, B.; Zhu, W. Effect of Minerals on the Threshold of Compacted Clay Material Liners and Service Life of Barriers in Municipal Solid Waste Landfills. Acta Geotech. 2023, 18, 1025–1037. [Google Scholar] [CrossRef]
- Tian, K.; Likos, W.J.; Benson, C.H. Polymer Elution and Hydraulic Conductivity of Bentonite–Polymer Composite Geosynthetic Clay Liners. J. Geotech. Geoenviron. Eng. 2019, 145, 04019071. [Google Scholar] [CrossRef] [Green Version]
- Srinivasan, R. Advances in Application of Natural Clay and Its Composites in Removal of Biological, Organic, and Inorganic Contaminants from Drinking Water. Adv. Mater. Sci. Eng. 2011, 2011, 872531. [Google Scholar] [CrossRef] [Green Version]
- Borah, D.; Nath, H.; Saikia, H. Modification of Bentonite Clay & Its Applications: A Review. Rev. Inorg. Chem. 2022, 42, 265–282. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Z.; Shao, D.; Li, Y.; Xu, Z.; Cheng, C.; Asiri, A.M.; Marwani, H.M.; Hu, S. Adsorption of U(VI) on Bentonite in Simulation Environmental Conditions. J. Mol. Liq. 2017, 242, 678–684. [Google Scholar] [CrossRef]
- Wu, W.; Lan, Y.; Zeng, Y.; Lin, D.; Yang, K. Nonlinear Sorption of Phenols and Anilines by Organobentonites: Nonlinear Partition and Space Limitation for Partitioning. Sci. Total Environ. 2020, 736, 139609. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, S.; Quan, X.; Yu, H.; Zhao, H.; Zhang, Y. Efficient Mineralization of Perfluorooctanoate by Electro-Fenton with H2O2 Electro-Generated on Hierarchically Porous Carbon. Environ. Sci. Technol. 2015, 49, 13528–13533. [Google Scholar] [CrossRef]
- Miranda-García, N.; Suárez, S.; Sánchez, B.; Coronado, J.M.; Malato, S.; Maldonado, M.I. Photocatalytic Degradation of Emerging Contaminants in Municipal Wastewater Treatment Plant Effluents Using Immobilized TiO2 in a Solar Pilot Plant. Appl. Catal. B Environ. 2011, 103, 294–301. [Google Scholar] [CrossRef]
- Zhou, G.; Wang, Y.; Zhou, R.; Wang, C.; Jin, Y.; Qiu, J.; Hua, C.; Cao, Y. Synthesis of Amino-Functionalized Bentonite/CoFe2O4@MnO2 Magnetic Recoverable Nanoparticles for Aqueous Cd2+ Removal. Sci. Total Environ. 2019, 682, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Mao, M.; Yan, T.; Chen, G.; Zhang, J.; Shi, L.; Zhang, D. Selective Capacitive Removal of Pb2+ from Wastewater over Redox-Active Electrodes. Environ. Sci. Technol. 2021, 55, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Kolluru, V.; Pal, D.; Papu John, A.M.S.; Ankem, M.K.; Freedman, J.H.; Damodaran, C. Induction of Plac8 Promotes Pro-Survival Function of Autophagy in Cadmium-Induced Prostate Carcinogenesis. Cancer Lett. 2017, 408, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Fernandes, J.; Jones, D.P.; Go, Y.-M. Cadmium Stimulates Myofibroblast Differentiation and Mouse Lung Fibrosis. Toxicology 2017, 383, 50–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, L.; Cao, H.; Luo, J.; Liu, P.; Wang, T.; Hu, G.; Zhang, C. Effects of Molybdenum and Cadmium on the Oxidative Damage and Kidney Apoptosis in Duck. Ecotoxicol. Environ. Saf. 2017, 145, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Özçoban, M.Ş.; Acarer, S.; Tüfekci, N. Effect of Solid Waste Landfill Leachate Contaminants on Hydraulic Conductivity of Landfill Liners. Water Sci. Technol. 2022, 85, 1581–1599. [Google Scholar] [CrossRef]
- Xie, H.; Chen, Y.; Lou, Z. An Analytical Solution to Contaminant Transport through Composite Liners with Geomembrane Defects. Sci. China Technol. Sci. 2010, 53, 1424–1433. [Google Scholar] [CrossRef]
- Katsumi, T.; Ishimori, H.; Onikata, M.; Fukagawa, R. Long-Term Barrier Performance of Modified Bentonite Materials against Sodium and Calcium Permeant Solutions. Geotext. Geomembr. 2008, 26, 14–30. [Google Scholar] [CrossRef]
- Salemi, N.; Abtahi, S.M.; Rowshanzamir, M.A.; Hejazi, S.M. Improving Hydraulic Performance and Durability of Sandwich Clay Liner Using Super-Absorbent Polymer. J. Sandw. Struct. Mater. 2019, 21, 1055–1071. [Google Scholar] [CrossRef]
- Cui, Q.; Chen, B. Review of Polymer-Amended Bentonite: Categories, Mechanism, Modification Processes and Application in Barriers for Isolating Contaminants. Appl. Clay Sci. 2023, 235, 106869. [Google Scholar] [CrossRef]
- Kolstad, D.C.; Benson, C.H.; Edil, T.B. Hydraulic Conductivity and Swell of Nonprehydrated Geosynthetic Clay Liners Permeated with Multispecies Inorganic Solutions. J. Geotech. Geoenviron. Eng. 2004, 130, 1236–1249. [Google Scholar] [CrossRef] [Green Version]
- Jo, H.Y.; Katsumi, T.; Benson, C.H.; Edil, T.B. Hydraulic Conductivity and Swelling of Nonprehydrated GCLs Permeated with Single-Species Salt Solutions. J. Geotech. Geoenviron. Eng. 2001, 127, 557–567. [Google Scholar] [CrossRef]
- Chen, J.-K.; Xia, L.-X.; Yang, Y.-X.; Dina, M.; Zhang, S.; Zhan, L.-T.; Chen, Y.-M.; Bate, B. Polymer-Modified Bentonites with Low Hydraulic Conductivity and Improved Chemical Compatibility as Barriers for Cu2+ Containment. Acta. Geotech. 2022, 18, 1629–1649. [Google Scholar] [CrossRef]
- Yu, C.; Liao, R.; Cai, X.; Yu, X. Sodium Polyacrylate Modification Method to Improve the Permeant Performance of Bentonite in Chemical Resistance. J. Clean. Prod. 2019, 213, 242–250. [Google Scholar] [CrossRef]
- Scalia, J.; Benson, C.H.; Bohnhoff, G.L.; Edil, T.B.; Shackelford, C.D. Long-Term Hydraulic Conductivity of a Bentonite-Polymer Composite Permeated with Aggressive Inorganic Solutions. J. Geotech. Geoenviron. Eng. 2014, 140, 04013025. [Google Scholar] [CrossRef]
- Ozhan, H.O. Determination of Mechanical and Hydraulic Properties of Polyacrylamide-Added Bentonite-Sand Mixtures. Bull. Eng. Geol. Environ. 2021, 80, 2557–2571. [Google Scholar] [CrossRef]
- Zhang, C.; Wei, X.; Zhang, C.; Li, Y.; Sheng, Y.; Peng, S. Study on Preparation of Polymer-Modified Bentonite and Sand Mixtures Based on Osmotic Pressure Principle. Materials 2022, 15, 3643. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.-H.; Sun, W.-J.; Liu, K.; Tan, Y.-Z. Effect of Sand Particle Size on Hydraulic-Mechanical Behavior of Bentonite-Sand Mixtures. KSCE J. Civ. Eng. 2022, 26, 3287–3300. [Google Scholar] [CrossRef]
- Bohnhoff, G.L.; Shackelford, C.D. Improving Membrane Performance via Bentonite Polymer Nanocomposite. Appl. Clay. Sci. 2013, 86, 83–98. [Google Scholar] [CrossRef]
- Ejezie, J.O.; Jefferis, S.A.; Lam, C.; Sedighi, M.; Ahmad, S.M. Permeation Behaviour of PHPA Polymer Fluids in Sand. Géotechnique 2021, 71, 561–570. [Google Scholar] [CrossRef]
- Yu, C.; Yang, Y.; Wu, Z.; Jiang, J.; Liao, R.; Deng, Y. Experimental Study on the Permeability and Self-Healing Capacity of Geosynthetic Clay Liners in Heavy Metal Solutions. Geotext. Geomembr. 2021, 49, 413–419. [Google Scholar] [CrossRef]
- Uddin, M.K. A Review on the Adsorption of Heavy Metals by Clay Minerals, with Special Focus on the Past Decade. Chem. Eng. J. 2017, 308, 438–462. [Google Scholar] [CrossRef]
- Dhar, A.K.; Himu, H.A.; Bhattacharjee, M.; Mostufa, M.G.; Parvin, F. Insights on Applications of Bentonite Clays for the Removal of Dyes and Heavy Metals from Wastewater: A Review. Environ. Sci. Pollut. Res. 2023, 30, 5440–5474. [Google Scholar] [CrossRef] [PubMed]
- Hank, D.; Azi, Z.; Ait Hocine, S.; Chaalal, O.; Hellal, A. Optimization of Phenol Adsorption onto Bentonite by Factorial Design Methodology. J. Ind. Eng. Chem. 2014, 20, 2256–2263. [Google Scholar] [CrossRef]
- My Linh, N.L.; Duong, T.; Van Duc, H.; Thi Anh Thu, N.; Khac Lieu, P.; Van Hung, N.; Hoa, L.T.; Quang Khieu, D. Phenol Red Adsorption from Aqueous Solution on the Modified Bentonite. J. Chem. 2020, 2020, 1504805. [Google Scholar] [CrossRef]
- Majdan, M.; Sabah, E.; Bujacka, M.; Pikus, S.; Płaska, A.-G. Spectral and Equillibrium Properties of Phenol–HDTMA- and Phenol–BDMHDA-Bentonite as a Response to the Molecular Arrangements of Surfactant Cations. J. Mol. Struct. 2009, 938, 29–34. [Google Scholar] [CrossRef]
- Yadav, V.B.; Gadi, R.; Kalra, S. Clay Based Nanocomposites for Removal of Heavy Metals from Water: A Review. J. Environ. Manag. 2019, 232, 803–817. [Google Scholar] [CrossRef]
- Al Kausor, M.; Sen Gupta, S.; Bhattacharyya, K.G.; Chakrabortty, D. Montmorillonite and Modified Montmorillonite as Adsorbents for Removal of Water Soluble Organic Dyes: A Review on Current Status of the Art. Inorg. Chem. Commun. 2022, 143, 109686. [Google Scholar] [CrossRef]
- Li, Y.; Hu, X.; Liu, X.; Zhang, Y.; Zhao, Q.; Ning, P.; Tian, S. Adsorption Behavior of Phenol by Reversible Surfactant-Modified Montmorillonite: Mechanism, Thermodynamics, and Regeneration. Chem. Eng. J. 2018, 334, 1214–1221. [Google Scholar] [CrossRef]
- He, H.; Xu, E.; Qiu, Z.; Wu, T.; Wang, S.; Lu, Y.; Chen, G. Phenol Adsorption Mechanism of Organically Modified Bentonite and Its Microstructural Changes. Sustainability 2022, 14, 1318. [Google Scholar] [CrossRef]
- Meng, B.; Guo, Q.; Men, X.; Ren, S.; Jin, W.; Shen, B. Modified Bentonite by Polyhedral Oligomeric Silsesquioxane and Quaternary Ammonium Salt and Adsorption Characteristics for Dye. J. Saudi Chem. Soc. 2020, 24, 334–344. [Google Scholar] [CrossRef]
- Andini, S.; Cioffi, R.; Montagnaro, F.; Pisciotta, F.; Santoro, L. Simultaneous Adsorption of Chlorophenol and Heavy Metal Ions on Organophilic Bentonite. Appl. Clay Sci. 2006, 31, 126–133. [Google Scholar] [CrossRef]
- Liu, C.; Wu, P.; Zhu, Y.; Tran, L. Simultaneous Adsorption of Cd2+ and BPA on Amphoteric Surfactant Activated Montmorillonite. Chemosphere 2016, 144, 1026–1032. [Google Scholar] [CrossRef]
- Liu, C.; Wu, P.; Tran, L.; Zhu, N.; Dang, Z. Organo-Montmorillonites for Efficient and Rapid Water Remediation: Sequential and Simultaneous Adsorption of Lead and Bisphenol A. Environ. Chem. 2018, 15, 286. [Google Scholar] [CrossRef]
- Li, W.B.; Liu, Z.; Meng, Z.F.; Ren, S.; Xu, S.E.; Zhang, Y.; Wang, M.Y. Composite Modification Mechanism of Cationic Modifier to Amphoteric Modified Kaolin and Its Effects on Surface Characteristics. Int. J. Environ. Sci. Technol. 2016, 13, 2639–2648. [Google Scholar] [CrossRef]
- Li, W.; Meng, Z.; Liu, Z.; Chen, H.; Wu, Q.; Xu, S. Chromium (VI) Adsorption Characteristics of Bentonite Under Different Modification Patterns. Pol. J. Environ. Stud. 2016, 25, 1075–1083. [Google Scholar] [CrossRef]
- Li, X.; Li, W.; Deng, H.; Li, T.; Wang, D. Enhanced Adsorption of Cu2+ on Purple Soil by Amphoteric-Modified Materials. DWT 2020, 196, 278–286. [Google Scholar] [CrossRef]
- Heidarzadeh, N.; Parhizi, P. Improving the Permeability and Adsorption of Phenol by Organophilic Clay in Clay Liners. Environ. Eng. Res. 2019, 25, 96–103. [Google Scholar] [CrossRef]
- Li, Y.; Gong, D.; Zhou, Y.; Zhang, C.; Zhang, C.; Sheng, Y.; Peng, S. Respiratory Adsorption of Organic Pollutants in Wastewater by Superhydrophobic Phenolic Xerogels. Polymers 2022, 14, 1596. [Google Scholar] [CrossRef] [PubMed]
- Taha, A.A.; Shreadah, M.A.; Ahmed, A.M.; Heiba, H.F. Multi-Component Adsorption of Pb(II), Cd(II), and Ni(II) onto Egyptian Na-Activated Bentonite; Equilibrium, Kinetics, Thermodynamics, and Application for Seawater Desalination. J. Environ. Chem. Eng. 2016, 4, 1166–1180. [Google Scholar] [CrossRef]
- Hu, X.; Meng, Z.; Cao, X.; Liu, Z.; Wu, Z.; Sun, H.; Sun, X.; Li, W. Effect of Double Carbon Chains on Enhanced Removal of Phenol from Wastewater by Amphoteric-Gemini Complex-Modified Bentonite. Environ. Pollut. 2023, 320, 121088. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liao, R.; Yu, C.; Yu, X. Sorption of Pb(II) on Sodium Polyacrylate Modified Bentonite. Adv. Powder Technol. 2020, 31, 3274–3286. [Google Scholar] [CrossRef]




| Montmorillonite | Kaolinite | Quartz | Halloysite |
|---|---|---|---|
| 63% | 19% | 14% | 3% |
| 0% | 1% | 3% | 5% | |
|---|---|---|---|---|
| Pc | 80 | 110 | 140 | 170 |
| πµ | 80.6 | 110.5 | 137 | 170.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, X.; Zhang, C.; Gong, D.; Tu, M.; Wu, L.; Chen, W.; Zhang, C. A Study on the Impermeability of Nanodispersible Modified Bentonite Based on Colloidal Osmotic Pressure Mechanisms and the Adsorption of Harmful Substances. Nanomaterials 2023, 13, 1840. https://doi.org/10.3390/nano13121840
Wei X, Zhang C, Gong D, Tu M, Wu L, Chen W, Zhang C. A Study on the Impermeability of Nanodispersible Modified Bentonite Based on Colloidal Osmotic Pressure Mechanisms and the Adsorption of Harmful Substances. Nanomaterials. 2023; 13(12):1840. https://doi.org/10.3390/nano13121840
Chicago/Turabian StyleWei, Xi, Chunyang Zhang, Depeng Gong, Mengdong Tu, Lili Wu, Wanyu Chen, and Chaocan Zhang. 2023. "A Study on the Impermeability of Nanodispersible Modified Bentonite Based on Colloidal Osmotic Pressure Mechanisms and the Adsorption of Harmful Substances" Nanomaterials 13, no. 12: 1840. https://doi.org/10.3390/nano13121840





