Effect of Electrolyte Concentration on the Electrochemical Performance of Spray Deposited LiFePO4
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Spray Deposited LiFePO4
2.3. Basic Characterization
2.4. Electrochemical Evaluation of LiFePO4
3. Results
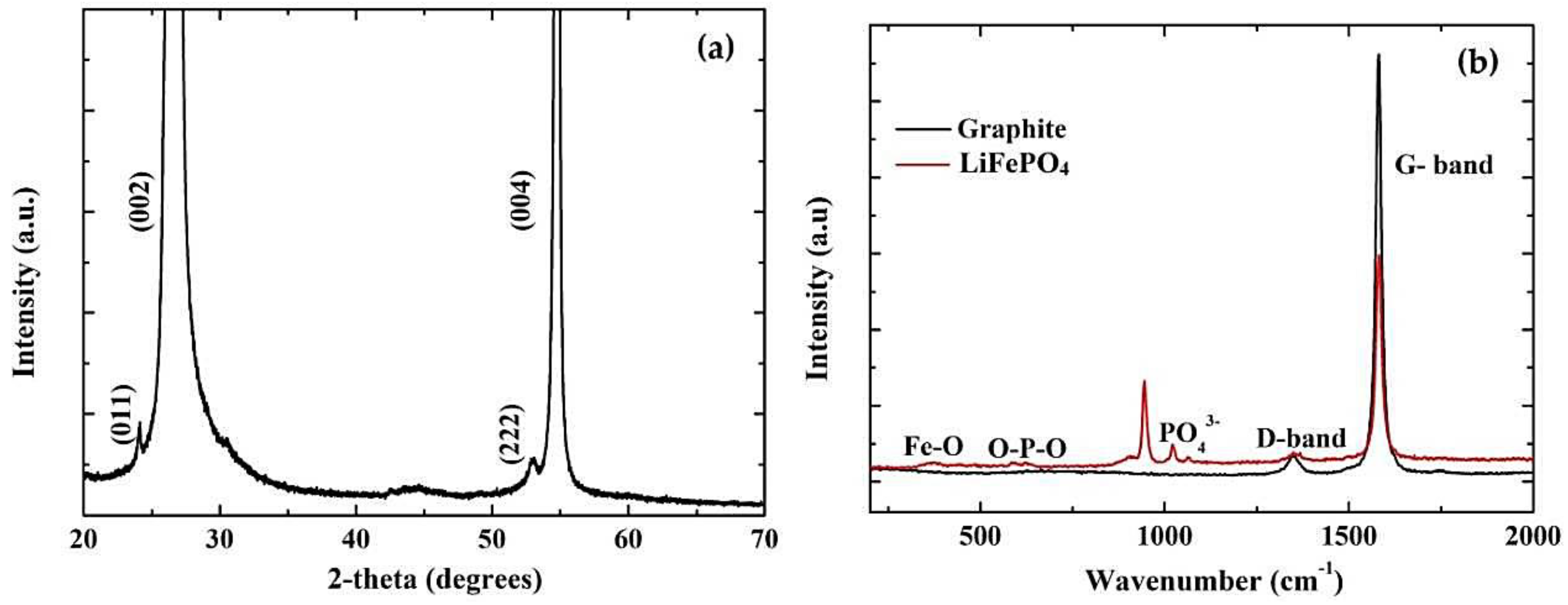
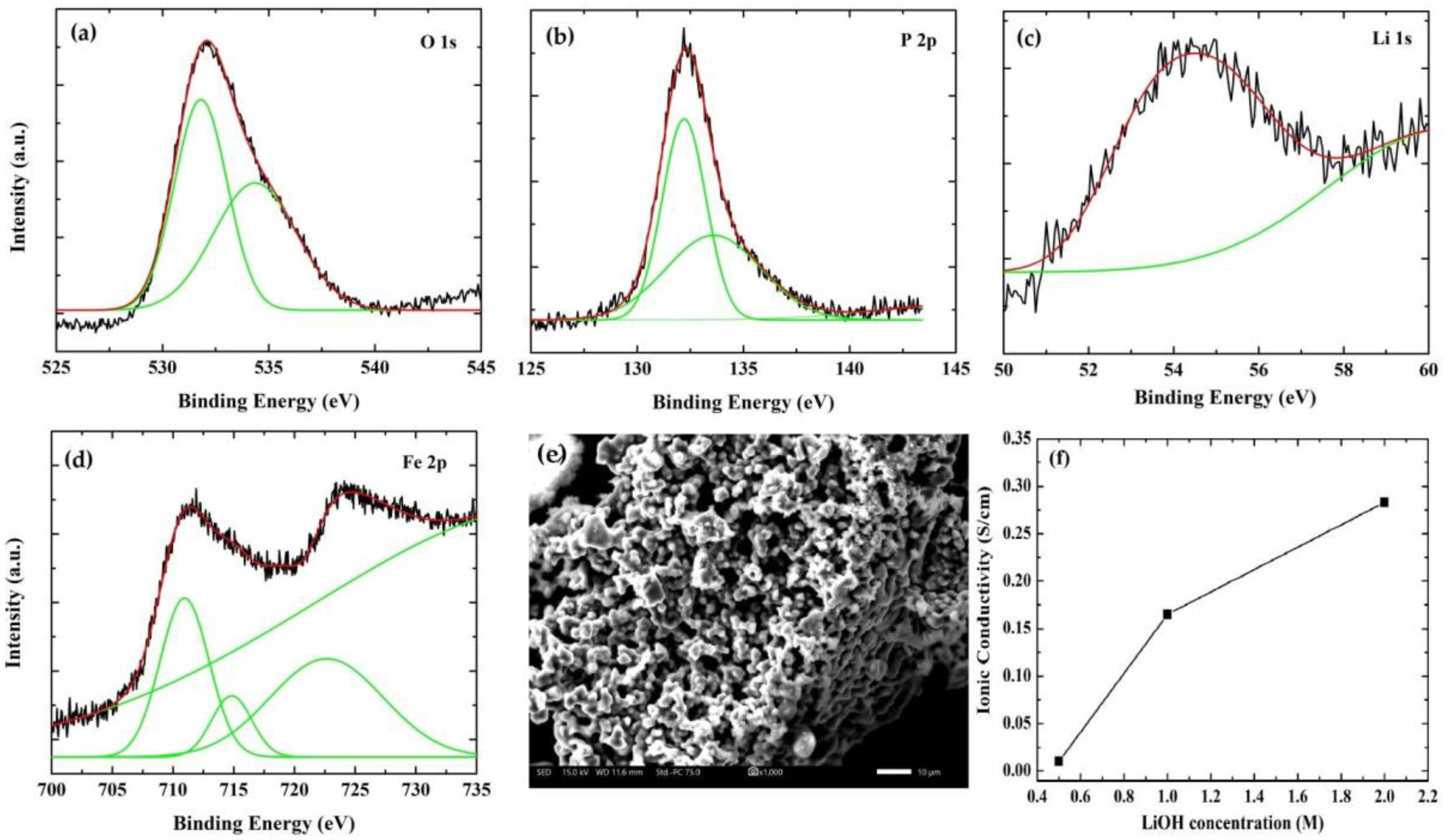
3.1. Structure and Morphology Evaluation
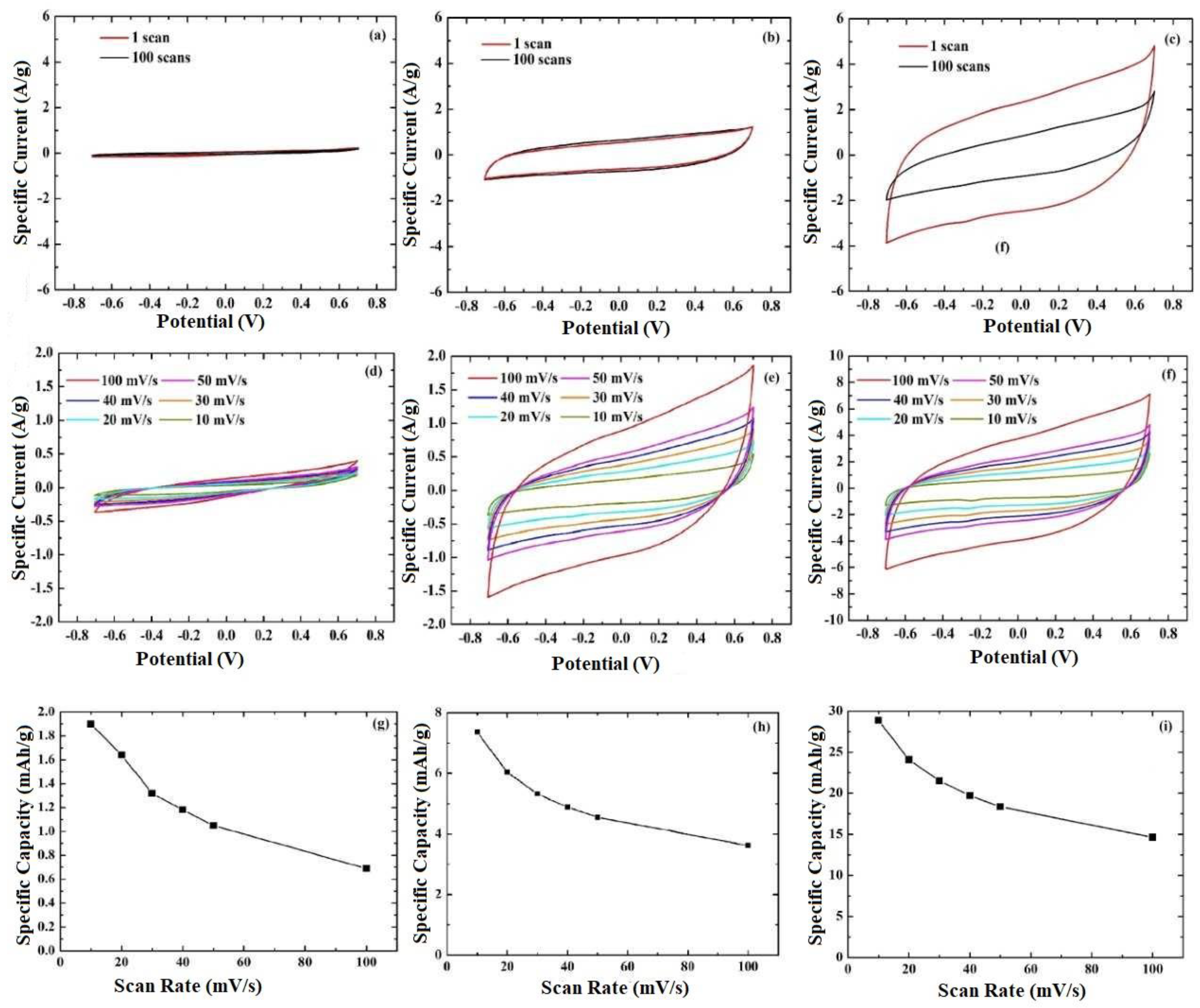
3.2. Electrochemical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wen, X.; Luo, J.; Xiang, K.; Zhou, W.; Zhang, C.; Chen, H. High-performance monoclinic WO3 nanospheres with the novel NH4+ diffusion behaviors for aqueous ammonium-ion batteries. Chem. Eng. J. 2023, 458, 141381. [Google Scholar] [CrossRef]
- Zhou, W.; Zeng, G.; Jin, H.; Jiang, S.; Huang, M.; Zhang, C.; Chen, H. Bio-Template Synthesis of V2O3@Carbonized Dictyophora Composites of Advanced Aqueous Zinc-Ion Batteries. Molecules 2023, 28, 2147. [Google Scholar] [CrossRef]
- Zhou, S.; Du, J.; Xiong, X.; Liu, L.; Wang, J.; Fu, L.; Ye, J.; Chen, Y.; Wu, Y. Direct recovery of scrapped LiFePO4 by a green and low-cost electrochemical re-lithiation method. Green Chem. 2022, 24, 6278–6286. [Google Scholar] [CrossRef]
- Kumar, S.; Chand, P.; Kumar, A.; Anand, H. Effect of different aqueous electrolytes on electrochemical behavior of LiFePO4 as a cathode material: Lithium ion battery and renewable energy nexus. Energy Nexus 2021, 1, 100005. [Google Scholar] [CrossRef]
- Zhou, N.; Liu, Y.; Li, J.; Uchaker, E.; Liu, S.; Huang, K.; Cao, G. Synthesis and characterization of high power LiFePO4/C nano-plate thin films. J. Power Sources 2012, 213, 100–105. [Google Scholar] [CrossRef]
- Vernardou, D. Recent report on the hydrothermal growth of LiFePO4 as a cathode material. Coatings 2022, 12, 1543. [Google Scholar] [CrossRef]
- Satyavani, T.V.S.L.; Kumar, A.S.; Rao, P.S.V.S. Methods of synthesis and performance improvement of lithium iron phosphate for high Li-ion batteries: A review. Eng. Sci. Technol. Int. J. 2016, 19, 178–188. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.-P.; Lv, D.; Chen, J.; Zhang, Y.-H.; Shi, F.-N. Review on defects and modification methods of LiFePO4 cathode material for lithium-ion batteries. Energy Fuels 2022, 36, 1232–1251. [Google Scholar] [CrossRef]
- Li, Z.; Yang, J.; Guang, T.; Fan, B.; Zhu, K.; Wang, X. Controlled hydrothermal/Solvothermal synthesis of high-performance LiFePO4 for Li-ion batteries. Small Methods 2021, 5, 2100193. [Google Scholar] [CrossRef]
- Wu, J.; Cai, W.; Shang, G. Electrochemical behaviour of LiFePO4 thin films prepared by rf magnetron sputtering in Li2SO4 aqueous electrolyte. Int. J. Nanosci. 2015, 14, 1460027. [Google Scholar] [CrossRef]
- Zhao, Q.-F.; Zhang, S.-Q.; Hu, M.-Y.; Wang, C.; Jiang, G.-H. Recent advances in LiFePO4 cathode materials for Li-ion batteries. First-principles research. Int. J. Electrochem. Sci. 2021, 16, 211226. [Google Scholar] [CrossRef]
- Kucinskis, G. Kinetic behaviour of LiFePO4/C thin film cathode material for lithium-ion batteries. Environ. Clim. Technol. 2010, 4, 53–57. [Google Scholar]
- Pat, S.; Yudar, H.H.; Korkmaz, Ş.; Özen, S.; Pat, Z. LiFePO4 thin deposition onto Ag coated glass by rf magnetron sputtering. Mater. Res. Express 2018, 5, 116401. [Google Scholar] [CrossRef]
- Hong, J.; Wang, C.; Dudney, N.J.; Lance, M.J. Characterization and performance of LiFePO4 thin-film cathodes prepared with radio-frequency magnetron-sputter deposition. J. Electrochem. Soc. 2007, 154, A805–A807. [Google Scholar] [CrossRef]
- Zhu, X.-J.; Cheng, L.-B.; Wang, C.-G.; Guo, Z.-P.; Zhang, P.; Du, G.-D.; Liu, H.-K. Preparation and characteristics of LiFePO4 thin film by radio frequency magnetron sputtering for lithium microbatteries. J. Phys. Chem. C 2009, 113, 14518–14522. [Google Scholar] [CrossRef]
- Sugiawati, V.A.; Vacandio, F.; Perrin-Pellegrino, C.; Galeyeva, A.; Kurbato, A.P.; Djenizian, T. Sputtered porous Li-Fe-P-O film cathodes prepared by radio frequency sputtering for Li-ion microbatteries. Sci. Rep. 2019, 9, 11172. [Google Scholar] [CrossRef] [Green Version]
- Mosa, J.; Aparicio, M.; Durán, A.; Laberty-Robert, C.; Sanchez, C. Nanocrystalline mesoporous LiFePO4 thin-films as cathodes for Li-ion microbatteries. J. Mater. Chem. A 2014, 2, 3038–3046. [Google Scholar] [CrossRef]
- Balakrishnan, T.; Sankarasubramanian, N.; Kathalingam, A. Studies on structural and optical properties of LiFePO4 thin films. Dig. J. Nanomater. Biostruct. 2017, 12, 659–667. [Google Scholar]
- Ramasubramanian, B.; Sundarrajan, S.; Chellappan, V.; Reddy, M.V.; Ramakrishna, S.; Zaghib, K. Recent development in carbon-LiFePO4 cathodes for Lithium-ion batteries: A Mini Review. Batteries 2022, 8, 133. [Google Scholar] [CrossRef]
- Nariyama, H.; Ito, S.; Okada, Y.; Inatomi, Y.; Ichikawa, K.; Masumoto, Y.; Fujimoto, M. High energy density 3V-class redox flow battery using LiFePO4 and graphite with organic bifunctional redox mediators. Electrochim. Acta 2022, 409, 139915. [Google Scholar] [CrossRef]
- Liao, Y.; Li, G.; Xu, N.; Chen, T.; Wang, X.; Li, W. Synergistic effect of electrolyte additives on the improvement in interfacial stability between ionic liquid based gel electrolyte and LiFePO4 cathode. Solid State Ion. 2019, 329, 31–39. [Google Scholar] [CrossRef]
- Zhang, Q.; Sha, Z.; Cui, X.; Qiu, S.; He, C.; Zhang, J.; Wang, X.; Yang, Y. Incorporation of redox-active polyimide binder into LiFePO4 cathode for high-rate electrochemical energy storage. Nanotechnol. Rev. 2020, 9, 1350–1358. [Google Scholar] [CrossRef]
- Yusuf, A.; Avvaru, V.S.; De la Vega, J.; Zhang, M.; Molleja, J.G.; Wang, D.-Y. Unveiling the structure, chemistry, and formation mechanism of an in-situ phosphazene flame retardant-derived interphase layer in LiFePO4 cathode. Chem. Eng. J. 2023, 455, 140678. [Google Scholar] [CrossRef]
- Pasta, M.; Wessels, C.D.; Huggins, R.A.; Cui, Y. A high-rate and long cycle life aqueous electrolyte battery for grid-scale energy storage. Nat. Commun. 2012, 3, 1149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sajjad, M.; Khan, M.I.; Cheng, F.; Lu, W. A review on selection criteria of aqueous electrolytes performance evaluation for advanced asymmetric supercapacitors. J. Energy Storage 2021, 40, 102729. [Google Scholar] [CrossRef]
- Zhang, A.; Yang, X.; Yang, F.; Zhang, C.; Zhang, Q.; Duan, G.; Jiang, S. Research Progress of the Ion Avtivity Coefficient of Polyelectrolytes: A Review. Molecules 2023, 28, 2042. [Google Scholar] [CrossRef]
- Mouratis, K.; Tudose, V.; Romanitan, C.; Pachiu, C.; Tutunaru, O.; Suchea, M.; Couris, S.; Vernardou, D.; Koudoumas, E. Electrochromic performance of V2O5 thin films grown by spray pyrolysis. Materials 2020, 13, 3859. [Google Scholar] [CrossRef]
- Oluwatosin Abegunde, O.; Titilayo Akinlabi, E.; Philip Oladijo, O.; Akinlabi, S.; Uchenna Ude, A. Overview of thin film deposition techniques. AIMS Mater. Sci. 2019, 6, 174–199. [Google Scholar] [CrossRef]
- Ikeda, S.; Fujikawa, S.; Harada, T.; Nguyen, T.H.; Nakanishi, S.; Takayama, T.; Iwase, A.; Kudo, A. Photocathode characteristics of a spray-deposited Cu2ZnGeS4 thin film for CO2 reduction in a CO2-saturated aqueous solution. ACS Appl. Energy Mater. 2019, 2, 6911–6918. [Google Scholar] [CrossRef]
- Vernardou, D.; Drosos, H.; Spanakis, E.; Koudoumas, E.; Katsarakis, N.; Pemble, M.E. Electrochemical properties of amorphous WO3 coatings grown on polycarbonate by aerosol-assisted CVD. Electrochim. Acta 2012, 65, 185–189. [Google Scholar] [CrossRef]
- Vernardou, D.; Apostolopoulou, M.; Louloudakis, D.; Katsarakis, N.; Koudoumas, E. Hydrothermally grown β-V2O5 electrode at 95 °C. J. Colloid Interface Sci. 2014, 424, 1–6. [Google Scholar] [PubMed]
- Liu, S.; Yan, P.; Li, H.; Zhang, X.; Sun, W. One-step microwave synthesis of micro/nanoscale performance for lithium-ion batteries. Front. Chem. 2020, 8, 104. [Google Scholar] [CrossRef]
- Tul Ain, Q.; Hyder Haq, S.; Alshammari, A.; Abdullah Al-Mutlaq, M.; Naeem Anjum, M. The synergetic effect of PEG-nGO-induced oxidative stree in vivo in a rodent model. Beilstein J. Nanotechnol. 2019, 10, 901–911. [Google Scholar]
- Wu, J.; Phani Dathar, G.K.; Sun, C.; Theivanayagam, M.G.; Applestone, D.; Dylla, A.G.; Manthiram, A.; Henkelman, G.; Goodenough, J.B.; Stevenson, K.J. In situ raman spectroscopy of LiFePO4: Size and morphology dependence during charge and self-discharge. Nanotechnology 2013, 24, 424009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, R.; Chen, J.; Li, Z.; Ding, Q.; An, X.; Pan, Y.; Zheng, Z.; Yang, M.; Fu, D. Preparation of LiFePO4/C cathode materials via a green synthesis route for lithium-ion battery applications. Materials 2018, 11, 2251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castro, L.; Dedryvère, R.; Ledeuil, J.-B.; Bréger, J.; Tessier, C.; Gonbeau, D. Aging mechanisms of LiFePO4//Graphite cells studied by XPS: Redox reaction and electrode/electrolyte interfaces. J. Electrochem. Soc. 2012, 159, A357–A363. [Google Scholar] [CrossRef]
- Schulz, N.; Hausbrand, R.; Dimesso, L.; Jaegermann, W. XPS surface analysis of SEI layers on Li-ion cathodes: Part I. Investigation of Initial Surface Chemistry. J. Electrochem. Soc. 2018, 165, A819–A832. [Google Scholar] [CrossRef]
- Gao, C.; Zhou, J.; Liu, G.; Wang, L. Lithium-ions diffusion kinetic in LiFePO4/carbon nanoparticles synthesized by microwave plasma chemical vapor deposition for lithium-ion batteries. Appl. Surf. Sci. 2018, 433, 35–44. [Google Scholar]
- Xiong, W.; Hu, Q.; Liu, S.A. A novel and accurate analytical method based on X-ray photoelectron spectroscopy for the quantitative detection of the lithium content in LiFePO4. Anal. Methods 2014, 6, 5708–5711. [Google Scholar]
- Castro, L.; Dedryvère, R.; El Khalifi, M.; Lippens, P.-E.; Bréger, J.; Tessier, C.; Gonbeau, D. The spin-polarized electronic structure of LiFePO4 and FePO4 evidenced by in-Lab XPS. J. Phys. Chem. C 2010, 114, 17995–18000. [Google Scholar] [CrossRef]
- Jin, Y.; Tang, X.; Wang, Y.; Dang, W.; Huang, J.; Fang, X. High-tap density LiFePO4 microsphere developed by comined computational and experimental approaches. R. Soc. Chem. 2018, 20, 6695–6703. [Google Scholar]
- Kumar, A.; Thomas, R.; Karan, N.K.; Saavedra-Arias, J.J.; Singh, M.K.; Majumder, S.B.; Tomar, M.S.; Katiyar, R.S. Structural and Electrochemical Characterization of Pure LiFePO4 and Nanocomposite C-LiFePO4 Cathodes for Lithium Ion Rechargeable Batteries. J. Nanotechnol. 2009, 2009, 176517. [Google Scholar] [CrossRef] [Green Version]
- Béléké, A.B.; Faure, C.; Röder, M.; Hovington, P.; Posset, U.; Guerfi, A.; Zaghib, K. Chemically fabricated LiFePO4 thin film electrode for transparent batteries and electrochromic devices. Mater. Sci. Eng. B 2016, 214, 81–86. [Google Scholar] [CrossRef]
- Karade, S.S.; Dubal, D.P.; Sankapal, B.R. MoS2 ultrathin nanoflakes for high performance supercpacitors: Room temperature chemical bath deposition (CBD). RSC Adv. 2016, 6, 39159–39165. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Liu, J. Definitions of pseudocapacitive materials: A Brief Review. Energy Environ. Mater. 2019, 2, 30–37. [Google Scholar] [CrossRef] [Green Version]
- Soon, J.M.; Loh, K.P. Electrochemical double-layer capacitance of MoS2 nanowall films. Electrochem. Solid-State Lett. 2007, 10, A250–A254. [Google Scholar] [CrossRef]
- Lee, S.; Jang, J.; Lee, D.; Kim, J.; Mun, J. Synergetic effect of aqueous electrolyte and ultra-thick millimeter-scale LiFePO4 cathode in aqueous lithium-ion batteries. Int. J. Energy Res. 2022, 46, 6480–6486. [Google Scholar] [CrossRef]
- Palanisamy, R.; Karuppiah, D.; Venkatesan, S.; Mani, S.; Kuppusamy, M.; Marimuthu, S.; Karuppanan, A.; Govindaraju, R.; Marimuthu, S.; Rengapillai, S.; et al. High-performance asymmetric supercapacitor fabricated with a novel MoS2/Fe2O3/Graphene composite electrode. Colloid Interface Sci. Commun. 2022, 46, 100573. [Google Scholar] [CrossRef]
- Luo, J.-Y.; Cui, W.-J.; He, P.; Xia, Y.-Y. Raising the cycling stability of aqueous lithium-ion batteries by eliminating oxygen in the electrolyte. Nat. Chem. 2010, 2, 760–765. [Google Scholar] [CrossRef]
- Majumder, M.; Thakur, A.K.; Bhushan, M.; Mohapatra, D. Polyaniline integration and interrogation on carbon nano-onions empowered supercapacitors. Electrochim. Acta 2021, 370, 137659. [Google Scholar] [CrossRef]
- Waseem, S.; Dubey, P.; Singh, M.; Sundriyal, S.; Maheshwari, P.H. Chemically oxidized carbon paper as a free-standing electrode for supercapacitor: An insight into surface and diffusion contribution. Chem. Sel. 2023, 8, e202204377. [Google Scholar] [CrossRef]
- Kurzweil, P. Gaston plante and his invention of the lead-acid battery-the genesis of the first practical rechargeable battery. J. Power Sources 2010, 195, 4424–4434. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, W.; Zhang, Y.; Lu, G. Highly conductive SiO2@PEDOT:PSS/LiFePO4 composite cathode for lithium-ion batteries. J. Power Sources 2015, 274, 1010–1015. [Google Scholar]
- Zhou, J.; Guo, X.; Liu, L.; Yang, Y. Enhanced conductivity of LiFePO4 cathode by coating with polyanailine for high-performance lithium-ion batteries. Electrochim. Acta 2013, 102, 62–67. [Google Scholar]
- Noerochim, L.; Yurwendra, A.O.; Susanti, D. Effect of carbon coating on the electrochemical performance of LiFePO4/C as cathode materials for aqueous electrolyte lithium-ion battery. Ionics 2016, 22, 341–346. [Google Scholar] [CrossRef]
- Wang, X.; Lee, P.S. Titanium doped niobium oxide for stable pseudocapacitive lithium ion storage and its application in 3 V non-aqueous supercapacitors. J. Mater. Chem. A 2015, 3, 21706–21712. [Google Scholar] [CrossRef]
- Wang, X.; Shen, G. Intercalation pseudo-capacitive TiNb2O7@carbon electrode for high performance lithium ion hybrid electrochemical supercapacitors with ultrahigh energy density. Nano Energy 2015, 15, 104–115. [Google Scholar] [CrossRef]
- Vujković, M.; Stojković, I.; Cvjetićanin, N.; Mentus, S. Gel-combustion synthesis of LiFePO4/C composite with improved capacity retention in aerated aqueous electrolyte solution. Electrochim. Acta 2013, 92, 248–256. [Google Scholar]
- Yin, Y.; Wen, Y.-H.; Lu, Y.-I.; Cheng, J.; Cao, G.-P.; Yang, Y.-S. Electrochemical performance and fading at different pH aqueous electrolyte solutions. Chin. J. Chem. Phys. 2015, 28, 315–322. [Google Scholar] [CrossRef] [Green Version]
- Mi, C.H.; Zhang, X.G.; Li, H.L. Electrochemical behaviors of solid LiFePO4 and Li0.99Nb0.01FePO4 in Li2SO4 aqueous electrolyte. J. Electroanal. Chem. 2007, 602, 245–254. [Google Scholar] [CrossRef]
- Zeng, X.; Liu, Q.; Chen, M.; Leng, L.; Shu, T.; Du, L.; Song, H. Electrochemical behavior of spherical LiFePO4/C nanomaterial in aqueous electrolyte, and novel aqueous rechargeable lithium battery with LiFePO4/C anode. Electrochim. Acta 2015, 177, 277–282. [Google Scholar] [CrossRef]
- He, P.; Liu, J.-L.; Cui, W.-J.; Luo, J.-Y.; Xia, Y.-Y. Investigation on capacity fading of LiFePO4 in aqueous electrolytes. Electrochim. Acta 2011, 56, 2351–2357. [Google Scholar] [CrossRef]
- Zhang, Y.; Xin, P.; Yao, Q. Electrochemical performance of LiFePO4/C synthesized by sol-gel method as cathode for aqueous lithium ion batteries. J. Alloys Compd. 2018, 741, 404–408. [Google Scholar] [CrossRef]
- Sun, J.; Ren, X.; Li, Z.; Wang, L.; Liang, G. Synthesis and electrochemical performance of LiFePO4/C composite based on xylitol-polyvinyl alcohol complex carbon sources. Ionics 2018, 4, 1567–1575. [Google Scholar] [CrossRef]
- Shen, F.; Liu, Y. LiFePO4 cathode material modification and its recycling research based on the development status of lithium-ion batteries. Acad. J. Environ. Earth Sci. 2021, 3, 4–8. [Google Scholar]
- Minaskhi, M. Lithium intercalation into amorphous FePO4 cathode in aqueous solutions. Electrochim. Acta 2010, 55, 9174–9178. [Google Scholar]



| Cathode | Diffusion Coefficient (cm2/s) | Aqueous Electrolytes |
|---|---|---|
| LiFePO4/C (gel-combustion synthesis) [59] | 0.8 × 10−14 | Saturated LiNO3 |
| LiFePO4 (commercial powder) [60] | 2.020 × 10−9 | Saturated LiNO3 |
| LiFePO4 (in situ synthesis technique) [61] | 1.5 × 10−11 | 1 M Li2SO4 |
| LiFePO4/C (spraying drying process) [62] | 1.22 × 10−14 | 0.5 M Li2SO4 |
| LiFePO4 on Graphite | 0.25 × 10−9 | 0.5 M LiOH This work |
| LiFePO4 on Graphite | 5.46 × 10−9 | 1 M LiOH This work |
| LiFePO4 on Graphite | 6.20 × 10−9 | 2 M LiOH This work |
| Cathode | Specific Capacity (mAh/g) | Aqueous Electrolytes |
|---|---|---|
| LiFePO4 (solid-state reaction process) [54] | 13.3 | 1 M Li2SO4 |
| LiFePO4 (hydrothermal growth) [60] | 2.8 1.75 | 1 M KOH 2 M NaOH |
| LiFePO4 (direct recovery of scrapped LiFePO4) [4] | 134 | 1 M Li2SO4 |
| LiFePO4 (commercial powder) [48] | 110 | 2 M Li2SO4 |
| LiFePO4 (mechanochemical activation) [59] | 130 | 0.5 M Li2SO4 |
| LiFePO4/C (sol–gel) [63] | 163.5 | 1 M Li2SO4 |
| LiFePO4/C (spray-drying) [62] | 140 | 0.5 M Li2SO4 |
| LiFePO4 (spraying deposition) | 36 | 2 M LiOH This work |
| LiFePO4 (solution method) [64] | 65 | 1 M LiOH |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Floraki, C.; Androulidaki, M.; Spanakis, E.; Vernardou, D. Effect of Electrolyte Concentration on the Electrochemical Performance of Spray Deposited LiFePO4. Nanomaterials 2023, 13, 1850. https://doi.org/10.3390/nano13121850
Floraki C, Androulidaki M, Spanakis E, Vernardou D. Effect of Electrolyte Concentration on the Electrochemical Performance of Spray Deposited LiFePO4. Nanomaterials. 2023; 13(12):1850. https://doi.org/10.3390/nano13121850
Chicago/Turabian StyleFloraki, Christina, Maria Androulidaki, Emmanuel Spanakis, and Dimitra Vernardou. 2023. "Effect of Electrolyte Concentration on the Electrochemical Performance of Spray Deposited LiFePO4" Nanomaterials 13, no. 12: 1850. https://doi.org/10.3390/nano13121850





