Recent Progress on Ligand-Protected Metal Nanoclusters in Photocatalysis
Abstract
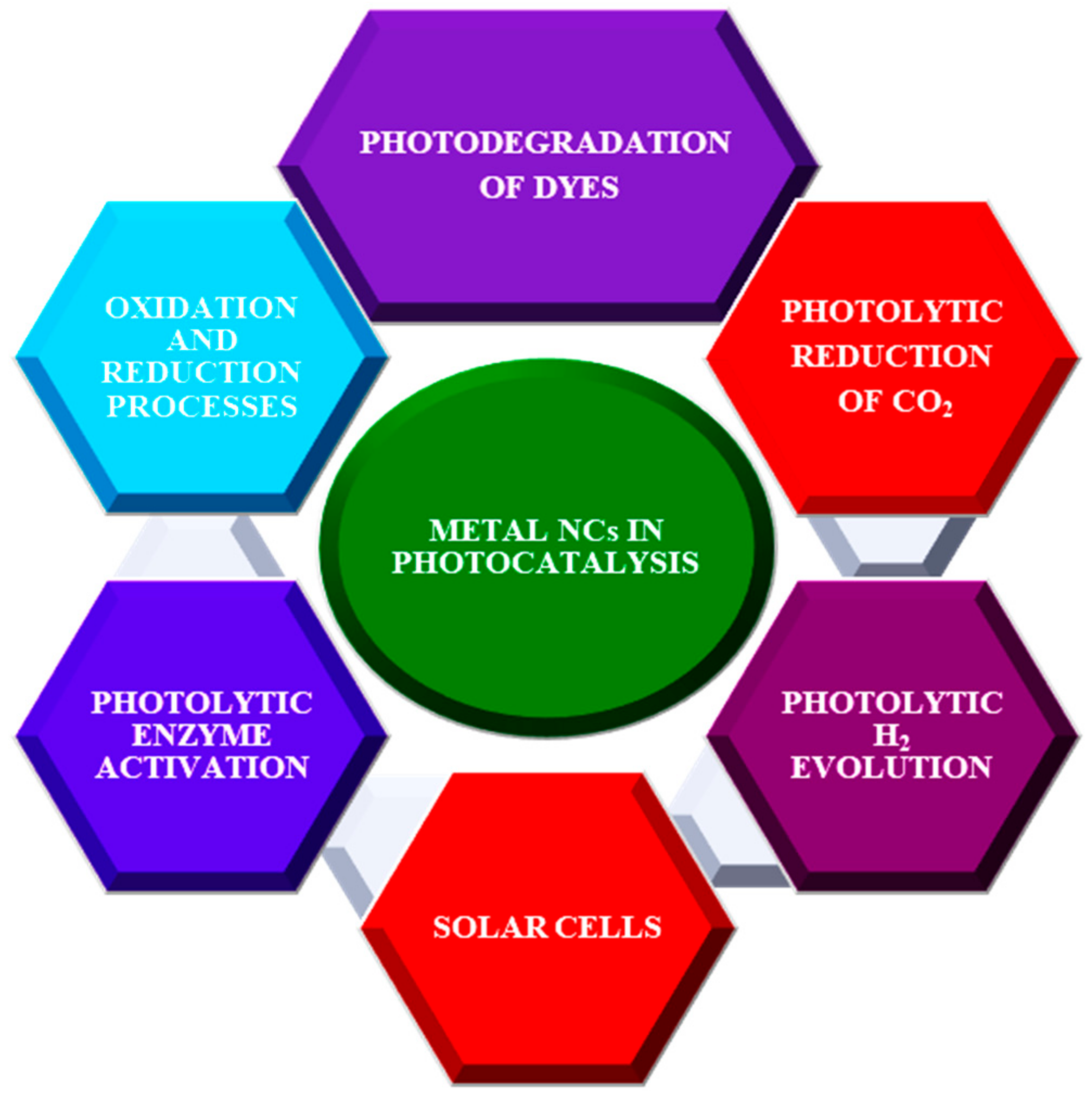
:1. Introduction
1.1. Chemical Composition and Structural Properties
1.2. Synthesis of Metal Nanocluster
1.2.1. General Synthetic Methods
1.2.2. Bottom-Up Method
1.2.3. Top-Down Method
1.2.4. Inter-Cluster Conversion Method
1.2.5. Monolayer-Protected Method
1.2.6. Etching Method
1.2.7. Template Method
1.2.8. Trends in Ligands Used for Nanocluster Stabilization
1.3. Key Physicochemical Properties of MNCs in Photocatalysis
1.4. Optical Properties
1.4.1. Optical Absorption
1.4.2. Photoluminescence
1.4.3. Two-Photon Absorption
1.4.4. Chirality
1.5. Stability
2. Application of Nanoclusters in Photocatalysis
2.1. Photodegradation of Organic Pollutants
2.2. Oxidation and Hydrogenation Processes
2.3. Photocatalytic H2 Production
2.3.1. Hydrogen Evolution Reaction
2.3.2. Photocatalytic Water Splitting
2.4. Photocatalytic CO2 Reduction
3. Conclusions and Future Perspective
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Bakbolat, B.; Daulbayev, C.; Sultanov, F.; Beissenov, R.; Umirzakov, A.; Mereke, A.; Bekbaev, A.; Chuprakov, I. Recent Developments of TiO2-Based Photocatalysis in the Hydrogen Evolution and Photodegradation: A Review. Nanomaterials 2020, 10, 1790. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Xiang, Q.; Liao, Y.; Zhang, H. CdS-Based Photocatalysts. Energy Environ. Sci. 2018, 11, 1362–1391. [Google Scholar] [CrossRef]
- Ong, C.B.; Ng, L.Y.; Mohammad, A.W. A Review of ZnO Nanoparticles as Solar Photocatalysts: Synthesis, Mechanisms and Applications. Renew. Sustain. Energy Rev. 2018, 81, 536–551. [Google Scholar] [CrossRef]
- Hitam, C.N.C.; Jalil, A.A. A Review on Exploration of Fe2O3 Photocatalyst towards Degradation of Dyes and Organic Contaminants. J. Environ. Manag. 2020, 258, 110050. [Google Scholar] [CrossRef]
- Lee, G.-J.; Wu, J.J. Recent Developments in ZnS Photocatalysts from Synthesis to Photocatalytic Applications—A Review. Powder Technol. 2017, 318, 8–22. [Google Scholar] [CrossRef]
- Li, J.; Lou, Z.; Li, B. Nanostructured Materials with Localized Surface Plasmon Resonance for Photocatalysis. Chin. Chem. Lett. 2022, 33, 1154–1168. [Google Scholar] [CrossRef]
- Song, X.-R.; Goswami, N.; Yang, H.-H.; Xie, J. Functionalization of Metal Nanoclusters for Biomedical Applications. Analyst 2016, 141, 3126–3140. [Google Scholar] [CrossRef] [Green Version]
- Goswami, N.; Zheng, K.; Xie, J. Bio-NCs—The Marriage of Ultrasmall Metal Nanoclusters with Biomolecules. Nanoscale 2014, 6, 13328–13347. [Google Scholar] [CrossRef]
- Basu, S.; Paul, A.; Antoine, R. Controlling the Chemistry of Nanoclusters: From Atomic Precision to Controlled Assembly. Nanomaterials 2021, 12, 62. [Google Scholar] [CrossRef]
- Wu, Z.; Jin, R. On the Ligand’s Role in the Fluorescence of Gold Nanoclusters. Nano Lett. 2010, 10, 2568–2573. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Corma, A. Metal Catalysts for Heterogeneous Catalysis: From Single Atoms to Nanoclusters and Nanoparticles. Chem. Rev. 2018, 118, 4981–5079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, X.; Jin, R. Atomically Precise Metal Nanoclusters for Catalysis. ACS Nano 2019, 13, 7383–7387. [Google Scholar] [CrossRef]
- Khandelwal, P.; Poddar, P. Fluorescent Metal Quantum Clusters: An Updated Overview of the Synthesis, Properties, and Biological Applications. J. Mater. Chem. B 2017, 5, 9055–9084. [Google Scholar] [CrossRef] [PubMed]
- Templeton, A.C.; Wuelfing, W.P.; Murray, R.W. Monolayer-Protected Cluster Molecules. Acc. Chem. Res. 2000, 33, 27–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qu, X.; Li, Y.; Li, L.; Wang, Y.; Liang, J.; Liang, J. Fluorescent Gold Nanoclusters: Synthesis and Recent Biological Application. J. Nanomater. 2015, 2015, 4. [Google Scholar] [CrossRef] [Green Version]
- Yang, T.-Q.; Peng, B.; Shan, B.-Q.; Zong, Y.-X.; Jiang, J.-G.; Wu, P.; Zhang, K. Origin of the Photoluminescence of Metal Nanoclusters: From Metal-Centered Emission to Ligand-Centered Emission. Nanomaterials 2020, 10, 261. [Google Scholar] [CrossRef] [Green Version]
- Antoine, R. Supramolecular Gold Chemistry: From Atomically Precise Thiolate-Protected Gold Nanoclusters to Gold-Thiolate Nanostructures. Nanomaterials 2020, 10, 377. [Google Scholar] [CrossRef] [Green Version]
- Kolay, S.; Bain, D.; Maity, S.; Devi, A.; Patra, A.; Antoine, R. Self-Assembled Metal Nanoclusters: Driving Forces and Structural Correlation with Optical Properties. Nanomaterials 2022, 12, 544. [Google Scholar] [CrossRef]
- Su, Y.; Xue, T.; Liu, Y.; Qi, J.; Jin, R.; Lin, Z. Luminescent Metal Nanoclusters for Biomedical Applications. Nano Res. 2019, 12, 1251–1265. [Google Scholar] [CrossRef]
- Bonačić-Koutecký, V.; Antoine, R. Enhanced Two-Photon Absorption of Ligated Silver and Gold Nanoclusters: Theoretical and Experimental Assessments. Nanoscale 2019, 11, 12436–12448. [Google Scholar] [CrossRef] [PubMed]
- Genji Srinivasulu, Y.; Yao, Q.; Goswami, N.; Xie, J. Interfacial Engineering of Gold Nanoclusters for Biomedical Applications. Mater. Horiz. 2020, 7, 2596–2618. [Google Scholar] [CrossRef]
- Antoine, R.; Maysinger, D.; Sancey, L.; Bonačić-Koutecký, V. Open Questions on Proteins Interacting with Nanoclusters. Commun. Chem. 2022, 5, 47. [Google Scholar] [CrossRef] [PubMed]
- Combes, G.F.; Vučković, A.-M.; Perić Bakulić, M.; Antoine, R.; Bonačić-Koutecky, V.; Trajković, K. Nanotechnology in Tumor Biomarker Detection: The Potential of Liganded Nanoclusters as Nonlinear Optical Contrast Agents for Molecular Diagnostics of Cancer. Cancers 2021, 13, 4206. [Google Scholar] [CrossRef]
- Rudzińska, M.; Daglioglu, C.; Savvateeva, L.V.; Kaci, F.N.; Antoine, R.; Zamyatnin, A.A., Jr. Current Status and Perspectives of Protease Inhibitors and Their Combination with Nanosized Drug Delivery Systems for Targeted Cancer Therapy. Drug Des. Dev. Ther. 2021, 15, 9–20. [Google Scholar] [CrossRef]
- Borghei, Y.; Hosseinkhani, S.; Ganjali, M.R. Bridging from Metallic Nanoclusters to Biomedical in Understanding Physicochemical Interactions at the Nano–Bio Interface. Part. Part. Syst. Charact. 2022, 39, 2100202. [Google Scholar] [CrossRef]
- Chai, O.J.H.; Liu, Z.; Chen, T.; Xie, J. Engineering Ultrasmall Metal Nanoclusters for Photocatalytic and Electrocatalytic Applications. Nanoscale 2019, 11, 20437–20448. [Google Scholar] [CrossRef]
- Liang, H.; Liu, B.-J.; Tang, B.; Zhu, S.-C.; Li, S.; Ge, X.-Z.; Li, J.-L.; Zhu, J.-R.; Xiao, F.-X. Atomically Precise Metal Nanocluster-Mediated Photocatalysis. ACS Catal. 2022, 12, 4216–4226. [Google Scholar] [CrossRef]
- Munir, A.; Joya, K.S.; Ul haq, T.; Babar, N.; Hussain, S.Z.; Qurashi, A.; Ullah, N.; Hussain, I. Metal Nanoclusters: New Paradigm in Catalysis for Water Splitting, Solar and Chemical Energy Conversion. ChemSusChem 2019, 12, 1517–1548. [Google Scholar] [CrossRef]
- Kauffman, D.R.; Thakkar, J.; Siva, R.; Matranga, C.; Ohodnicki, P.R.; Zeng, C.; Jin, R. Efficient Electrochemical CO 2 Conversion Powered by Renewable Energy. ACS Appl. Mater. Interfaces 2015, 7, 15626–15632. [Google Scholar] [CrossRef]
- Al Dosari, H.M.; Ayesh, A.I. Nanocluster Production for Solar Cell Applications. J. Appl. Phys. 2013, 114, 054305. [Google Scholar] [CrossRef]
- Fang, J.; Zhang, B.; Yao, Q.; Yang, Y.; Xie, J.; Yan, N. Recent Advances in the Synthesis and Catalytic Applications of Ligand-Protected, Atomically Precise Metal Nanoclusters. Coord. Chem. Rev. 2016, 322, 1–29. [Google Scholar] [CrossRef]
- Serhan, M.; Jackemeyer, D.; Long, M.; Sprowls, M.; Diez Perez, I.; Maret, W.; Chen, F.; Tao, N.; Forzani, E. Total Iron Measurement in Human Serum With a Novel Smartphone-Based Assay. IEEE J. Transl. Eng. Health Med. 2020, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Yuan, X.; Chen, T.; Leong, D.T.; Xie, J. Engineering Functional Metal Materials at the Atomic Level. Adv. Mater. 2018, 30, 1802751. [Google Scholar] [CrossRef] [PubMed]
- Mathew, A.; Pradeep, T. Noble Metal Clusters: Applications in Energy, Environment, and Biology. Part. Part. Syst. Charact. 2014, 31, 1017–1053. [Google Scholar] [CrossRef]
- Jadzinsky, P.D.; Calero, G.; Ackerson, C.J.; Bushnell, D.A.; Kornberg, R.D. Structure of a Thiol Monolayer-Protected Gold Nanoparticle at 1.1 Å Resolution. Science 2007, 318, 430–433. [Google Scholar] [CrossRef] [Green Version]
- Qian, H.; Eckenhoff, W.T.; Zhu, Y.; Pintauer, T.; Jin, R. Total Structure Determination of Thiolate-Protected Au38 Nanoparticles. J. Am. Chem. Soc. 2010, 132, 8280–8281. [Google Scholar] [CrossRef]
- Zeng, C.; Qian, H.; Li, T.; Li, G.; Rosi, N.L.; Yoon, B.; Barnett, R.N.; Whetten, R.L.; Landman, U.; Jin, R. Total Structure and Electronic Properties of the Gold Nanocrystal Au36(SR)24. Angew. Chem. Int. Ed. 2012, 51, 13114–13118. [Google Scholar] [CrossRef]
- Das, A.; Li, T.; Nobusada, K.; Zeng, Q.; Rosi, N.L.; Jin, R. Total Structure and Optical Properties of a Phosphine/Thiolate-Protected Au24 Nanocluster. J. Am. Chem. Soc. 2012, 134, 20286–20289. [Google Scholar] [CrossRef]
- Zeng, C.; Li, T.; Das, A.; Rosi, N.L.; Jin, R. Chiral Structure of Thiolate-Protected 28-Gold-Atom Nanocluster Determined by X-Ray Crystallography. J. Am. Chem. Soc. 2013, 135, 10011–10013. [Google Scholar] [CrossRef]
- Jin, R.; Zeng, C.; Zhou, M.; Chen, Y. Atomically Precise Colloidal Metal Nanoclusters and Nanoparticles: Fundamentals and Opportunities. Chem. Rev. 2016, 116, 10346–10413. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Chen, W. Application of Mass Spectrometry in the Synthesis and Characterization of Metal Nanoclusters. Anal. Chem. 2015, 87, 10659–10667. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, P.; Pradeep, T. The Emerging Interface of Mass Spectrometry with Materials. NPG Asia Mater. 2019, 11, 48. [Google Scholar] [CrossRef] [Green Version]
- Comby-Zerbino, C.; Dagany, X.; Chirot, F.; Dugourd, P.; Antoine, R. The Emergence of Mass Spectrometry for Characterizing Nanomaterials. Atomically Precise Nanoclusters and Beyond. Mater. Adv. 2021, 2, 4896–4913. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhou, H.; Zhang, S.; Xu, J. The Synthesis of Metal Nanoclusters and Their Applications in Bio-Sensing and Imaging. Methods Appl. Fluoresc. 2019, 8, 012001. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wu, Z.; Yao, Q.; Cao, Y.; Chai, O.J.H.; Xie, J. Correlations between the Fundamentals and Applications of Ultrasmall Metal Nanoclusters: Recent Advances in Catalysis and Biomedical Applications. Nano Today 2021, 36, 101053. [Google Scholar] [CrossRef]
- Ling, S.; Cui, X.; Zhang, X.; Liu, B.; He, C.; Wang, J.; Qin, W.; Zhang, Y.; Gao, Y.; Bai, G. Glutathione-Protected Gold Nanocluster Decorated Cadmium Sulfide with Enhanced Photostability and Photocatalytic Activity. J. Colloid Interface Sci. 2018, 530, 120–126. [Google Scholar] [CrossRef]
- Mathew, M.S.; Joseph, K. Green Synthesis of Gluten-Stabilized Fluorescent Gold Quantum Clusters: Application As Turn-On Sensing of Human Blood Creatinine. ACS Sustain. Chem. Eng. 2017, 5, 4837–4845. [Google Scholar] [CrossRef]
- Zhou, S.; Duan, Y.; Wang, F.; Wang, C. Fluorescent Au Nanoclusters Stabilized by Silane: Facile Synthesis, Color-Tunability and Photocatalytic Properties. Nanoscale 2017, 9, 4981–4988. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zaluzhna, O.; Xu, B.; Gao, Y.; Modest, J.M.; Tong, Y.J. Mechanistic Insights into the Brust−Schiffrin Two-Phase Synthesis of Organo-Chalcogenate-Protected Metal Nanoparticles. J. Am. Chem. Soc. 2011, 133, 2092–2095. [Google Scholar] [CrossRef] [PubMed]
- Dhanalakshmi, L.; Udayabhaskararao, T.; Pradeep, T. Conversion of Double Layer Charge-Stabilized Ag@citrate Colloids to Thiol Passivated Luminescent Quantum Clusters. Chem. Commun. 2012, 48, 859–861. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Gharib, M.; Becker, C.; Zeng, Y.; Ziefuß, A.R.; Chen, L.; Alkilany, A.M.; Rehbock, C.; Barcikowski, S.; Parak, W.J.; et al. Synthesis of Fluorescent Silver Nanoclusters: Introducing Bottom-Up and Top-Down Approaches to Nanochemistry in a Single Laboratory Class. J. Chem. Educ. 2020, 97, 239–243. [Google Scholar] [CrossRef]
- Meng, X.; Xu, Q.; Wang, S.; Zhu, M. Ligand-Exchange Synthesis of Selenophenolate-Capped Au25 Nanoclusters. Nanoscale 2012, 4, 4161. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Bürgi, T. Ligand Exchange Reactions on Thiolate-Protected Gold Nanoclusters. Nanoscale Adv. 2021, 3, 2710–2727. [Google Scholar] [CrossRef] [PubMed]
- Heinecke, C.L.; Ni, T.W.; Malola, S.; Mäkinen, V.; Wong, O.A.; Häkkinen, H.; Ackerson, C.J. Structural and Theoretical Basis for Ligand Exchange on Thiolate Monolayer Protected Gold Nanoclusters. J. Am. Chem. Soc. 2012, 134, 13316–13322. [Google Scholar] [CrossRef] [Green Version]
- Udaya Bhaskara Rao, T.; Pradeep, T. Luminescent Ag7 and Ag8 Clusters by Interfacial Synthesis. Angew. Chem. Int. Ed. 2010, 49, 3925–3929. [Google Scholar] [CrossRef]
- Bootharaju, M.S.; Burlakov, V.M.; Besong, T.M.D.; Joshi, C.P.; AbdulHalim, L.G.; Black, D.M.; Whetten, R.L.; Goriely, A.; Bakr, O.M. Reversible Size Control of Silver Nanoclusters via Ligand-Exchange. Chem. Mater. 2015, 27, 4289–4297. [Google Scholar] [CrossRef] [Green Version]
- Antonello, S.; Dainese, T.; Pan, F.; Rissanen, K.; Maran, F. Electrocrystallization of Monolayer-Protected Gold Clusters: Opening the Door to Quality, Quantity, and New Structures. J. Am. Chem. Soc. 2017, 139, 4168–4174. [Google Scholar] [CrossRef] [Green Version]
- Brust, M.; Walker, M.; Bethell, D.; Schiffrin, D.J.; Whyman, R. Synthesis of Thiol-Derivatised Gold Nanoparticles in a Two-Phase Liquid–Liquid System. J. Chem. Soc. Chem. Commun. 1994, 801–802. [Google Scholar] [CrossRef]
- Negishi, Y.; Nobusada, K.; Tsukuda, T. Glutathione-Protected Gold Clusters Revisited: Bridging the Gap between Gold(I)−Thiolate Complexes and Thiolate-Protected Gold Nanocrystals. J. Am. Chem. Soc. 2005, 127, 5261–5270. [Google Scholar] [CrossRef]
- Chen, S.; Templeton, A.C.; Murray, R.W. Monolayer-Protected Cluster Growth Dynamics. Langmuir 2000, 16, 3543–3548. [Google Scholar] [CrossRef]
- Edinger, K.; Goelzhaeuser, A.; Demota, K.; Woell, C.; Grunze, M. Formation of Self-Assembled Monolayers of n-Alkanethiols on Gold: A Scanning Tunneling Microscopy Study on the Modification of Substrate Morphology. Langmuir 1993, 9, 4–8. [Google Scholar] [CrossRef] [Green Version]
- Habeeb Muhammed, M.A.; Ramesh, S.; Sinha, S.S.; Pal, S.K.; Pradeep, T. Two Distinct Fluorescent Quantum Clusters of Gold Starting from Metallic Nanoparticles by PH-Dependent Ligand Etching. Nano Res. 2008, 1, 333–340. [Google Scholar] [CrossRef] [Green Version]
- Yuan, X.; Luo, Z.; Zhang, Q.; Zhang, X.; Zheng, Y.; Lee, J.Y.; Xie, J. Synthesis of Highly Fluorescent Metal (Ag, Au, Pt, and Cu) Nanoclusters by Electrostatically Induced Reversible Phase Transfer. ACS Nano 2011, 5, 8800–8808. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S. Luminescent Metal Nanoclusters: Synthesis, Characterisation and Applications; Woodhead Publishing: Cambridge, UK, 2022; ISBN 978-0-323-88641-3. [Google Scholar]
- Woodruff, D.P. (Ed.) Atomic Clusters: From Gas Phase to Deposited. In The Chemical Physics of Solid Surfaces, 1st ed.; Elsevier: Amsterdam, The Netherlands; Boston, MA, USA, 2007; ISBN 978-0-444-52756-1. [Google Scholar]
- Briant, C.E.; Theobald, B.R.C.; White, J.W.; Bell, L.K.; Mingos, D.M.P.; Welch, A.J. Synthesis and X-ray Structural Characterization of the Centred Icosahedral Gold Cluster Compound [Aul3(PMe2Ph)10Cl2](PF6)3; the Realization of a Theoretical Prediction. J. Chem. Soc. Chem. Commun. 1981, 201. [Google Scholar] [CrossRef]
- Akola, J.; Walter, M.; Whetten, R.L.; Häkkinen, H.; Grönbeck, H. On the Structure of Thiolate-Protected Au25. J. Am. Chem. Soc. 2008, 130, 3756–3757. [Google Scholar] [CrossRef]
- Xavier, P.L.; Chaudhari, K.; Baksi, A.; Pradeep, T. Protein-Protected Luminescent Noble Metal Quantum Clusters: An Emerging Trend in Atomic Cluster Nanoscience. Nano Rev. 2012, 3, 14767. [Google Scholar] [CrossRef] [Green Version]
- Alex, A.M.; Mathew, M.S.; Kuruvilla, K.J.; Appukuttan, S.; Joseph, K.; Thomas, S. Protein and Enzyme Protected Metal Nanoclusters. In Luminescent Metal Nanoclusters; Elsevier: Amsterdam, The Netherlands, 2022; pp. 303–348. ISBN 978-0-323-88657-4. [Google Scholar]
- Halawa, M.I.; Lai, J.; Xu, G. Gold Nanoclusters: Synthetic Strategies and Recent Advances in Fluorescent Sensing. Mater. Today Nano 2018, 3, 9–27. [Google Scholar] [CrossRef]
- Chen, L.-Y.; Wang, C.-W.; Yuan, Z.; Chang, H.-T. Fluorescent Gold Nanoclusters: Recent Advances in Sensing and Imaging. Anal. Chem. 2015, 87, 216–229. [Google Scholar] [CrossRef]
- Hayyan, M.; Hashim, M.A.; AlNashef, I.M. Superoxide Ion: Generation and Chemical Implications. Chem. Rev. 2016, 116, 3029–3085. [Google Scholar] [CrossRef] [Green Version]
- Xie, J.; Zheng, Y.; Ying, J.Y. Protein-Directed Synthesis of Highly Fluorescent Gold Nanoclusters. J. Am. Chem. Soc. 2009, 131, 888–889. [Google Scholar] [CrossRef] [PubMed]
- Le Guével, X.; Hötzer, B.; Jung, G.; Hollemeyer, K.; Trouillet, V.; Schneider, M. Formation of Fluorescent Metal (Au, Ag) Nanoclusters Capped in Bovine Serum Albumin Followed by Fluorescence and Spectroscopy. J. Phys. Chem. C 2011, 115, 10955–10963. [Google Scholar] [CrossRef]
- Stamplecoskie, K.G.; Swint, A. Optimizing Molecule-like Gold Clusters for Light Energy Conversion. J. Mater. Chem. A 2016, 4, 2075–2081. [Google Scholar] [CrossRef]
- Robinson, D. Synthesis and Characterization of Metal Nanoclusters Stabilized by Dithiolates. Master’s Thesis, Georgia State University, Atlanta, GA, USA, 19 July 2011. [Google Scholar] [CrossRef]
- Kreibig, U.; Vollmer, M. Optical Properties of Metal Clusters; Springer Series in Materials Science; Springer: Berlin/Heidelberg, Germany, 1995; Volume 25, ISBN 978-3-642-08191-0. [Google Scholar]
- Antoine, R.; Bonačić-Koutecký, V. Liganded Silver and Gold Quantum Clusters. Towards a New Class of Nonlinear Optical Nanomaterials; SpringerBriefs in Materials; Springer International Publishing: Cham, Switzerland, 2018; ISBN 978-3-319-64742-5. [Google Scholar]
- Jin, R.; Higaki, T. Open Questions on the Transition between Nanoscale and Bulk Properties of Metals. Commun. Chem. 2021, 4, 28. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Li, Z.; Yu, Z.; Deng, X.; Xin, Y. Recent Advances in the Synthesis, Properties, and Biological Applications of Platinum Nanoclusters. J. Nanomater. 2019, 2019, 6248725. [Google Scholar] [CrossRef] [Green Version]
- Muhammed, M.A.H.; Verma, P.K.; Pal, S.K.; Kumar, R.C.A.; Paul, S.; Omkumar, R.V.; Pradeep, T. Bright, NIR-Emitting Au23 from Au25: Characterization and Applications Including Biolabeling. Chem. Eur. J. 2009, 15, 10110–10120. [Google Scholar] [CrossRef]
- Zhu, M.; Aikens, C.M.; Hollander, F.J.; Schatz, G.C.; Jin, R. Correlating the Crystal Structure of A Thiol-Protected Au 25 Cluster and Optical Properties. J. Am. Chem. Soc. 2008, 130, 5883–5885. [Google Scholar] [CrossRef]
- Du, Y.; Sheng, H.; Astruc, D.; Zhu, M. Atomically Precise Noble Metal Nanoclusters as Efficient Catalysts: A Bridge between Structure and Properties. Chem. Rev. 2020, 120, 526–622. [Google Scholar] [CrossRef]
- Li, S.; Du, X.; Liu, Z.; Li, Y.; Shao, Y.; Jin, R. Size Effects of Atomically Precise Gold Nanoclusters in Catalysis. Precis. Chem. 2023, 1, 14–28. [Google Scholar] [CrossRef]
- Shang, L.; Dong, S.; Nienhaus, G.U. Ultra-Small Fluorescent Metal Nanoclusters: Synthesis and Biological Applications. Nano Today 2011, 6, 401–418. [Google Scholar] [CrossRef]
- Bertorelle, F.; Wegner, D.; Bakulić, M.P.; Fakhouri, H.; Comby-Zerbino, C.; Sagar, A.; Bernadó, P.; Resch-Genger, U.; Koutecky, V.B.; Guével, X.L.; et al. Tailoring NIR-II Photoluminescence of Single Thiolated Au25 Nanoclusters by Selective Binding to Proteins. Chem. A Eur. J. 2022, 28, e202200570. [Google Scholar]
- Tang, J.-H.; Han, G.; Li, G.; Yan, K.; Sun, Y. Near-Infrared Light Photocatalysis Enabled by a Ruthenium Complex-Integrated Metal–Organic Framework via Two-Photon Absorption. iScience 2022, 25, 104064. [Google Scholar] [CrossRef]
- Russier-Antoine, I.; Bertorelle, F.; Calin, N.; Sanader, Ž.; Krstić, M.; Comby-Zerbino, C.; Dugourd, P.; Brevet, P.-F.; Bonačić-Koutecký, V.; Antoine, R. Ligand-Core NLO-Phores: A Combined Experimental and Theoretical Approach to the Two-Photon Absorption and Two-Photon Excited Emission Properties of Small-Ligated Silver Nanoclusters. Nanoscale 2017, 9, 1221–1228. [Google Scholar] [CrossRef] [Green Version]
- Antoine, R. Ligand-Core NLO-Phores: Two-Photon Absorption and Two-Photon Excited Emission Properties of Atomically Precise Clusters of Gold and Silver. In Molecular Spectroscopy—Experiment and Theory; Koleżyński, A., Król, M., Eds.; Challenges and Advances in Computational Chemistry and Physics; Springer International Publishing: Cham, Switzerland, 2019; Volume 26, pp. 139–160. ISBN 978-3-030-01354-7. [Google Scholar]
- Sanader, Ž.; Krstić, M.; Russier-Antoine, I.; Bertorelle, F.; Dugourd, P.; Brevet, P.-F.; Antoine, R.; Bonačić-Koutecký, V. Two-Photon Absorption of Ligand-Protected Ag 15 Nanoclusters. Towards a New Class of Nonlinear Optics Nanomaterials. Phys. Chem. Chem. Phys. 2016, 18, 12404–12408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hao, C.; Xu, L.; Ma, W.; Wu, X.; Wang, L.; Kuang, H.; Xu, C. Unusual Circularly Polarized Photocatalytic Activity in Nanogapped Gold-Silver Chiroplasmonic Nanostructures. Adv. Funct. Mater. 2015, 25, 5816–5822. [Google Scholar] [CrossRef]
- Schaaff, T.G.; Knight, G.; Shafigullin, M.N.; Borkman, R.F.; Whetten, R.L. Isolation and Selected Properties of a 10.4 KDa Gold:Glutathione Cluster Compound. J. Phys. Chem. B 1998, 102, 10643–10646. [Google Scholar] [CrossRef]
- Yao, H. Chiral Ligand-Protected Gold Nanoclusters: Considering the Optical Activity from a Viewpoint of Ligand Dissymmetric Field. Prog. Nat. Sci. Mater. Int. 2016, 26, 428–439. [Google Scholar] [CrossRef] [Green Version]
- Tsukuda, T.; Häkkinen, H. Introduction. In Frontiers of Nanoscience; Elsevier: Amsterdam, The Netherlands, 2015; Volume 9, pp. 1–7. ISBN 978-0-08-100086-1. [Google Scholar]
- Shichibu, Y.; Negishi, Y.; Tsunoyama, H.; Kanehara, M.; Teranishi, T.; Tsukuda, T. Extremely High Stability of Glutathionate-Protected Au25 Clusters Against Core Etching. Small 2007, 3, 835–839. [Google Scholar] [CrossRef]
- Boyen, H.-G.; Kästle, G.; Weigl, F.; Koslowski, B.; Dietrich, C.; Ziemann, P.; Spatz, J.P.; Riethmüller, S.; Hartmann, C.; Möller, M.; et al. Oxidation-Resistant Gold-55 Clusters. Science 2002, 297, 1533–1536. [Google Scholar] [CrossRef]
- Tracy, J.B.; Kalyuzhny, G.; Crowe, M.C.; Balasubramanian, R.; Choi, J.-P.; Murray, R.W. Poly(Ethylene Glycol) Ligands for High-Resolution Nanoparticle Mass Spectrometry. J. Am. Chem. Soc. 2007, 129, 6706–6707. [Google Scholar] [CrossRef]
- Kumar, S.; Jin, R. Water-Soluble Au25(Capt)18 Nanoclusters: Synthesis, Thermal Stability, and Optical Properties. Nanoscale 2012, 4, 4222. [Google Scholar] [CrossRef]
- Negishi, Y.; Kurashige, W.; Niihori, Y.; Iwasa, T.; Nobusada, K. Isolation, Structure, and Stability of a Dodecanethiolate-Protected Pd1Au24 Cluster. Phys. Chem. Chem. Phys. 2010, 12, 6219. [Google Scholar] [CrossRef] [PubMed]
- Fakhouri, H.; Salmon, E.; Wei, X.; Joly, S.; Moulin, C.; Russier-Antoine, I.; Brevet, P.-F.; Kang, X.; Zhu, M.; Antoine, R. Effects of Single Platinum Atom Doping on Stability and Nonlinear Optical Properties of Ag29 Nanoclusters. J. Phys. Chem. C 2022, 126, 21094–21100. [Google Scholar] [CrossRef]
- Jiang, D.; Dai, S. From Superatomic Au25(SR)18− to Superatomic M@Au24 (SR)18 q Core−Shell Clusters. Inorg. Chem. 2009, 48, 2720–2722. [Google Scholar] [CrossRef]
- Negishi, Y.; Munakata, K.; Ohgake, W.; Nobusada, K. Effect of Copper Doping on Electronic Structure, Geometric Structure, and Stability of Thiolate-Protected Au25 Nanoclusters. J. Phys. Chem. Lett. 2012, 3, 2209–2214. [Google Scholar] [CrossRef]
- Tlahuice-Flores, A.; Muñoz-Castro, A. Bonding and Properties of Superatoms. Analogs to Atoms and Molecules and Related Concepts from Superatomic Clusters. Int. J. Quantum Chem. 2019, 119, e25756. [Google Scholar] [CrossRef] [Green Version]
- De Heer, W.A.; Knight, W.D.; Chou, M.Y.; Cohen, M.L. Electronic Shell Structure and Metal Clusters. In Solid State Physics; Elsevier: Amsterdam, The Netherlands, 1987; Volume 40, pp. 93–181. ISBN 978-0-12-607740-7. [Google Scholar]
- Bertorelle, F.; Russier-Antoine, I.; Comby-Zerbino, C.; Chirot, F.; Dugourd, P.; Brevet, P.-F.; Antoine, R. Isomeric Effect of Mercaptobenzoic Acids on the Synthesis, Stability, and Optical Properties of Au25(MBA)18 Nanoclusters. ACS Omega 2018, 3, 15635–15642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakai, N.; Tatsuma, T. Photovoltaic Properties of Glutathione-Protected Gold Clusters Adsorbed on TiO2 Electrodes. Adv. Mater. 2010, 22, 3185–3188. [Google Scholar] [CrossRef]
- Wang, H.; Liu, X.; Yang, W.; Mao, G.; Meng, Z.; Wu, Z.; Jiang, H.-L. Surface-Clean Au25 Nanoclusters in Modulated Microenvironment Enabled by Metal–Organic Frameworks for Enhanced Catalysis. J. Am. Chem. Soc. 2022, 144, 22008–22017. [Google Scholar] [CrossRef]
- Duan, Y.; Luo, J.; Zhou, S.; Mao, X.; Shah, M.W.; Wang, F.; Chen, Z.; Wang, C. TiO2-Supported Ag Nanoclusters with Enhanced Visible Light Activity for the Photocatalytic Removal of NO. Appl. Catal. B Environ. 2018, 234, 206–212. [Google Scholar] [CrossRef]
- Yu, C.; Li, G.; Kumar, S.; Kawasaki, H.; Jin, R. Stable Au25(SR)18/TiO2 Composite Nanostructure with Enhanced Visible Light Photocatalytic Activity. J. Phys. Chem. Lett. 2013, 4, 2847–2852. [Google Scholar] [CrossRef]
- Das, K.; Roychoudhury, A. Reactive Oxygen Species (ROS) and Response of Antioxidants as ROS-Scavengers during Environmental Stress in Plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef] [Green Version]
- Sacco, O.; Stoller, M.; Vaiano, V.; Ciambelli, P.; Chianese, A.; Sannino, D. Photocatalytic Degradation of Organic Dyes under Visible Light on N-Doped TiO2 Photocatalysts. Int. J. Photoenergy 2012, 2012, 626759. [Google Scholar] [CrossRef] [Green Version]
- Guo, W.; Yang, L.; Lu, J.; Gao, P.; Li, W.; Feng, Z. An Accurate Growth Mechanism and Photocatalytic Degradation Rhodamine B of Crystalline Nb2O5 Nanotube Arrays. Catalysts 2020, 10, 1480. [Google Scholar] [CrossRef]
- Goswami, T.; Singh, M.; Reddy, K.M.; Mishra, A.K. Facile Synthesis of Ag-TiO2 Hybrid Nanocluster:A Comprehensive Experimental and Computational Insight into the Role of Surface Ligands on Enhanced Visible Light Photo-Catalysis. ChemistrySelect 2018, 3, 10892–10899. [Google Scholar] [CrossRef]
- Yang, J.; Wang, D.; Han, H.; Li, C. Roles of Cocatalysts in Photocatalysis and Photoelectrocatalysis. Acc. Chem. Res. 2013, 46, 1900–1909. [Google Scholar] [CrossRef]
- Zhu, H.; Goswami, N.; Yao, Q.; Chen, T.; Liu, Y.; Xu, Q.; Chen, D.; Lu, J.; Xie, J. Cyclodextrin–Gold Nanocluster Decorated TiO2 Enhances Photocatalytic Decomposition of Organic Pollutants. J. Mater. Chem. A 2018, 6, 1102–1108. [Google Scholar] [CrossRef]
- Sharma, V.; Kumar, S.; Krishnan, V. Clustered Au on TiO2 Snowman-Like Nanoassemblies for Photocatalytic Applications. ChemistrySelect 2016, 1, 2963–2970. [Google Scholar] [CrossRef]
- Goswami, T.; Reddy, K.M.; Bheemaraju, A. Silver Nanocluster Anchored TiO2/Nb2O5 Hybrid Nanocomposite as Highly Efficient and Selective Visible-Light Sensitive Photocatalyst. ChemistrySelect 2019, 4, 6790–6799. [Google Scholar] [CrossRef]
- Samai, B.; Chall, S.; Mati, S.S.; Bhattacharya, S.C. Role of Silver Nanoclusters in the Enhanced Photocatalytic Activity of Cerium Oxide Nanoparticles. Eur. J. Inorg. Chem. 2018, 2018, 3224–3231. [Google Scholar] [CrossRef]
- González-Rodríguez, J.; Fernández, L.; Bava, Y.B.; Buceta, D.; Vázquez-Vázquez, C.; López-Quintela, M.A.; Feijoo, G.; Moreira, M.T. Enhanced Photocatalytic Activity of Semiconductor Nanocomposites Doped with Ag Nanoclusters Under UV and Visible Light. Catalysts 2019, 10, 31. [Google Scholar] [CrossRef] [Green Version]
- Vilar-Vidal, N.; Rey, J.R.; López Quintela, M.A. Green Emitter Copper Clusters as Highly Efficient and Reusable Visible Degradation Photocatalysts. Small 2014, 10, 3632–3636. [Google Scholar] [CrossRef] [PubMed]
- Veziroglu, S.; Obermann, A.-L.; Ullrich, M.; Hussain, M.; Kamp, M.; Kienle, L.; Leißner, T.; Rubahn, H.-G.; Polonskyi, O.; Strunskus, T.; et al. Photodeposition of Au Nanoclusters for Enhanced Photocatalytic Dye Degradation over TiO2 Thin Film. ACS Appl. Mater. Interfaces 2020, 12, 14983–14992. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Pang, R.; Wang, Q.-Y.; Han, Z.; Wang, Z.-Y.; Dong, X.-Y.; Li, S.-F.; Zang, S.-Q.; Mak, T.C.W. Porphyrinic Silver Cluster Assembled Material for Simultaneous Capture and Photocatalysis of Mustard-Gas Simulant. J. Am. Chem. Soc. 2019, 141, 14505–14509. [Google Scholar] [CrossRef] [PubMed]
- Weng, B.; Lu, K.-Q.; Tang, Z.; Chen, H.M.; Xu, Y.-J. Stabilizing Ultrasmall Au Clusters for Enhanced Photoredox Catalysis. Nat. Commun. 2018, 9, 1543. [Google Scholar] [CrossRef] [Green Version]
- Al-Shankiti, B.; Al-Maksoud, W.; Habeeb Muhammed, M.A.; Anjum, D.H.; Moosa, B.; Basset, J.-M.; Khashab, N.M. Ligand-Free Gold Nanoclusters Confined in Mesoporous Silica Nanoparticles for Styrene Epoxidation. Nanoscale Adv. 2020, 2, 1437–1442. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Liu, C.; Wang, M.; Zhang, C.; Luo, N.; Wang, Y.; Abroshan, H.; Li, G.; Wang, F. Visible Light Gold Nanocluster Photocatalyst: Selective Aerobic Oxidation of Amines to Imines. ACS Catal. 2017, 7, 3632–3638. [Google Scholar] [CrossRef]
- Liu, L.; Li, H.; Tan, Y.; Chen, X.; Lin, R.; Yang, W.; Huang, C.; Wang, S.; Wang, X.; Liu, X.Y.; et al. Metal-Support Synergy of Supported Gold Nanoclusters in Selective Oxidation of Alcohols. Catalysts 2020, 10, 107. [Google Scholar] [CrossRef] [Green Version]
- Haruta, M.; Kobayashi, T.; Sano, H.; Yamada, N. Novel Gold Catalysts for the Oxidation of Carbon Monoxide at a Temperature Far Below 0 °C. Chem. Lett. 1987, 16, 405–408. [Google Scholar] [CrossRef] [Green Version]
- Kogo, A.; Sakai, N.; Tatsuma, T. Photocatalysis of Au25-Modified TiO2 under Visible and near Infrared Light. Electrochem. Commun. 2010, 12, 996–999. [Google Scholar] [CrossRef]
- Zhu, Y.; Qian, H.; Zhu, M.; Jin, R. Thiolate-Protected Aun Nanoclusters as Catalysts for Selective Oxidation and Hydrogenation Processes. Adv. Mater. 2010, 22, 1915–1920. [Google Scholar] [CrossRef] [PubMed]
- Tsunoyama, H.; Ichikuni, N.; Sakurai, H.; Tsukuda, T. Effect of Electronic Structures of Au Clusters Stabilized by Poly(N -Vinyl-2-Pyrrolidone) on Aerobic Oxidation Catalysis. J. Am. Chem. Soc. 2009, 131, 7086–7093. [Google Scholar] [CrossRef] [PubMed]
- Hamoud, H.I.; Douma, F.; Lafjah, M.; Djafri, F.; Lebedev, O.; Valtchev, V.; El-Roz, M. Size-Dependent Photocatalytic Activity of Silver Nanoparticles Embedded in ZX-Bi Zeolite Supports. ACS Appl. Nano Mater. 2022, 5, 3866–3877. [Google Scholar] [CrossRef]
- Jain, I.P. Hydrogen the Fuel for 21st Century. Int. J. Hydrog. Energy 2009, 34, 7368–7378. [Google Scholar] [CrossRef]
- Ahluwalia, R.K.; Wang, X.; Rousseau, A.; Kumar, R. Fuel Economy of Hydrogen Fuel Cell Vehicles. J. Power Sources 2004, 130, 192–201. [Google Scholar] [CrossRef]
- Thoi, V.S.; Sun, Y.; Long, J.R.; Chang, C.J. Complexes of Earth-Abundant Metals for Catalytic Electrochemical Hydrogen Generation under Aqueous Conditions. Chem. Soc. Rev. 2013, 42, 2388–2400. [Google Scholar] [CrossRef]
- Xiao, F.-X.; Hung, S.-F.; Miao, J.; Wang, H.-Y.; Yang, H.; Liu, B. Metal-Cluster-Decorated TiO2 Nanotube Arrays: A Composite Heterostructure toward Versatile Photocatalytic and Photoelectrochemical Applications. Small 2015, 11, 554–567. [Google Scholar] [CrossRef]
- Kurashige, W.; Kumazawa, R.; Mori, Y. Au25 Cluster-Loaded SrTiO3 Water-Splitting Photocatalyst; Preparation and Elucidation of the Effect of Cocatalyst Refinement on Photocatalytic Activity. J. Mater. Appl. 2018, 7, 41–46. [Google Scholar] [CrossRef]
- Attia, Y.A.; Buceta, D.; Blanco-Varela, C.; Mohamed, M.B.; Barone, G.; López-Quintela, M.A. Structure-Directing and High-Efficiency Photocatalytic Hydrogen Production by Ag Clusters. J. Am. Chem. Soc. 2014, 136, 1182–1185. [Google Scholar] [CrossRef]
- Shen, P.; Zhao, S.; Su, D.; Li, Y.; Orlov, A. Outstanding Activity of Sub-Nm Au Clusters for Photocatalytic Hydrogen Production. Appl. Catal. B Environ. 2012, 126, 153–160. [Google Scholar] [CrossRef]
- Wang, H.; Luo, S.; Song, Y.; Shi, Y.; Wang, Z.; Guo, B.; Wu, L. Enhanced Photocatalytic Hydrogen Evolution over Monolayer HTi2NbO7 Nanosheets with Highly Dispersed Pt Nanoclusters. Appl. Surf. Sci. 2020, 511, 145501. [Google Scholar] [CrossRef]
- Naveen, M.H.; Khan, R.; Bang, J.H. Gold Nanoclusters as Electrocatalysts: Atomic Level Understanding from Fundamentals to Applications. Chem. Mater. 2021, 33, 7595–7612. [Google Scholar] [CrossRef]
- Du, X.L.; Wang, X.L.; Li, Y.H.; Wang, Y.L.; Zhao, J.J.; Fang, L.J.; Zheng, L.R.; Tong, H.; Yang, H.G. Isolation of Single Pt Atoms in a Silver Cluster: Forming Highly Efficient Silver-Based Cocatalysts for Photocatalytic Hydrogen Evolution. Chem. Commun. 2017, 53, 9402–9405. [Google Scholar] [CrossRef] [Green Version]
- Miyauchi, M.; Irie, H.; Liu, M.; Qiu, X.; Yu, H.; Sunada, K.; Hashimoto, K. Visible-Light-Sensitive Photocatalysts: Nanocluster-Grafted Titanium Dioxide for Indoor Environmental Remediation. J. Phys. Chem. Lett. 2016, 7, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Yin, G.; Nishikawa, M.; Nosaka, Y.; Srinivasan, N.; Atarashi, D.; Sakai, E.; Miyauchi, M. Photocatalytic Carbon Dioxide Reduction by Copper Oxide Nanocluster-Grafted Niobate Nanosheets. ACS Nano 2015, 9, 2111–2119. [Google Scholar] [CrossRef]
- Sagadevan, A.; Ghosh, A.; Maity, P.; Mohammed, O.F.; Bakr, O.M.; Rueping, M. Visible-Light Copper Nanocluster Catalysis for the C–N Coupling of Aryl Chlorides at Room Temperature. J. Am. Chem. Soc. 2022, 144, 12052–12061. [Google Scholar] [CrossRef]
- Negishi, Y.; Mizuno, M.; Hirayama, M.; Omatoi, M.; Takayama, T.; Iwase, A.; Kudo, A. Enhanced Photocatalytic Water Splitting by BaLa4Ti4O15 Loaded with ∼1 Nm Gold Nanoclusters Using Glutathione-Protected Au25 Clusters. Nanoscale 2013, 5, 7188. [Google Scholar] [CrossRef]
- Mousavi, H.; Small, T.D.; Sharma, S.K.; Golovko, V.B.; Shearer, C.J.; Metha, G.F. Graphene Bridge for Photocatalytic Hydrogen Evolution with Gold Nanocluster Co-Catalysts. Nanomaterials 2022, 12, 3638. [Google Scholar] [CrossRef]
- Schweinberger, F.F.; Berr, M.J.; Döblinger, M.; Wolff, C.; Sanwald, K.E.; Crampton, A.S.; Ridge, C.J.; Jäckel, F.; Feldmann, J.; Tschurl, M.; et al. Cluster Size Effects in the Photocatalytic Hydrogen Evolution Reaction. J. Am. Chem. Soc. 2013, 135, 13262–13265. [Google Scholar] [CrossRef]
- Kurashige, W.; Hayashi, R.; Wakamatsu, K.; Kataoka, Y.; Hossain, S.; Iwase, A.; Kudo, A.; Yamazoe, S.; Negishi, Y. Atomic-Level Understanding of the Effect of Heteroatom Doping of the Cocatalyst on Water-Splitting Activity in AuPd or AuPt Alloy Cluster-Loaded BaLa4Ti4O15. ACS Appl. Energy Mater. 2019, 2, 4175–4187. [Google Scholar] [CrossRef]
- Méndez-Medrano, M.G.; Kowalska, E.; Lehoux, A.; Herissan, A.; Ohtani, B.; Rau, S.; Colbeau-Justin, C.; Rodríguez-López, J.L.; Remita, H. Surface Modification of TiO2 with Au Nanoclusters for Efficient Water Treatment and Hydrogen Generation under Visible Light. J. Phys. Chem. C 2016, 120, 25010–25022. [Google Scholar] [CrossRef]
- Tawalbeh, M.; Al-Othman, A.; Kafiah, F.; Abdelsalam, E.; Almomani, F.; Alkasrawi, M. Environmental Impacts of Solar Photovoltaic Systems: A Critical Review of Recent Progress and Future Outlook. Sci. Total Environ. 2021, 759, 143528. [Google Scholar] [CrossRef] [PubMed]
- Kondratenko, E.V.; Mul, G.; Baltrusaitis, J.; Larrazábal, G.O.; Pérez-Ramírez, J. Status and Perspectives of CO2 Conversion into Fuels and Chemicals by Catalytic, Photocatalytic and Electrocatalytic Processes. Energy Environ. Sci. 2013, 6, 3112. [Google Scholar] [CrossRef] [Green Version]
- Roy, S.C.; Varghese, O.K.; Paulose, M.; Grimes, C.A. Toward Solar Fuels: Photocatalytic Conversion of Carbon Dioxide to Hydrocarbons. ACS Nano 2010, 4, 1259–1278. [Google Scholar] [CrossRef] [PubMed]
- Alper, E.; Yuksel Orhan, O. CO2 Utilization: Developments in Conversion Processes. Petroleum 2017, 3, 109–126. [Google Scholar] [CrossRef]
- Du, C.; Wang, X.; Chen, W.; Feng, S.; Wen, J.; Wu, Y.A. CO2 Transformation to Multicarbon Products by Photocatalysis and Electrocatalysis. Mater. Today Adv. 2020, 6, 100071. [Google Scholar] [CrossRef]
- Halmann, M. Photoelectrochemical Reduction of Aqueous Carbon Dioxide on P-Type Gallium Phosphide in Liquid Junction Solar Cells. Nature 1978, 275, 115–116. [Google Scholar] [CrossRef]
- Olah, G.A. Beyond Oil and Gas: The Methanol Economy. Angew. Chem. Int. Ed. 2005, 44, 2636–2639. [Google Scholar] [CrossRef]
- Lu, Q.; Jiao, F. Electrochemical CO2 Reduction: Electrocatalyst, Reaction Mechanism, and Process Engineering. Nano Energy 2016, 29, 439–456. [Google Scholar] [CrossRef] [Green Version]
- Philip Colombo, D.; Roussel, K.A.; Saeh, J.; Skinner, D.E.; Cavaleri, J.J.; Bowman, R.M. Femtosecond Study of the Intensity Dependence of Electron-Hole Dynamics in TiO2 Nanoclusters. Chem. Phys. Lett. 1995, 232, 207–214. [Google Scholar] [CrossRef]
- Kauffman, D.R.; Alfonso, D.; Matranga, C.; Qian, H.; Jin, R. Experimental and Computational Investigation of Au25 Clusters and CO2: A Unique Interaction and Enhanced Electrocatalytic Activity. J. Am. Chem. Soc. 2012, 134, 10237–10243. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.-X.; MacFarlane, D.R.; Zhang, J. Bioinspired Electrocatalytic CO2 Reduction by Bovine Serum Albumin-Capped Silver Nanoclusters Mediated by [α-SiW12O40]4−. ChemSusChem 2016, 9, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Corma, A.; Garcia, H. Photocatalytic Reduction of CO2 for Fuel Production: Possibilities and Challenges. J. Catal. 2013, 308, 168–175. [Google Scholar] [CrossRef]
- Qin, L.; Ma, G.; Wang, L.; Tang, Z. Atomically Precise Metal Nanoclusters for (Photo)Electroreduction of CO2: Recent Advances, Challenges and Opportunities. J. Energy Chem. 2021, 57, 359–370. [Google Scholar] [CrossRef]
- Linsebigler, A.L.; Lu, G.; Yates, J.T. Photocatalysis on TiO2 Surfaces: Principles, Mechanisms, and Selected Results. Chem. Rev. 1995, 95, 735–758. [Google Scholar] [CrossRef]
- Liu, M.; Qiu, X.; Miyauchi, M.; Hashimoto, K. Cu(II) Oxide Amorphous Nanoclusters Grafted Ti3+ Self-Doped TiO2: An Efficient Visible Light Photocatalyst. Chem. Mater. 2011, 23, 5282–5286. [Google Scholar] [CrossRef]
- Shoji, S.; Yin, G.; Nishikawa, M.; Atarashi, D.; Sakai, E.; Miyauchi, M. Photocatalytic Reduction of CO2 by CuO Nanocluster Loaded SrTiO3 Nanorod Thin Film. Chem. Phys. Lett. 2016, 658, 309–314. [Google Scholar] [CrossRef] [Green Version]
- Jin, J.; Luo, J.; Zan, L.; Peng, T. One-Pot Synthesis of Cu-Nanocluster-Decorated Brookite TiO2 Quasi -Nanocubes for Enhanced Activity and Selectivity of CO2 Photoreduction to CH4. ChemPhysChem 2017, 18, 3230–3239. [Google Scholar] [CrossRef] [Green Version]
- Cui, X.; Wang, J.; Liu, B.; Ling, S.; Long, R.; Xiong, Y. Turning Au Nanoclusters Catalytically Active for Visible-Light-Driven CO2 Reduction through Bridging Ligands. J. Am. Chem. Soc. 2018, 140, 16514–16520. [Google Scholar] [CrossRef]
- Jiang, Y.; Yu, Y.; Zhang, X.; Weinert, M.; Song, X.; Ai, J.; Han, L.; Fei, H. N-Heterocyclic Carbene-Stabilized Ultrasmall Gold Nanoclusters in a Metal-Organic Framework for Photocatalytic CO2 Reduction. Angew. Chem. Int. Ed. 2021, 60, 17388–17393. [Google Scholar] [CrossRef]
- Billo, T.; Fu, F.-Y.; Raghunath, P.; Shown, I.; Chen, W.-F.; Lien, H.-T.; Shen, T.-H.; Lee, J.-F.; Chan, T.-S.; Huang, K.-Y.; et al. Ni-Nanocluster Modified Black TiO2 with Dual Active Sites for Selective Photocatalytic CO2 Reduction. Small 2018, 14, 1702928. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-S.; Kamat, P.V. Glutathione-Capped Gold Nanoclusters as Photosensitizers. Visible Light-Induced Hydrogen Generation in Neutral Water. J. Am. Chem. Soc. 2014, 136, 6075–6082. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Can, M.; Ragsdale, S.W.; Armstrong, F.A. Fast and Selective Photoreduction of CO2 to CO Catalyzed by a Complex of Carbon Monoxide Dehydrogenase, TiO2, and Ag Nanoclusters. ACS Catal. 2018, 8, 2789–2795. [Google Scholar] [CrossRef] [PubMed]
- El-Roz, M.; Telegeiev, I.; Mordvinova, N.E.; Lebedev, O.I.; Barrier, N.; Behilil, A.; Zaarour, M.; Lakiss, L.; Valtchev, V. Uniform Generation of Sub-Nanometer Silver Clusters in Zeolite Cages Exhibiting High Photocatalytic Activity under Visible Light. ACS Appl. Mater. Interfaces 2018, 10, 28702–28708. [Google Scholar] [CrossRef] [PubMed]















| Sl NO | Photocatalyst | Co Catalyst | Application | Efficiency | Reference |
|---|---|---|---|---|---|
| 1 | TiO2-Au NCs@β-CD | AuNC coupled with per-6-thio-β-cyclodextrin (SH-β-CD) | Photodegradation of dyes | 98% degradation in 10 min of exposure | [116] |
| 2 | Ag/TiO2/Nb | AgNC stabilized by Captopril | Photodegradation of dyes | 100% degradation | [118] |
| 3 | AgNC/CeO2 | AgNC stabilized by Polyethylene imine (PEI) | Photodegradation of dyes | 80% degradation in 2 h | [119] |
| 4 | ZnO–Ag NCs. | AgNC | Photodegradation of dyes | 100% degradation in 1 h | [120] |
| 5 | CuNC: [Cu18(CH3COO)(OH)]−2 and [Cu34O2(CH3COO)3N(C4H9)3Na]−2. | No cocatalyst | Photodegradation of dyes | 100% degradation in 69 h | [121] |
| 6 | AuNC@MPTS (MPTS-3-Mercaptopropyl trimethoxysilane) | No cocatalyst | Photodegradation of dyes | 100% degradation in 1 h | [49] |
| 7 | (Ag12TPyP) | No cocatalyst | Photodegradation of dyes | 98% degradation | [123] |
| 8 | SiO2-Au GSH clusters-BPEI@TiO2 | SiO2-Au GSH clusters-BEPI | Photodegradation of organic dyes | 99.1% degradation in 0.5 h | [124] |
| 9 | Au25 NC-TiO2 | Au25 NC | Oxidation of phenol derivatives and ferrocyanide and reduction of Ag+, Cu2+, and oxygen | [129] | |
| 10 | Au25NC | No Catalyst | Oxidation of styrene and hydrogenation of α,β-unsaturated ketone | 27 ± 1.0% | [130] |
| 11 | [Au25(PPh3)10(SR)5Cl2]-TiO2 | AuNC: [Au25(PPh3)10(SR)5Cl2] | Oxidation of benzylamines to imines | 73–99% | [126] |
| 12 | Ag/ZX-Bi_200 | Ag NC: (Ag/ZX-Bi_200) | Photooxidation of methanol | 49.60 mmol·g−1·cm−2 after 12 h of reaction | [132] |
| 13 | Aux-GSH NCs @TiO2 | Aux-GSH NCs | Production of H2 | 0.3 mmol of hydrogen/h/g | [171] |
| 14 | Aux/NP-TNTA NP-TNTAs-TiO2 nanotube arrays | AuNC | Photodegradation of organic dyes, photocatalytic reduction of aromatic nitro compounds, and photoelectrochemical water splitting | [136] | |
| 15 | (Au25(SG)18)-BaLa4Ti4O15 | AuNC: (Au25(SG)18) | Photocatalytic water splitting | 190 µmol/h | [146] |
| 16 | Au25/SrTiO3 | AuNC | Hydrogen evolution reaction | 41.2 µmol/h of H | [137] |
| 17 | Au101NCs-AlSrTiO3-rGO | Au101NCs | Photocatalytic production of H2, photocatalytic water splitting | 385 ± 22 µmol h−1 | [147] |
| 18 | GNRs-AgNCs GNRS-Gold nanorods | AgNCs | Hydrogen evolution reaction | 10% | [138] |
| 19 | Pt/HTi2NbO7 Monolayer niobate (HTi2NbO7) | Pt NC | Higher H2 production | 10 μmol h−1 | [140] |
| 20 | Pt46NC-CdS Modified cadmium sulfide (CdS) nanorod | Pt46NC | Photocatalytic water splitting | 1.5‰ h−1 | [148] |
| 21 | Au24Pd NCs-BaLa4Ti4O15 & Au24Pt NCs- BaLa4Ti4O15 | Au24Pd NCs and Au24Pt NCs | Photocatalytic H2 evolution | 100–150 µmol h−1 | [149] |
| 22 | PtAg24 NC-g-C3N4 | PtAg24 NC | Photocatalytic H2 production | 39.7 µmol h−1 | [142] |
| 23 | Cu-BTN-TiO2 | CuNCs: (Cu-BTN) | Photocatalytic CO2 reduction | 150.9 μmol g−1 h−1 | [167] |
| 24 | Metal cations-Fe2+, Co2+, Ni2+ and Cu2+ | Au NCs: (Auc-C-Co) & (Auc-MPA-Co) | Photocatalytic CO2 reduction | 3.45 µmol⋅g−1⋅h−1 | [168] |
| 25 | CODH/AgNCs-PMAA/TiO2 CODH-carbon monoxide dehydrogenase | Ag NC coupled with PMAA | Photocatalytic CO2 reduction | turnover frequency of 20 s−1 | [172] |
| 26 | Au-NCs@MOF | Photocatalytic CO2 reduction | 57.6 μmol g−1 h−1 | [169] | |
| 27 | Ni-NCs-TiO2 | Ni-NCs | Photocatalytic CO2 reduction | 10 µmol g-cat−1 | [170] |
| 28 | AgNC@ZX-V | No Cocatalyst | Reforming of formic acid to H2 and CO2 | 99% selectivity | [173] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mathew, M.S.; Krishnan, G.; Mathews, A.A.; Sunil, K.; Mathew, L.; Antoine, R.; Thomas, S. Recent Progress on Ligand-Protected Metal Nanoclusters in Photocatalysis. Nanomaterials 2023, 13, 1874. https://doi.org/10.3390/nano13121874
Mathew MS, Krishnan G, Mathews AA, Sunil K, Mathew L, Antoine R, Thomas S. Recent Progress on Ligand-Protected Metal Nanoclusters in Photocatalysis. Nanomaterials. 2023; 13(12):1874. https://doi.org/10.3390/nano13121874
Chicago/Turabian StyleMathew, Meegle S., Greeshma Krishnan, Amita Aanne Mathews, Kevin Sunil, Leo Mathew, Rodolphe Antoine, and Sabu Thomas. 2023. "Recent Progress on Ligand-Protected Metal Nanoclusters in Photocatalysis" Nanomaterials 13, no. 12: 1874. https://doi.org/10.3390/nano13121874






