Novel Sol-Gel Synthesis of TiO2 Spherical Porous Nanoparticles Assemblies with Photocatalytic Activity
Abstract
:1. Introduction
2. Materials and Methods
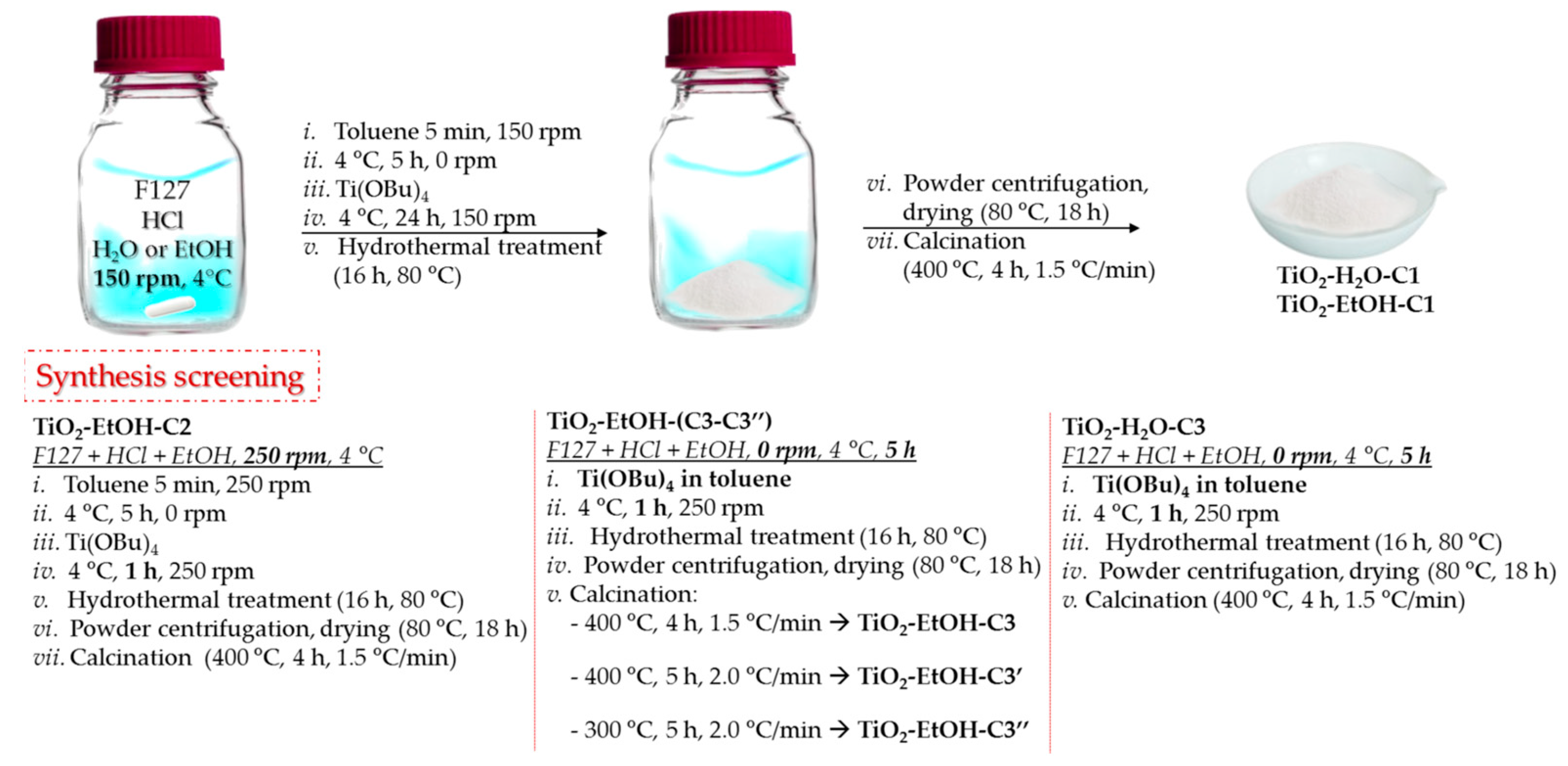
2.1. Synthesis of TiO2-Based Materials
2.1.1. Synthesis of TiO2-H2O-D1
2.1.2. Synthesis of TiO2-EtOH-D1
2.1.3. Synthesis of TiO2-EtOH-D2
2.1.4. Synthesis of TiO2-H2O-D3
2.1.5. Synthesis of TiO2-EtOH-D3
2.1.6. Synthesis of TiO2-H2O-C1, TiO2-EtOH-C1, TiO2-EtOH-C2, TiO2-H2O-C3, and TiO2-EtOH-C3
2.1.7. Synthesis of TiO2-EtOH-C3′
2.1.8. Synthesis of TiO2-EtOH-C3″
2.2. Characterization
2.3. Photocatalytic Assessment
2.4. Photodegradation in the UV-A Range
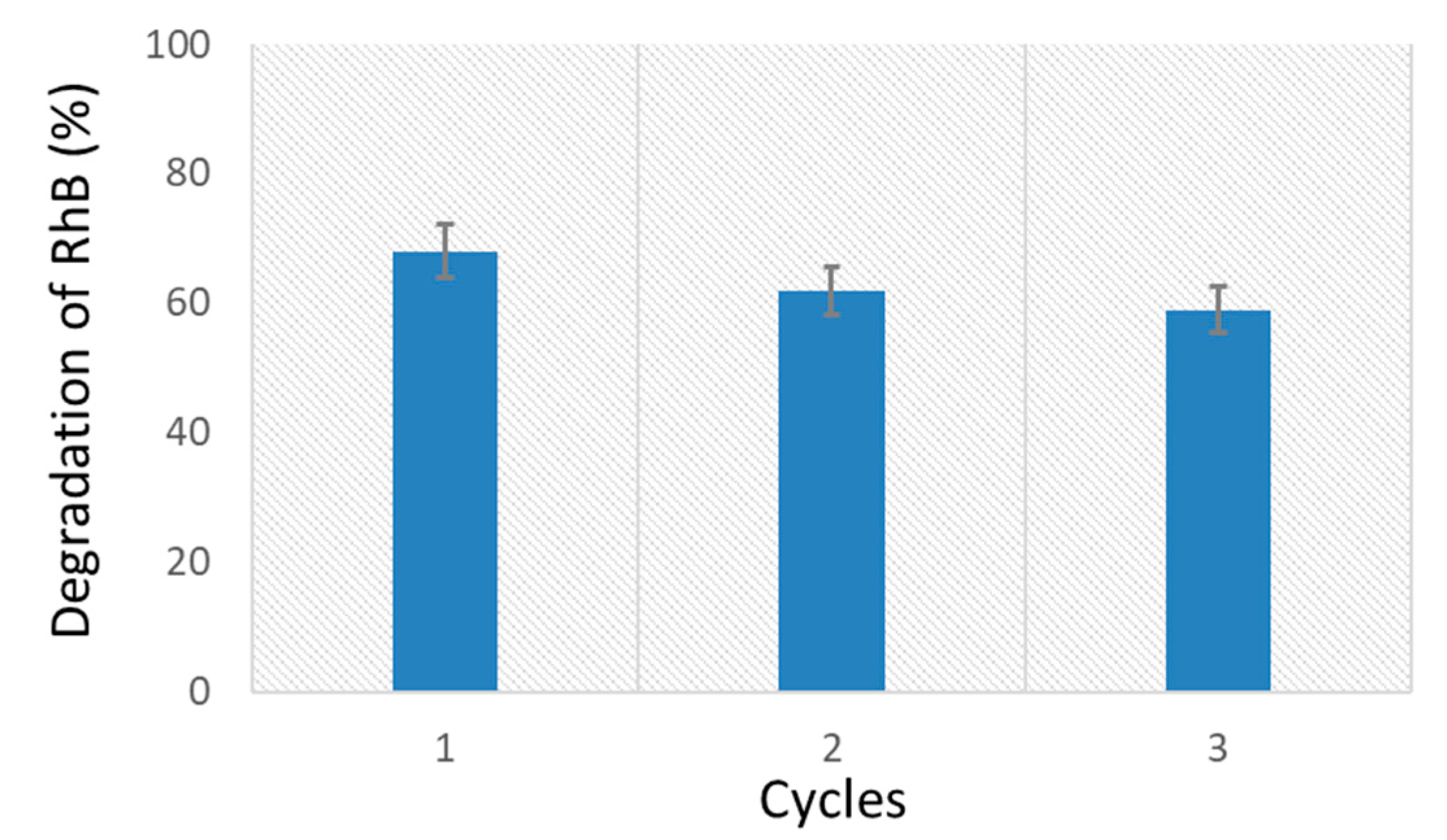
2.5. Recycling Tests
3. Results and Discussion
Catalytic Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, S.; Song, P.; Cui, J.; Liang, S. Amorphous TiO2 nanostructures: Synthesis, fundamental properties and photocatalytic applications. Catal. Sci. Technol. 2019, 9, 4198–4215. [Google Scholar] [CrossRef]
- Shang, C.; Bu, J.; Song, C. Preparation, Antimicrobial Properties under Different Light Sources, Mechanisms and Applications of TiO2: A Review. Materials 2022, 15, 5820. [Google Scholar] [CrossRef]
- Sippel, C.; Guaglianoni, W.C.; Bergmann, C.P. Titanium Dioxide Nanomaterials for Renewable Energy Applications. In Environmental Applications of Nanomaterials; Kopp Alves, A., Ed.; Springer International Publishing: Cham, Switzerland, 2022; pp. 73–96. [Google Scholar] [CrossRef]
- Kang, X.; Liu, S.; Dai, Z.; He, Y.; Song, X.; Tan, Z. Titanium Dioxide: From Engineering to Applications. Catalysts 2019, 9, 191. [Google Scholar] [CrossRef] [Green Version]
- Ziental, D.; Czarczynska-Goslinska, B.; Mlynarczyk, D.T.; Glowacka-Sobotta, A.; Stanisz, B.; Goslinski, T.; Sobotta, L. Titanium Dioxide Nanoparticles: Prospects and Applications in Medicine. Nanomaterials 2020, 10, 387. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Wang, Z.; Wang, W.; Wang, Y.; Hu, X.; Liu, J.; Gong, X.; Miao, W.; Ding, L.; Li, X.; et al. Synthesis, modification and application of titanium dioxide nanoparticles: A review. Nanoscale 2022, 14, 6709–6734. [Google Scholar] [CrossRef]
- Gagliardi, S.; Rondino, F.; Paoletti, C.; Falconieri, M. On the Morphology of Nanostructured TiO2 for Energy Applications: The Shape of the Ubiquitous Nanomaterial. Nanomaterials 2022, 12, 2608. [Google Scholar] [CrossRef]
- Anucha, C.B.; Altin, I.; Bacaksiz, E.; Stathopoulos, V.N. Titanium dioxide (TiO2)-based photocatalyst materials activity enhancement for contaminants of emerging concern (CECs) degradation: In the light of modification strategies. Chem. Eng. J. Adv. 2022, 10, 100262. [Google Scholar] [CrossRef]
- Armaković, S.J.; Savanović, M.M.; Armaković, S. Titanium Dioxide as the Most Used Photocatalyst for Water Purification: An Overview. Catalysts 2023, 13, 26. [Google Scholar] [CrossRef]
- Nasr, M.; Eid, C.; Habchi, R.; Miele, P.; Bechelany, M. Recent Progress on Titanium Dioxide Nanomaterials for Photocatalytic Applications. ChemSusChem 2018, 11, 3023–3047. [Google Scholar] [CrossRef]
- Mavračić, J.; Mocanu, F.C.; Deringer, V.L.; Csányi, G.; Elliott, S.R. Similarity Between Amorphous and Crystalline Phases: The Case of TiO2. J. Phys. Chem. Lett. 2018, 9, 2985–2990. [Google Scholar] [CrossRef] [PubMed]
- Balayeva, N.O.; Mamiyev, Z.; Dillert, R.; Zheng, N.; Bahnemann, D.W. Rh/TiO2-Photocatalyzed Acceptorless Dehydrogenation of N-Heterocycles upon Visible-Light Illumination. ACS Catal. 2020, 10, 5542–5553. [Google Scholar] [CrossRef]
- Balayeva, N.O.; Zheng, N.; Dillert, R.; Bahnemann, D.W. Visible-Light-Mediated Photocatalytic Aerobic Dehydrogenation of N-heterocycles by Surface-Grafted TiO2 and 4-amino-TEMPO. ACS Catal. 2019, 9, 10694–10704. [Google Scholar] [CrossRef]
- Sang, L.; Zhao, Y.; Burda, C. TiO2 Nanoparticles as Functional Building Blocks. Chem. Rev. 2014, 114, 9283–9318. [Google Scholar] [CrossRef]
- Li, W.; Wu, Z.; Wang, J.; Elzatahry, A.A.; Zhao, D. A Perspective on Mesoporous TiO2 Materials. Chem. Mater. 2014, 26, 287–298. [Google Scholar] [CrossRef]
- Su, C.; Hong, B.Y.; Tseng, C.M. Sol–gel preparation and photocatalysis of titanium dioxide. Catal. Today 2004, 96, 119–126. [Google Scholar] [CrossRef]
- Dionigi, C.; Calestani, G.; Ferraroni, T.; Ruani, G.; Liotta, L.F.; Migliori, A.; Nozar, P.; Palles, D. Template evaporation method for controlling anatase nanocrystal size in ordered macroporous TiO2. J. Colloid Interface Sci. 2005, 290, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Dominguez, M.; Liotta, L.F.; Di Carlo, G.; Pantaleo, G.; Venezia, A.M.; Solans, C.; Boutonnet, M. Synthesis of CeO2, ZrO2, Ce0.5Zr0.5O2, and TiO2 nanoparticles by a novel oil-in-water microemulsion reaction method and their use as catalyst support for CO oxidation. Catal. Today 2010, 158, 35–43. [Google Scholar] [CrossRef]
- Truppi, A.; Petronella, F.; Placido, T.; Margiotta, V.; Lasorella, G.; Giotta, L.; Giannini, C.; Sibillano, T.; Murgolo, S.; Mascolo, G.; et al. Gram-scale synthesis of UV–vis light active plasmonic photocatalytic nanocomposite based on TiO2/Au nanorods for degradation of pollutants in water. Appl. Catal. B Environ. 2019, 243, 604–613. [Google Scholar] [CrossRef]
- Macwan, D.P.; Dave, P.N.; Chaturvedi, S. A review on nano-TiO2 sol–gel type syntheses and its applications. J. Mater. Sci. 2011, 46, 3669–3686. [Google Scholar] [CrossRef]
- Ullattil, S.G.; Periyat, P. Sol-Gel Synthesis of Titanium Dioxide. In Sol-Gel Materials for Energy, Environment and Electronic Applications; Pillai, S.C., Hehir, S., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 271–283. [Google Scholar] [CrossRef]
- Gan, W.Y.; Lee, M.W.; Amal, R.; Zhao, H.; Chiang, K. Photoelectrocatalytic activity of mesoporous TiO2 films prepared using the sol–gel method with tri-block copolymer as structure directing agent. J. Appl. Electrochem. 2008, 38, 703–712. [Google Scholar] [CrossRef]
- Li, H.; Wang, J.; Li, H.; Yin, S.; Sato, T. High thermal stability thick wall mesoporous titania thin films. Mater. Lett. 2009, 63, 1583–1585. [Google Scholar] [CrossRef]
- Das, S.K.; Bhunia, M.K.; Bhaumik, A. Self-assembled TiO2 nanoparticles: Mesoporosity, optical and catalytic properties. Dalton Trans. 2010, 39, 4382–4390. [Google Scholar] [CrossRef]
- Bleta, R.; Alphonse, P.; Lorenzato, L. Nanoparticle Route for the Preparation in Aqueous Medium of Mesoporous TiO2 with Controlled Porosity and Crystalline Framework. J. Phys. Chem. C 2010, 114, 2039–2048. [Google Scholar] [CrossRef]
- Oveisi, H.; Suzuki, N.; Beitollahi, A.; Yamauchi, Y. Aerosol-assisted fabrication of mesoporous titania spheres with crystallized anatase structures and investigation of their photocatalitic properties. J. Sol-Gel Sci. Technol. 2010, 56, 212–218. [Google Scholar] [CrossRef]
- Samsudin, E.M.; Hamid, S.B.A.; Juan, J.C.; Basirun, W.J. Influence of triblock copolymer (pluronic F127) on enhancing the physico-chemical properties and photocatalytic response of mesoporous TiO2. Appl. Surf. Sci. 2015, 355, 959–968. [Google Scholar] [CrossRef]
- Oseghe, E.O.; Maddila, S.; Ndungu, P.G.; Jonnalagadda, S.B. Effect of surfactant concentration on active species generation and photocatalytic properties of TiO2. Appl. Catal. B Environ. 2015, 176, 288–297. [Google Scholar] [CrossRef]
- Zhang, W.; He, H.; Tian, Y.; Lan, K.; Liu, Q.; Wang, C.; Liu, Y.; Elzatahry, A.; Che, R.; Li, W.; et al. Synthesis of uniform ordered mesoporous TiO2 microspheres with controllable phase junctions for efficient solar water splitting. Chem. Sci. 2019, 10, 1664–1670. [Google Scholar] [CrossRef] [Green Version]
- Tsotetsi, D.; Dhlamini, M.; Mbule, P. Sol-gel derived mesoporous TiO2: Effects of non-ionic co-polymers on the pore size, morphology, specific surface area and optical properties analysis. Results Mater. 2022, 14, 100266. [Google Scholar] [CrossRef]
- Mrowiec-Bialoń, J. Determination of hydroxyls density in the silica-mesostructured cellular foams by thermogravimetry. Thermochim. Acta 2006, 443, 49–52. [Google Scholar] [CrossRef]
- Piras, A.; Olla, C.; Reekmans, G.; Kelchtermans, A.-S.; De Sloovere, D.; Elen, K.; Carbonaro, C.M.; Fusaro, L.; Adriaensens, P.; Hardy, A.; et al. Photocatalytic Performance of Undoped and Al-Doped ZnO Nanoparticles in the Degradation of Rhodamine B under UV-Visible Light:The Role of Defects and Morphology. Int. J. Mol. Sci. 2022, 23, 15459. [Google Scholar] [CrossRef]
- Soumoy, L.; Fusaro, L.; Debecker, D.P.; Aprile, C. Low-Dimensional Hollow Nanostructures: From Morphology Control to the Release of an Active Pharmaceutical Ingredient. Chem. Mater. 2023, 35, 1877–1890. [Google Scholar] [CrossRef]
- Shi, L.; Weng, D. Highly active mixed-phase TiO2 photocatalysts fabricated at low temperatureand the correlation between phase compositionand photocatalytic activity. J. Environ. Sci. 2008, 20, 1263–1267. [Google Scholar] [CrossRef]
- Kumar, K.-N.P.; Kumar, J.; Keizer, K. Effect of Peptization on Densification and Phase-Transformation Behavior of Sol–Gel-Derived Nanostructured Titania. J. Am. Ceram. Soc. 1994, 77, 1396–1400. [Google Scholar] [CrossRef] [Green Version]
- EDELSON, L.H.; GLAESER, A.M. Role of Particle Substructure in the Sintering of Monosized Titania. J. Am. Ceram. Soc. 1988, 71, 225–235. [Google Scholar] [CrossRef] [Green Version]
- Kumar, K.-N.P.; Keizer, K.; Burggraaf, A.J.; Okubo, T.; Nagamoto, H.; Morooka, S. Densification of nanostructured titania assisted by a phase transformation. Nature 1992, 358, 48–51. [Google Scholar] [CrossRef]
- Zhang, H.; Banfield, J.F. New kinetic model for the nanocrystalline anatase-to-rutile transformation revealing rate dependence on number of particles. Am. Mineral. 1999, 84, 528–535. [Google Scholar] [CrossRef]
- Penn, R.L.; Banfield, J.F. Formation of rutile nuclei at anatase {112} twin interfaces and the phase transformation mechanism in nanocrystalline titania. Am. Mineral. 1999, 84, 871–876. [Google Scholar] [CrossRef]
- Wang, C.-C.; Ying, J.Y. Sol−Gel Synthesis and Hydrothermal Processing of Anatase and Rutile Titania Nanocrystals. Chem. Mater. 1999, 11, 3113–3120. [Google Scholar] [CrossRef]
- Ivanova, R.; Alexandridis, P.; Lindman, B. Interaction of poloxamer block copolymers with cosolvents and surfactants. Colloids Surf. A Physicochem. Eng. Asp. 2001, 183, 41–53. [Google Scholar] [CrossRef]
- Natarajan, T.S.; Thomas, M.; Natarajan, K.; Bajaj, H.C.; Tayade, R.J. Study on UV-LED/TiO2 process for degradation of Rhodamine B dye. Chem. Eng. J. 2011, 169, 126–134. [Google Scholar] [CrossRef]
- Žerjav, G.; Žižek, K.; Zavašnik, J.; Pintar, A. Brookite vs. rutile vs. anatase: What’s behind their various photocatalytic activities? J. Environ. Chem. Eng. 2022, 10, 107722. [Google Scholar] [CrossRef]
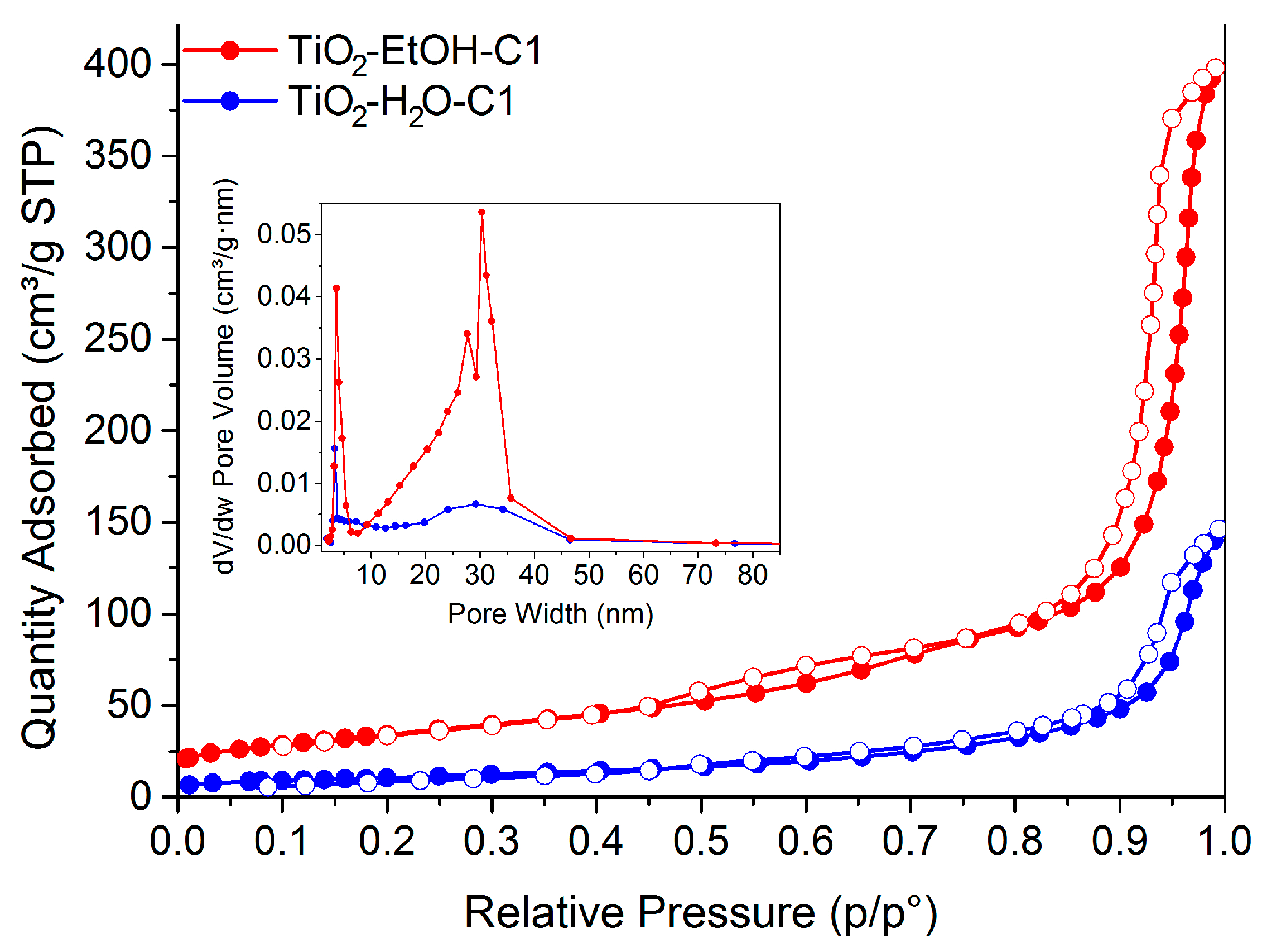

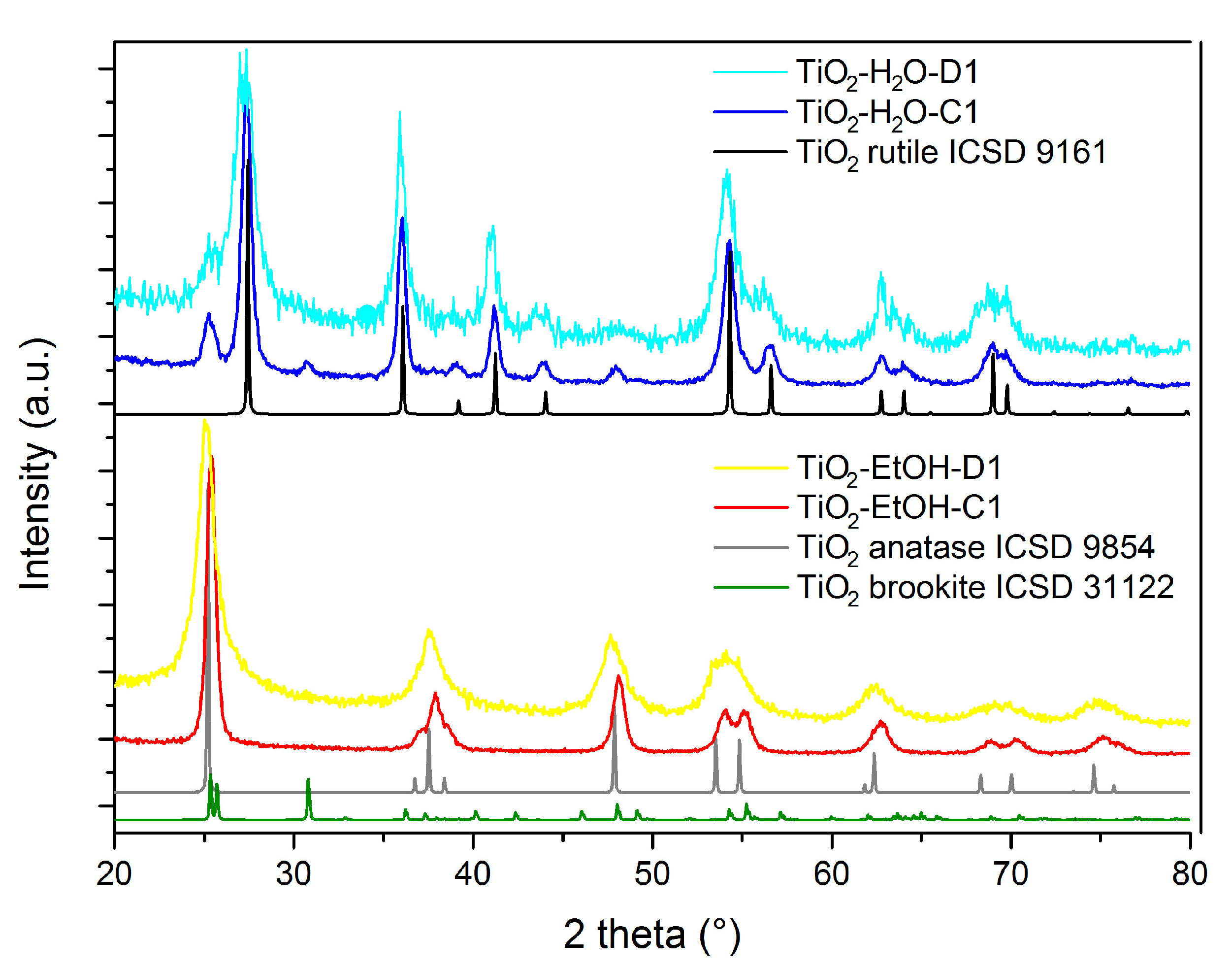
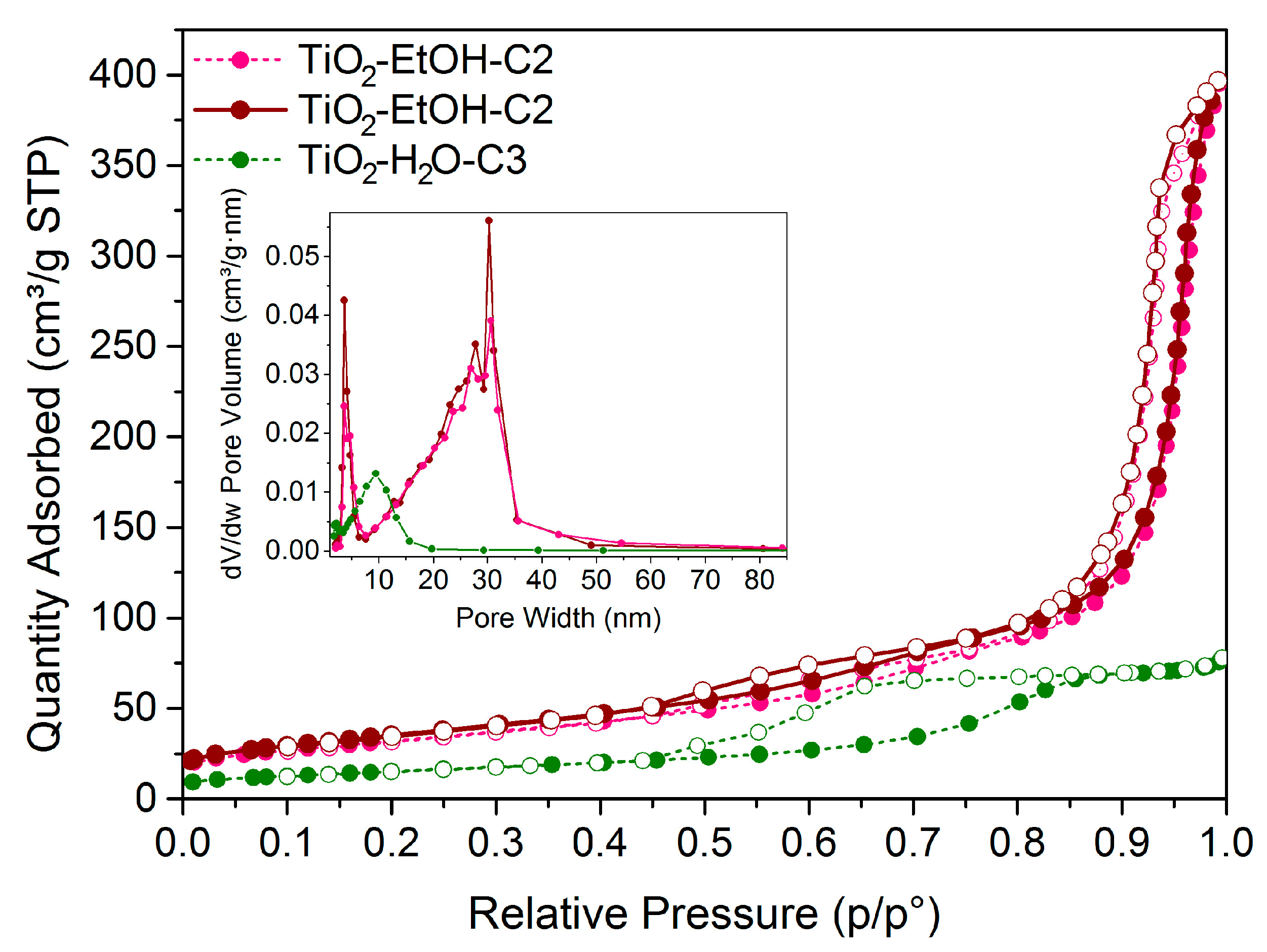
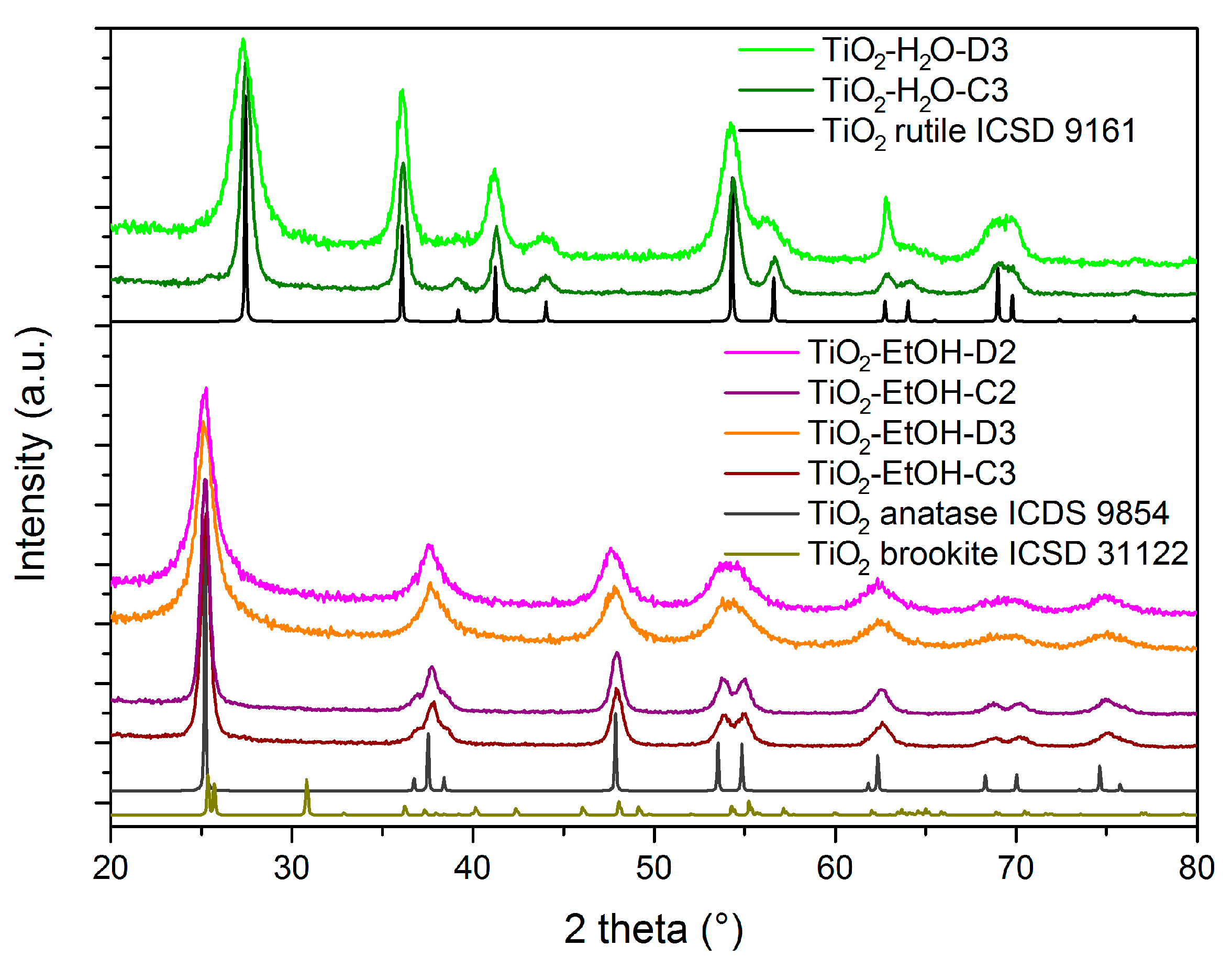

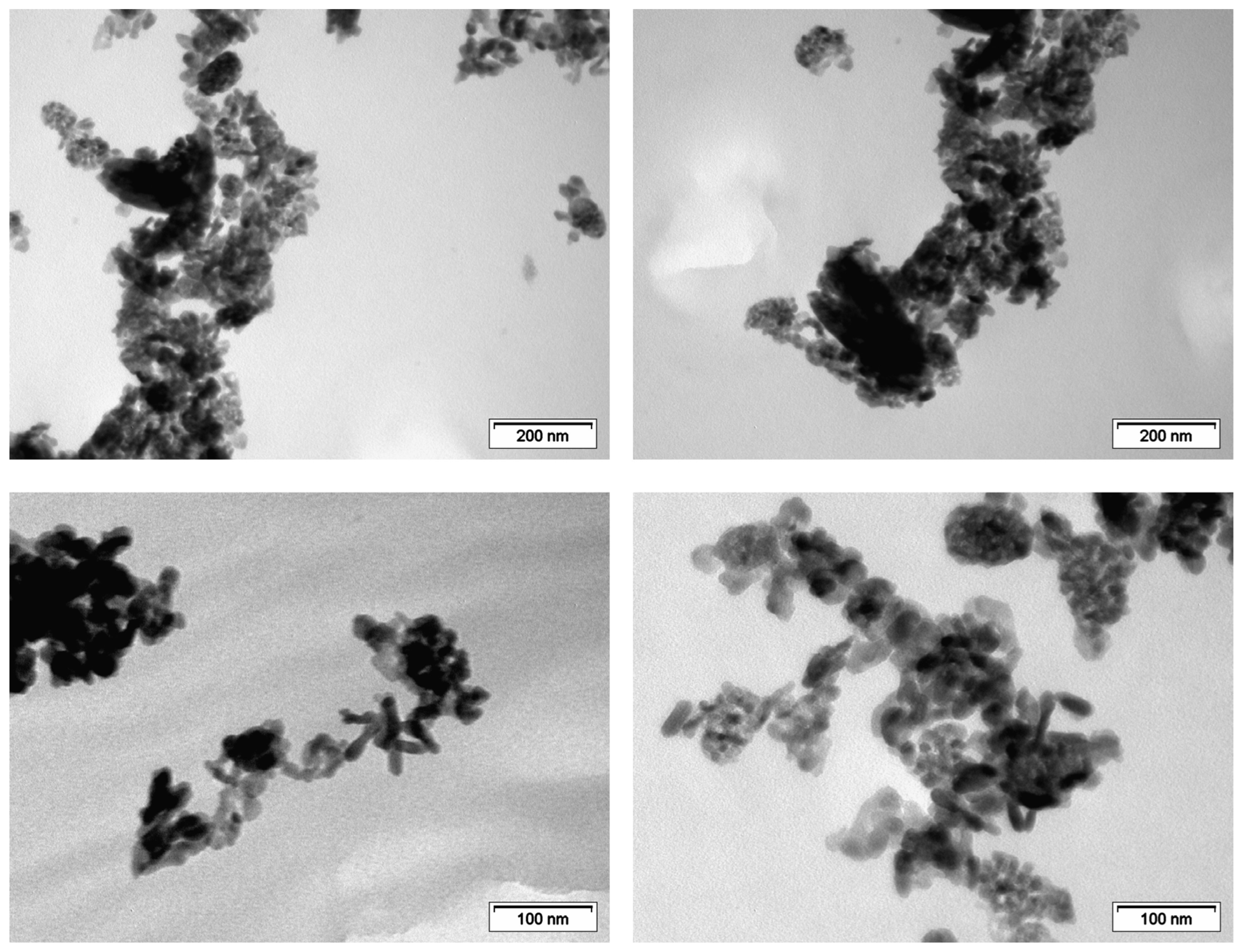
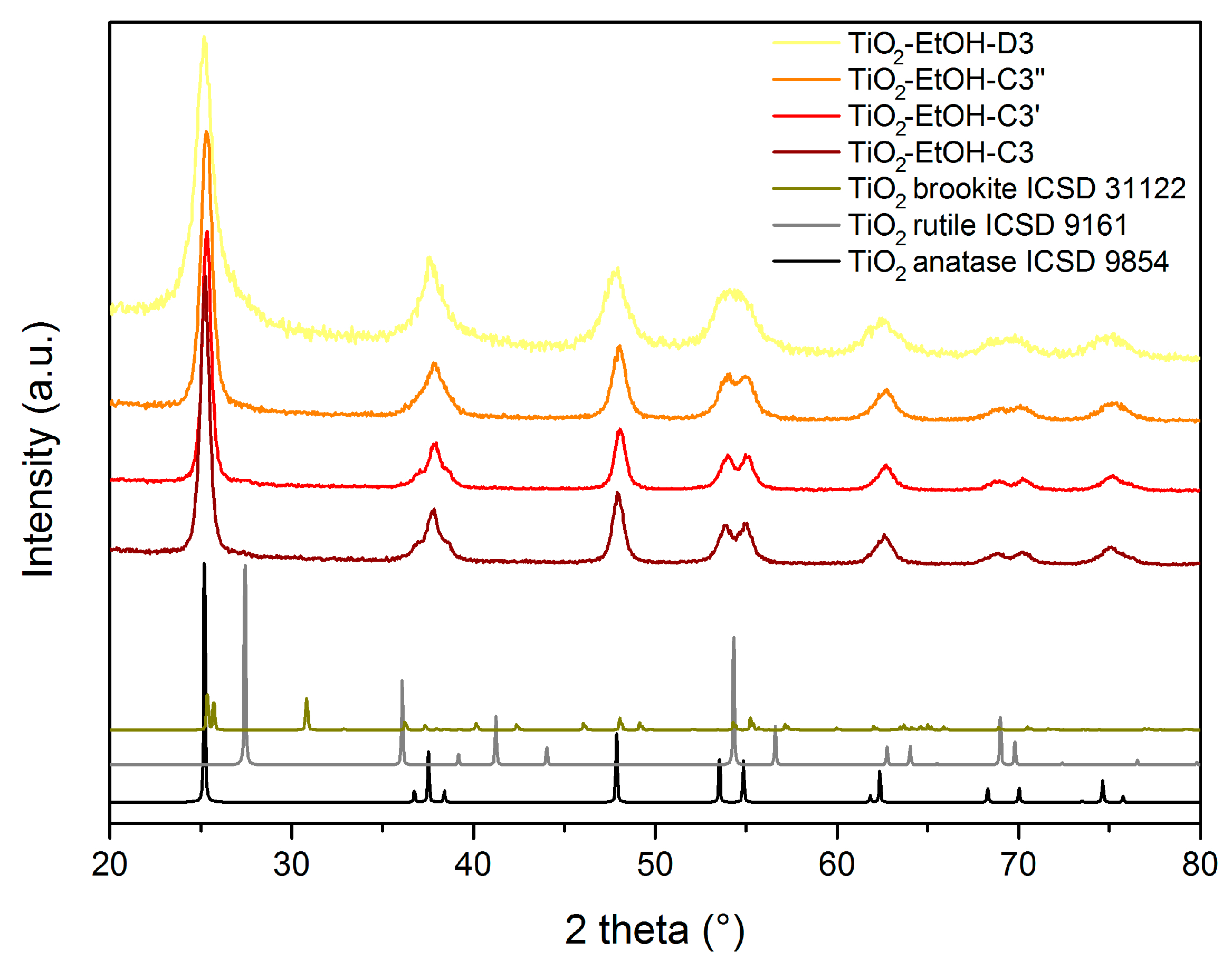
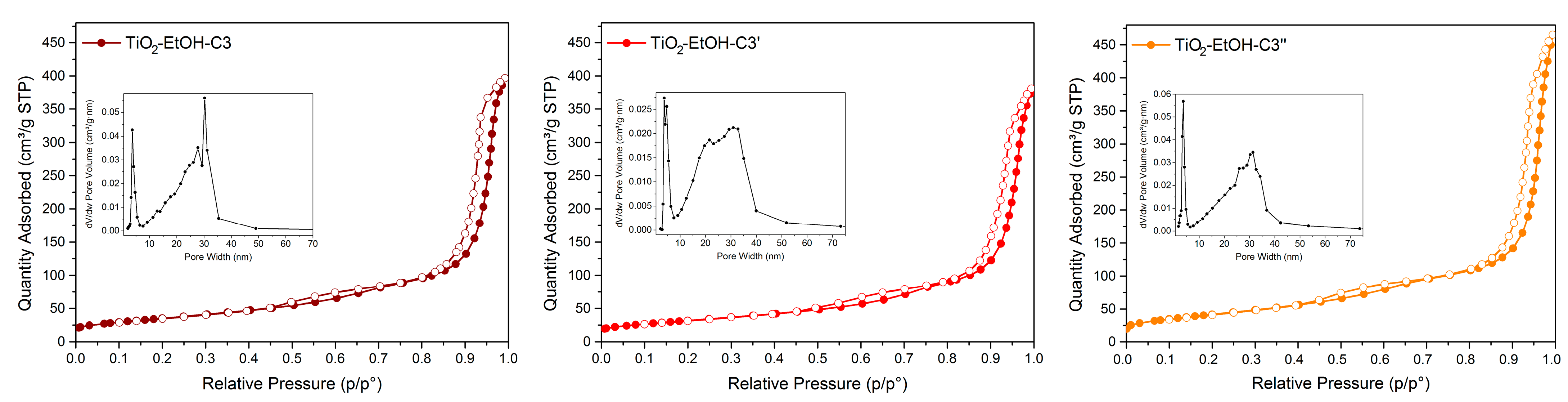
| Material | Calcination Conditions | SBET (m2/g) | Pore Volume (BJHAds.) (cm3/g) | Main Crystalline Phase |
|---|---|---|---|---|
| TiO2-H2O-C1 | 400 °C, 1.5 °C/min, 4 h | 38 | 0.23 | Rutile |
| TiO2-H2O-C3 | 400 °C, 1.5 °C/min, 4 h | 55 | 0.12 | Rutile |
| TiO2-EtOH-C1 | 400 °C, 1.5 °C/min, 4 h | 124 | 0.62 | Anatase |
| TiO2-EtOH-C2 | 400 °C, 1.5 °C/min, 4 h | 116 | 0.62 | Anatase |
| TiO2-EtOH-C3 | 400 °C, 1.5 °C/min, 4 h | 129 | 0.63 | Anatase |
| TiO2-EtOH-C3’ | 400 °C, 2 °C/min, 5 h | 116 | 0.59 | Anatase |
| TiO2-EtOH-C3’’ | 300 °C, 2 °C/min, 5 h | 153 | 0.72 | Anatase |
| Material | Particle Size Distribution (nm) via TEM Analysis |
|---|---|
| TiO2-H2O-C1 | 44 nm ± 11 nm |
| TiO2-H2O-C3 | n.d.* |
| TiO2-EtOH-C1 | n.d.* |
| TiO2-EtOH-C3 | 37 nm ± 10 nm |
| Material | Main TiO2 Crystalline Phase | Photodegradation RhB (%) |
|---|---|---|
| TiO2-H2O-C1 | Rutile | 25 ± 4 |
| TiO2-H2O-C3 | Rutile | 15 ± 1 |
| TiO2-EtOH-C1 | Anatase | 37 ± 3 |
| TiO2-EtOH-C3 | Anatase | 68 ± 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calabrese, C.; Maertens, A.; Piras, A.; Aprile, C.; Liotta, L.F. Novel Sol-Gel Synthesis of TiO2 Spherical Porous Nanoparticles Assemblies with Photocatalytic Activity. Nanomaterials 2023, 13, 1928. https://doi.org/10.3390/nano13131928
Calabrese C, Maertens A, Piras A, Aprile C, Liotta LF. Novel Sol-Gel Synthesis of TiO2 Spherical Porous Nanoparticles Assemblies with Photocatalytic Activity. Nanomaterials. 2023; 13(13):1928. https://doi.org/10.3390/nano13131928
Chicago/Turabian StyleCalabrese, Carla, Amélie Maertens, Alessandra Piras, Carmela Aprile, and Leonarda Francesca Liotta. 2023. "Novel Sol-Gel Synthesis of TiO2 Spherical Porous Nanoparticles Assemblies with Photocatalytic Activity" Nanomaterials 13, no. 13: 1928. https://doi.org/10.3390/nano13131928
APA StyleCalabrese, C., Maertens, A., Piras, A., Aprile, C., & Liotta, L. F. (2023). Novel Sol-Gel Synthesis of TiO2 Spherical Porous Nanoparticles Assemblies with Photocatalytic Activity. Nanomaterials, 13(13), 1928. https://doi.org/10.3390/nano13131928









