Preliminary Study on the Preparation of Conductive Nanosized Calcium Carbonate Utilizing Biogas Slurry by a Synchronous Double Decomposition Coating Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials and Chemical Reagents
2.2. Preparation of CNCC
2.3. Characterization Methods
3. Results
3.1. HA-NCC Formed in Biogas Slurry
3.2. Morphological Features of CNCC
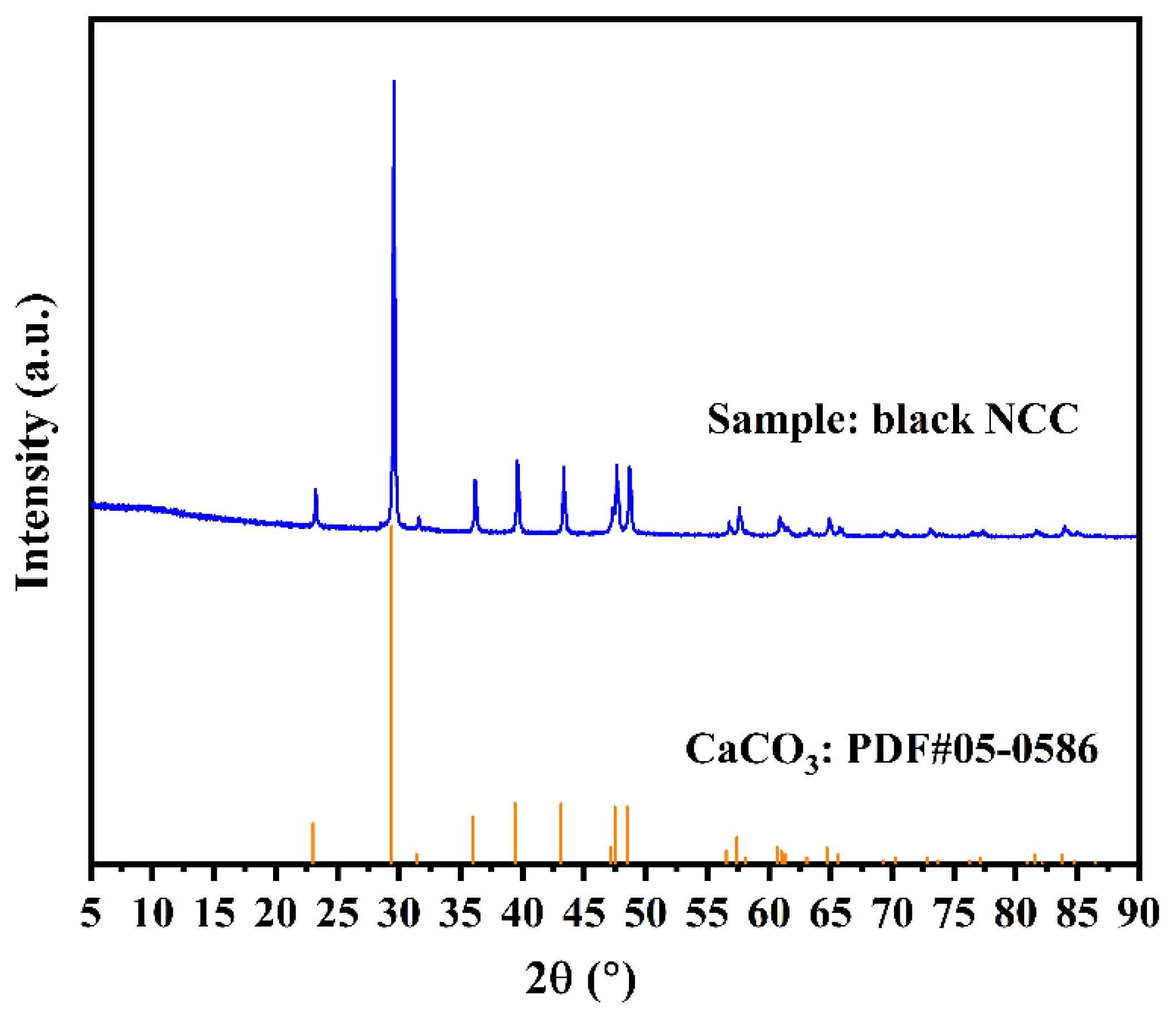
3.3. Crystal Structure and Composition of CNCC
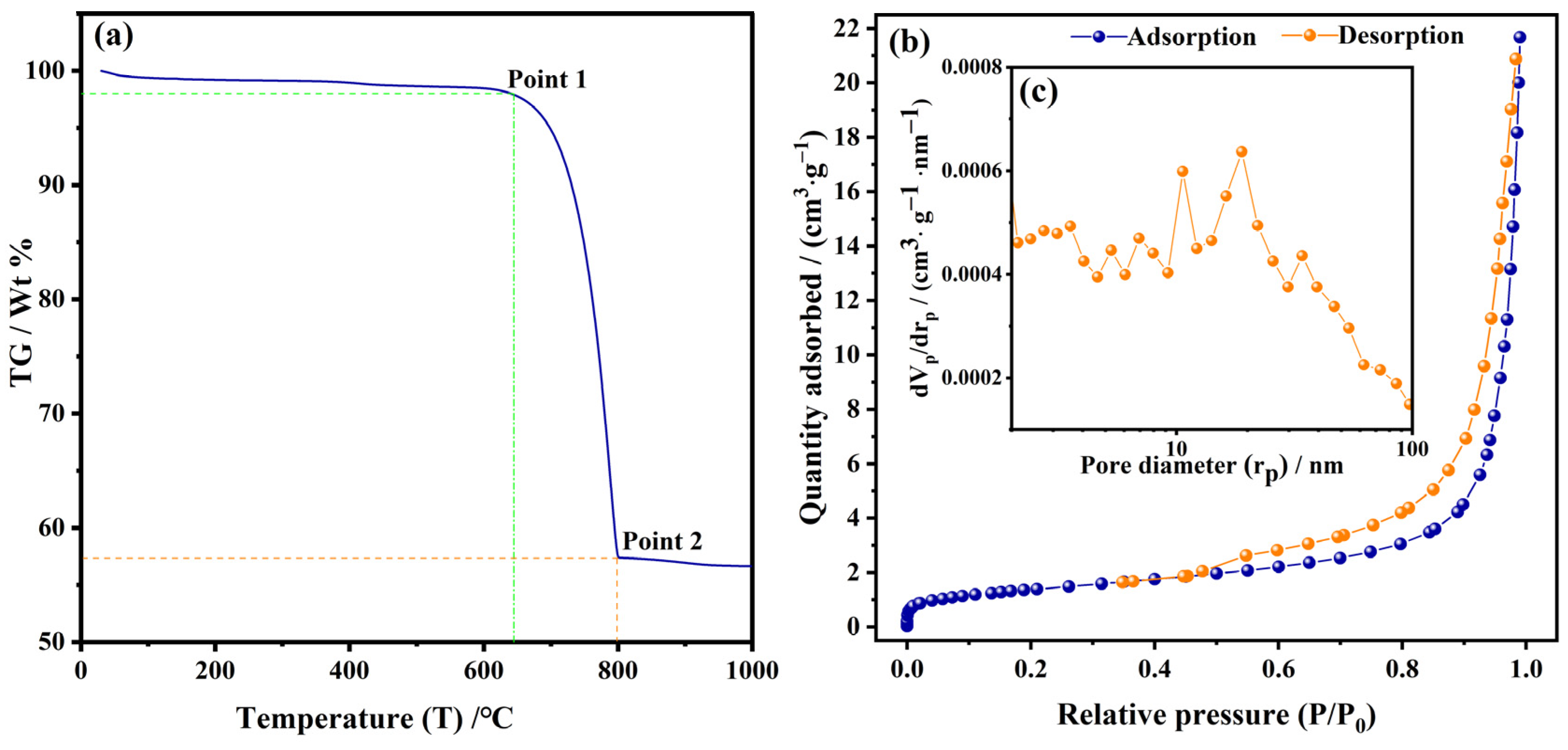
3.4. TGA and BET
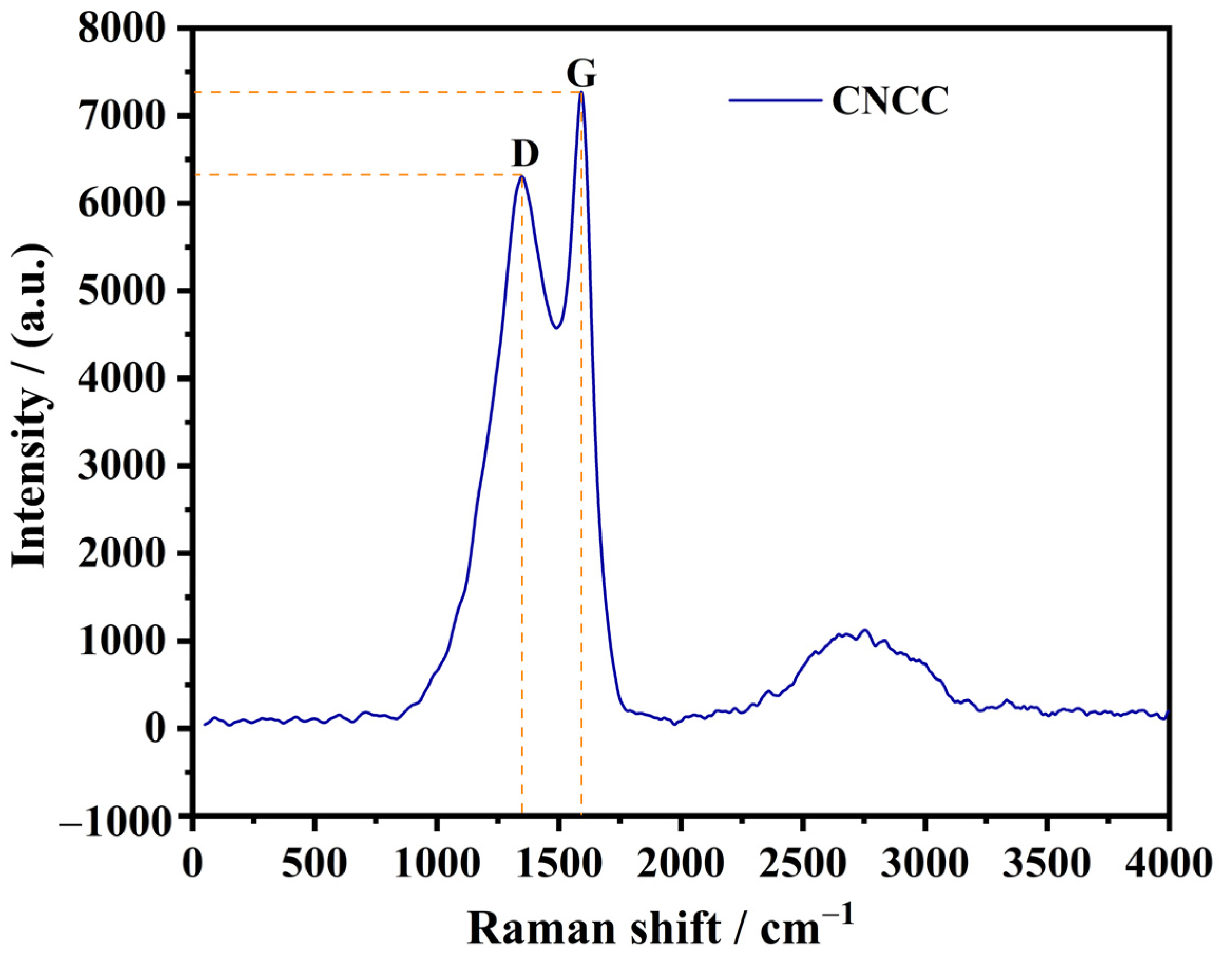
3.5. Graphitization and Conductivity of CNCC
4. Discussion
4.1. A Meaningful Discovery in Biogas Slurry
4.2. Synchronous Double Decomposition Coating Method
4.3. CNCC
4.4. Limitations and Development Prospects
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nooririnah, A.; Azwar, M.A.; Yusliza, Y.; Abreeza, M.; Ashadi, M.J. Effect of CO2 Flow Rate on the Synthesis Nanosized Precipitated Calcium Carbonate, PCC. Appl. Mech. Mater. 2014, 481, 72–75. [Google Scholar] [CrossRef]
- Wu, G.; Wang, Y.; Zhu, S.; Wang, J. Preparation of ultrafine calcium carbonate particles with micropore dispersion method. Powder Technol. 2007, 172, 82–88. [Google Scholar] [CrossRef]
- Ding, J.; Ma, W.; Song, F.; Zhong, Q. Effect of nano-Calcium Carbonate on microcellular foaming of polypropylene. J. Mater. Sci. 2013, 48, 2504–2511. [Google Scholar] [CrossRef]
- Ramakrishna, C.; Thenepalli, T.; Ahn, J.W. A Brief review of Aragonite Precipitated Calcium Carbonate (PCC) Synthesis Methods and Its Applications. Korean Chem. Eng. Res. 2017, 55, 443–455. [Google Scholar]
- Ramakrishna, C.; Thenepalli, T.; Ahn, J.W. Evaluation of Various Synthesis Methods for Calcite-Precipitated Calcium Carbonate (PCC) Formation. Korean Chem. Eng. Res. 2017, 55, 279–286. [Google Scholar]
- McDonald, L.J.; Afzal, W.; Glasser, F.P. Evidence of scawtite and tilleyite formation at ambient conditions in hydrated Portland cement blended with freshly-precipitated nano-size calcium carbonate to reduce greenhouse gas emissions. J. Build. Eng. 2022, 48, 103906. [Google Scholar] [CrossRef]
- Polat, S. Evaluation of the effects of sodium laurate on calcium carbonate precipitation: Characterization and optimization studies. J. Cryst. Growth 2019, 508, 8–18. [Google Scholar] [CrossRef]
- Melotti, R.; Santagata, E.; Bassani, M.; Salvo, M.; Rizzo, S. A preliminary investigation into the physical and chemical properties of biomass ashes used as aggregate fillers for bituminous mixtures. Waste Manag. 2013, 33, 1906–1917. [Google Scholar] [CrossRef]
- Swain, S.K.; Dash, S.; Kisku, S.K.; Singh, R.K. Thermal and Oxygen Barrier Properties of Chitosan Bionanocomposites by Reinforcement of Calcium Carbonate Nanopowder. J. Mater. Sci. Technol. 2014, 30, 791–795. [Google Scholar] [CrossRef]
- Kim, M.K.; Lee, J.A.; Jo, M.R.; Kim, M.K.; Kim, H.M.; Oh, J.M.; Song, N.W.; Choi, S.J. Cytotoxicity, Uptake Behaviors, and Oral Absorption of Food Grade Calcium Carbonate Nanomaterials. Nanomaterials 2015, 5, 1938–1954. [Google Scholar] [CrossRef] [Green Version]
- Andricic, B.; Kovacic, T.; Klaric, I. Properties of recycled material containing poly(vinyl chloride), polypropylene, and calcium carbonate nanofiller. Polym. Eng. Sci. 2008, 48, 572–577. [Google Scholar] [CrossRef]
- Ari, G.A.; Aydin, I. Nanocomposites prepared by solution blending: Microstructure and mechanical properties. J. Macromol. Sci. B 2008, 47, 260–267. [Google Scholar]
- Liang, J.Z.; Tang, C.Y.; Zhou, L.; Tsui, C.P.; Li, F.J. Melt flow behavior in capillary extrusion of nanosized calcium carbonate-filled poly(L-lactic acid) biocomposites. Polym. Eng. Sci. 2012, 52, 1839–1844. [Google Scholar] [CrossRef]
- Bao, L.; Lei, J.; Wang, J. Preparation and characterization of a novel antistatic poly(vinyl chloride)/quaternary ammonium based ion-conductive acrylate copolymer composites. J. Electrostat. 2013, 71, 987–993. [Google Scholar] [CrossRef]
- Kuo, Y.C.; Lee, C.H.; Rajesh, R. Iron oxide-entrapped solid lipid nanoparticles and poly(lactide-co-glycolide) nanoparticles with surfactant stabilization for antistatic application. J. Mater. Res. Technol. 2019, 8, 887–895. [Google Scholar] [CrossRef]
- Li, J.; Wu, G.; Xu, Z. Tribo-charging properties of waste plastic granules in process of tribo-electrostatic separation. Waste Manag. 2015, 35, 36–41. [Google Scholar] [CrossRef]
- Zhang, J.; Ciampi, S. The Position of Solid Carbon Dioxide in the Triboelectric Series. Aust. J. Chem. 2019, 72, 633–636. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Che, R.; Yang, W.; Lei, J. Biodegradable antistatic plasticizer based on citrate electrolyte doped with alkali metal salt and its poly(vinyl chloride) composites. Polym. Int. 2011, 60, 344–352. [Google Scholar] [CrossRef]
- Jenbanphue, T.; Hongesombut, K.; Jitkajornwanich, K.; Srisonphan, S. Electrostatic Properties and Ions Elimination Effect of Polyvinyl Chloride (PVC) Adhesive Tape Manufacturing. In Proceedings of the 2020 8th International Electrical Engineering Congress (IEECON), Chiang Mai, Thailand, 4–6 March 2020; pp. 1–4. [Google Scholar]
- Almeida, S.; Ozkan, S.; Goncalves, D.; Paulo, I.; Queiros, C.; Ferreira, O.; Bordado, J.; dos Santos, R.G. A Brief Evaluation of Antioxidants, Antistatics, and Plasticizers Additives from Natural Sources for Polymers Formulation. Polymers 2023, 15, 6. [Google Scholar] [CrossRef]
- Yang, P.; Yu, F.; Yang, Z.; Zhang, X.; Ma, J. Graphene oxide modified κ-carrageenan/sodium alginate double-network hydrogel for effective adsorption of antibiotics in a batch and fixed-bed column system. Sic. Total Environ. 2022, 837, 155662. [Google Scholar] [CrossRef]
- de Souza Vieira, L.; dos Anjos, E.G.R.; Verginio, G.E.A.; Oyama, I.C.; Braga, N.F.; da Silva, T.F.; Montagna, L.S.; Rezende, M.C.; Passador, F.R. Carbon-based materials as antistatic agents for the production of antistatic packaging: A review. J. Mater. Sci.-Mater. El. 2021, 32, 3929–3947. [Google Scholar] [CrossRef]
- Ma, J.; Sun, Y.; Zhang, M.; Yang, M.; Gong, X.; Yu, F.; Zheng, J. Comparative Study of Graphene Hydrogels and Aerogels Reveals the Important Role of Buried Water in Pollutant Adsorption. Environ. Sci. Technol. 2017, 51, 12283–12292. [Google Scholar] [CrossRef]
- Jyoti, J.; Kumar, A.; Dhakate, S.R.; Singh, B.P. Dielectric and impedance properties of three dimension graphene oxide carbon nanotube acrylonitrile butadiene styrene hybrid composites. Polym. Test. 2018, 68, 456–466. [Google Scholar] [CrossRef]
- Zhou, Z.; Yu, F.; Ma, J. Nanoconfinement engineering for enchanced adsorption of carbon materials, metal-organic frameworks, mesoporous silica, MXenes and porous organic polymers: A review. Environ. Chem. Lett. 2022, 20, 563–595. [Google Scholar] [CrossRef]
- Chathuranga Senarathna, K.G.; Mantilaka, M.M.M.G.P.G.; Nirmal Peiris, T.A.; Pitawala, H.M.T.G.A.; Karunaratne, D.G.G.P.; Rajapakse, R.M.G. Convenient routes to synthesize uncommon vaterite nanoparticles and the nanocomposites of alkyd resin/polyanifine/vaterite: The latter possessing superior anticorrosive performance on mild steel surfaces. Electrochim. Acta 2014, 117, 460–469. [Google Scholar] [CrossRef]
- Huang, G.; Liu, Q.; Kang, W.; Xing, B.; Chen, L.; Zhang, C. Potassium humate based reduced graphite oxide materials for supercapacitor applications. Electrochim. Acta 2016, 196, 450–456. [Google Scholar] [CrossRef]
- Barhoum, A.; Van Lokeren, L.; Rahier, H.; Dufresne, A.; Van Assche, G. Roles of in situ surface modification in controlling the growth and crystallization of CaCO3 nanoparticles, and their dispersion in polymeric materials. J. Mater. Sci. 2015, 50, 7908–7918. [Google Scholar] [CrossRef]
- Shen, Q.; Wei, H.; Zhao, Y.; Wang, D.J.; Zheng, L.Q.; Xu, D.F. Morphological control of calcium carbonate crystals by polyvinylpyrrolidone and sodium dodecyl benzene sulfonate. Colloid. Surface. A 2004, 251, 87–91. [Google Scholar] [CrossRef]
- Liu, X.Y.; Zhu, H.G. Treatment of Low C/N Ratio Wastewater by a Carbon Cloth Bipolar Plate Multicompartment Electroenhanced Bioreactor (CBM-EEB). ACS Omega 2020, 5, 27823–27832. [Google Scholar] [CrossRef]
- Hu, Z.H.; Zhu, H.G. Effects of Combined Technologies on Environmental Prevention against Livestock Pollution in Biogas Projects. J. Environ. Eng. 2019, 145, 1–7. [Google Scholar] [CrossRef]
- Pan, F.H.; Xiao, H.; Huang, F.; Lei, J.J.; Zhu, H.G.; Ma, J. Macroscale preparation nanosized calcium carbonate exploiting biogas slurry synchronous metathesis encapsulation method. New J. Chem. 2023. [Google Scholar] [CrossRef]
- Gu, L.P.; Tang, X.; Sun, Y.; Kou, H.J. Bioavailability of dissolved organic matter in biogas slurry enhanced by catalytic ozonation combined with membrane separation. Ecotoxicol. Environ. Saf. 2020, 196, 110547. [Google Scholar] [CrossRef]
- Falini, G.; Fermani, S.; Tosi, G.; Dinelli, E. Calcium Carbonate Morphology and Structure in the Presence of Seawater Ions and Humic Acids. Crys. Growth Des. 2009, 9, 2065–2072. [Google Scholar] [CrossRef]
- Chen, M.; Gowthaman, S.; Nakashima, K.; Kawasaki, S. Influence of humic acid on microbial induced carbonate precipitation for organic soil improvement. Environ. Sci. Pollut. R 2023, 30, 15230–15240. [Google Scholar] [CrossRef]
- Neira-Carrillo, A.; Vasquez-Quitral, P.; Sanchez, M.; Farhadi-Khouzani, M.; Aguilar-Bolados, H.; Yazdani-Pedram, M.; Colfen, H. Functionalized Multiwalled CNTs in Classical and Nonclassical CaCO3 Crystallization. Nanomaterials 2019, 9, 1169. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Song, J.; Ji, Z.; Liu, J.; Guo, X.; Zhao, Y.; Yuan, J. The preparation of calcium carbonate with different morphologies under the effect of alkanolamide. Colloid. Surface. A 2020, 588, 124392. [Google Scholar] [CrossRef]
- Cizer, O.; Rodriguez-Navarro, C.; Ruiz-Agudo, E.; Elsen, R.J.; Van Gemert, D.; Van Balen, K. Phase and morphology evolution of calcium carbonate precipitated by carbonation of hydrated lime. J. Mater. Sci. 2012, 47, 6151–6165. [Google Scholar] [CrossRef] [Green Version]
- Hai, J.; Yang, J.X.; Cheng, J.; Yang, Z.R. Preparation and Performance of RTV Coating for Anti-pollution Flashover with Superhydrophobicity by Filling CaCO3/SiO2 Composite Particles into Silicone Rubber. Adv. Mater. Res. 2011, 328, 1263–1267. [Google Scholar] [CrossRef]
- Zhu, A.; Wang, H.; Sun, S.; Zhang, C. The synthesis and antistatic, anticorrosive properties of polyaniline composite coating. Prog. Org. Coat. 2018, 122, 270–279. [Google Scholar] [CrossRef]
- Zhang, Y.; Chu, W.; Zhou, Q.; Li, S.; Li, N.; Zheng, J. Electrochemical behaviors of polyaniline confined in highly ordered micro-sized SiO2 cavities. Electroanal. Chem. 2016, 775, 105–109. [Google Scholar] [CrossRef]
- Kim, K.N.; Kim, Y.D. Synthesis of Calcium Carbonate Particles Modified by Coating with Nickel Precursor. Mater. Sci. Forum 2005, 486, 546–549. [Google Scholar] [CrossRef]
- Huang, G.; Kang, W.; Xing, B.; Chen, L.; Zhang, C. Oxygen-rich and hierarchical porous carbons prepared from coal based humic acid for supercapacitor electrodes. Fuel Process. Technol. 2016, 142, 1–5. [Google Scholar] [CrossRef]
- Jimoh, O.A.; Ariffin, K.S.; Bin Hussin, H.; Temitope, A.E. Synthesis of precipitated calcium carbonate: A review. Carbonates Evaporites 2018, 33, 331–346. [Google Scholar] [CrossRef]
- Lan, T.; Wu, P.; Liu, Z.; Stroet, M.; Liao, J.; Chai, Z.; Mark, A.E.; Liu, N.; Wang, D. Understanding the Effect of pH on the Solubility and Aggregation Extent of Humic Acid in Solution by Combining Simulation and the Experiment. Environ. Sci. Technol. 2022, 56, 917–927. [Google Scholar] [CrossRef]
- Costa, V.L.D.; Simoes, R.M.S. Hydrophobicity improvement of cellulose nanofibrils films by stearic acid and modified precipitated calcium carbonate coating. J. Mater. Sci. 2022, 57, 11443–11459. [Google Scholar] [CrossRef]


| Chemical Element | Mass Percentage of Chemical Element (%) | Oxide Component | Mass Percentage of Oxide Component (%) |
|---|---|---|---|
| Ca | 57.45 | CaO | 50.35 |
| O | 22.01 | CO2 | 47.09 |
| C | 17.97 | P2O5 | 0.95 |
| Cl | 0.84 | Cl | 0.61 |
| P | 0.56 | K2O | 0.29 |
| Sr | 0.36 | SrO | 0.21 |
| K | 0.34 | SO3 | 0.15 |
| Na | 0.12 | Na2O | 0.12 |
| Zn | 0.12 | ZnO | 0.07 |
| Fe | 0.09 | SiO2 | 0.07 |
| S | 0.08 | Fe2O3 | 0.06 |
| Si | 0.04 | Al2O3 | 0.03 |
| Al | 0.02 | - | - |
| Total | 100.00 | Total | 100.00 |
| Functional Materials | Resistivity/Ω·cm | Conductivity/S·cm−1 | References |
|---|---|---|---|
| CNCC | 2.62 × 106 | 3.8 × 10−7 | This study |
| CaCO3 | >107 | <10−7 | [39] |
| CaCO3-SiO2 | >107 | <10−7 | [39] |
| Polymers | 1015~1017 | 10−15~10−17 | [15] |
| Pure polyaniline (PANI) | 2.63 × 102 | 3.8 × 10−1 | [40] |
| CaCO3-SiO2-PANI (1:1~3:1) | 1.79 × 103~1.75 × 104 | 5.6 × 10−2~5.7 × 10−3 | [41] |
| PANI/CaCO3 composites | 1.0 × 105 | 1.0 × 10−5 | [26] |
| CaCO3-Ni (0.47:1~1:1) | 1.2 × 106~3.4 × 1011 | 8.3 × 10−7~2.9 × 10−12 | [42] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, F.; Xiao, H.; Huang, F.; Zhu, H.; Lei, J.; Ma, J. Preliminary Study on the Preparation of Conductive Nanosized Calcium Carbonate Utilizing Biogas Slurry by a Synchronous Double Decomposition Coating Method. Nanomaterials 2023, 13, 1938. https://doi.org/10.3390/nano13131938
Pan F, Xiao H, Huang F, Zhu H, Lei J, Ma J. Preliminary Study on the Preparation of Conductive Nanosized Calcium Carbonate Utilizing Biogas Slurry by a Synchronous Double Decomposition Coating Method. Nanomaterials. 2023; 13(13):1938. https://doi.org/10.3390/nano13131938
Chicago/Turabian StylePan, Fanghui, Han Xiao, Fei Huang, Hongguang Zhu, Jingjing Lei, and Jie Ma. 2023. "Preliminary Study on the Preparation of Conductive Nanosized Calcium Carbonate Utilizing Biogas Slurry by a Synchronous Double Decomposition Coating Method" Nanomaterials 13, no. 13: 1938. https://doi.org/10.3390/nano13131938





