Recent Advances of Triglyceride Catalytic Pyrolysis via Heterogenous Dolomite Catalyst for Upgrading Biofuel Quality: A Review
Abstract
1. Introduction
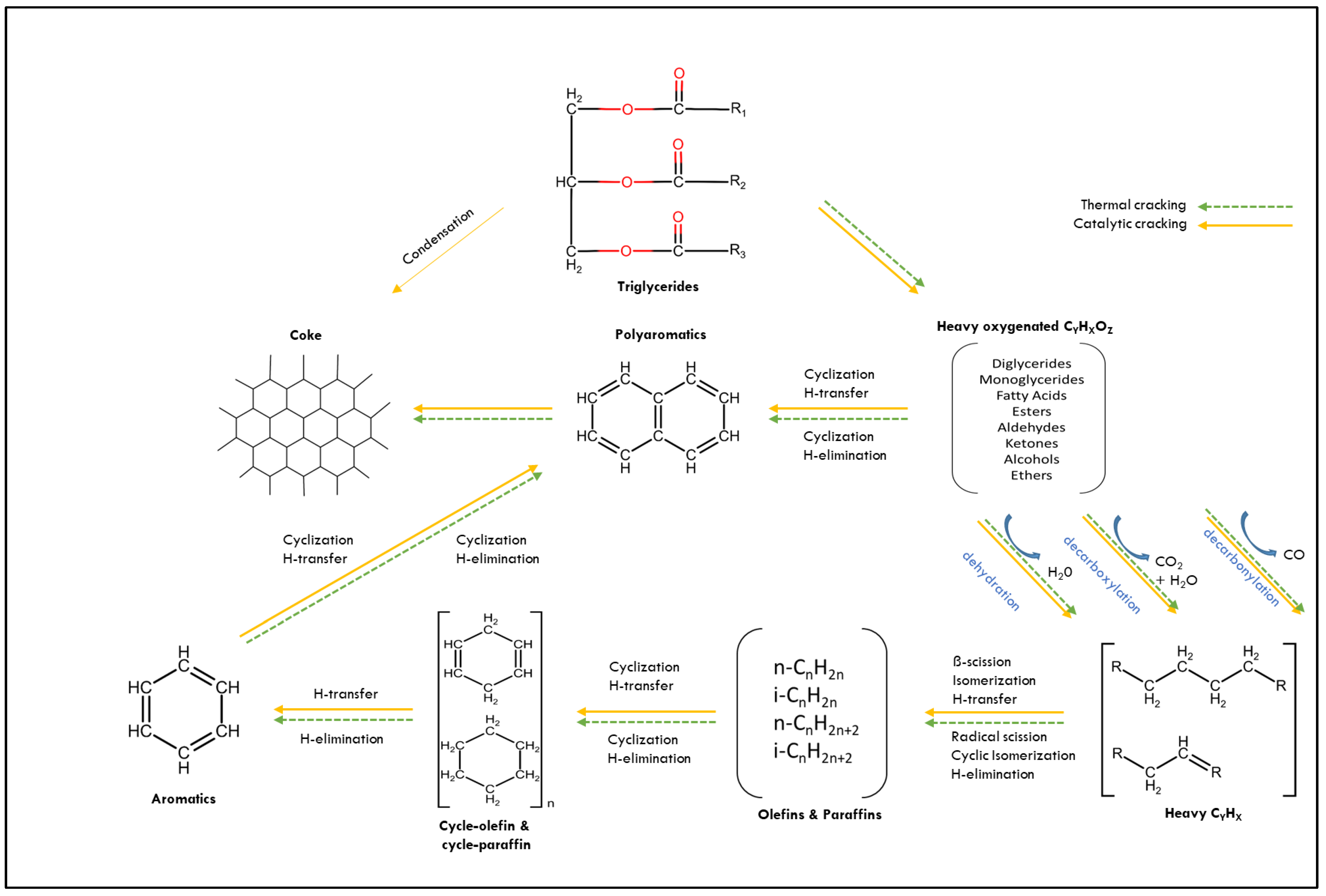
2. Catalysis in Catalytic Pyrolysis of Triglyceride Feedstocks
| Feedstock | Condition | Catalyst | Reactor | Product/Yield | References |
|---|---|---|---|---|---|
| Sunflower Oil | 330–380 °C WHSV: 0.66/h | Vanadium pentoxide/1–2 wt% | Fixed-fluidized bed | Hydrocarbon (89.6–90.2 wt%) Gas (9.2–11.2 wt%) Coke (2–4 wt%) | [61] |
| Palm Oil | 450 °C WHSV: 1.6–4.58/h | Na2CO3 | Stirred sludge bed reactor | Hydrocarbon (61–88 wt%) Oxygenates (11.9–38.9 wt%) Coke (2–4 wt%) | [62] |
| Canola Oil | 375–500 °C WHSV: 2–4/h | HZSM-5 | Fixed bed tubular reactor | Hydrocarbon (74.9–93.6 wt%) Aromatic (0.66 to 1.7 wt%) | [63] |
| Woody Oil | 480 °C Catalyst: 5% Flow rate: 50 g/h | CaO | Glass Vessel Reactor | Gasoline, Kerosene, Diesel (77%) | [64] |
| Soybean Oil | 500 °C WHSV: 0.01/h | ZSM-5 | Fixed bed reactor | Hydrocarbon (3–54%) Coke (30%) | [65] |
| Waste Cooking Oil | 400 °C 1.5 h Heating rate: 20 °C/min | K2O/Ba-MCM-41 | Pyrolysis Reactor | Cracking oil | [66] |
| Triolein | 380 °C 0.5 g catalyst 2 h | Ni/HMS | Glass made reactor | 95% of diesel range (C11–C20) | [67] |
| Used Vegetable Oil (UVO) | 400 °C Catalyst: 1 g 3 h WHSV: 4/h | Ni, Mo, NiMo functionalized zeolite | Fixed bed reactor | Conversion to hydrocarbons: palmitic acid (84.72%) and stearic acid (74.10%) | [68] |
| Waste Cooking Oil | 400–600 °C 2 g catalyst | Ni/Corn Activated Carbon (AC) | Fixed bed reactor | Hydrocarbons (68.47–86.38%) Syngas (CO and H2) (70%) | [69] |
| Jatropha Oil | 600 °C | Ni2P/Zr-SBA-15 | High-pressure fixed bed reactor | C3 to C14 (52.92 wt%) C15 (12.93 wt%) C16 (4.56 wt%) C17 (22.27 wt%) C18 (3.01 wt%) C19 to C25(4.32 wt%) | [70] |
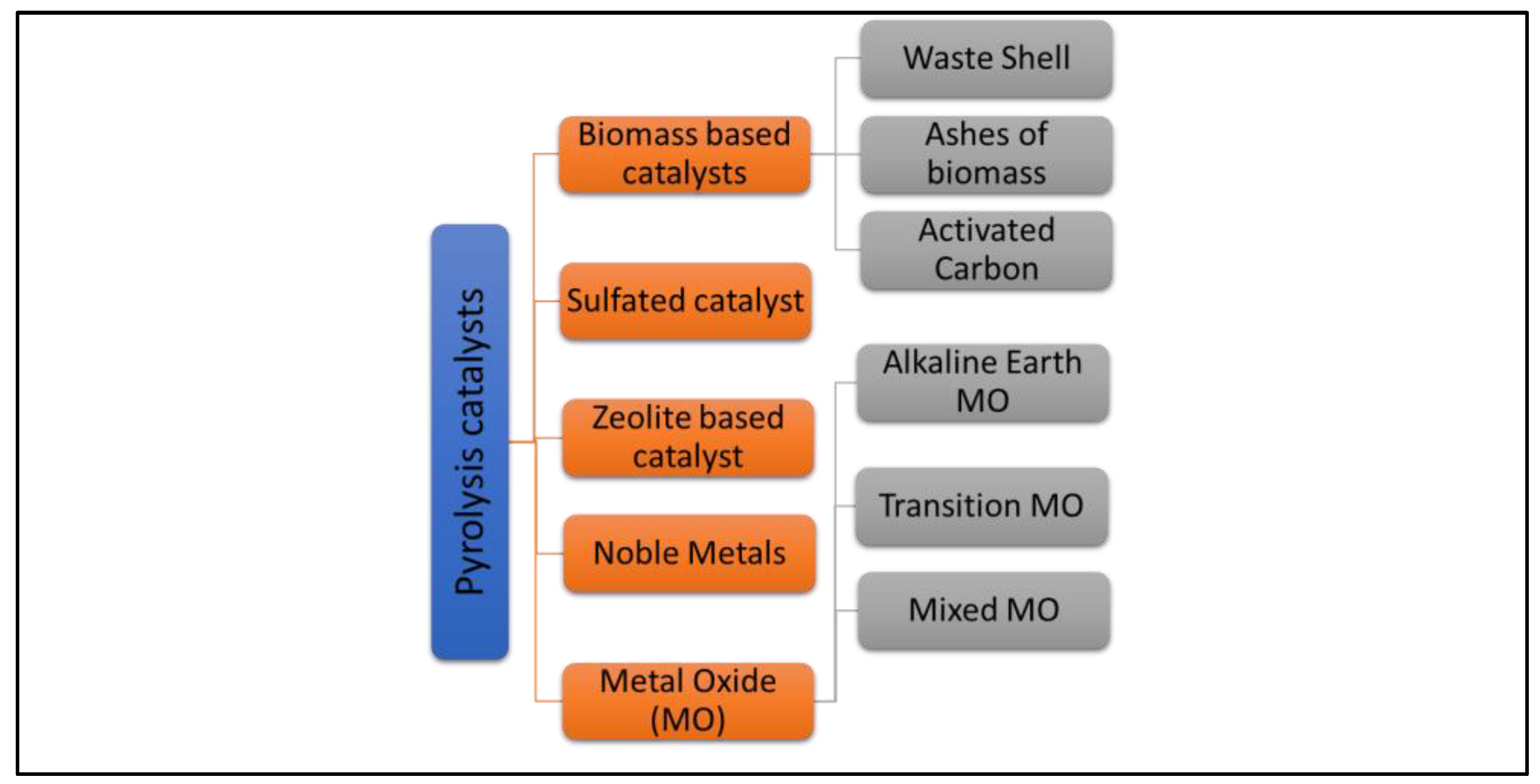
3. Dolomite in Catalytic Pyrolysis
3.1. Raw Dolomite Catalyst
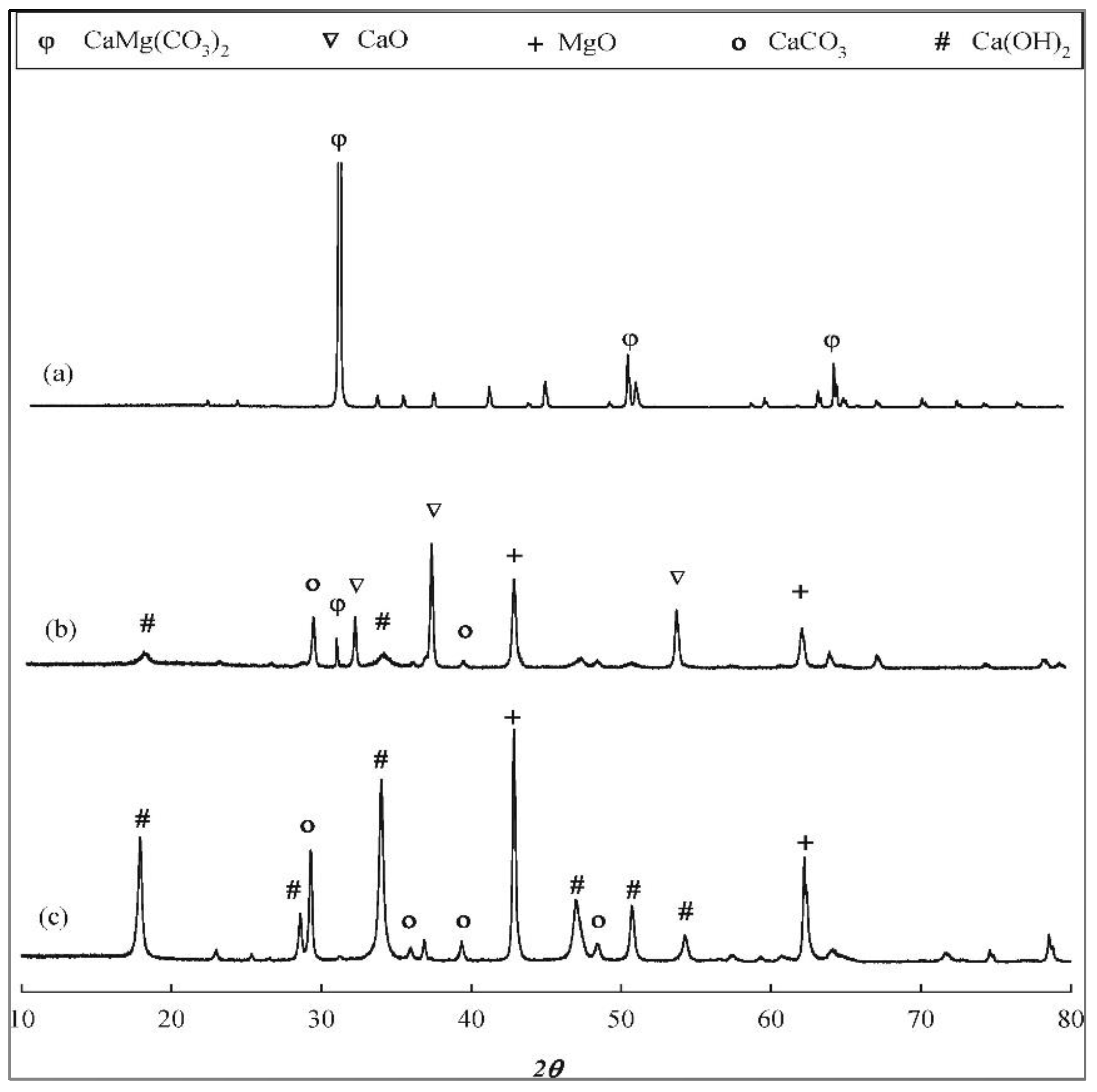
3.2. Thermal-Activated (Calcined) Dolomite Catalysts
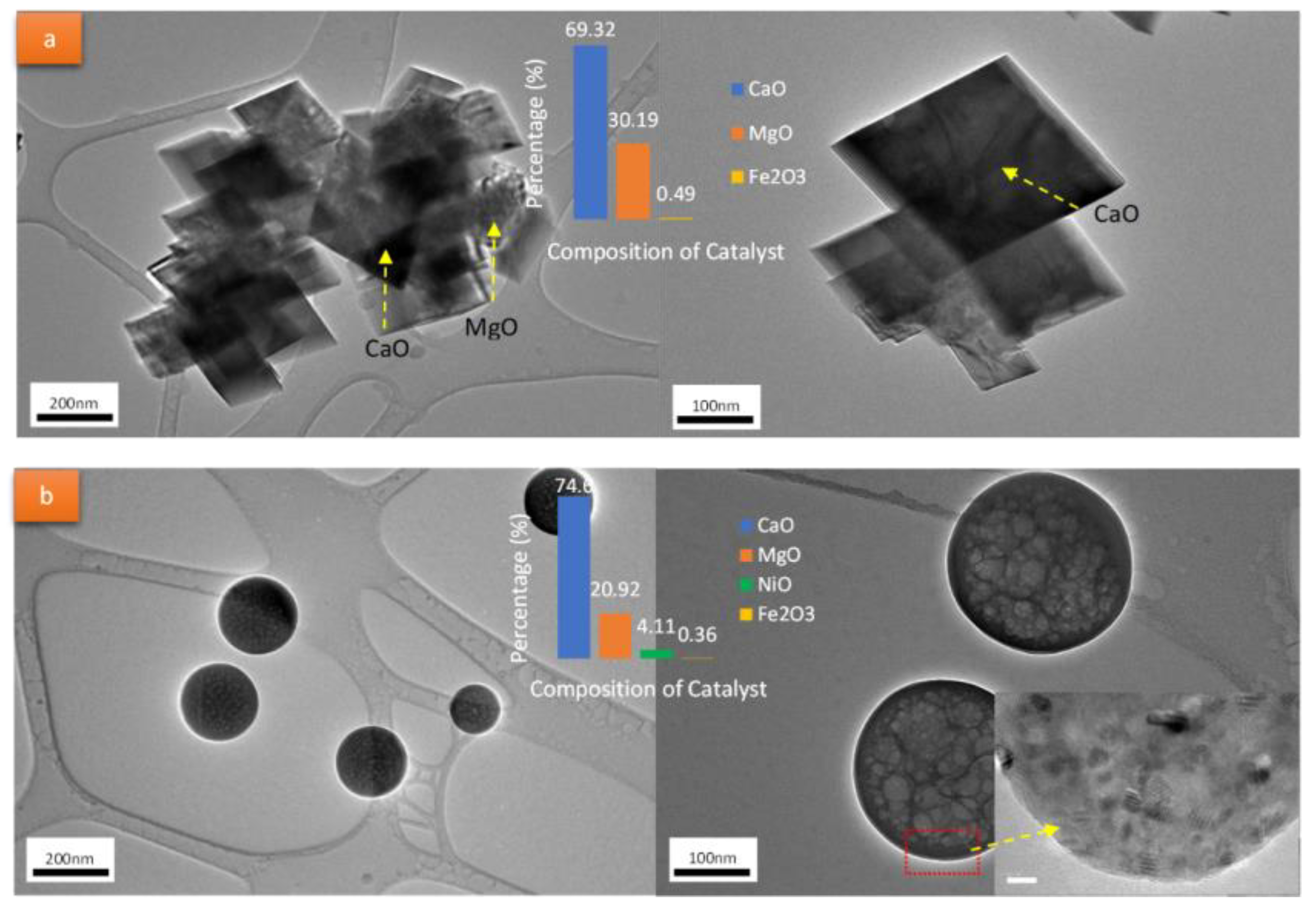
3.3. Metal-Activated Dolomite Catalysts
4. Dolomite Catalyst Key Elements for Upgrading Biofuel Quality
4.1. Catalyst Structure, Surface Area, and Porosity
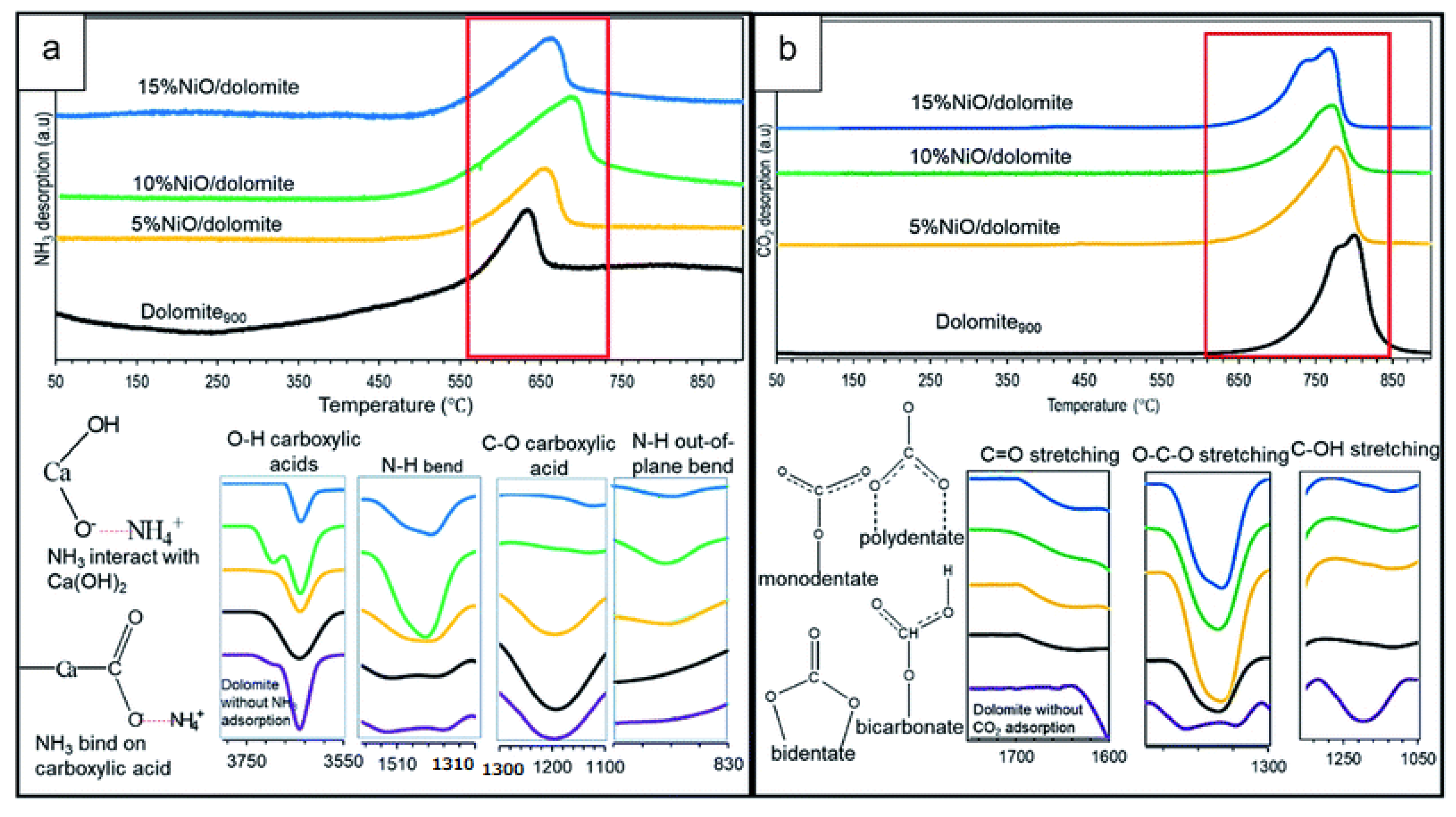
4.2. Catalyst Bifunctional Acid–Base Properties
4.3. Catalyst Activity and Selectivity
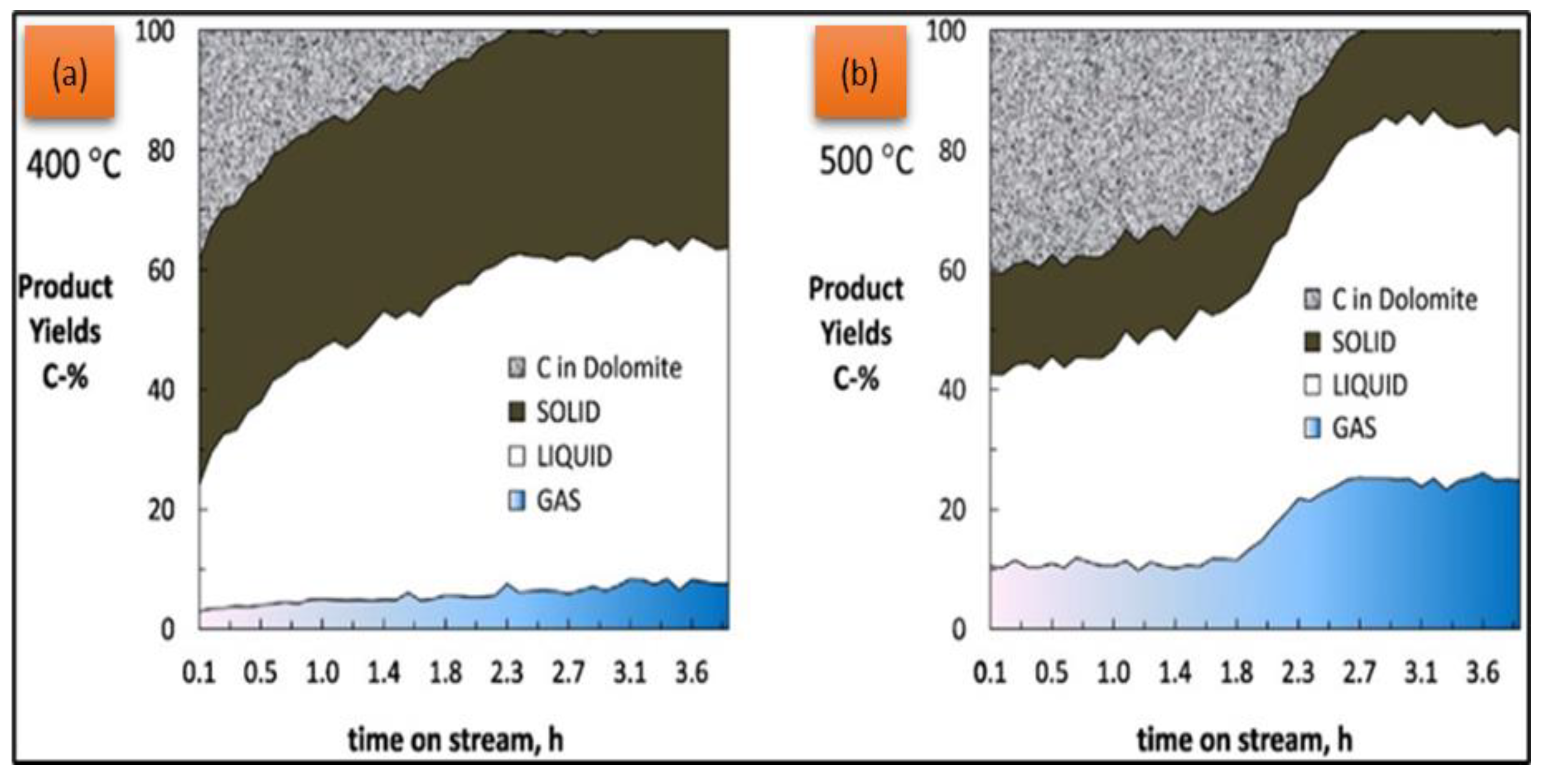
4.4. Catalyst Stability
5. Challenges and Future Direction
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guo, M.X.; Song, W.P.; Buhain, J. Bioenergy and biofuels: History, status, and perspective. Renew. Sust. Energ. Rev. 2015, 42, 712–725. [Google Scholar] [CrossRef]
- Ramos, M.; Dias, A.P.; Puna, J.F.; Gomes, J.; Bordado, J.C. Biodiesel production processes and sustainable raw materials. Energies 2019, 12, 4408. [Google Scholar] [CrossRef]
- Catarino, M.; Martins, S.; Soares Dias, A.P.; Costa Pereira, M.F.; Gomes, J. Calcium diglyceroxide as a catalyst for biodiesel production. J. Environ. Chem. Eng. 2019, 7, 103099. [Google Scholar] [CrossRef]
- Razeghi, M.; Hajinezhad, A.; Naseri, A.; Noorollahi, Y.; Moosavian, S.F. An overview of renewable energy technologies for the simultaneous production of high-performance power and heat. Future Energy 2023, 2, 1–11. [Google Scholar]
- Hosseini, S.E. Transition away from fossil fuels toward renewables: Lessons from Russia-Ukraine crisis. Future Energy 2022, 1, 2–5. [Google Scholar] [CrossRef]
- Serrano, D.P.; Melero, J.A.; Morales, G.; Iglesias, J.; Pizarro, P. Progress in the design of zeolite catalysts for biomass conversion into biofuels and bio-based chemicals. Catal. Rev. 2018, 60, 1–70. [Google Scholar] [CrossRef]
- Mardiana, S.; Azhari, N.J.; Ilmi, T.; Kadja, G.T.M. Hierarchical zeolite for biomass conversion to biofuel: A review. Fuel 2022, 309, 122119. [Google Scholar] [CrossRef]
- Bridgwater, A.V. Review of fast pyrolysis of biomass and product upgrading. Biomass Bioenergy 2012, 38, 68–94. [Google Scholar] [CrossRef]
- Hossain, N.; Hasan, M.H.; Mahlia, T.M.I.; Shamsuddin, A.H.; Silitonga, A.S. Feasibility of microalgae as feedstock for alternative fuel in Malaysia: A review. Energy Strategy Rev. 2020, 32, 100536. [Google Scholar] [CrossRef]
- Gholkar, P.; Shastri, Y.; Tanksale, A. Renewable hydrogen and methane production from microalgae: A techno-economic and life cycle assessment study. J. Clean. Prod. 2021, 279, 123726. [Google Scholar] [CrossRef]
- Kim, T.-H.; Lee, K.; Oh, B.-R.; Lee, M.-E.; Seo, M.; Li, S.; Kim, J.-K.; Choi, M.; Chang, Y.K. A novel process for the coproduction of biojet fuel and high-value polyunsaturated fatty acid esters from heterotrophic microalgae Schizochytrium sp. ABC101. Renew. Energy 2021, 165, 481–490. [Google Scholar] [CrossRef]
- Brennan, L.; Owende, P. Biofuels from microalgae-A review of technologies for production, processing, and extractions of biofuels and co-products. Renew. Sust. Energ. Rev. 2010, 14, 557–577. [Google Scholar] [CrossRef]
- Kumar, A.; Anushree; Kumar, J.; Bhaskar, T. Utilization of lignin: A sustainable and eco-friendly approach. J. Energy Inst. 2020, 93, 235–271. [Google Scholar] [CrossRef]
- Ragauskas, A.J.; Beckham, G.T.; Biddy, M.J.; Chandra, R.; Chen, F.; Davis, M.F.; Davison, B.H.; Dixon, R.A.; Gilna, P.; Keller, M.; et al. Lignin Valorization: Improving Lignin Processing in the Biorefinery. Science 2014, 344, 1246843. [Google Scholar] [CrossRef] [PubMed]
- Kumari, D.; Singh, R. Pretreatment of lignocellulosic wastes for biofuel production: A critical review. Renew. Sust. Energ. Rev. 2018, 90, 877–891. [Google Scholar] [CrossRef]
- Chen, W.H.; Peng, J.H.; Bi, X.T.T. A state-of-the-art review of biomass torrefaction, densification and applications. Renew. Sust. Energ. Rev. 2015, 44, 847–866. [Google Scholar] [CrossRef]
- Rajanna, K.C.; Krishnaiah, G.; Pasnoori, S.; Santhoshi, P.S.; Rajeshwer Rao, Y.; Rao Patnaik, K.S.K. Ultrasonic and microwave effects on Prussian blue catalysed high-quality biodiesel production using Watermelon (Citrullus vulgaris) seed oil and alcohol extract (from fibrous flesh) as an exclusive green feedstock. Biofuels 2021, 12, 597–603. [Google Scholar] [CrossRef]
- Kayode, B.; Hart, A. An overview of transesterification methods for producing biodiesel from waste vegetable oils. Biofuels 2019, 10, 419–437. [Google Scholar] [CrossRef]
- Chu, S.; Majumdar, A. Opportunities and challenges for a sustainable energy future. Nature 2012, 488, 294–303. [Google Scholar] [CrossRef]
- Lampridi, M.; Kateris, D. Chapter 1—Primary production sustainability. In Bio-Economy and Agri-Production; Bochtis, D., Achillas, C., Banias, G., Lampridi, M., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 3–30. [Google Scholar] [CrossRef]
- Bond, T.C.; Doherty, S.J.; Fahey, D.W.; Forster, P.M.; Berntsen, T.; DeAngelo, B.J.; Flanner, M.G.; Ghan, S.; Karcher, B.; Koch, D.; et al. Bounding the role of black carbon in the climate system: A scientific assessment. J. Geophys. Res.-Atmos. 2013, 118, 5380–5552. [Google Scholar] [CrossRef]
- Long, F.; Liu, W.; Jiang, X.; Zhai, Q.; Cao, X.; Jiang, J.; Xu, J. State-of-the-art technologies for biofuel production from triglycerides: A review. Renew. Sustain. Energy Rev. 2021, 148, 111269. [Google Scholar] [CrossRef]
- Thanh, N.T.; Mostapha, M.; Lam, M.K.; Ishak, S.; Dasan, Y.K.; Lim, J.W.; Tan, I.S.; Lau, S.Y.; Chin, B.L.F.; Hadibarata, T.; et al. Fundamental understanding of in-situ transesterification of microalgae biomass to biodiesel: A critical review. Energy Convers. Manag. 2022, 270, 116212. [Google Scholar] [CrossRef]
- Umar, Y.; Aboelazayem, O.; Gadalla, M.A.; Saha, B. Enhanced biodiesel production with improved oxidation stability by water addition to supercritical methanolysis. Can. J. Chem. Eng. 2022, 100, 2587–2607. [Google Scholar] [CrossRef]
- Musthafa, M.M.; Kumar, T.A.; Mohanraj, T.; Chandramouli, R. A comparative study on performance, combustion and emission characteristics of diesel engine fuelled by biodiesel blends with and without an additive. Fuel 2018, 225, 343–348. [Google Scholar] [CrossRef]
- Lapuerta, M.N.; Hernández, J.J.; Rodríguez-Fernández, J.; Calle-Asensio, A. Vehicle emissions from a glycerol-derived biofuel under cold and warm conditions. Energy Fuels 2020, 34, 6020–6029. [Google Scholar] [CrossRef]
- Singh, D.; Sharma, D.; Soni, S.; Inda, C.S.; Sharma, S.; Sharma, P.K.; Jhalani, A. A comprehensive review of biodiesel production from waste cooking oil and its use as fuel in compression ignition engines: 3rd generation cleaner feedstock. J. Clean. Prod. 2021, 307, 127299. [Google Scholar] [CrossRef]
- Sadaf, S.; Iqbal, J.; Ullah, I.; Bhatti, H.N.; Nouren, S.; Nisar, J.; Iqbal, M. Biodiesel production from waste cooking oil: An efficient technique to convert waste into biodiesel. Sustain. Cities Soc. 2018, 41, 220–226. [Google Scholar]
- Zhang, Y.; Alvarez-Majmutov, A. Production of Renewable Liquid Fuels by Coprocessing HTL Biocrude Using Hydrotreating and Fluid Catalytic Cracking. Energy Fuels 2021, 35, 19535–19542. [Google Scholar] [CrossRef]
- Bozell, J.J.; Petersen, G.R. Technology development for the production of biobased products from biorefinery carbohydrates-the US Department of Energy’s “Top 10” revisited. Green Chem. 2010, 12, 539–554. [Google Scholar] [CrossRef]
- Aslam, M.; Kumar, H.; Sarma, A.K.; Kumar, P. Current Status of the Green Diesel Industry. In Green Diesel: An Alternative to Biodiesel and Petrodiesel; Springer: Berlin/Heidelberg, Germany, 2022; pp. 265–283. [Google Scholar]
- Tuli, D.; Kasture, S. Chapter 5—Biodiesel and green diesel. In Advanced Biofuel Technologies; Tuli, D., Kasture, S., Kuila, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 119–133. [Google Scholar] [CrossRef]
- Mohan, S.V.; Nikhil, G.N.; Chiranjeevi, P.; Reddy, C.N.; Rohit, M.V.; Kumar, A.N.; Sarkar, O. Waste biorefinery models towards sustainable circular bioeconomy: Critical review and future perspectives. Bioresour. Technol. 2016, 215, 2–12. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Q.; Ke, L.; Peng, Y.; Liu, Y.; Wu, Q.; Tian, X.; Dai, L.; Ruan, R.; Jiang, L. Review on the catalytic pyrolysis of waste oil for the production of renewable hydrocarbon fuels. Fuel 2021, 283, 119170. [Google Scholar] [CrossRef]
- Long, F.; Zhai, Q.; Liu, P.; Cao, X.; Jiang, X.; Wang, F.; Wei, L.; Liu, C.; Jiang, J.; Xu, J. Catalytic conversion of triglycerides by metal-based catalysts and subsequent modification of molecular structure by ZSM-5 and Raney Ni for the production of high-value biofuel. Renew. Energy 2020, 157, 1072–1080. [Google Scholar] [CrossRef]
- Hassan, N.; Jalil, A.; Hitam, C.; Vo, D.; Nabgan, W. Biofuels and renewable chemicals production by catalytic pyrolysis of cellulose: A review. Environ. Chem. Lett. 2020, 18, 1625–1648. [Google Scholar] [CrossRef]
- Rahimi, M.; Tajmirriahi, M.; Saadatinavaz, F.; Lam, S.S. Esterification/Transesterification of Lipidic Wastes for Biodiesel Production. In Waste-to-Energy; Springer: Berlin/Heidelberg, Germany, 2022; pp. 227–273. [Google Scholar]
- Di Luca, G.; Pipicelli, M.; Ianniello, R.; Belgiorno, G.; Di Blasio, G. Alcohol Fuels in Spark Ignition Engines. In Application of Clean Fuels in Combustion Engines; Di Blasio, G., Agarwal, A.K., Belgiorno, G., Shukla, P.C., Eds.; Springer Nature: Singapore, 2022; pp. 33–54. [Google Scholar] [CrossRef]
- Sahu, T.K.; Shukla, P.C.; Belgiorno, G.; Maurya, R.K. Alcohols as alternative fuels in compression ignition engines for sustainable transportation: A review. Energy Sources Part A Recovery Util. Environ. Eff. 2022, 44, 8736–8759. [Google Scholar] [CrossRef]
- Agarwal, A.K. Biofuels (alcohols and biodiesel) applications as fuels for internal combustion engines. Prog. Energy Combust. Sci. 2007, 33, 233–271. [Google Scholar] [CrossRef]
- Agarwal, D.; Sinha, S.; Agarwal, A.K. Experimental investigation of control of NOx emissions in biodiesel-fueled compression ignition engine. Renew. Energy 2006, 31, 2356–2369. [Google Scholar] [CrossRef]
- Nayab, R.; Imran, M.; Ramzan, M.; Tariq, M.; Taj, M.B.; Akhtar, M.N.; Iqbal, H.M. Sustainable biodiesel production via catalytic and non-catalytic transesterification of feedstock materials–A review. Fuel 2022, 328, 125254. [Google Scholar] [CrossRef]
- Bohlouli, A.; Mahdavian, L. Catalysts used in biodiesel production: A review. Biofuels 2021, 12, 885–898. [Google Scholar] [CrossRef]
- Hsiao, M.-C.; Kuo, J.-Y.; Hsieh, S.-A.; Hsieh, P.-H.; Hou, S.-S. Optimized conversion of waste cooking oil to biodiesel using modified calcium oxide as catalyst via a microwave heating system. Fuel 2020, 266, 117114. [Google Scholar] [CrossRef]
- Ling, J.S.J.; Tan, Y.H.; Mubarak, N.M.; Kansedo, J.; Saptoro, A.; Nolasco-Hipolito, C. A review of heterogeneous calcium oxide based catalyst from waste for biodiesel synthesis. SN Appl. Sci. 2019, 1, 810. [Google Scholar] [CrossRef]
- Marwaha, A.; Dhir, A.; Mahla, S.K.; Mohapatra, S.K. An overview of solid base heterogeneous catalysts for biodiesel production. Catal. Rev. 2018, 60, 594–628. [Google Scholar] [CrossRef]
- Jayakumar, M.; Karmegam, N.; Gundupalli, M.P.; Gebeyehu, K.B.; Asfaw, B.T.; Chang, S.W.; Ravindran, B.; Awasthi, M.K. Heterogeneous base catalysts: Synthesis and application for biodiesel production–A review. Bioresour. Technol. 2021, 331, 125054. [Google Scholar] [CrossRef]
- Niu, S.; Zhang, X.; Ning, Y.; Zhang, Y.; Qu, T.; Hu, X.; Gong, Z.; Lu, C. Dolomite incorporated with cerium to enhance the stability in catalyzing transesterification for biodiesel production. Renew. Energy 2020, 154, 107–116. [Google Scholar] [CrossRef]
- Mishra, R.K.; Mohanty, K. Pyrolysis kinetics and thermal behavior of waste sawdust biomass using thermogravimetric analysis. Bioresour. Technol. 2018, 251, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Sankaran, R.; Chew, K.W.; Tan, C.H.; Krishnamoorthy, R.; Chu, D.T.; Show, P.L. Waste to bioenergy: A review on the recent conversion technologies. BMC Energy 2019, 1, 4. [Google Scholar] [CrossRef]
- Islam, M.W. A review of dolomite catalyst for biomass gasification tar removal. Fuel 2020, 267, 117095. [Google Scholar] [CrossRef]
- Hafriz, R.S.R.M.; Shafizah, I.N.; Arifin, N.A.; Salmiaton, A.; Yunus, R.; Yap, Y.H.T.; Shamsuddin, A.H. Effect of Ni/Malaysian dolomite catalyst synthesis technique on deoxygenation reaction activity of waste cooking oil. Renew. Energy 2021, 178, 128–143. [Google Scholar] [CrossRef]
- Trabelsi, A.B.H.; Zaafouri, K.; Baghdadi, W.; Naoui, S.; Ouerghi, A. Second generation biofuels production from waste cooking oil via pyrolysis process. Renew. Energy 2018, 126, 888–896. [Google Scholar] [CrossRef]
- Mohamed, M.; Tan, C.-K.; Fouda, A.; Gad, M.S.; Abu-Elyazeed, O.; Hashem, A.-F. Diesel engine performance, emissions and combustion characteristics of biodiesel and its blends derived from catalytic pyrolysis of waste cooking oil. Energies 2020, 13, 5708. [Google Scholar] [CrossRef]
- Martinez-Villarreal, S.; Breitenstein, A.; Nimmegeers, P.; Saura, P.P.; Hai, B.; Asomaning, J.; Eslami, A.A.; Billen, P.; Van Passel, S.; Bressler, D.C.; et al. Drop-in biofuels production from microalgae to hydrocarbons: Microalgal cultivation and harvesting, conversion pathways, economics and prospects for aviation. Biomass Bioenergy 2022, 165, 106555. [Google Scholar] [CrossRef]
- Amenaghawon, A.N.; Anyalewechi, C.L.; Okieimen, C.O.; Kusuma, H.S. Development; Sustainability. Biomass pyrolysis technologies for value-added products: A state-of-the-art review. Environ. Dev. Sustain. 2021, 23, 14324–14378. [Google Scholar] [CrossRef]
- Bartoli, M.; Giorcelli, M.; Vigliaturo, R.; Jagdale, P.; Rovere, M.; Tagliaferro, A. Bio-derived and Waste Fats Use for the Production of Drop-In Fuels. In Clean Fuels for Mobility; Springer: Berlin/Heidelberg, Germany, 2022; pp. 125–139. [Google Scholar]
- Zakzeski, J.; Bruijnincx, P.C.A.; Jongerius, A.L.; Weckhuysen, B.M. The Catalytic Valorization of Lignin for the Production of Renewable Chemicals. Chem. Rev. 2010, 110, 3552–3599. [Google Scholar] [CrossRef] [PubMed]
- Zamri, M.F.M.A.; Milano, J.; Shamsuddin, A.H.; Roslan, M.E.M.; Salleh, S.F.; Rahman, A.A.; Bahru, R.; Fattah, I.M.R.; Mahlia, T.M.I. An overview of palm oil biomass for power generation sector decarbonization in Malaysia: Progress, challenges, and prospects. WIREs Energy Environ. 2022, 11, e437. [Google Scholar] [CrossRef]
- Leng, T.Y.; Mohamed, A.R.; Bhatia, S. Catalytic conversion of palm oil to fuels and chemicals. Can. J. Chem. Eng. 1999, 77, 156–162. [Google Scholar] [CrossRef]
- Yigezu, Z.D.; Muthukumar, K. Biofuel production by catalytic cracking of sunflower oil using vanadium pentoxide. J. Anal. Appl. Pyrolysis 2015, 112, 341–347. [Google Scholar] [CrossRef]
- Mancio, A.A.; da Costa, K.M.B.; Ferreira, C.C.; Santos, M.C.; Lhamas, D.E.L.; da Mota, S.A.P.; Leão, R.A.C.; de Souza, R.O.M.A.; Araújo, M.E.; Borges, L.E.P.; et al. Thermal catalytic cracking of crude palm oil at pilot scale: Effect of the percentage of Na2CO3 on the quality of biofuels. Ind. Crops Prod. 2016, 91, 32–43. [Google Scholar] [CrossRef]
- Bayat, A.; Sadrameli, S.M. Conversion of canola oil and canola oil methyl ester (CME) to green aromatics over a HZSM-5 catalyst: A comparative study. RSC Adv. 2015, 5, 28360–28368. [Google Scholar] [CrossRef]
- Xu, J.; Jiang, J.; Chen, J.; Sun, Y. Biofuel production from catalytic cracking of woody oils. Bioresour. Technol. 2010, 101, 5586–5591. [Google Scholar] [CrossRef]
- Ishihara, A.; Tsukamoto, T.; Hashimoto, T.; Nasu, H. Catalytic cracking of soybean oil by ZSM-5 zeolite-containing silica-aluminas with three layered micro-meso-meso-structure. Catal. Today 2018, 303, 123–129. [Google Scholar] [CrossRef]
- Li, L.; Quan, K.; Xu, J.; Liu, F.; Liu, S.; Yu, S.; Xie, C.; Zhang, B.; Ge, X. Liquid Hydrocarbon Fuels from Catalytic Cracking of Waste Cooking Oils Using Basic Mesoporous Molecular Sieves K2O/Ba-MCM-41 as Catalysts. ACS Sustain. Chem. Eng. 2013, 1, 1412–1416. [Google Scholar] [CrossRef]
- Zulkepli, S.; Juan, J.C.; Lee, H.V.; Rahman, N.S.A.; Show, P.L.; Ng, E.P. Management. Modified mesoporous HMS supported Ni for deoxygenation of triolein into hydrocarbon-biofuel production. Energy Convers. Manag. 2018, 165, 495–508. [Google Scholar] [CrossRef]
- Majed, M.A.A.; Tye, C.T. Catalytic Cracking Of Used Vegetable Oil To Green Fuel With Metal Functionalized Zsm-5 Catalysts. Malays. J. Anal. Sci. 2018, 22, 8–16. [Google Scholar]
- Li, P.; Niu, B.; Pan, H.; Zhang, Y.; Long, D. Production of hydrocarbon-rich bio-oil from catalytic pyrolysis of waste cooking oil over nickel monoxide loaded corn cob-derived activated carbon. J. Clean. Prod. 2022, 384, 135653. [Google Scholar] [CrossRef]
- Tan, Q.; Cao, Y.; Li, J. Prepared multifunctional catalyst Ni2P/Zr-SBA-15 and catalyzed Jatropha Oil to produce bio-aviation fuel. Renew. Energy 2020, 150, 370–381. [Google Scholar] [CrossRef]
- Dupain, X.; Costa, D.J.; Schaverien, C.J.; Makkee, M.; Moulijn, J.A. Cracking of a rapeseed vegetable oil under realistic FCC conditions. Appl. Catal. B Environ. 2007, 72, 44–61. [Google Scholar] [CrossRef]
- Kissin, Y.V. Chemical mechanisms of catalytic cracking over solid acidic catalysts: Alkanes and alkenes. Catal. Rev. 2001, 43, 85–146. [Google Scholar] [CrossRef]
- Ganesan, R.; Narasimhalu, P.; Joseph, A.I.J.; Pugazhendhi, A. Synthesis of silver nanoparticle from X-ray film and its application in production of biofuel from jatropha oil. Int. J. Energy Res. 2021, 45, 17378–17388. [Google Scholar] [CrossRef]
- Naji, S.Z.; Tye, C.T.; Abd, A.A. State of the art of vegetable oil transformation into biofuels using catalytic cracking technology: Recent trends and future perspectives. Process Biochem. 2021, 109, 148–168. [Google Scholar] [CrossRef]
- Bhoi, P.; Ouedraogo, A.; Soloiu, V.; Quirino, R. Recent advances on catalysts for improving hydrocarbon compounds in bio-oil of biomass catalytic pyrolysis. Renew. Sustain. Energy Rev. 2020, 121, 109676. [Google Scholar] [CrossRef]
- Praserttaweeporn, K.; Vitidsant, T.; Charusiri, W. Ni-modified dolomite for the catalytic deoxygenation of pyrolyzed softwood and non-wood to produce bio-oil. Results Eng. 2022, 14, 100461. [Google Scholar] [CrossRef]
- Hafriz, R.S.R.M.; Nor Shafizah, I.; Salmiaton, A.; Arifin, N.A.; Yunus, R.; Taufiq Yap, Y.H.; Abd Halim, S. Comparative study of transition metal-doped calcined Malaysian dolomite catalysts for WCO deoxygenation reaction. Arab. J. Chem. 2020, 13, 8146–8159. [Google Scholar] [CrossRef]
- Oyama, S.T.; Gott, T.; Zhao, H.; Lee, Y.-K. Transition metal phosphide hydroprocessing catalysts: A review. Catal. Today 2009, 143, 94–107. [Google Scholar] [CrossRef]
- Priecel, P.; Kubička, D.; Vázquez-Zavala, A.; Antonio de Los Reyes, J.; Pouzar, M.; Čapek, L. Alternative Preparation of Improved NiMo-Alumina Deoxygenation Catalysts. Front Chem 2020, 8, 216. [Google Scholar] [CrossRef]
- Yao, L.; Wang, Y.; Gálvez, M.E.; Hu, C.; Costa, P.D. γ-Alumina-Supported Ni-Mo Carbides as Promising Catalysts for CO2 Methanation. Mod. Res. Catal. 2017, 6, 135–145. [Google Scholar] [CrossRef]
- Priecel, P.; Čapek, L.; Kubička, D.; Homola, F.; Ryšánek, P.; Pouzar, M. The role of alumina support in the deoxygenation of rapeseed oil over NiMo–alumina catalysts. Catal. Today 2011, 176, 409–412. [Google Scholar] [CrossRef]
- Kanchanatip, E.; Chansiriwat, W.; Palalerd, S.; Khunphonoi, R.; Kumsaen, T.; Wantala, K. Light biofuel production from waste cooking oil via pyrolytic catalysis cracking over modified Thai dolomite catalysts. Carbon Resour. Convers. 2022, 5, 177–184. [Google Scholar] [CrossRef]
- Correia, L.M.; de Sousa Campelo, N.; Novaes, D.S.; Cavalcante, C.L.; Cecilia, J.A.; Rodríguez-Castellón, E.; Vieira, R.S. Characterization and application of dolomite as catalytic precursor for canola and sunflower oils for biodiesel production. Chem. Eng. J. 2015, 269, 35–43. [Google Scholar] [CrossRef]
- Jaiyen, S.; Naree, T.; Ngamcharussrivichai, C. Comparative study of natural dolomitic rock and waste mixed seashells as heterogeneous catalysts for the methanolysis of palm oil to biodiesel. Renew. Energy 2015, 74, 433–440. [Google Scholar] [CrossRef]
- Ngamcharussrivichai, C.; Wiwatnimit, W.; Wangnoi, S. Modified dolomites as catalysts for palm kernel oil transesterification. J. Mol. Catal. A Chem. 2007, 276, 24–33. [Google Scholar] [CrossRef]
- Taufiq-Yap, Y.H.; Nur-Faizal, A.R.; Sivasangar, S.; Hussein, M.Z.; Aishah, A. Modification of Malaysian dolomite using mechanochemical treatment via different media for oil palm fronds gasification. Int. J. Energy Res. 2014, 38, 1008–1015. [Google Scholar] [CrossRef]
- Weber, J.; Thompson, A.; Wilmoth, J.; Batra, V.S.; Janulaitis, N.; Kastner, J.R. Effect of metal oxide redox state in red mud catalysts on ketonization of fast pyrolysis oil derived oxygenates. Appl. Catal. B Environ. 2019, 241, 430–441. [Google Scholar] [CrossRef]
- Kastner, J.R.; Hilten, R.; Weber, J.; McFarlane, A.R.; Hargreaves, J.S.J.; Batra, V.S. Continuous catalytic upgrading of fast pyrolysis oil using iron oxides in red mud. RSC Adv. 2015, 5, 29375–29385. [Google Scholar] [CrossRef]
- Zheng, Y.; Tao, L.; Huang, Y.; Liu, C.; Wang, Z.; Zheng, Z. Improving aromatic hydrocarbon content from catalytic pyrolysis upgrading of biomass on a CaO/HZSM-5 dual-catalyst. J. Anal. Appl. Pyrolysis 2019, 140, 355–366. [Google Scholar] [CrossRef]
- Buyang, Y.; Suprapto, S.; Nugraha, R.E.; Holilah, H.; Bahruji, H.; Hantoro, R.; Jalil, A.A.; Oetami, T.P.; Prasetyoko, D. Catalytic pyrolysis of Reutealis trisperma oil using raw dolomite for bio-oil production. J. Anal. Appl. Pyrolysis 2023, 169, 105852. [Google Scholar] [CrossRef]
- Duanguppama, K.; Pannucharoenwong, N.; Echaroj, S.; Pham, L.K.H.; Samart, C.; Rattanadecho, P. Integrated catalytic pyrolysis and catalytic upgrading of Leucaena leucocephala over natural catalysts. J. Energy Inst. 2023, 106, 101155. [Google Scholar] [CrossRef]
- Valle, B.; García-Gómez, N.; Remiro, A.; Gayubo, A.G.; Bilbao, J. Cost-effective upgrading of biomass pyrolysis oil using activated dolomite as a basic catalyst. Fuel Process. Technol. 2019, 195, 106142. [Google Scholar] [CrossRef]
- Ly, H.V.; Lim, D.-H.; Sim, J.W.; Kim, S.-S.; Kim, J. Catalytic pyrolysis of tulip tree (Liriodendron) in bubbling fluidized-bed reactor for upgrading bio-oil using dolomite catalyst. Energy 2018, 162, 564–575. [Google Scholar] [CrossRef]
- Charusiri, W.; Vitidsant, T. Upgrading bio-oil produced from the catalytic pyrolysis of sugarcane (Saccharum officinarum L) straw using calcined dolomite. Sustain. Chem. Pharm. 2017, 6, 114–123. [Google Scholar] [CrossRef]
- Hafriz, R.S.R.M.; Arifin, N.A.; Salmiaton, A.; Yunus, R.; Taufiq-Yap, Y.H.; Saifuddin, N.M.; Shamsuddin, A.H. Multiple-objective optimization in green fuel production via catalytic deoxygenation reaction with NiO-dolomite catalyst. Fuel 2022, 308, 122041. [Google Scholar] [CrossRef]
- Zhou, C.; Yrjas, P.; Engvall, K. Reaction mechanisms for H2O-enhanced dolomite calcination at high pressure. Fuel Process. Technol. 2021, 217, 106830. [Google Scholar] [CrossRef]
- Yahaya, M.; Ramli, I.; Muhamad, E.; Ishak, N.; Nda-Umar, U.; Taufiq-Yap, Y. K2O Doped Dolomite as Heterogeneous Catalyst for Fatty Acid Methyl Ester Production from Palm Oil. Catalysts 2020, 10, 791. [Google Scholar] [CrossRef]
- Asikin-Mijan, N.; Lee, H.V.; Taufiq-Yap, Y.H.; Juan, J.C.; Rahman, N.A. Pyrolytic–deoxygenation of triglyceride via natural waste shell derived Ca(OH)2 nanocatalyst. J. Anal. Appl. Pyrolysis 2016, 117, 46–55. [Google Scholar] [CrossRef]
- Kesica, Z.; Lukic, I.; Zdujic, M.; Liu, H.; Skala, D. Mechanochemically Synthesized CaO ZnO Catalyst For Biodiesel Production. Procedia Eng. 2012, 42, 1169–1178. [Google Scholar] [CrossRef]
- Wu, Q.; Ke, L.; Wang, Y.; Zhou, N.; Li, H.; Yang, Q.; Xu, J.; Dai, L.; Zou, R.; Liu, Y.; et al. Pulse pyrolysis of waste cooking oil over CaO: Exploration of catalyst deactivation pathway based on feedstock characteristics. Appl. Catal. B Environ. 2022, 304, 120968. [Google Scholar] [CrossRef]
- Raja Shahruzzaman, R.M.H.; Ali, S.; Yunus, R.; Taufiq-Yap, Y.H. Green Biofuel Production via Catalytic Pyrolysis of Waste Cooking Oil using Malaysian Dolomite Catalyst. Bull. Chem. React. Eng. Catal. 2018, 13, 489–501. [Google Scholar] [CrossRef]
- Tani, H.; Hasegawa, T.; Shimouchi, M.; Asami, K.; Fujimoto, K. Selective catalytic decarboxy-cracking of triglyceride to middle-distillate hydrocarbon. Catal. Today 2011, 164, 410–414. [Google Scholar] [CrossRef]
- Diwald, O.; Berger, T. Metal Oxide Nanoparticles: Formation, Functional Properties, and Interfaces; Wiley: Hoboken, NJ, USA, 2021. [Google Scholar]
- Kuchonthara, P.; Puttasawat, B.; Piumsomboon, P.; Mekasut, L.; Vitidsant, T. Catalytic steam reforming of biomass-derived tar for hydrogen production with K2CO3/NiO/γ-Al2O3 catalyst. Korean J. Chem. Eng. 2012, 29, 1525–1530. [Google Scholar] [CrossRef]
- Alsultan, G.A.; Asikin-Mijan, N.; Lee, H.V.; Albazzaz, A.S.; Taufiq-Yap, Y.H. Deoxygenation of waste cooking to renewable diesel over walnut shell-derived nanorode activated carbon supported CaO-La2O3 catalyst. Energy Convers. Manag. 2017, 151, 311–323. [Google Scholar] [CrossRef]
- Kozliak, E.; Mota, R.; Rodriguez, D.; Overby, P.; Kubátová, A.; Stahl, D.; Niri, V.; Ogden, G.; Seames, W. Non-catalytic cracking of jojoba oil to produce fuel and chemical by-products. Ind. Crops Prod. 2013, 43, 386–392. [Google Scholar] [CrossRef]
- Chansiriwat, W.; Wantala, K.; Khunphonoi, R.; Khemthong, P.; Suwannaruang, T.; Rood, S.C. Enhancing the catalytic performance of calcium-based catalyst derived from gypsum waste for renewable light fuel production through a pyrolysis process: A study on the effect of magnesium content. Chemosphere 2022, 292, 133516. [Google Scholar] [CrossRef]
- Srinakruang, J.; Sato, K.; Vitidsant, T.; Fujimoto, K. Highly efficient sulfur and coking resistance catalysts for tar gasification with steam. Fuel 2006, 85, 2419–2426. [Google Scholar] [CrossRef]
- Ruiz Puigdollers, A.; Schlexer, P.; Tosoni, S.; Pacchioni, G. Increasing Oxide Reducibility: The Role of Metal/Oxide Interfaces in the Formation of Oxygen Vacancies. ACS Catal. 2017, 7, 6493–6513. [Google Scholar] [CrossRef]
- Shamsuddin, M.R.; Asikin-Mijan, N.; Marliza, T.S.; Miyamoto, M.; Uemiya, S.; Yarmo, M.A.; Taufiq-Yap, Y.H. Promoting dry reforming of methane via bifunctional NiO/dolomite catalysts for production of hydrogen-rich syngas. RSC Adv. 2021, 11, 6667–6681. [Google Scholar] [CrossRef] [PubMed]
- Hervy, M.; Olcese, R.; Bettahar, M.M.; Mallet, M.; Renard, A.; Maldonado, L.; Remy, D.; Mauviel, G.; Dufour, A. Evolution of dolomite composition and reactivity during biomass gasification. Appl. Catal. A Gen. 2019, 572, 97–106. [Google Scholar] [CrossRef]
- Asikin-Mijan, N.; Lee, H.V.; Juan, J.C.; Noorsaadah, A.R.; Taufiq-Yap, Y.H. Catalytic deoxygenation of triglycerides to green diesel over modified CaO-based catalysts. RSC Adv. 2017, 7, 46445–46460. [Google Scholar] [CrossRef]
- Chandra Mouli, K.; Soni, K.; Dalai, A.; Adjaye, J. Effect of pore diameter of Ni–Mo/Al-SBA-15 catalysts on the hydrotreating of heavy gas oil. Appl. Catal. A: Gen. 2011, 404, 21–29. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, R.; Gou, Q.; Lai, J.; Zhang, R.; Li, X.; Guo, Z. Developments in late transition metal catalysts with high thermal stability for ethylene polymerization: A crucial aspect from laboratory to industrialization. Eur. Polym. J. 2022, 181, 111693. [Google Scholar] [CrossRef]



| Chemical Formula | Key Constituents | Trace Elements | Color | Specific Gravity |
|---|---|---|---|---|
| CaMg(CO3)2 | CaO, MgO and CO2 | SiO2, Fe2O3 and Al2O3 | White, Pink, Green, Brown, and Black | 2.8 to 2.9 g/cm3 |
| Elemental Analysis (wt%) | Sweden Dolomite | Thailand Dolomite | China Dolomite | Malaysia Dolomite |
|---|---|---|---|---|
| CaO | 30.50 | 65.30 | 30.72 | 38.97 |
| MgO | 20.20 | 34.60 | 20.12 | 39.79 |
| Al2O3 | 0.11 | - | 0.14 | 0.16 |
| Fe2O3 | 0.54 | 623 ppm | 0.03 | 0.08 |
| SiO2 | 2.21 | - | 2.01 | 0.098 |
| K2O | 0.04 | - | 0.02 | - |
| MnO | 0.06 | - | 0.002 | - |
| Feedstock | Catalyst Type | Process Condition | Findings | Reference |
|---|---|---|---|---|
| Leucaena leucocephala Oil | Calcined dolomite and zeolite catalyst |
| Dolomite facilitated the cracking reaction and increased the light bio-oil yield. The viscosities of the bio-oil products obtained using the catalysts were lower by 60% of that of the bio-oil produced without dolomite or zeolite. | [91] |
| Pine sawdust Bio-Oil | Calcined-activated dolomite catalyst |
| 48% high quality biofuel conversion: Ketones (37%) Cyclo pentanones (30%) | [92] |
| Liriodendron Oil | Calcined-activated dolomite catalyst |
| Dolomite HHVs (23.09–28.02 MJ/kg) higher than those of bio-oil from the sand (21.64–24.37 MJ/kg). High H2/CO ratio in the gas product, which can be used in the synthesis of liquid fuel | [93] |
| Saccharum officinarum LOil | Calcined dolomite |
| Calcined dolomite upgraded bio-oil with a lower oxygen content, higher gross calorific value, and decreased acid corrosion | [94] |
| Waste Cooking Oil | Ni-doped-calcined Malaysiadolomite (Ni/CMD900) catalyst |
| PRE/Ni/CMD900 catalyst resulted superior deoxygenation reaction activity with high conversion of WCO (68.0%), high yield of pyrolysis oil (36.4%), and less coke formation (32.0%) | [52] |
| Waste Cooking Oil | Ni, Fe, Zn, Cu, Co/CMD900 |
| Ni/CMD900 catalyst exhibited highest conversion (67.0%) and high selectivity (80.2%) with high proportion of saturated linear hydrocarbons | [77] |
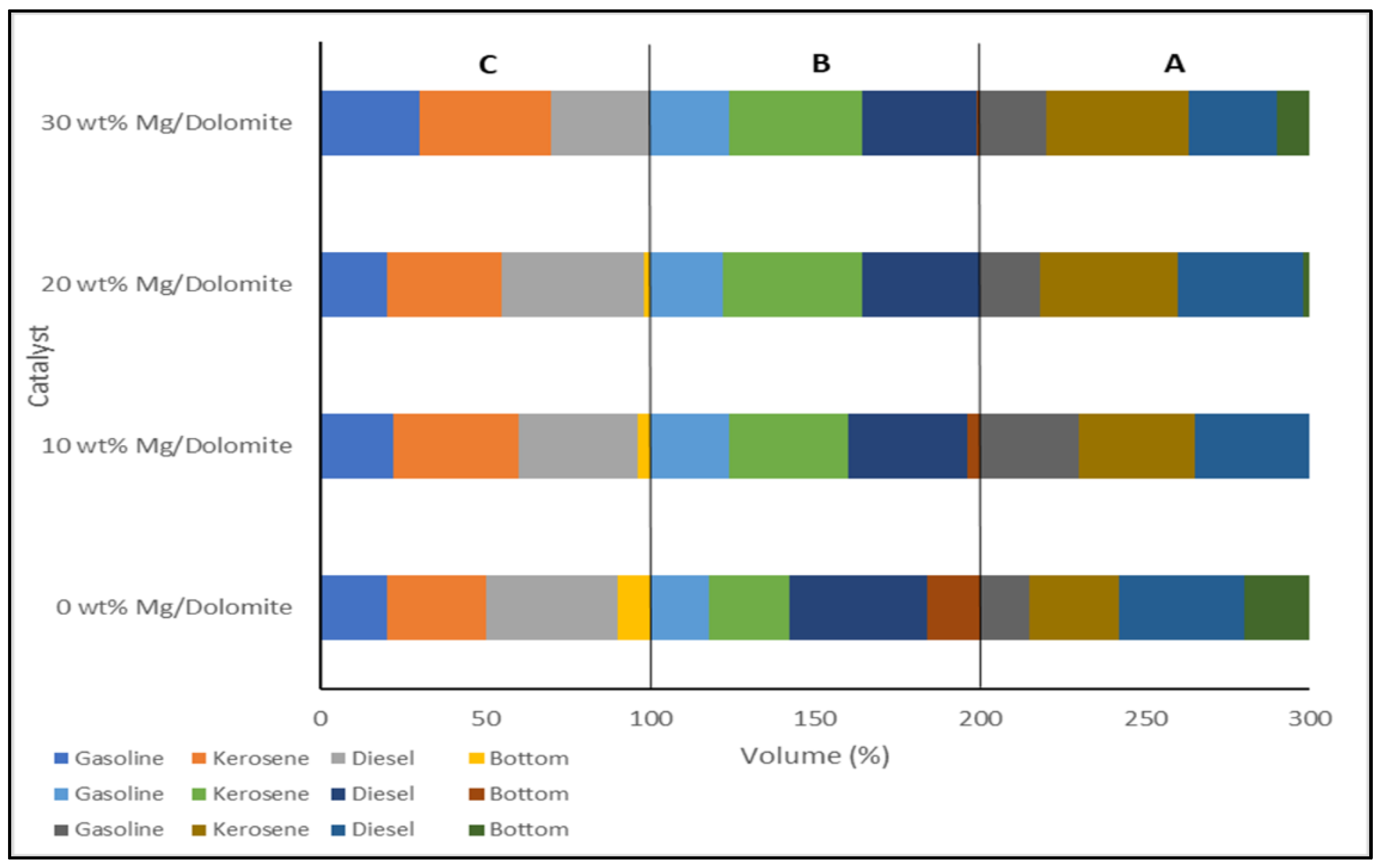
| Waste Cooking Oil | Dolomite added with magnesium.carbonate (MgCO3) (0–30 wt%), |
| The highest production yield 84 vol% Light biofuels yield 65 vol%. | [82] |
| Waste Cooking Oil | NiO-dolomite catalyst |
| The bio-oil meets the requirements of diesel fuel. The biofuel characterization is tested with several standard parameters | [95] |
| Catalyst | Heating Value | Acid Value | Reference |
|---|---|---|---|
| ASTM 44–45 (MJ/Kg) | ASTM <0.01 mg (KOH/g) | ||
| Activated Dolomite | Not Available | 33 | [101] |
| Mg/Activated Dolomite | 42.14 | 2.81 | [82] |
| Ni/Activated Dolomite (Precipitation) | Not Available | 47 | [52] |
| Ni/Activated Dolomite(Co-Precipitation) | Not Available | 49 | [52] |
| Ni/Activated Dolomite (Impregnation) | Not Available | 58 | [52] |
| NiO/Activated Dolomite | 43.83 | 26.6 | [95] |
| Fe/Activated Dolomite | Not Available | 40 | [77] |
| Co/Activated Dolomite | Not Available | 64 | [77] |
| Cu/Activated Dolomite | Not Available | 75 | [77] |
| Zn/Activated Dolomite | Not Available | 78 | [77] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zamri, M.F.M.A.; Shamsuddin, A.H.; Ali, S.; Bahru, R.; Milano, J.; Tiong, S.K.; Fattah, I.M.R.; Raja Shahruzzaman, R.M.H. Recent Advances of Triglyceride Catalytic Pyrolysis via Heterogenous Dolomite Catalyst for Upgrading Biofuel Quality: A Review. Nanomaterials 2023, 13, 1947. https://doi.org/10.3390/nano13131947
Zamri MFMA, Shamsuddin AH, Ali S, Bahru R, Milano J, Tiong SK, Fattah IMR, Raja Shahruzzaman RMH. Recent Advances of Triglyceride Catalytic Pyrolysis via Heterogenous Dolomite Catalyst for Upgrading Biofuel Quality: A Review. Nanomaterials. 2023; 13(13):1947. https://doi.org/10.3390/nano13131947
Chicago/Turabian StyleZamri, Mohd Faiz Muaz Ahmad, Abd Halim Shamsuddin, Salmiaton Ali, Raihana Bahru, Jassinnee Milano, Sieh Kiong Tiong, Islam Md Rizwanul Fattah, and Raja Mohd Hafriz Raja Shahruzzaman. 2023. "Recent Advances of Triglyceride Catalytic Pyrolysis via Heterogenous Dolomite Catalyst for Upgrading Biofuel Quality: A Review" Nanomaterials 13, no. 13: 1947. https://doi.org/10.3390/nano13131947
APA StyleZamri, M. F. M. A., Shamsuddin, A. H., Ali, S., Bahru, R., Milano, J., Tiong, S. K., Fattah, I. M. R., & Raja Shahruzzaman, R. M. H. (2023). Recent Advances of Triglyceride Catalytic Pyrolysis via Heterogenous Dolomite Catalyst for Upgrading Biofuel Quality: A Review. Nanomaterials, 13(13), 1947. https://doi.org/10.3390/nano13131947







