A Hexadecanuclear Cobalt-Added Tungstogermanate Containing Counter Cobalt Hydrates: Synthesis, Structure and Photocatalytic Properties
Abstract
:1. Introduction
2. Experiments Section
2.1. General Procedure
2.2. Synthesis
2.3. X-ray Crystallography
2.4. Photocatalytic Water Reduction Tests
3. Results and Discussion
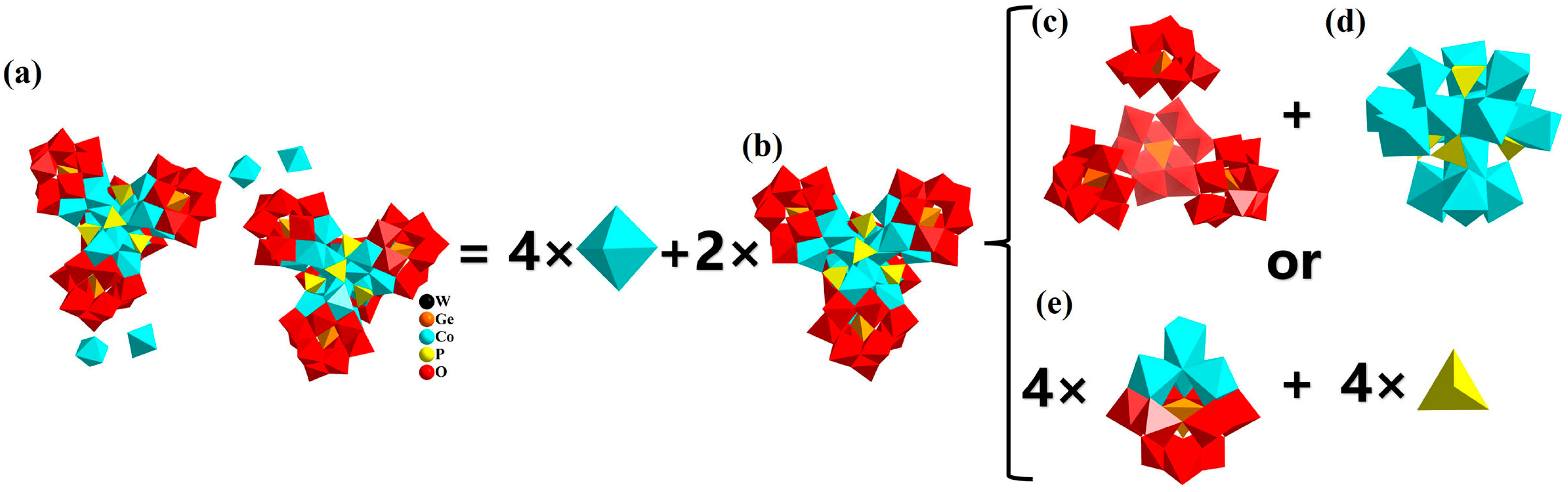
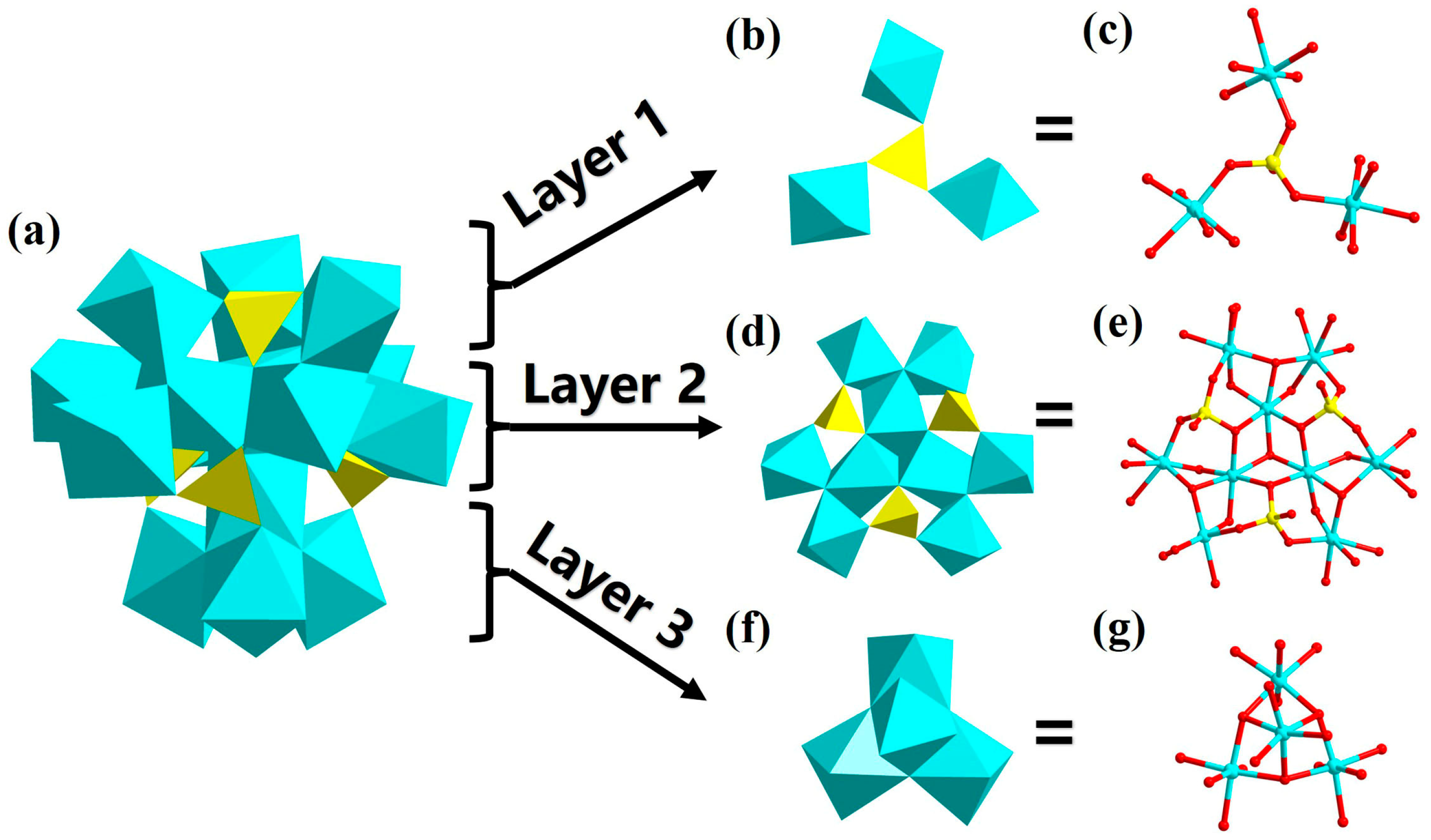
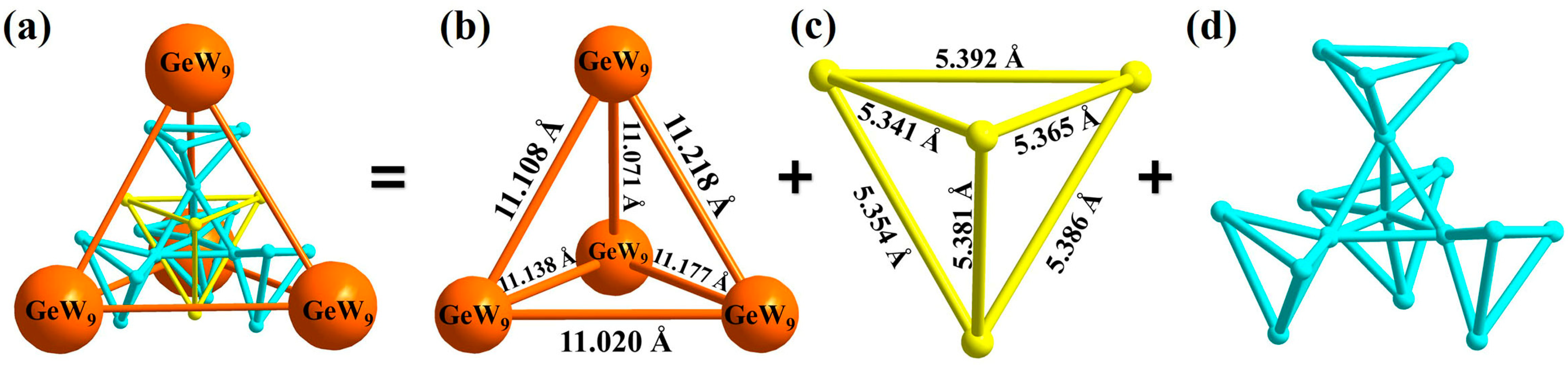
3.1. Structure of Compound 1
3.2. FT-IR Measurement
3.3. Powder X-ray Diffraction Patterns
3.4. UV-Vis Diffuse Reflectance Test
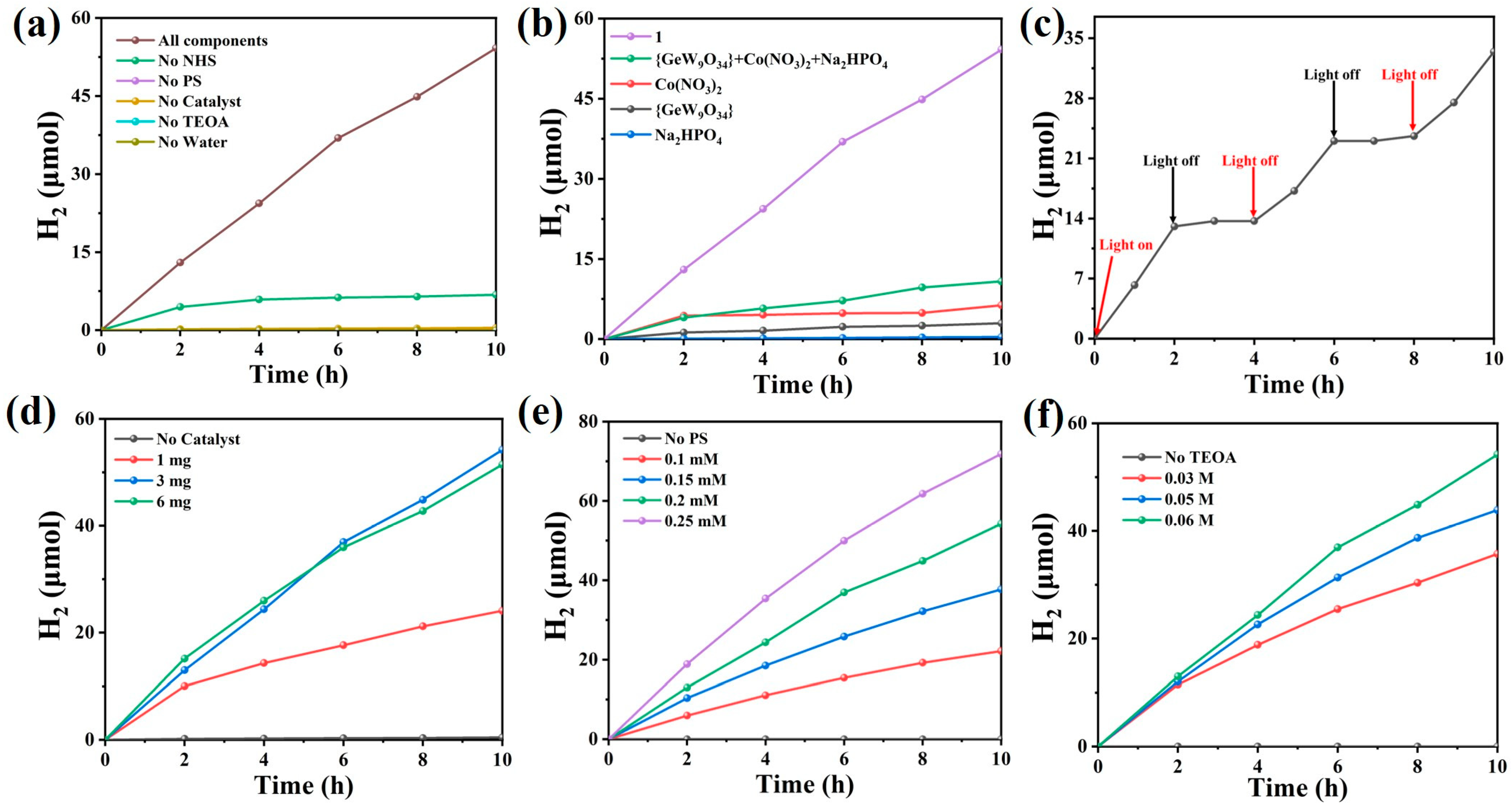
3.5. Photocatalytic Hydrogen Generation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zheng, S.-T.; Yang, G.-Y. Recent advances in paramagnetic-TM-substituted polyoxometalates (TM = Mn, Fe, Co, Ni, Cu). Chem. Soc. Rev. 2012, 41, 7623–7646. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-S.; Yang, G.-Y. Recent Advances in Polyoxometalate-Catalyzed Reactions. Chem. Rev. 2015, 115, 4893–4962. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.-W.; Li, Y.-Z.; Chen, L.-J.; Yang, G.-Y. Research progress on polyoxometalate-based transition-metal-rare-earth hetero-metallic derived materials: Synthetic strategies, structural overview and functional applications. Chem. Commun. 2016, 52, 4418–4445. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-M.; Xin, X.; Zhang, M.; Li, Z.; Lv, H.-J.; Yang, G.-Y. Recent advances of mixed-transition-metal-substituted polyoxometalates. Sci. China Chem. 2022, 65, 1515–1525. [Google Scholar] [CrossRef]
- Kim, N.; Nam, J.-S.; Jo, J.; Seong, J.; Kim, H.; Kwon, Y.; Lah, M.-S.; Lee, J.-H.; Kwon, T.-H.; Ryu, J. Selective photocatalytic production of CH4 using Zn-based polyoxometalate as a nonconventional CO2 reduction catalyst. Nanoscale Horiz. 2021, 6, 379–385. [Google Scholar] [CrossRef]
- Zhao, W.; Zeng, X.; Huang, L.; Qiu, S.; Xie, J.; Yu, H.; Wei, Y. Oxidative dehydrogenation of hydrazines and diarylamines using a polyoxomolybdate-based iron catalyst. Chem. Commun. 2021, 57, 7677–7680. [Google Scholar] [CrossRef]
- Li, N.; Liu, J.; Dong, B.-X.; Lan, Y.-Q. Polyoxometalate-based Compounds for Photo- and Electrocatalytic Applications. Angew. Chem. Int. Ed. 2020, 59, 2–17. [Google Scholar] [CrossRef]
- Neumann, R.; Azaiza-Dabbah, D.; Vogt, C.; Wang, F.; Masip-Sanchez, A.; de Graaf, C.; Poblet, J.M.; Haviv, E. Molecular Transition Metal Oxide Electrocatalysts for the Reversible Carbon Dioxide-Carbon Monoxide Transformation. Angew. Chem. Int. Ed. 2022, 61, e202112915. [Google Scholar] [CrossRef]
- Blasco-Ahicart, M.; Soriano-López, J.; Carbó, J.-J.; Poblet, J.-M.; Galan-Mascaros, J.-R. Polyoxometalate Electrocatalysts Based on Earth-abundant Metals for Efficient Water Oxidation in Acidic Media. Nat. Chem. 2017, 10, 24–30. [Google Scholar] [CrossRef]
- Ni, L.-B.; Yang, G.; Liu, Y.; Wu, Z.; Ma, Z.; Shen, C.; Lv, Z.; Wang, Q.; Gong, X.; Xie, J.; et al. Self-assembled Polyoxometalate Nanodots as Bidirectional Cluster Catalysts for Polysulfide/Sulfide Redox Conversion in Lithium–sulfur Batteries. ACS Nano 2021, 15, 12222–12236. [Google Scholar] [CrossRef]
- Li, X.-X.; Ji, T.; Gao, J.-Y.; Chen, W.-C.; Yuan, Y.; Sha, H.-Y.; Faller, R.; Shan, G.-G.; Shao, K.-Z.; Wang, X.-L.; et al. An Unprecedented Fully Reduced {Mo60V} Polyoxometalate: From All-inorganic Molecular Light-absorber Model to Improved Photoelectronic Performance. Chem. Sci. 2022, 13, 4573–4580. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.; Lan, Y.; Bassil, B.-S.; Xiang, Y.; Suchopar, A.; Powell, A.-K.; Kortz, U. Hexadecacobalt(II)-containing Polyoxo-metalate-Based Single-molecule Magnet. Angew. Chem. Int. Ed. 2011, 50, 4708–4711. [Google Scholar] [CrossRef]
- Zheng, S.-T.; Yuan, D.-Q.; Jia, H.-P.; Zhang, J.; Yang, G.-Y. Combination between Lacunary Polyoxometalates and High-nuclear Transition Metal Clusters under Hydrothermal Conditions: I. from Isolated Cluster to 1-D Chain. Chem. Commun. 2007, 18, 1858–1860. [Google Scholar] [CrossRef]
- Rhule, J.-T.; Hill, C.-L.; Judd, D.-A.; Schinazi, R.-F. Polyoxometalates in medicine. Chem. Rev. 1998, 98, 327–357. [Google Scholar] [CrossRef]
- Zhao, J.-W.; Jia, H.-P.; Zhang, J.; Zheng, S.-T.; Yang, G.-Y. A Combination of Lacunary Polyoxometalates and High-nuclear Transition-metal Clusters under Hydrothermal Conditions. Part II: From Double Cluster, Dimer, and Tetramer to Three-dimensional Frameworks. Chem. Eur. J. 2007, 13, 10030–10045. [Google Scholar] [CrossRef]
- Zheng, S.-T.; Zhang, J.; Yang, G.-Y. Designed Synthesis of POM-organic Frameworks from {Ni6PW9} Building Blocks under Hydrothermal Conditions. Angew. Chem. Int. Ed. 2008, 47, 3909–3913. [Google Scholar] [CrossRef]
- Zheng, S.-T.; Zhang, J.; Clemente-Juan, J.-M.; Da-Qiang, Y.; Yang, G.-Y. Poly(polyoxotungstate)s with 20 Nickel Centers: From Nanoclusters to One-dimensional Chains. Angew. Chem. Int. Ed. 2009, 121, 7312–7315. [Google Scholar] [CrossRef]
- Zheng, S.-T.; Zhang, J.; Li, X.-X.; Fang, W.-H.; Yang, G.-Y. Cubic Polyoxometalate-organic Molecular Cage. J. Am. Chem. Soc. 2010, 132, 15102–15103. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, J.; Cheng, L.; Yang, G.-Y. Poly(polyoxometalate)s Assembled by {Ni6PW9} Units: From Ring-shaped Ni24-tetramers to Rod-shaped Ni40-octamers. Chem. Commun. 2012, 48, 9658–9660. [Google Scholar] [CrossRef]
- Han, X.-B.; Zhang, Z.-M.; Zhang, T.; Li, Y.-G.; Lin, W.-B.; You, W.-S.; Su, Z.-M.; Wang, E.-B. Polyoxometalate-based Cobalt-phosphate Molecular Catalysts for Visible Light-driven Water Oxidation. J. Am. Chem. Soc. 2014, 136, 5359–5366. [Google Scholar] [CrossRef]
- Han, X.-B.; Li, Y.-G.; Zhang, Z.-M.; Tan, H.-Q.; Lu, Y.; Wang, E.-B. Polyoxometalate-Based Nickel Clusters as Visible Light-driven Water Oxidation Catalysts. J. Am. Chem. Soc. 2015, 137, 5486–5493. [Google Scholar] [CrossRef] [PubMed]
- Goura, J.; Bassil, B.; Ma, X.; Rajan, A.; Moreno-Pineda, E.; Schnack, J.; Ibrahim, M.; Powell, A.; Ruben, M.; Wang, J.-J.; et al. Ni36II-containing 54-tungsto-6-silicate: Synthesis, Structure, Magnetic and Electrochemical Studies. Chem. Eur. J. 2021, 27, 15081–15085. [Google Scholar] [CrossRef] [PubMed]
- Chang, Q.; Meng, X.-Y.; Ruan, W.-J.; Feng, Y.-Q.; Li, R.; Zhu, J.-Y.; Ding, Y.; Lv, H.-J.; Wang, W.; Chen, G.-Y.; et al. Metal-organic Cages with {SiW9Ni4} Polyoxotungstate Nodes. Angew. Chem. Int. Ed. 2022, 134, e202117637. [Google Scholar] [CrossRef]
- Chen, Y.; Guo, Z.-W.; Li, X.-X.; Zheng, S.-T.; Yang, G.-Y. Multicomponent Cooperative Assembly of Nanoscale Boron-rich Polyoxotungstates with 22 and 30 Boron Atoms. CCS Chem. 2022, 4, 1305–1314. [Google Scholar] [CrossRef]
- Hu, Q.-Y.; Chen, S.-S.; Wågberg, T.; Zhou, H.-S.; Li, S.-J.; Li, Y.-D.; Tan, Y.-L.; Hu, W.-Q.; Ding, Y.; Han, X.-B. Developing Insoluble Polyoxometalate Clusters to Bridge Homogeneous and Heterogeneous Water Oxidation Photocatalysis. Angew. Chem. Int. Ed. 2023, 2023, e202303290. [Google Scholar] [CrossRef]
- Galán-Mascarós, J.-R.; Gómez-Garcia, C.-J.; Borrás-Almenar, J.-J.; Coronado, E. High Nuclearity Magnetic Clusters: Magnetic Properties of a Nine Cobalt Cluster Encapsulated in a Polyoxometalate, [Co9(OH)3(H2O)6(HPO4)2(PW9O34)3]16−. Adv. Mater. 1994, 6, 221–223. [Google Scholar] [CrossRef]
- Bassil, B.-S.; Nellutla, S.; Kortz, U.; Stowe, A.-C.; Van Tol, J.; Dalal, N.-S.; Keita, B.; Nadjo, L. The Satellite-shaped Co-15 Polyoxotungstate, [Co6(H2O)30{Co9Cl2(OH)3(H2O)9(β-SiW8O31)3}]5−. Inorg. Chem. 2005, 44, 2659–2665. [Google Scholar] [CrossRef]
- Shi, D.-Y.; Cui, C.-J.; Sun, C.-X.; Du, J.-P.; Liu, C.-S. A New [Co21(H2O)4(OH)12]30+ Unit-incorporating Polyoxotungstate for Sensitive Detection of dichlorvos. New J. Chem. 2020, 44, 11336–11341. [Google Scholar] [CrossRef]
- Dempsey, J.-L.; Brunschwig, B.-S.; Winkler, J.-R.; Gray, H.-B. Hydrogen Evolution Catalyzed by Cobaloximes. Acc. Chem. Res. 2009, 42, 1995–2004. [Google Scholar] [CrossRef] [Green Version]
- Artero, V.; Chavarot-Kerlidou, M.; Fontecave, M. Splitting Water with Cobalt. Angew. Chem. Int. Ed. 2011, 50, 7238–7266. [Google Scholar] [CrossRef]
- Jiao, Y.; Zheng, Y.; Jaroniecb, M.; Qiao, S.-Z. Design of electrocatalysts for oxygen- and hydrogen-involving energy conversion reactions. Chem. Soc. Rev. 2015, 44, 2060–2086. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.-Q.; Luo, Z.; Geletii, Y.-V.; Vickers, J.-W.; Yin, Q.; Wu, D.; Hou, Y.; Ding, Y.; Song, J.; Musaev, D.-G.; et al. Efficient light-driven carbon-free cobalt-based molecular catalyst for water oxidation. J. Am. Chem. Soc. 2011, 133, 2068–2071. [Google Scholar] [CrossRef]
- Lv, H.-J.; Geletii, Y.-V.; Zhao, C.-C.; Vickers, J.-W.; Zhu, G.-B.; Luo, Z.; Song, J.; Lian, T.-Q.; Musaev, D.-G.; Hill, C.-L. Polyoxo-metalate water oxidation catalysts and the production of green fuel. Chem. Soc. Rev. 2012, 41, 7572–7589. [Google Scholar] [CrossRef]
- Lv, H.-J.; Song, J.; Geletii, Y.-V.; Vickers, J.-W.; Sumliner, J.-M.; Musaev, D.-G.; Kögerler, P.; Zhuk, P.-F.; Bacsa, J.; Zhu, G.-B.; et al. An Exceptionally Fast Homogeneous Carbon-free Cobalt-based Water Oxidation Catalyst. J. Am. Chem. Soc. 2014, 136, 9268–9271. [Google Scholar] [CrossRef]
- Stracke, J.-J.; Finke, R.-G. Water Oxidation Catalysis Beginning with [Co4(H2O)2(PW9O34)2]10− When Driven by the Chemical Oxidant Ruthenium(III)tris(2,2′-bipyridine): Stoichiometry, Kinetic, and Mechanistic Studies en Route to Identifying the True Catalyst. ACS Catal. 2014, 4, 79–89. [Google Scholar] [CrossRef]
- Haider, A.; Bassil, B.-S.; Soriano-López, J.; Qasim, H.-M.; De Pipaón, C.S.; Ibrahim, M.; Dutta, D.; Koo, Y.-S.; Carbó, J.-J.; Poblet, J.-M.; et al. 9-Cobalt(II)-Containing 27-Tungsto-3-germanate(IV): Synthesis, Structure, Computational Modeling, and Heterogeneous Water Oxidation Catalysis. Inorg. Chem. 2019, 58, 11308–11316. [Google Scholar] [CrossRef]
- Dong, K.-L.; Ma, P.-T.; Wu, H.-C.; Wu, Y.-K.; Niu, J.-Y.; Wang, J.-P. Cobalt- and Nickel-Containing Germanotungstates Based on Open Wells–Dawson Structure: Synthesis and Characterization of Tetrameric Anion. Inorg. Chem. 2019, 58, 6000–6007. [Google Scholar] [CrossRef]
- Al-Sayed, E.; Nandan, S.-P.; Tanuhadi, E.; Giester, G.; Arrigoni, M.; Madsen, G.-K.-H.; Cherevan, A.; Eder, D.; Rompel, A. Phosphate-Templated Encapsulation of a {CoII4O4} Cubane in Germanotungstates as Carbon-Free Homogeneous Water Oxidation Photocatalysts. ChemSusChem 2021, 14, 2529–2536. [Google Scholar] [CrossRef]
- Zhao, J.-L.; Ding, Y.; Wei, J.; Du, X.-Q.; Yu, Y.-Z.; Han, R.-X. A Molecular Keggin Polyoxometalate Catalyst with High Efficiency for Visible-light Driven Hydrogen Evolution. Int. J. Hydrog. Energy 2014, 39, 18908–18918. [Google Scholar] [CrossRef]
- Wu, W.-M.; Teng, T.; Wu, X.-Y.; Dui, X.-J.; Zhang, L.; Xiong, J.-H.; Wu, L.; Lu, C.-Z. A Cobalt-based Polyoxometalate Catalyst for Efficient Visible-light-driven H2 Evolution from Water Splitting. Catal. Commun. 2015, 64, 44–47. [Google Scholar] [CrossRef]
- Wang, Z.-W.; Yang, G.-Y. A {Co9}-Added Polyoxometalate for Efficient Visible-light-driven Hydrogen Evolution. Molecules 2023, 28, 664. [Google Scholar] [CrossRef]
- Lv, H.-J.; Guo, W.-W.; Wu, K.-F.; Chen, Z.-Y.; Bacsa, J.; Musaev, D.-G.; Geletii, Y.-V.; Lauinger, S.-M.; Lian, T.-Q.; Hill, C.-L. A Noble-metal-free, Tetra-nickel Polyoxotungstate Catalyst for Efficient Photocatalytic Hydrogen Evolution. J. Am. Chem. Soc. 2014, 136, 14015–14018. [Google Scholar] [CrossRef]
- Lv, H.-J.; Gao, Y.-Z.; Guo, W.-W.; Lauinger, S.M.; Chi, Y.-N.; Bacsa, J.; Sullivan, K.P.; Wieliczko, M.; Musaev, D.G.; Hill, C.L. Cu-based Polyoxometalate Catalyst for Efficient Catalytic Hydrogen Evolution. Inorg. Chem. 2016, 55, 6750–6758. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.-S.; Wang, H.-Y.; Ding, Y. Visible-light-driven hydrogen evolution using a polyoxometalate-based copper molecular catalyst. Dalton Trans. 2020, 49, 3457–3462. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.-B.; Gómez-García, C.-J.; Sun, W.-L.; Lai, X.-Y.; Pang, H.-J.; Ma, H.-Y. Improving the Photocatalytic H2 Evolution Activity of Keggin Polyoxometalates Anchoring Copper-azole Complexes. Green Chem. 2021, 23, 3104–3114. [Google Scholar] [CrossRef]
- Haraguchi, N.; Okaue, Y.; Isobe, T.; Matsuda, Y. Stabilization of Tetravalent Cerium upon Coordination of Unsaturated Heteropolytungstate Anions. Inorg. Chem. 1994, 33, 1015–1020. [Google Scholar] [CrossRef]
- Bi, L.-H.; Kortz, U.; Nellutla, S.; Stowe, A.-C.; Van Tol, J.; Dalal, N.-S.; Keita, B.; Nadjo, L. Structure, Electrochemistry, and Magnetism of the Iron(III)-substituted Keggin Dimer, [Fe6(OH)3(A-α-GeW9O34(OH)3)2]11−. Inorg. Chem. 2005, 44, 896–903. [Google Scholar] [CrossRef]
- Dolomanov, O.-V.; Bourhis, L.-J.; Gildea, R.-J.; Howard, J.-A.-K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G. SHELXT-Integrated space-group and crystal-structure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Spek, A. PLATON SQUEEZE: A tool for the calculation of the disordered solvent contribution to the calculated structure factors. Acta Cryst. 2015, C71, 9–18. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, M.; Haider, A.; Lan, Y.-H.; Bassil, B.-S.; Carey, A.-M.; Liu, R.-J.; Zhang, G.-J.; Keita, B.; Li, W.-H.; Kostakis, G.E.; et al. Multinuclear Cobalt(II)-containing Heteropolytungstates: Structure, Magnetism, and Electrochemistry. Inorg. Chem. 2014, 53, 5179–5188. [Google Scholar] [CrossRef] [PubMed]
- Brown, I.-D.; Altermatt, D. Bond-valence Parameters Obtained from a Systematic Analysis of the Inorganic Crystal Structure Database. Acta Cryst. 1985, B41, 244–247. [Google Scholar] [CrossRef] [Green Version]
- Li, H.-L.; Zhang, M.; Lian, C.; Lang, Z.-L.; Lv, H.; Yang, G.-Y. Ring-Shaped Polyoxometalate Built by {Mn4PW9} and PO4 Units for Efficient Visible-Light-Driven Hydrogen Evolution. CCS Chem. 2021, 3, 2095–2103. [Google Scholar] [CrossRef]
- Paille, G.; Boulmier, A.; Bensaid, A.; Ha-Thi, M.-H.; Tran, T.-T.; Pino, T.; Marrot, J.; Rivière, E.; Hendon, C.H.; Oms, O.; et al. An Unprecedented {Ni14SiW9} Hybrid Polyoxometalate with High Photocatalytic Hydrogen Evolution Activity. Chem. Commun. 2019, 55, 4166–4169. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.-L.; Lu, Y.; Li, Y.-G.; Wang, S.-M.; Wang, E.-B. Visible-light photocatalytic H2 evolution over a series of transition metal substituted Keggin-structure heteropoly blues. Chin. Sci. Bull. 2012, 57, 2265–2268. [Google Scholar] [CrossRef] [Green Version]
- Shang, X.-K.; Liu, R.-J.; Zhang, G.-J.; Zhang, S.-J.; Cao, H.-B.; Gu, Z.-J. Artificial photosynthesis for solar hydrogen generation over transition-metal substituted Keggin-type titanium tungstate. New J. Chem. 2014, 38, 1315–1320. [Google Scholar] [CrossRef]


| 1 | |
|---|---|
| Empirical formula | H169N5O375Na16P8Co36Ge8W72 |
| Formula weight | 22,794.97 |
| Temperature/K | 293(2) |
| Crystal system | Triclinic |
| Space group | P-1 |
| a/Å | 24.4950 (8) |
| b/Å | 30.9701 (17) |
| c/Å | 36.8329 (17) |
| α/° | 72.049 (4) |
| β/° | 70.58 |
| γ/° | 66.70 |
| Volume/Å3 | 23,694.9 (19) |
| Z | 2 |
| ρcalcg/cm3 | 3.113 |
| μ/mm−1 | 19.239 |
| F(000) | 19,358.0 |
| Goodness-of-fit on F2 | 0.900 |
| Final R indexes [I ≥ 2σ (I)] a,b | R1 = 0.0643, wR2 = 0.1158 |
| Final R indexes [all data] a,b | R1 = 0.1428, wR2 = 0.1420 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Q.; Li, X.; Wang, Y.; Lv, H.; Yang, G. A Hexadecanuclear Cobalt-Added Tungstogermanate Containing Counter Cobalt Hydrates: Synthesis, Structure and Photocatalytic Properties. Nanomaterials 2023, 13, 2009. https://doi.org/10.3390/nano13132009
Zhao Q, Li X, Wang Y, Lv H, Yang G. A Hexadecanuclear Cobalt-Added Tungstogermanate Containing Counter Cobalt Hydrates: Synthesis, Structure and Photocatalytic Properties. Nanomaterials. 2023; 13(13):2009. https://doi.org/10.3390/nano13132009
Chicago/Turabian StyleZhao, Qing, Xuyan Li, Yu Wang, Hongjin Lv, and Guoyu Yang. 2023. "A Hexadecanuclear Cobalt-Added Tungstogermanate Containing Counter Cobalt Hydrates: Synthesis, Structure and Photocatalytic Properties" Nanomaterials 13, no. 13: 2009. https://doi.org/10.3390/nano13132009





