Carbon Capture Using Porous Silica Materials
Abstract
:1. Introduction
2. CO2 Capture
2.1. CO2 Capture Technologies
2.2. Criteria for Selecting CO2 Sorbent Material
- Adsorption capacity for CO2:
- Selectivity for CO2:
- Adsorption and desorption kinetics:
- Mechanical strength of sorbent particles:
- Chemical stability/tolerance towards impurities:
- Regeneration of sorbents:
- Sorbent costs:
2.3. Liquid Amine for CO2 Capture
| Criteria | Alkanolamines | Sterically Hindered Amines | ||
|---|---|---|---|---|
| Primary | Secondary | Tertiary | ||
| Examples | Monoethanolamine (MEA) | Diethanolamine (DEA) | N-methyldiethanolamine (MEDA) | 2-amino-2-methyl-1- propanol (AMP) |
| Structure |  | 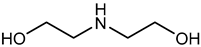 | 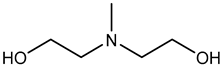 | 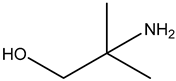 |
| CO2 loading at 59.85 °C (mol CO2/mol amine) | 0.426 (MEA 30 wt%) | 0.404 (DEA 30 wt%) | 0.141 (TEA 30 wt%) | 0.466 (AMP 30 wt%) |
| Regeneration efficiency (%) at 90 °C | 75.5 | 84.89 | 95.09 | |
| Advantages |
|
|
|
|
| Disadvantages |
|
|
|
|
2.4. Comparison between Major Non-Carbonaceous Solid Sorbents for CO2 Capture and Importance of Silica Materials
3. CO2 Capture Methods
4. CO2 Adsorption Using Mesoporous Silica Materials (Physisorbents)
4.1. Mesoporous Silica Materials
4.2. Synthesis Procedures of Mesoporous Silica
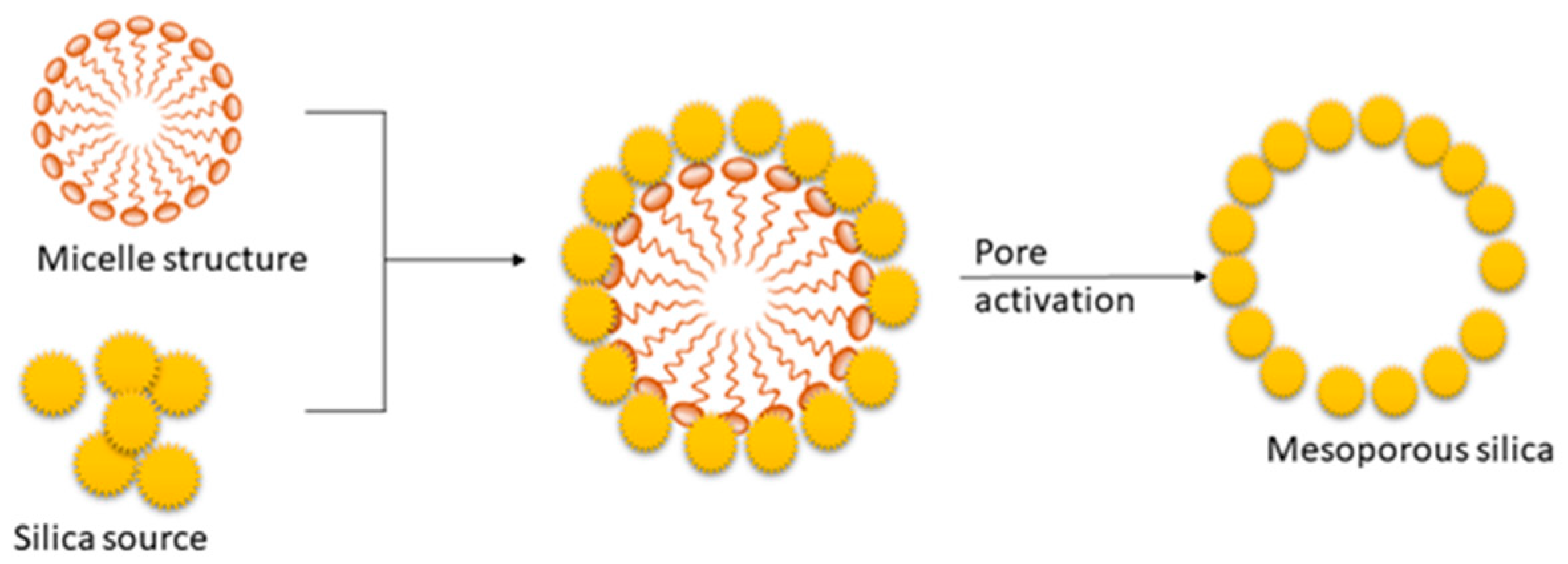
4.3. Importance of Micro-Porosity and CO2 Adsorption Capacity of Mesoporous Silica Materials
| Types of Mesoporous Silica | Mesostructure | Silica Source | Surfactant/ Block Co-Polymer | BET Specific Surface Area (m2/g) | Pore Volume (cm3/g) | Pore Size (nm) | Adsorption Capacity (mmol/g) | Adsorption Conditions | Ref. | |
|---|---|---|---|---|---|---|---|---|---|---|
| Temp. (°C) | Pressure (Bar) | |||||||||
| KIT-5 | 3D-cubic | TEOS | Pluronic P123 | 711 | 1.05 | 8.04 | 0.48 | 30 | 1 | [97] |
| KIT-6 | 3D-cubic | TEOS | Pluronic P123 | 895 | 1.22 | 6.0 | - | - | - | [94] |
| MCM-41 | Hexagonal | Na2SiO3 | CTAB | 994 | 1.00 | 3.03 | 0.63 | 25 | 1 | [93] |
| Na2SiO3 | CTAB | 993 | 1.00 | 3.1 | 0.63 | 25 | 1 | [98] | ||
| Na2SiO3 | CTAB | 980 | 0.92 | 4.08 | [90] | |||||
| MCM 48 | Cubic | SiO2 | CTAB | 1287 | 1.1 | 3.5 | 25 | 1 | [99] | |
| SBA-15 | 2D hexagonal | TEOS | P123 | 1254 | 2.44 | 11.4 | - | - | - | [100] |
| SBA-16 | Cubic cage | TEOS | Pluronic F127 | 736 | 0.75 | 4.1 | - | - | - | [94] |
| SNS | TEOS | Pluronic F127 | 394 | 0.10 | 21.1 | 2.06 | 25 | 1 | [101] | |
| SNT | TEOS | Pluronic F127 | 319 | 0.07 | 26.0 | 2.46 | 25 | 1 | [101] | |
5. Chemisorbents (Amine Functionalized Si-Based Materials)—Application at Low and High Temperature CO2 Sorption
5.1. Synthesis of Amine-Functionalized Silica

5.2. Comparison of Adsorption Capacities of Silica-Based Sorbents
| Silica-Based Sorbent | Amine Types | CO2 Adsorption Performance Capacity (mmol/g) | Conditions | BET Specific Surface Area (m2/g) | Pore Volume (cm3/g) | Pore Size (nm) | Preparation Methods | Ref. | |
|---|---|---|---|---|---|---|---|---|---|
| Temperature (°C) | Pressure (Bar) | ||||||||
| DWSNT | - | 0.1 | 25 | 83 | 0.58 | Immobilization | [124] | ||
| DWSNT | APTMS | 1.0 | 25 | 112 | 0.72 | Immobilization | [124] | ||
| DWSNT | MAPTMS | 1.5 | 25 | 114 | 0.79 | Immobilization | [124] | ||
| DWSNT | DEAPTMS | 1.8 | 25 | 68.9 | 0.49 | Immobilization | [124] | ||
| DWSNT | AEAPTMS | 2.25 | 25 | 60.9 | 0.45 | Immobilization | [124] | ||
| HAS | Aziridines | 3.25 | 25 | 71 | 5 | 0.15 | [125] | ||
| HPS | PEI | 2.44 | 75 | 1 | 0.5 | 0.009 | Impregnation | [126] | |
| HVMCM-41 | PEHA | 4.07 | 105 | 1 | Impregnation | [123] | |||
| KIT-6 | PEHA | 4.48 | 105 | 1 | Impregnation | [123] | |||
| MCM-41 | EDA | 1.19 | 35 | Impregnation | [127] | ||||
| MCM-41 | DETA | 1.43 | 35 | Impregnation | [127] | ||||
| MCM-41 | TEPA | 1.96 | 35 | Impregnation | [127] | ||||
| MCM-41 | PEHA | 2.34 | 35 | Impregnation | [127] | ||||
| MCM-41 | MEA (3%) | 11.39 | 25 | 426 | 0.42 | 3.12 | Impregnation | [128] | |
| MCM-41 | PEI | 0.39 | 40 | 0.15 | 443 | 0.340 | 2.95 | Impregnation | [49] |
| MCM-41 | PEI | 0.22 | 75 | 1 | 590 | 1.4 | 13.6 | Impregnation | [120] |
| MCM-41 | PEI Aziridine | 0.98 | 75 | 1 | In-situ grafted polymerization | [129] | |||
| MCM-41 | APTS | 94 | 25 | 1 | 10 | 0.01 | Grafting | [114] | |
| MCM-41 | APTS | 0.70 | 30 | 0.1 | [130] | ||||
| MCM-41 | APTS | 2.48 | 20 | 1 | 17 | 0.04 | 20.1 | Grafting | [131] |
| MCM-41 | PEHA | 4.5 | 105 | 1 | Impregnation | [120] | |||
| MCM-41 | MEA | 0.89 | 25 | 1 | 19 | 0.82 | Impregnation | [98] | |
| MCM-41 | DEA | 0.80 | 25 | 1 | 13 | 0.07 | Impregnation | [98] | |
| MCM-41 | TEA | 0.63 | 25 | 1 | 213 | 0.17 | Impregnation | [98] | |
| MCM-41 | Branched PEI | 1.08 | 100 | 1 | 6 | 0 | - | Impregnation | [93] |
| MCM-41 | Branched PEI | 0.79 | 100 | 1 | 12 | 0.04 | - | Impregnation | [93] |
| MCM-41 | Branched PEI—(30 wt%) | 0.70 | 100 | 1 | 80 | 0.14 | - | Impregnation | [93] |
| MCM-41 | Branched PEI | 28 | 100 | 1 | 104 | 0.12 | 2.05 | Impregnation | [93] |
| MCM-41 | Branched PEI | 17.5 | 100 | 1 | 291 | 0.17 | 2.05 | Impregnation | [93] |
| MCM-41 | TEPA | 1.24 | 25 | 1 | 11 | 0.05 | 1.8 | Impregnation | [132] |
| MCM-48 | APTES | 0.62 | 25 | 1.01 | 1072 | 0.52 | 2.9 | Grafting | [99] |
| MCM-48 | TRI | 0.46 | 25 | 1.01 | 698 | 0.39 | 2.6 | Grafting | [99] |
| MCM-48 | TRI | 0.44 | 25 | 1.01 | 463 | 0.23 | 2.5 | Grafting | [99] |
| MSiNTs | PEI | 2.75 | 92 | 52.4 | 0.17 | 12.4 | Impregnation | [133] | |
| OMS | PEI | 1.4 | 25 | 352 | 0.79 | Grafting | [120] | ||
| SAB-15 | PEHA | 4.0 | 105 | 1 | Impregnation | [123] | |||
| SBA-15 | PEI | 0.65 | 25 | 683 | 1.19 | 8.5 | Impregnation | [122] | |
| SBA-15 | PEI/Zr4 | 1.34 | 25 | 642 | 1.08 | 8.6 | Impregnation | [122] | |
| SBA-15 | PEI/Zr7 | 1.56 | 25 | 674 | 1.23 | 9.5 | Impregnation | [122] | |
| SBA-15 | PEI/Zr14 | 1.41 | 25 | 601 | 0.69 | 7.0 | Impregnation | [122] | |
| SBA-15 | PEI/Ti1.4 | 0.24 | 25 | 510 | 0.39 | 4.4 | Impregnation | [122] | |
| SBA-15 | NH2OH | 1.65 | 25 | 1 | 435.6 | 0.54 | 6.85 | Grafting | [134] |
| SBA-15 | APTMS | 1.46 | 25 | 0.15 | 82 | 0.16 | 5 | Grafting | [135] |
| SBA-15 | TEPA | 2.45 | 70 | 5 | 0.03 | Grafting | [100] | ||
| SBA-15 | AMP | 1.79 | 70 | 372 | 0.21 | Grafting | [120] | ||
| SBA-15 (0.2 µm) | PEI | 5.84 | 100 | 1 | 590 | 1.44 | 13.6 | Impregnation | [120] |
| SBA-15 (1.5 µm) | PEI | - | 100 | 1 | 746 | 0.80 | 7.2 | Impregnation | [120] |
| SBA-15 (25 µm) | PEI | 5.81 | 100 | 1 | 580 | 0.95 | 10.5 | Impregnation | [120] |
| SiO2 | APTES | 4.3 | 30 | 67 | 0.51 | In-situ polymerization | [29] | ||
| SiO2 | AEAPTMS | 5.7 | 30 | 45 | 0.37 | In-situ polymerization | [29] | ||
| SiO2 | TRI | 5.6 | 30 | 25 | 0.22 | In-situ polymerization | [29] | ||
| SiO2 | APTES | 0.5 | 30 | 216 | 1.11 | Grafting | [29] | ||
| SiO2 | AEAPTMS | 0.3 | 30 | 206 | 1.10 | Grafting | [29] | ||
| SiO2 | TRI | 0.8 | 30 | 172 | 0.99 | Grafting | [29] | ||
| SMCM-41 | MEA | 10.40 | 25 | 405 | 0.39 | 3.01 | Impregnation | [128] | |
| SBA-15 | TEPA | 4.5 | 75 | 1 | 121.1 | 0.327 | Impregnation | [136] | |
| MPSM | TEA | 4.27 | 75 | 1 | 34 | 0.08 | 9.5 | Impregnation | [50] |
| MCM-41 | TRI | 1.74 | 25 | 0.05 | 678.3 | 1.47 | Grafting | [137] | |
| MCM-41 | APTES | 1.20 | 30 | 1 | 1045.21 | 2.59 | 30 | Grafting | [138] |
| MCM-41 | PEI | 0.98 | 30 | 1 | 6.6 | 0.01 | 0.8 | Grafting | [139] |
| MCM-41 | PEI | 4.68 | 45 | 1 | 894 | 1.28 | 5.1 | Grafting | [116] |
| MCM-41 | PEI | 2.92 | 50 | 0.1 | 508 | 0.98 | 2.54 | Impregnation | [140] |
| MCM-41 | TEPA | 2.25 | 50 | 0.1 | 431 | 0.83 | 2.21 | Impregnation | [140] |
| MCM-41-KOH | PEI- | 3.38 | 50 | 0.1 | 391 | 1.08 | 2.33 | Impregnation | [140] |
| MCM-41-Ca(OH)2 | PEI- | 3.81 | 50 | 0.1 | 411 | 1.12 | 2.50 | Impregnation | [140] |
| MCM-41-CsOH | PEI- | 5.02 | 50 | 0.1 | 306 | 0.91 | 2.14 | Impregnation | [140] |
| MCM-41-KOH | TEPA- | 3.93 | 50 | 0.1 | 322 | 0.97 | 2.15 | Impregnation | [140] |
| MCM-41-Ca(OH)2 | TEPA- | 3.76 | 50 | 0.1 | 405 | 0.94 | 2.31 | Impregnation | [140] |
| PET-CsOH | TEPA- | 5.42 | 50 | 0.1 | 293 | 0.97 | 2.61 | Impregnation | [140] |
| MCM 48 | PEI | 1.09 | 80 | 0.24 | 79.3 | 0.02 | 1.68 | Impregnation | [141] |
| MCM-41 | PEI | 1.23 | 80 | 0.24 | 59.1 | 0.02 | 1.80 | Impregnation | [141] |
| SBA-15 | PEI | 1.07 | 80 | 0.24 | 62.1 | 0.01 | 5.2 | Impregnation | [141] |
| SBA-15 | PEI | 1.77 | 0 | 1 | 783 | 0.03 | 7.0 | Impregnation | [142] |
| SBA-15 | PEI | 1.26 | 45 | 0.15 | 399 | 0.79 | 8.2 | Impregnation | [143] |
| MCM 41 | PEI | 3.53 | 25 | 1 | 24 | 0.012 | Impregnation | [144] | |
| MCM 41 | APTS | 2.41 | 25 | 1 | 736 | 0.37 | Grafting | [144] | |
| SBA-15 | PEI | 1.84 | 25 | 1.2 | 195 | 0.39 | 7.0 | Grafting | [145] |
| SBA-15-APES | 1.78 | 25 | 1.2 | 190 | 0.37 | 7.2 | Grafting | [145] | |
| SBA-15-APES | PEI | 1.54 | 25 | 1.2 | 24 | 0.21 | 2.7 | Grafting | [145] |
| OMS | PEI | 2.43 | 25 | 1.2 | 167 | 0.33 | 7.6 | Grafting | [145] |
| OMS-APES | 3.03 | 25 | 1.2 | 180 | 0.37 | 7.2 | Grafting | [145] | |
| OMS-APES | PEI | 1.18 | 25 | 1.2 | 39 | 0.18 | 2.3 | Grafting | [145] |
| OMS-NCC | Amidoxime | 5.54 | 120 | 1 | 315 | 0.69 | 9.3 | [146] | |
| MPS-MCC * | 2.41 | 120 | 302 | 0.44 | 7.0 | [147] | |||
| MPS-MCC ** | 3.85 | 120 | 285 | 0.40 | 6.7 | [147] | |||
| OMS-MgO | 4.71 | 120 | 1 | 261 | 0.48 | 7.25 | [148] | ||
| OMS-CaO | 3.85 | 120 | 1 | 163 | 0.25 | 6.76 | [148] | ||
| SiO2-Al2O3 | APTS | 2.64 | 25 | 1 | 740 | 1.24 | 5.1 | Grafting | [149] |
| SiO2-Al(NO3)3 | APTS | 0.78 | 25 | 1 | 319 | 0.63 | 2.9 | Grafting | [149] |
| OMS-Ti | 0.81 | 25 | 1 | 487 | [88] | ||||
| MSiNTs | APTES | 2.87 | 25 | 1.2 | 293 | 0.79 | 22 | Grafting | [101] |
| SNS | APTES | 2.13 | 25 | 1.2 | 210 | 0.31 | 19.6 | Grafting | [101] |
| Al(NO3)3 | AP | 0.98 | 25 | 1 | 359 | 0.62 | 10.0 | [150] | |
| OMS-Al-Zr | 2.60 | 60 | 1 | 441 | 0.61 | 6.9 | [151] | ||
5.3. Sorbent Selectivity, Regeneration, and Stability in the Cyclic CO2 Adsorption–Desorption
6. Technical Challenges and Future Trends
7. Summary
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations and Acronyms
| Abbreviation | Definition |
| CO2 | Carbon dioxide |
| Li4SiO4 | Lithium orthosilicate |
| CCS | Carbon dioxide capture |
| PEHA | Pentaethylenehexamine |
| HSSP | Hollow silica spherical particles |
| PEI | Polyethylenimine |
| APTS | 3-aminopropyltriethoxysilane |
| MCC | Mesoporous silica with amidoxime functionalities |
| APTMS | 3-[2-(2-aminoethylamino)ethylamino]propyltrimethoxysilane, |
| TRI | 3-[2-(2-aminoethylamino)ethylamino]propyltrimethoxysilane |
| TEPA | Tetraethylenepentamine |
| TEA | Triethanolamine |
| SNS | Silica nano spheres |
| OMS | Oxide-templated silica |
| NCC | Nanocrystalline cellulose |
| MSiNTs | Mesoporous silica nanotubes |
| MPSM | Monodispersed porous silica microspheres |
| MCC | Microcrystalline cellulose |
| HPS | Hierarchically porous silica |
| MSPD | Matrix solid phase dispersion |
| FCC | Face-centered cubic |
| SBA | Santa Barbara amorphous family |
| MWSA | Microwave-swing adsorption |
| PSA | Pressure swing adsorption |
| TSA | Temperature swing adsorption |
| ESA | Electric swing adsorption |
| VSA | Vacuum swing adsorption |
References
- Osman, A.I.; Hefny, M.; Maksoud, M.A.; Elgarahy, A.M.; Rooney, D.W. Recent advances in carbon capture storage and utilisation technologies: A review. Environ. Chem. Lett. 2020, 19, 797–849. [Google Scholar] [CrossRef]
- Ahn, D. Quantifying the Emissions of Carbon Dioxide (CO2), Carbon Monoxide (Co), and Nitrogen Oxides (Nx) from Human Activities: Top-Down and Bottom-Up Approaches Doctoral Dissertation; University of Maryland: College Park, ML, USA, 2021. [Google Scholar]
- Gunawardene, O.H.; Gunathilake, C.A.; Vikrant, K.; Amaraweera, S.M. Carbon Dioxide Capture through Physical and Chemical Adsorption Using Porous Carbon Materials: A Review. Atmosphere 2022, 13, 397. [Google Scholar] [CrossRef]
- Leung, D.Y.; Caramanna, G.; Maroto-Valer, M.M. An overview of current status of carbon dioxide capture and storage technologies. Renew. Sustain. Energy Rev. 2014, 39, 426–443. [Google Scholar] [CrossRef] [Green Version]
- Omoregbe, O.; Mustapha, A.N.; Steinberger-Wilckens, R.; El-Kharouf, A.; Onyeaka, H. Carbon capture technologies for climate change mitigation: A bibliometric analysis of the scientific discourse during 1998–2018. Energy Rep. 2020, 6, 1200–1212. [Google Scholar] [CrossRef]
- Lee, S.Y.; Park, S.J. A review on solid adsorbents for carbon dioxide capture. J. Ind. Eng. Chem. 2015, 23, 1–11. [Google Scholar] [CrossRef]
- Bui, M.; Gunawan, I.; Verheyen, V.; Feron, P.; Meuleman, E.; Adeloju, S. Dynamic modelling and optimisation of flexible operation in post-combustion CO2 capture plants—A review. Comput. Chem. Eng. 2014, 61, 245–265. [Google Scholar] [CrossRef]
- Cotton, A.; Patchigolla, K.; Oakey, J.E. Minor and trace element emissions from post-combustion CO2 capture from coal: Experimental and equilibrium calculations. Fuel 2014, 117, 391–407. [Google Scholar] [CrossRef] [Green Version]
- Goto, K.; Yogo, K.; Higashii, T. A review of efficiency penalty in a coal-fired power plant with post-combustion CO2 capture. Appl. Energy 2013, 111, 710–720. [Google Scholar] [CrossRef]
- Zhang, N.; Pan, Z.; Zhang, Z.; Zhang, W.; Zhang, L.; Baena-Moreno, F.M.; Lichtfouse, E. CO2 capture from coalbed methane using membranes: A review. Environ. Chem. Lett. 2020, 18, 79–96. [Google Scholar] [CrossRef]
- Sreenivasalu, B.; Gayatri, D.V.; Sreedhar, I.; Ragharan, K.V. A journey into the process and engineering aspects of carbon capture technologies. Renew. Sustain. Energy Rev. 2015, 41, 1324–1350. [Google Scholar] [CrossRef]
- Singh, J.; Bhunia, H.; Basu, S. Development of sulfur-doped carbon monolith derived from phenol-formaldehyde resin for fixed bed CO2 adsorption. Environ. Innov. 2020, 20, 101104. [Google Scholar] [CrossRef]
- Sánchez, J.M.; Maroño, M.; Cillero, D.; Montenegro, L.; Ruiz, E. Laboratory-and bench-scale studies of a sweet water–gas-shift catalyst for H2 and CO2 production in pre-combustion CO2 capture. Fuel 2013, 114, 191–198. [Google Scholar] [CrossRef]
- Babu, P.; Kumar, R.; Linga, P. A new porous material to enhance the kinetics of clathrate process: Application to precombustion carbon dioxide capture. Environ. Sci. Technol. 2013, 47, 13191–13198. [Google Scholar] [CrossRef]
- Wienchol, P.; Szlȩk, A.; Ditaranto, M. Waste-to-energy technology integrated with carbon capture—Challenges and opportunities. Energy 2020, 198, 117352. [Google Scholar] [CrossRef]
- Kim, Y.E.; Moon, S.J.; Yoon, Y.I.; Jeong, S.K.; Park, K.T.; Bae, S.T.; Nam, S.C. Heat of absorption and absorption capacity of CO2 in aqueous solutions of amine containing multiple amino groups. Sep. Purif. Technol. 2014, 122, 112–118. [Google Scholar] [CrossRef]
- Roth, E.A.; Agarwal, S.; Gupta, R.K. Nanoclay-based solid sorbents for CO2 capture. Energy Fuels 2013, 27, 4129–4136. [Google Scholar] [CrossRef]
- Kenarsari, S.D.; Yang, D.; Jiang, G.; Zhang, S.; Wang, J.; Russell, A.G.; Wei, Q.; Fan, M. Review of recent advances in carbon dioxide separation and capture. RSC Adv. 2013, 3, 22739–22773. [Google Scholar] [CrossRef]
- Zhang, X.; He, X.; Gundersen, T. Post-combustion carbon capture with a gas separation membrane: Parametric study, capture cost, and exergy analysis. Energy Fuels 2013, 27, 4137–4149. [Google Scholar] [CrossRef]
- Scholes, C.A.; Ho, M.T.; Wiley, D.E.; Stevens, G.W.; Kentish, S.E. Cost competitive membrane—Cryogenic post-combustion carbon capture. Int. J. Greenh. Gas Control 2013, 17, 341–348. [Google Scholar] [CrossRef]
- Khraisheh, M.; Mukherjee, S.; Kumar, A.; Al Momani, F.; Walker, G.; Zaworotko, M.J. An overview on trace CO2 removal by advanced physisorbent materials. J. Environ. Manag. 2020, 255, 109874. [Google Scholar] [CrossRef]
- Anwar, M.N.; Fayyaz, A.; Sohail, N.F.; Khokhar, M.F.; Baqar, M.; Khan, W.D.; Rasool, K.; Rehan, M.; Nizami, A.S. CO2 capture and storage: A way forward for sustainable environment. J. Environ. Manag. 2018, 226, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Liang, F.; Yang, Y.; Hu, Y.; Zhang, K.; Liu, W. An improved CO2 separation and purification system based on cryogenic separation and distillation theory. Energies 2014, 7, 3484–3502. [Google Scholar] [CrossRef] [Green Version]
- Samanta, A.; Zhao, A.; Shimizu, G.K.; Sarkar, P.; Gupta, R. Post-combustion CO2 capture using solid sorbents: A review. Ind. Eng. Chem. Res. 2012, 51, 1438–1463. [Google Scholar] [CrossRef]
- Patel, H.A.; Byun, J.; Yavuz, C.T. Carbon dioxide capture adsorbents: Chemistry and methods. ChemSusChem 2017, 10, 1303–1317. [Google Scholar] [CrossRef]
- Sumida, K.; Rogow, D.L.; Mason, J.A.; McDonald, T.M.; Bloch, E.D.; Herm, Z.R.; Bae, T.H.; Long, J.R. Carbon dioxide capture in metal–organic frameworks. Chem. Rev. 2012, 112, 724–781. [Google Scholar] [CrossRef]
- Reynolds, A.J.; Verheyen, T.V.; Adeloju, S.B.; Meuleman, E.; Feron, P. Towards commercial scale postcombustion capture of CO2 with monoethanolamine solvent: Key considerations for solvent management and environmental impacts. Environ. Sci. Technol. 2012, 46, 3643–3654. [Google Scholar] [CrossRef]
- Mumford, K.A.; Wu, Y.; Smith, K.H.; Stevens, G.W. Review of solvent based carbon-dioxide capture technologies. Front. Chem. Sci. Eng. 2015, 9, 125–141. [Google Scholar] [CrossRef]
- Park, J.H.; Celedonio, J.M.; Seo, H.; Park, Y.K.; Ko, Y.S. A study on the effect of the amine structure in CO2 dry sorbents on CO2 capture. Catal. Today 2016, 265, 68–76. [Google Scholar] [CrossRef]
- Gunathilake, C.; Gangoda, M.; Jaroniec, M. Mesoporous alumina with amidoxime groups for CO2 sorption at ambient and elevated temperatures. Ind. Eng. Chem. Res. 2016, 55, 5598–5607. [Google Scholar] [CrossRef]
- Lv, B.; Guo, B.; Zhou, Z.; Jing, G. Mechanisms of CO2 capture into monoethanolamine solution with different CO2 loading during the absorption/desorption processes. Environ. Sci. Technol. 2015, 49, 10728–10735. [Google Scholar] [CrossRef]
- García-Abuín, A.; Gomez-Diaz, D.; Lopez, A.B.; Navaza, J.M.; Rumbo, A. NMR characterization of carbon dioxide chemical absorption with monoethanolamine, diethanolamine, and triethanolamine. Ind. Eng. Chem. Res. 2013, 52, 13432–13438. [Google Scholar] [CrossRef]
- Kim, Y.E.; Lim, J.A.; Jeong, S.K.; Yoon, Y.I.; Bae, S.T.; Nam, S.C. Comparison of carbon dioxide absorption in aqueous MEA, DEA, TEA, and AMP solutions. Bull. Korean Chem. Soc. 2013, 34, 783–787. [Google Scholar] [CrossRef] [Green Version]
- Rinprasertmeechai, S.; Chavadej, S.; Rangsunvigit, P.; Kulprathipanja, S. Carbon dioxide removal from flue gas using amine-based hybrid solvent absorption. Int. J. Chem. Eng. 2012, 6, 296–300. [Google Scholar]
- Aaron, D.; Tsouris, C. Separation of CO2 from flue gas: A review. Sep. Sci. Technol. 2005, 40, 321–348. [Google Scholar] [CrossRef]
- Songolzadeh, M.; Soleimani, M.; Takht Ravanchi, M.; Songolzadeh, R. Carbon dioxide separation from flue gases: A technological review emphasizing reduction in greenhouse gas emissions. Sci. World J. 2014, 2014, 828131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.; Lawal, A.; Stephenson, P.; Sidders, J.; Ramshaw, C. Post-combustion CO2 capture with chemical absorption: A state-of-the-art review. Chem. Eng. Res. Des. 2011, 89, 1609–1624. [Google Scholar] [CrossRef] [Green Version]
- Dave, N.; Do, T.; Puxty, G.; Rowland, R.; Feron, P.H.M.; Attalla, M.I. CO2 capture by aqueous amines and aqueous ammonia–A Comparison. Energy Procedia 2009, 1, 949–954. [Google Scholar] [CrossRef] [Green Version]
- Zeng, S.; Zhang, X.; Bai, L.; Zhang, X.; Wang, H.; Wang, J.; Bao, D.; Li, M.; Liu, X.; Zhang, S. Ionic-liquid-based CO2 capture systems: Structure, interaction and process. Chem. Rev. 2017, 117, 9625–9673. [Google Scholar] [CrossRef]
- Borhani, T.N.; Wang, M. Role of solvents in CO2 capture processes: The review of selection and design methods. Renew. Sustain. Energy Rev. 2019, 114, 109299. [Google Scholar] [CrossRef]
- Aghaie, M.; Rezaei, N.; Zendehboudi, S. A systematic review on CO2 capture with ionic liquids: Current status and future prospects. Renew. Sustain. Energy Rev. 2018, 96, 502–525. [Google Scholar] [CrossRef]
- Jin, X.; Ge, J.; Zhang, L.; Wu, Z.; Zhu, L.; Xiong, M. Synthesis of Hierarchically Ordered Porous Silica Materials for CO2 Capture: The Role of Pore Structure and Functionalized Amine. Inorganics 2022, 10, 87. [Google Scholar] [CrossRef]
- Zagho, M.M.; Hassan, M.K.; Khraisheh, M.; Al-Maadeed, M.A.A.; Nazarenko, S. A review on recent advances in CO2 separation using zeolite and zeolite-like materials as adsorbents and fillers in mixed matrix membranes (MMMs). Chem. Eng. J. Adv. 2021, 6, 100091. [Google Scholar] [CrossRef]
- Yu, C.H.; Huang, C.H.; Tan, C.S. A review of CO2 capture by absorption and adsorption. Aerosol Air Qual. Res. 2012, 12, 745–769. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Bera, R.; Das, N.; Koh, J. Chitosan-based Zeolite-Y and ZSM-5 porous biocomposites for H2 and CO2 storage. Carbohydr. Polym. 2020, 232, 115808. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Srivastava, R.; Koh, J. Utilization of zeolites as CO2 capturing agents: Advances and future perspectives. J. CO2 Util. 2020, 41, 101251. [Google Scholar] [CrossRef]
- Balashankar, V.S.; Rajendran, A. Process optimization-based screening of zeolites for post-combustion CO2 capture by vacuum swing adsorption. ACS Sustain. Chem. Eng. 2019, 7, 17747–17755. [Google Scholar] [CrossRef]
- Avci, G.; Erucar, I.; Keskin, S. Do new MOFs perform better for CO2 capture and H2 purification? Computational screening of the updated MOF database. ACS Appl. Mater. Interfaces 2020, 12, 41567–41579. [Google Scholar] [CrossRef]
- Modak, A.; Jana, S. Advancement in porous adsorbents for post-combustion CO2 capture. Microporous Mesoporous Mater. 2019, 276, 107–132. [Google Scholar] [CrossRef]
- Le, M.U.T.; Lee, S.Y.; Park, S.J. Preparation and characterization of PEI-loaded MCM-41 for CO2 capture. Int. J. Hydrogen Energy 2014, 39, 12340–12346. [Google Scholar]
- Kim, M.I.; Choi, S.J.; Kim, D.W.; Park, D.W. Catalytic performance of zinc containing ionic liquids immobilized on silica for the synthesis of cyclic carbonates. J. Ind. Eng. Chem. 2014, 20, 3102–3107. [Google Scholar] [CrossRef]
- Alkhabbaz, M.A.; Khunsupat, R.; Jones, C.W. Guanidinylated poly (allylamine) supported on mesoporous silica for CO2 capture from flue gas. Fuel 2014, 121, 79–85. [Google Scholar] [CrossRef]
- Reddy, M.S.B.; Ponnamma, D.; Sadasivuni, K.K.; Kumar, B.; Abdullah, A.M. Carbon dioxide adsorption based on porous materials. RSC Adv. 2021, 11, 12658–12681. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.S.; Shi, X.D.; Wang, C.T.; Gao, Y.Z.; Xu, S.; Hao, G.P.; Chen, S.; Lu, A.H. Advances in Post-Combustion CO2 Capture by Physical Adsorption: From Materials Innovation to Separation Practice. ChemSusChem 2021, 14, 1428–1471. [Google Scholar] [CrossRef] [PubMed]
- Boot-Handford, M.E.; Abanades, J.C.; Anthony, E.J.; Blunt, M.J.; Brandani, S.; Mac Dowell, N.; Fernández, J.R.; Ferrari, M.C.; Gross, R.; Hallett, J.P.; et al. Carbon capture and storage update. Energy Environ. Sci. 2014, 7, 130–189. [Google Scholar] [CrossRef]
- MacFarlane, D.R.; Tachikawa, N.; Forsyth, M.; Pringle, J.M.; Howlett, P.C.; Elliott, G.D.; Davis, J.H.; Watanabe, M.; Simon, P.; Angell, C.A. Energy applications of ionic liquids. Energy Environ. Sci. 2014, 7, 232–250. [Google Scholar] [CrossRef] [Green Version]
- Tuci, G.; Iemhoff, A.; Ba, H.; Luconi, L.; Rossin, A.; Papaefthimiou, V.; Palkovits, R.; Artz, J.; Pham-Huu, C.; Giambastiani, G. Playing with covalent triazine framework tiles for improved CO2 adsorption properties and catalytic performance. Beilstein J. Nanotechnol. 2019, 10, 1217–1227. [Google Scholar] [CrossRef] [Green Version]
- Madden, D.G.; O’Nolan, D.; Chen, K.J.; Hua, C.; Kumar, A.; Pham, T.; Forrest, K.A.; Space, B.; Perry, J.J.; Khraisheh, M.; et al. Highly selective CO2 removal for one-step liquefied natural gas processing by physisorbents. Chem. Commun. 2019, 55, 3219–3222. [Google Scholar] [CrossRef]
- Balsamo, M.; Budinova, T.; Erto, A.; Lancia, A.; Petrova, B.; Petrov, N.; Tsyntsarski, B. CO2 adsorption onto synthetic activated carbon: Kinetic, thermodynamic and regeneration studies. Sep. Purif. Technol. 2013, 116, 214–221. [Google Scholar] [CrossRef]
- Cherbanski, R.; Komorowska-Durka, M.; Stefanidis, G.D.; Stankiewicz, A.I. Microwave Swing Regeneration Vs Temperature Swing Regeneration Comparison of Desorption Kinetics. Ind. Eng. Chem. Res. 2011, 50, 8632–8644. [Google Scholar] [CrossRef]
- Maity, A.; Belgamwar, R.; Polshettiwar, V. Facile synthesis to tune size, textural properties and fiber density of dendritic fibrous nanosilica for applications in catalysis and CO2 capture. Nat. Protoc. 2019, 14, 2177–2204. [Google Scholar] [CrossRef]
- Miricioiu, M.G.; Niculescu, V.C. Fly ash, from recycling to potential raw material for mesoporous silica synthesis. Nanomaterials 2020, 10, 474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ying, W.; Zhao, D. On the controllable soft-templating approach to mesoporous silicates. Chem. Rev. 2007, 107, 2821–2860. [Google Scholar]
- Narayan, R.; Nayak, U.Y.; Raichur, A.M.; Garg, S. Mesoporous silica nanoparticles: A comprehensive review on synthesis and recent advances. Pharmaceutics 2018, 10, 118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, J.A.S.; de Jesus, R.A.; Santos, D.O.; Mano, J.F.; Romao, L.P.; Paranhos, C.M. Recent progresses in the adsorption of organic, inorganic, and gas compounds by MCM-41-based mesoporous materials. Microporous Mesoporous Mater. 2020, 291, 109698. [Google Scholar] [CrossRef]
- Brezoiu, A.M.; Deaconu, M.; Nicu, I.; Vasile, E.; Mitran, R.A.; Matei, C.; Berger, D. Heteroatom modified MCM-41-silica carriers for Lomefloxacin delivery systems. Microporous Mesoporous Mater. 2019, 275, 214–222. [Google Scholar] [CrossRef]
- Jesus, R.A.; Rabelo, A.S.; Figueiredo, R.T.; Da Silva, L.C.; Codentino, I.C.; Fantini, M.C.D.A.; Araújo, G.L.B.D.; Araújo, A.A.S.; Mesquita, M.E. Synthesis and application of the MCM-41 and SBA-15 as matrices for in vitro efavirenz release study. J. Drug Deliv. Sci. Technol. 2016, 31, 153–159. [Google Scholar] [CrossRef]
- Liu, Y.; Li, C.; Peyravi, A.; Sun, Z.; Zhang, G.; Rahmani, K.; Zheng, S.; Hashisho, Z. Mesoporous MCM-41 derived from natural Opoka and its application for organic vapors removal. J. Hazard. Mater. 2021, 408, 124911. [Google Scholar] [CrossRef]
- Costa, J.A.S.; Paranhos, C.M. Mitigation of silica-rich wastes: An alternative to the synthesis eco-friendly silica-based mesoporous materials. Microporous Mesoporous Mater. 2020, 309, 110570. [Google Scholar] [CrossRef]
- Björk, E.M. Synthesizing and characterizing mesoporous silica SBA-15: A hands-on laboratory experiment for undergraduates using various instrumental techniques. J. Chem. Educ. 2017, 94, 91–94. [Google Scholar] [CrossRef] [Green Version]
- Martínez, M.L.; Ponte, M.V.; Beltramone, A.R.; Anunziata, O.A. Synthesis of ordered mesoporous SBA-3 materials using silica gel as silica source. Mater. Lett. 2014, 134, 95–98. [Google Scholar] [CrossRef]
- López-Mendoza, M.A.; Nava, R.; Peza-Ledesma, C.; Millán-Malo, B.; Huirache-Acuña, R.; Skewes, P.; Rivera-Muñoz, E.M. Characterization and catalytic performance of Co-Mo-W sulfide catalysts supported on SBA-15 and SBA-16 mechanically mixed. Catal. Today 2016, 271, 114–126. [Google Scholar] [CrossRef]
- Gonzalez, G.; Sagarzazu, A.; Cordova, A.; Gomes, M.E.; Salas, J.; Contreras, L.; Noris-Suarez, K.; Lascano, L. Comparative study of two silica mesoporous materials (SBA-16 and SBA-15) modified with a hydroxyapatite layer for clindamycin controlled delivery. Microporous Mesoporous Mater. 2018, 256, 251–265. [Google Scholar] [CrossRef]
- Wu, Q.; Li, Y.; Hou, Z.; Xin, J.; Meng, Q.; Han, L.; Xiao, C.; Hu, D.; Duan, A.; Xu, C. Synthesis and characterization of Beta-FDU-12 and the hydrodesulfurization performance of FCC gasoline and diesel. Fuel Process. Technol. 2018, 172, 55–64. [Google Scholar] [CrossRef]
- Sanaeishoar, H.; Sabbaghan, M.; Mohave, F.; Nazarpour, R. Disordered mesoporous KIT-1 synthesized by DABCO-based ionic liquid and its characterization. Microporous Mesoporous Mater. 2016, 228, 305–309. [Google Scholar] [CrossRef]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Choma, J.; Jamioła, D.; Augustynek, K.; Marszewski, M.; Gao, M.; Jaroniec, M. New opportunities in Stöber synthesis: Preparation of microporous and mesoporous carbon spheres. J. Mater. Chem. 2012, 22, 12636–12642. [Google Scholar] [CrossRef]
- Wu, S.H.; Mou, C.Y.; Lin, H.P. Synthesis of mesoporous silica nanoparticles. Chem. Soc. Rev. 2013, 42, 3862–3875. [Google Scholar] [CrossRef]
- Sakamoto, S.; Yoshikawa, M.; Ozawa, K.; Kuroda, Y.; Shimojima, A.; Kuroda, K. Formation of single-digit nanometer scale silica nanoparticles by evaporation-induced self-assembly. Langmuir 2018, 34, 1711–1717. [Google Scholar] [CrossRef]
- Sihler, S.; Nguyen, P.L.; Lindén, M.; Ziener, U. Green chemistry in red emulsion: Interface of dye stabilized emulsions as a powerful platform for the formation of sub-20-nm SiO2 nanoparticles. ACS Appl. Mater. Interfaces 2018, 10, 24310–24319. [Google Scholar] [CrossRef]
- Murray, E.; Born, P.; Weber, A.; Kraus, T. Synthesis of Monodisperse Silica Nanoparticles Dispersable in Non-Polar Solvents. Adv. Eng. Mater. 2010, 12, 374–378. [Google Scholar] [CrossRef]
- Mandal, M.; Kruk, M. Family of single-micelle-templated organosilica hollow nanospheres and nanotubes synthesized through adjustment of organosilica/surfactant ratio. Chem. Mater. 2012, 24, 123–132. [Google Scholar] [CrossRef]
- Savic, S.; Vojisavljevic, K.; Počuča-Nešić, M.; Zivojevic, K.; Mladenovic, M.; Knezevic, N. Hard Template Synthesis of Nanomaterials Based on Mesoporous Silica. Metall. Mater. Eng. 2018, 24, 225–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, T.; Elzatahry, A.; Li, X.; Zhao, D. Single-micelle-directed synthesis of mesoporous materials. Nat. Rev. Mater. 2019, 4, 775–791. [Google Scholar] [CrossRef]
- Khan, A.H.; Ghosh, S.; Pradhan, B.; Dalui, A.; Shrestha, L.K.; Acharya, S.; Ariga, K. Two-dimensional (2D) nanomaterials towards electrochemical nanoarchitectonics in energy-related applications. Bull. Chem. Soc. Jpn. 2017, 90, 627–648. [Google Scholar] [CrossRef]
- Yamamoto, E.; Kuroda, K. Colloidal mesoporous silica nanoparticles. Bull. Chem. Soc. Jpn. 2016, 89, 501–539. [Google Scholar] [CrossRef]
- Kim, H.J.; Yang, H.C.; Chung, D.Y.; Yang, I.H.; Choi, Y.J.; Moon, J.K. Functionalized mesoporous silica membranes for CO2 separation applications. J. Chem. 2015, 2015, 202867. [Google Scholar] [CrossRef] [Green Version]
- Gunathilake, C.; Kalpage, C.; Kadanapitiye, M.; Dassanayake, R.S.; Manchanda, A.S.; Gangoda, M. Facile synthesis and surface characterization of titania-incorporated mesoporous organosilica materials. J. Compos. Sci. 2019, 3, 77. [Google Scholar] [CrossRef] [Green Version]
- Hao, P.; Peng, B.; Shan, B.Q.; Yang, T.Q.; Zhang, K. Comprehensive understanding of the synthesis and formation mechanism of dendritic mesoporous silica nanospheres. Nanoscale Adv. 2020, 2, 1792–1810. [Google Scholar] [CrossRef]
- Panek, R.; Wdowin, M.; Franus, W.; Czarna, D.; Stevens, L.A.; Deng, H.; Liu, J.; Sun, C.; Liu, H.; Snape, C.E. Fly ash-derived MCM-41 as a low-cost silica support for polyethyleneimine in post-combustion CO2 capture. J. CO2 Util. 2017, 22, 81–90. [Google Scholar] [CrossRef]
- Singh, B.; Polshettiwar, V. Solution-phase synthesis of two-dimensional silica nanosheets using soft templates and their applications in CO2 capture. Nanoscale 2019, 11, 5365–5376. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Cao, M. Three-dimensional porous carbon frameworks derived from mangosteen peel waste as promising materials for CO2 capture and supercapacitors. J. CO2 Util. 2018, 27, 204–216. [Google Scholar] [CrossRef]
- Ahmed, S.; Ramli, A.; Yusup, S. Development of polyethylenimine-functionalized mesoporous Si-MCM-41 for CO2 adsorption. Fuel Process. Technol. 2017, 167, 622–630. [Google Scholar] [CrossRef]
- Son, W.J.; Choi, J.S.; Ahn, W.S. Adsorptive removal of carbon dioxide using polyethyleneimine-loaded mesoporous silica materials. Microporous Mesoporous Mater. 2008, 113, 31–40. [Google Scholar] [CrossRef]
- Zeleňák, V.; Badaničová, M.; Halamova, D.; Čejka, J.; Zukal, A.; Murafa, N.; Goerigk, G. Amine-modified ordered mesoporous silica: Effect of pore size on carbon dioxide capture. Chem. Eng. J. 2008, 144, 336–342. [Google Scholar] [CrossRef]
- Lashaki, M.J.; Sayari, A. CO2 capture using triamine-grafted SBA-15: The impact of the support pore structure. Chem. Eng. J. 2018, 334, 1260–1269. [Google Scholar] [CrossRef]
- Kishor, R.; Ghoshal, A.K. APTES grafted ordered mesoporous silica KIT-6 for CO2 adsorption. Chem. Eng. J. 2015, 262, 882–890. [Google Scholar] [CrossRef]
- Ahmed, S.; Ramli, A.; Yusup, S. CO2 adsorption study on primary, secondary and tertiary amine functionalized Si-MCM-41. Int. J. Greenh. Gas Control 2016, 51, 230–238. [Google Scholar] [CrossRef]
- Nigar, H.; Garcia-Baños, B.; Peñaranda-Foix, F.L.; Catalá-Civera, J.M.; Mallada, R.; Santamaría, J. Amine-functionalized mesoporous silica: A material capable of CO2 adsorption and fast regeneration by microwave heating. AIChE J. 2016, 62, 547–555. [Google Scholar] [CrossRef]
- Heydari-Gorji, A.; Yang, Y.; Sayari, A. Effect of the pore length on CO2 adsorption over amine-modified mesoporous silicas. Energy Fuels 2011, 25, 4206–4210. [Google Scholar] [CrossRef]
- Gunathilake, C.; Manchanda, A.S.; Ghimire, P.; Kruk, M.; Jaroniec, M. Amine-modified silica nanotubes and nanospheres: Synthesis and CO2 sorption properties. Environ. Sci. 2016, 3, 806–817. [Google Scholar] [CrossRef]
- Cabriga, C.K.C.; Clarete, K.V.R.; Zhang, J.A.T.; Pacia, R.M.P.; Ko, Y.S.; Castro, J.C. Evaluation of biochar derived from the slow pyrolysis of rice straw as a potential adsorbent for carbon dioxide. Biomass Convers. Biorefin. 2021, 13, 7887–7894. [Google Scholar] [CrossRef]
- Wang, J.; Yuan, X.; Deng, S.; Zeng, X.; Yu, Z.; Li, S.; Lia, K. Waste polyethylene terephthalate (PET) plastics-derived activated carbon for CO2 capture: A route to a closed carbon loop. Green Chem. 2020, 22, 6836–6845. [Google Scholar] [CrossRef]
- Berger, A.H.; Bhown, A.S. Comparing physisorption and chemisorption solid sorbents for use separating CO2 from flue gas using temperature swing adsorption. Energy Proc. 2011, 4, 562–567. [Google Scholar] [CrossRef] [Green Version]
- Moni, P.; Chaves, W.F.; Wilhelm, M.; Rezwan, K. Polysiloxane microspheres encapsulated in carbon allotropes: A promising material for supercapacitor and carbon dioxide capture. J. Colloid Interface Sci. 2019, 542, 91–101. [Google Scholar] [CrossRef]
- Hahn, M.W.; Jelic, J.; Berger, E.; Reuter, K.; Jentys, A.; Lercher, J.A. Role of amine functionality for CO2 chemisorption on silica. J. Phys. Chem. B 2016, 120, 1988–1995. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Yang, S.T.; Ahn, W.S.; Ryoo, R. Amine-impregnated silica monolith with a hierarchical pore structure: Enhancement of CO2 capture capacity. Chem. Commun. 2009, 120, 627–3629. [Google Scholar] [CrossRef]
- Chen, C.; Son, W.J.; You, K.S.; Ahn, J.W.; Ahn, W.S. Carbon dioxide capture using amine-impregnated HMS having textural mesoporosity. Chem. Eng. J. 2010, 161, 46–52. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, S.; Row, K.H.; Ahn, W.S. Amine–silica composites for CO2 capture: A short review. J. Energy Chem. 2017, 26, 868–880. [Google Scholar] [CrossRef] [Green Version]
- Wei, J.; Liao, L.; Xiao, Y.; Zhang, P.; Shi, Y. Capture of carbon dioxide by amine-impregnated as-synthesized MCM-41. J. Environ. Sci. 2010, 22, 1558–1563. [Google Scholar] [CrossRef]
- Chew, T.L.; Ahmad, A.L.; Bhatia, S. Ordered mesoporous silica (OMS) as an adsorbent and membrane for separation of carbon dioxide (CO2). Adv. Colloid Interface Sci. 2010, 153, 43–57. [Google Scholar] [CrossRef]
- Li, W.; Choi, S.; Drese, J.H.; Hornbostel, M.; Krishnan, G.; Eisenberger, P.M.; Jones, C.W. Steam-stripping for regeneration of supported amine-based CO2 adsorbents. ChemSusChem 2010, 3, 899–903. [Google Scholar] [CrossRef] [PubMed]
- Bollini, P.; Didas, S.A.; Jones, C.W. Amine-oxide hybrid materials for acid gas separations. J. Mater. Chem. 2011, 21, 15100–15120. [Google Scholar] [CrossRef]
- Li, Y.; Sun, N.; Li, L.; Zhao, N.; Xiao, F.; Wei, W.; Sun, Y.; Huang, W. Grafting of amines on ethanol-extracted SBA-15 for CO2 adsorption. Materials 2013, 6, 981–999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez-Aranguren, P.; Builes, S.; Fraile, J.; Vega, L.F.; Domingo, C. Understanding the performance of new amine-functionalized mesoporous silica materials for CO2 adsorption. Ind. Eng. Chem. Res. 2014, 53, 15611–15619. [Google Scholar] [CrossRef]
- Sanz, R.; Calleja, G.; Arencibia, A.; Sanz-Perez, E.S. CO2 capture with pore-expanded MCM-41 silica modified with amino groups by double functionalization. Microporous Mesoporous Mater. 2015, 209, 165–171. [Google Scholar] [CrossRef]
- Wang, X.; Guo, Q.; Zhao, J.; Chen, L. Mixed amine-modified MCM-41 sorbents for CO2 capture. Int. J. Greenh. Gas Control 2015, 37, 90–98. [Google Scholar] [CrossRef]
- Jung, H.; Lee, C.H.; Jeon, S.; Jo, D.H.; Huh, J.; Kim, S.H. Effect of amine double-functionalization on CO2 adsorption behaviors of silica gel-supported adsorbents. Adsorption 2016, 22, 1137–1146. [Google Scholar] [CrossRef]
- Fillerup, E.; Zhang, Z.; Peduzzi, E.; Wang, D.; Guo, J.; Ma, X.; Wang, X.; Song, C. CO Capture from Flue Gas Using Solid Molecular Basket Sorbents; The Pennsylvania State University: State College, PA, USA, 2012. [Google Scholar]
- Gargiulo, N.; Peluso, A.; Aprea, P.; Pepe, F.; Caputo, D. CO2 adsorption on polyethylenimine-functionalized SBA-15 mesoporous silica: Isotherms and modeling. J. Chem. Eng. Data 2014, 59, 896–902. [Google Scholar] [CrossRef]
- Heydari-Gorji, A.; Sayari, A. Thermal, oxidative, and CO2-induced degradation of supported polyethylenimine adsorbents. Ind. Eng. Chem. Res. 2012, 51, 6887–6894. [Google Scholar] [CrossRef]
- Kuwahara, Y.; Kang, D.Y.; Copeland, J.R.; Brunelli, N.A.; Didas, S.A.; Bollini, P.; Sievers, C.; Kamegawa, T.; Yamashita, H.; Jones, C.W. Dramatic enhancement of CO2 uptake by poly (ethyleneimine) using zirconosilicate supports. J. Am. Chem. Soc. 2012, 134, 10757–10760. [Google Scholar] [CrossRef]
- Kishor, R.; Ghoshal, A.K. Amine-modified mesoporous silica for CO2 adsorption: The role of structural parameters. Ind. Eng. Chem. Res. 2017, 56, 6078–6087. [Google Scholar] [CrossRef]
- Ko, Y.G.; Lee, H.J.; Oh, H.C.; Choi, U.S. Amines immobilized double-walled silica nanotubes for CO2 capture. J. Hazard. Mater. 2013, 250, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Drese, J.H.; Eisenberger, P.M.; Jones, C.W. Application of amine-tethered solid sorbents for direct CO2 capture from the ambient air. Environ. Sci. Technol. 2011, 45, 2420–2427. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Ding, L.; Kanamori, K.; Nakanishi, K.; Yang, H. Functionalization of hierarchically porous silica monoliths with polyethyleneimine (PEI) for CO2 adsorption. Microporous Mesoporous Mater. 2017, 245, 51–57. [Google Scholar] [CrossRef]
- Liu, Z.; Teng, Y.; Zhang, K.; Chen, H.; Yang, Y. CO2 adsorption performance of different amine-based siliceous MCM-41 materials. J. Energy Chem. 2015, 24, 322–330. [Google Scholar] [CrossRef]
- de Carvalho, L.S.; Silva, E.; Andrade, J.C.; Silva, J.A.; Urbina, M.; Nascimento, P.F.; Carvalho, F.; Ruiz, J.A. Low-cost mesoporous adsorbents amines-impregnated for CO2 capture. Adsorption 2015, 21, 597–609. [Google Scholar] [CrossRef]
- López-Aranguren, P.; Builes, S.; Fraile, J.; López-Periago, A.; Vega, L.F.; Domingo, C. Hybrid aminopolymer–silica materials for efficient CO2 adsorption. RSC Adv. 2015, 5, 104943–104953. [Google Scholar] [CrossRef]
- Mello, M.R.; Phanon, D.; Silveira, G.Q.; Llewellyn, P.L.; Ronconi, C.M. Amine-modified MCM-41 mesoporous silica for carbon dioxide capture. Microporous Mesoporous Mater. 2011, 143, 174–179. [Google Scholar] [CrossRef]
- Ahmed, S.; Ramli, A.; Yusup, S.; Farooq, M. Adsorption behavior of tetraethylenepentamine-functionalized Si-MCM-41 for CO2 adsorption. Chem. Eng. Res. Des. 2017, 122, 33–42. [Google Scholar] [CrossRef]
- Niu, M.; Yang, H.; Zhang, X.; Wang, Y.; Tang, A. Amine-impregnated mesoporous silica nanotube as an emerging nanocomposite for CO2 capture. ACS Appl. Mater. Interfaces 2016, 8, 17312–17320. [Google Scholar] [CrossRef]
- Ullah, R.; Atilhan, M.; Aparicio, S.; Canlier, A.; Yavuz, C.T. Insights of CO2 adsorption performance of amine impregnated mesoporous silica (SBA-15) at wide range pressure and temperature conditions. Int. J. Greenh. Gas Control 2015, 43, 22–32. [Google Scholar] [CrossRef]
- Sánchez-Vicente, Y.; Stevens, L.A.; Pando, C.; Torralvo, M.J.; Snape, C.E.; Drage, T.C.; Cabañas, A. A new sustainable route in supercritical CO2 to functionalize silica SBA-15 with 3-aminopropyltrimethoxysilane as material for carbon capture. J. Chem. Eng. 2015, 264, 886–898. [Google Scholar] [CrossRef]
- Zhao, A.; Samanta, A.; Sarkar, P.; Gupta, R. Carbon dioxide adsorption on amine-impregnated mesoporous SBA-15 sorbents: Experimental and kinetics study. Ind. Eng. Chem. Res. 2013, 52, 6480–6491. [Google Scholar] [CrossRef]
- Gholami, M.; Talaie, M.R.; Aghamiri, S.F. Direct synthesis of bi-modal porous structure MCM-41 and its application in CO2 capturing through amine-grafting. Korean J. Chem. Eng. 2014, 31, 322–326. [Google Scholar] [CrossRef]
- Loganathan, S.; Tikmani, M.; Ghoshal, A.K. Novel pore-expanded MCM-41 for CO2 capture: Synthesis and characterization. Langmuir 2013, 29, 3491–3499. [Google Scholar] [CrossRef]
- Kassab, H.; Maksoud, M.; Aguado, S.; Pera-Titus, M.; Albela, B.; Bonneviot, L. Polyethylenimine covalently grafted on mesostructured porous silica for CO2 capture. RSC Adv. 2012, 2, 2508–2516. [Google Scholar] [CrossRef]
- Teng, Y.; Li, L.; Xu, G.; Zhang, K.; Li, K. Promoting effect of inorganic alkali on carbon dioxide adsorption in amine-modified MCM-41. Energies 2016, 9, 667. [Google Scholar] [CrossRef] [Green Version]
- Sharma, P.; Seong, J.K.; Jung, Y.H.; Choi, S.H.; Park, S.D.; Yoon, Y.I.; Baek, I.H. Amine modified and pelletized mesoporous materials: Synthesis, textural–mechanical characterization and application in adsorptive separation of carbondioxide. Powder Technol. 2012, 219, 86–98. [Google Scholar] [CrossRef]
- Yan, X.; Komarneni, S.; Yan, Z. CO2 adsorption on Santa Barbara Amorphous-15 (SBA-15) and amine-modified Santa Barbara Amorphous-15 (SBA-15) with and without controlled microporosity. J. Colloid Interface Sci. 2013, 390, 217–224. [Google Scholar] [CrossRef]
- Sanz, R.; Calleja, G.; Arencibia, A.; Sanz-Pérez, E.S. Amino functionalized mesostructured SBA-15 silica for CO2 capture: Exploring the relation between the adsorption capacity and the distribution of amino groups by TEM. Microporous Mesoporous Mater. 2012, 158, 309–317. [Google Scholar] [CrossRef]
- Rao, N.; Wang, M.; Shang, Z.; Hou, Y.; Fan, G.; Li, J. CO2 adsorption by amine-functionalized MCM-41: A comparison between impregnation and grafting modification methods. Energy Fuels 2018, 32, 670–677. [Google Scholar] [CrossRef]
- Sim, K.; Lee, N.; Kim, J.; Cho, E.B.; Gunathilake, C.; Jaroniec, M. CO2 adsorption on amine-functionalized periodic mesoporous benzenesilicas. ACS Appl. Mater. Interfaces 2015, 7, 6792–6802. [Google Scholar] [CrossRef] [PubMed]
- Dassanayake, R.S.; Gunathilake, C.; Dassanayake, A.C.; Abidi, N.; Jaroniec, M. Amidoxime-functionalized nanocrystalline cellulose–mesoporous silica composites for carbon dioxide sorption at ambient and elevated temperatures. J. Mater. Chem. A 2017, 5, 7462–7473. [Google Scholar] [CrossRef]
- Gunathilake, C.; Dassanayake, R.S.; Abidi, N.; Jaroniec, M. Amidoxime-functionalized microcrystalline cellulose–mesoporous silica composites for carbon dioxide sorption at elevated temperatures. J. Mater. Chem. A 2016, 4, 4808–4819. [Google Scholar] [CrossRef]
- Gunathilake, C.; Jaroniec, M. Mesoporous calcium oxide–silica and magnesium oxide–silica composites for CO2 capture at ambient and elevated temperatures. J. Mater. Chem. A 2016, 4, 10914–10924. [Google Scholar] [CrossRef]
- Gunathilake, C.; Dassanayake, R.S.; Kalpage, C.S.; Jaroniec, M. Development of Alumina–Mesoporous Organosilica Hybrid Materials for Carbon Dioxide Adsorption at 25 °C. Materials 2018, 11, 2301. [Google Scholar] [CrossRef] [Green Version]
- Gunathilake, C.; Gangoda, M.; Jaroniec, M. Mesoporous isocyanurate-containing organosilica–alumina composites and their thermal treatment in nitrogen for carbon dioxide sorption at elevated temperatures. J. Mater. Chem. A 2013, 1, 8244–8252. [Google Scholar] [CrossRef]
- Gunathilake, C.; Jaroniec, M. Mesoporous alumina–zirconia–organosilica composites for CO2 capture at ambient and elevated temperatures. J. Mater. Chem. A 2015, 3, 2707–2716. [Google Scholar] [CrossRef]
- Choi, W.; Min, K.; Kim, C.; Ko, Y.S.; Jeon, J.W.; Seo, H.; Park, Y.K.; Choi, M. Epoxide-functionalization of polyethyleneimine for synthesis of stable carbon dioxide adsorbent in temperature swing adsorption. Nat. Commun. 2016, 7, 12640. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Liu, W.; Yang, Y.; Qu, M.; Li, H. CO2 capture by Li4SiO4 sorbents and their applications: Current developments and new trends. J. Chem. Eng. 2019, 359, 604–625. [Google Scholar] [CrossRef]
- Wang, L.; Yang, R.T. Increasing selective CO2 adsorption on amine-grafted SBA-15 by increasing silanol density. J. Phys. Chem. C 2011, 115, 21264–21272. [Google Scholar] [CrossRef]
- Rafigh, S.M.; Heydarinasab, A. Mesoporous chitosan–SiO2 nanoparticles: Synthesis, characterization, and CO2 adsorption capacity. ACS Sustain. Chem. Eng. 2017, 5, 10379–10386. [Google Scholar] [CrossRef]
- Yang, J.; Li, J.; Wang, W.; Li, L.; Li, J. Adsorption of CO2, CH4, and N2 on 8-, 10-, and 12-membered ring hydrophobic microporous high-silica zeolites: DDR, silicalite-1, and beta. Ind. Eng. Chem. Res. 2013, 52, 17856–17864. [Google Scholar] [CrossRef]
- Zohdi, S.; Anbia, M.; Salehi, S. Improved CO2 adsorption capacity and CO2/CH4 and CO2/N2 selectivity in novel hollow silica particles by modification with multi-walled carbon nanotubes containing amine groups. Polyhedron 2019, 166, 175–185. [Google Scholar] [CrossRef]
- Fernandes, J.; Fernandes, A.C.; Echeverría, J.C.; Moriones, P.; Garrido, J.J.; Pires, J. Adsorption of gases and vapours in silica based xerogels. Colloids Surf. A Physicochem. Eng. Asp. 2019, 561, 128–135. [Google Scholar] [CrossRef]
- Liu, X.; Gao, F.; Xu, J.; Zhou, L.; Liu, H.; Hu, J. Zeolite@ Mesoporous silica-supported-amine hybrids for the capture of CO2 in the presence of water. Microporous Mesoporous Mater. 2016, 222, 113–119. [Google Scholar] [CrossRef]
- Wei, L.; Jing, Y.; Gao, Z.; Wang, Y. Development of a pentaethylenehexamine-modified solid support adsorbent for CO2 capture from model flue gas. Chin. J. Chem. Eng. 2015, 23, 366–371. [Google Scholar] [CrossRef]
- Yan, X.; Zhang, L.; Zhang, Y.; Yang, G.; Yan, Z. Amine-modified SBA-15: Effect of pore structure on the performance for CO2 capture. Ind. Eng. Chem. Res. 2011, 50, 3220–3226. [Google Scholar] [CrossRef]
- Zhang, X.; Qin, H.; Zheng, X.; Wu, W. Development of efficient amine-modified mesoporous silica SBA-15 for CO2 capture. Mater. Res. Bull. 2013, 48, 3981–3986. [Google Scholar] [CrossRef]
- Subagyono, D.J.; Marshall, M.; Knowles, G.P.; Chaffee, A.L. CO2 adsorption by amine modified siliceous mesostructured cellular foam (MCF) in humidified gas. Microporous Mesoporous Mater. 2014, 186, 84–93. [Google Scholar] [CrossRef]
- Ma, J.; Liu, Q.; Chen, D.; Wen, S.; Wang, T. CO2 adsorption on amine-modified mesoporous silicas. J. Porous Mater. 2014, 21, 859–867. [Google Scholar] [CrossRef]
- Zhang, H.; Goeppert, A.; Prakash, G.S.; Olah, G. Applicability of linear polyethylenimine supported on nano-silica for the adsorption of CO2 from various sources including dry air. RSC Adv. 2015, 5, 52550–52562. [Google Scholar] [CrossRef]
- Wang, X.; Chen, L.; Guo, Q. Development of hybrid amine-functionalized MCM-41 sorbents for CO2 capture. Chem. Eng. J. 2015, 260, 573–581. [Google Scholar] [CrossRef]
- Liu, J.L.; Lin, R.B. Structural properties and reactivities of amino-modified silica fume solid sorbents for low-temperature CO2 capture. Powder Technol. 2013, 241, 188–195. [Google Scholar] [CrossRef]
- Li, K.; Jiang, J.; Tian, S.; Yan, F.; Chen, X. Polyethyleneimine–nano silica composites: A low-cost and promising adsorbent for CO2 capture. J. Mater. Chem. A 2015, 3, 2166–2175. [Google Scholar] [CrossRef]
- Li, K.; Jiang, J.; Yan, F.; Tian, S.; Chen, X. The influence of polyethyleneimine type and molecular weight on the CO2 capture performance of PEI-nano silica adsorbents. Appl. Energy 2014, 136, 750–755. [Google Scholar] [CrossRef]
- Quang, D.V.; Hatton, T.A.; Abu-Zahra, M.R. Thermally stable amine-grafted adsorbent prepared by impregnating 3-aminopropyltriethoxysilane on mesoporous silica for CO2 capture. Ind. Eng. Chem. Res. 2016, 55, 7842–7852. [Google Scholar] [CrossRef]
- Zeng, W.; Bai, H. High-performance CO2 capture on amine-functionalized hierarchically porous silica nanoparticles prepared by a simple template-free method. Adsorption 2016, 22, 117–127. [Google Scholar] [CrossRef]
- Linneen, N.N.; Pfeffer, R.; Lin, Y.S. CO2 adsorption performance for amine grafted particulate silica aerogels. Chem. Eng. J. 2014, 254, 190–197. [Google Scholar] [CrossRef]
- Le, Y.; Guo, D.; Cheng, B.; Yu, J. Amine-functionalized monodispersed porous silica microspheres with enhanced CO2 adsorption performance and good cyclic stability. J. Colloid Interface Sci. 2013, 408, 173–180. [Google Scholar] [CrossRef]
- Singh, B.; Polshettiwar, V. Design of CO2 sorbents using functionalized fibrous nanosilica (KCC-1): Insights into the effect of the silica morphology (KCC-1 vs. MCM-41). J. Mater. Chem. A 2016, 4, 7005–7019. [Google Scholar] [CrossRef]
- Zhang, L.; Zhan, N.; Jin, Q.; Liu, H.; Hu, J. Impregnation of polyethylenimine in mesoporous multilamellar silica vesicles for CO2 capture: A kinetic study. Ind. Eng. Chem. Res. 2016, 55, 5885–5891. [Google Scholar] [CrossRef]
- Zhou, L.; Fan, J.; Cui, G.; Shang, X.; Tang, Q.; Wang, J.; Fan, M. Highly efficient and reversible CO2 adsorption by amine-grafted platelet SBA-15 with expanded pore diameters and short mesochannels. Green Chem. 2014, 16, 4009–4016. [Google Scholar] [CrossRef]
- Mittal, N.; Samanta, A.; Sarkar, P.; Gupta, R. Postcombustion CO2 capture using N-(3-trimethoxysilylpropyl) diethylenetriamine-grafted solid adsorbent. Energy Sci. Eng. 2015, 3, 207–220. [Google Scholar] [CrossRef]
- Yao, M.; Dong, Y.; Feng, X.; Hu, X.; Jia, A.; Xie, G.; Hu, G.; Lu, J.; Luo, M.; Fan, M. The effect of post-processing conditions on aminosilane functionalizaiton of mesocellular silica foam for post-combustion CO2 capture. Fuel 2014, 123, 66–72. [Google Scholar] [CrossRef]
- Ko, Y.G.; Lee, H.J.; Kim, J.Y.; Choi, U.S. Hierarchically porous aminosilica monolith as a CO2 adsorbent. ACS Appl. Mater. Interfaces 2014, 6, 12988–12996. [Google Scholar] [CrossRef]
- Shi, X.; Xiao, H.; Azarabadi, H.; Song, J.; Wu, X.; Chen, X.; Lackner, K.S. Sorbents for the direct capture of CO2 from ambient air. Angew. Chem. Int. Ed. 2020, 59, 6984–7006. [Google Scholar] [CrossRef]
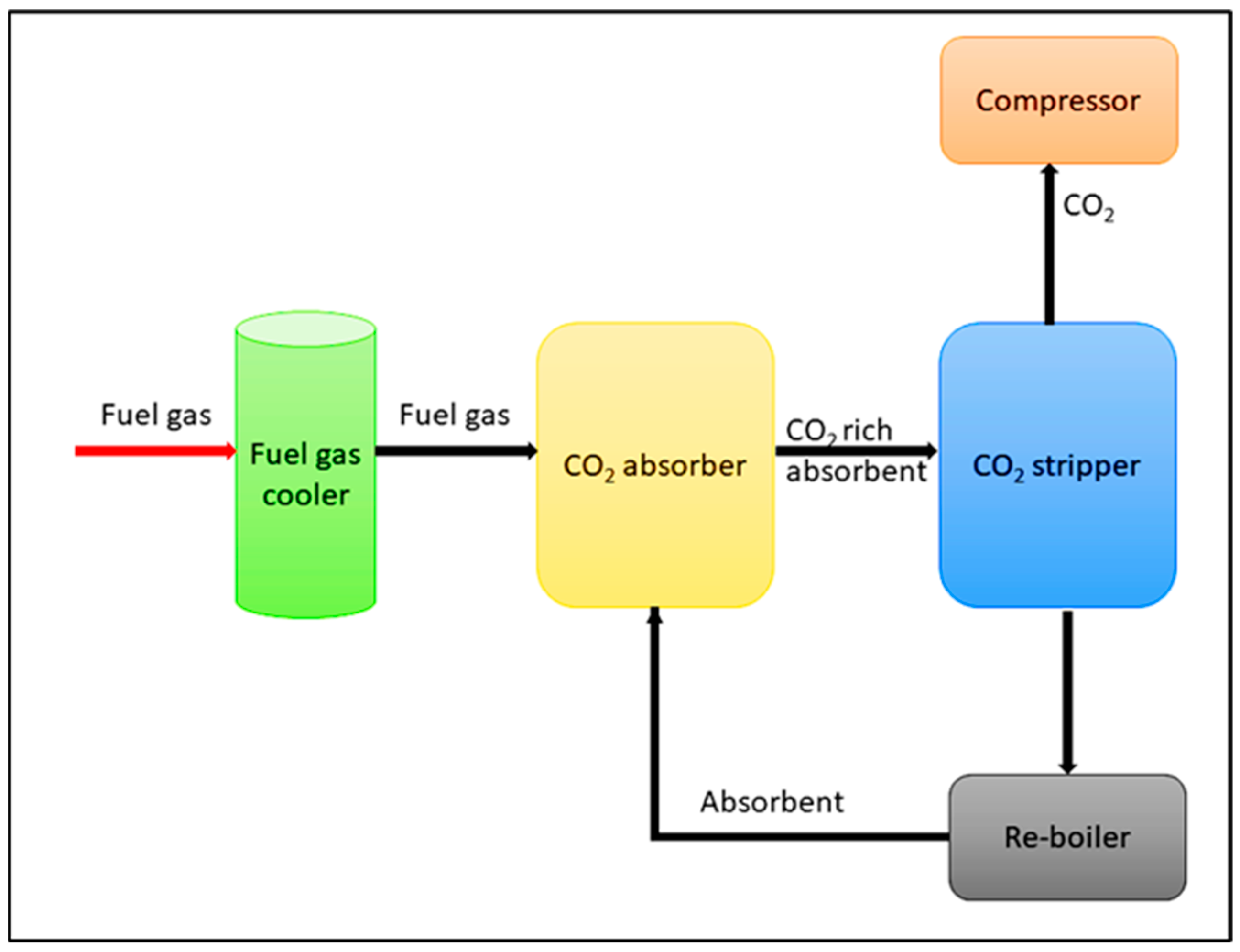

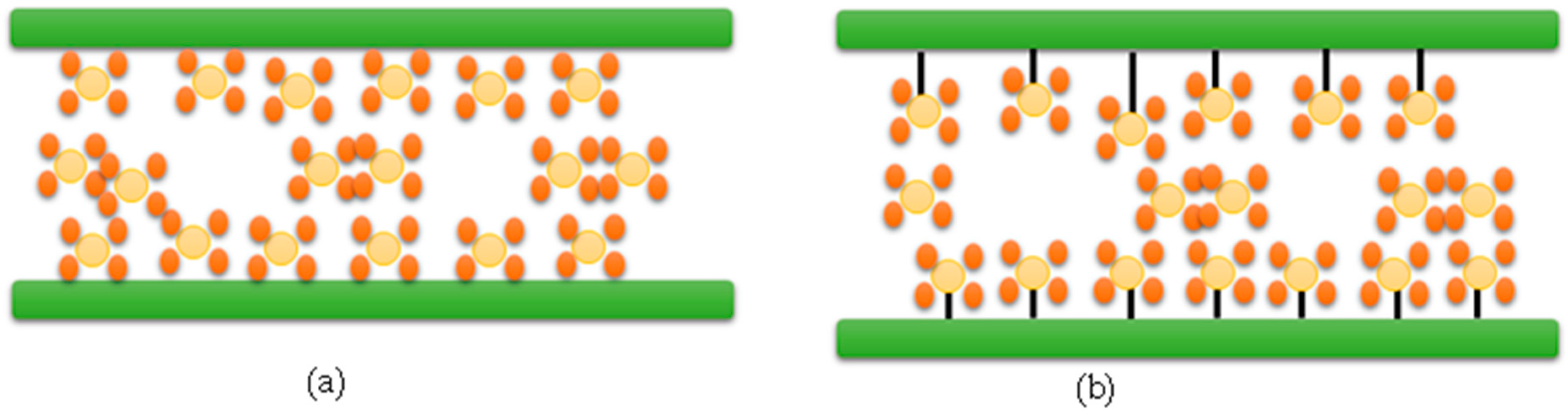
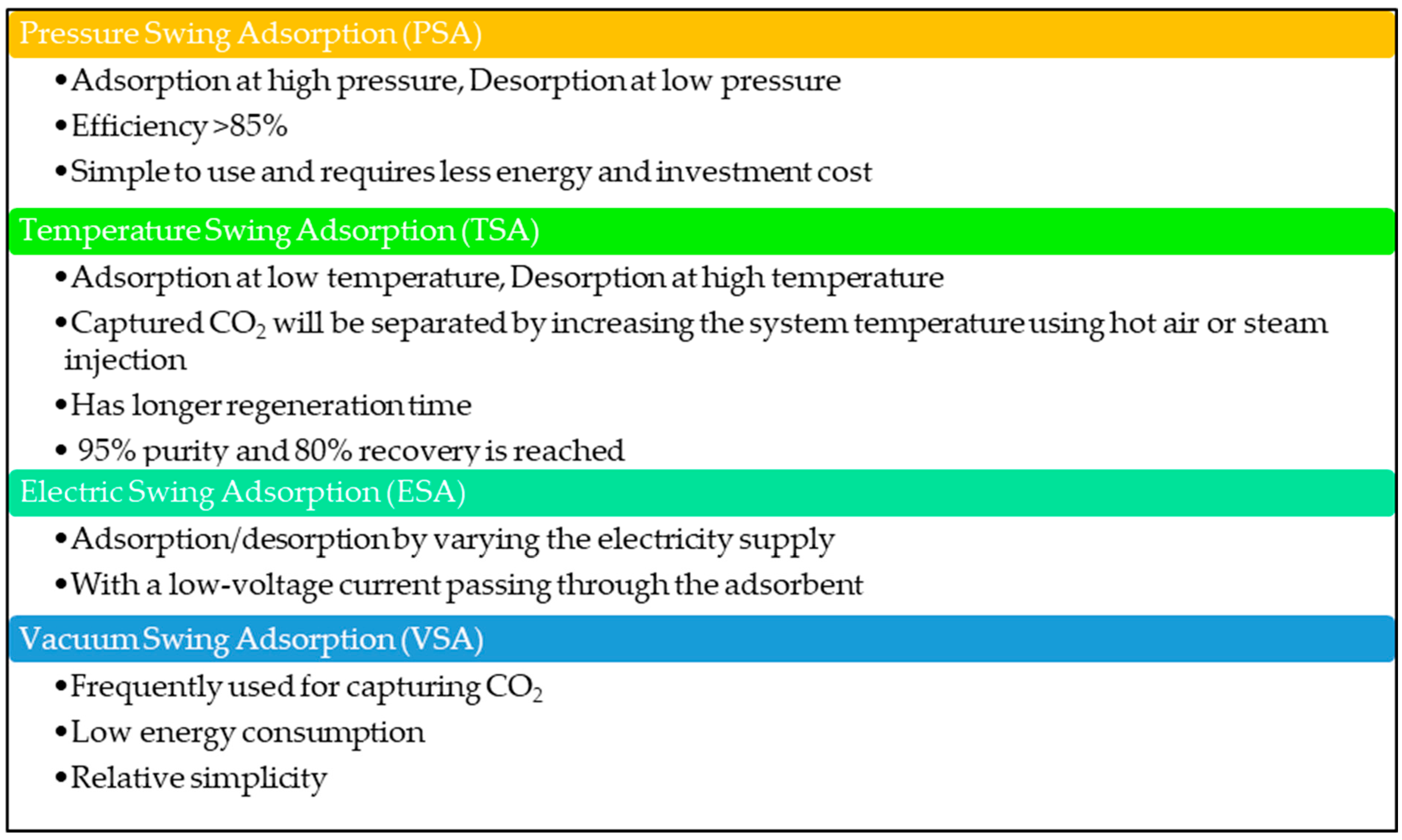
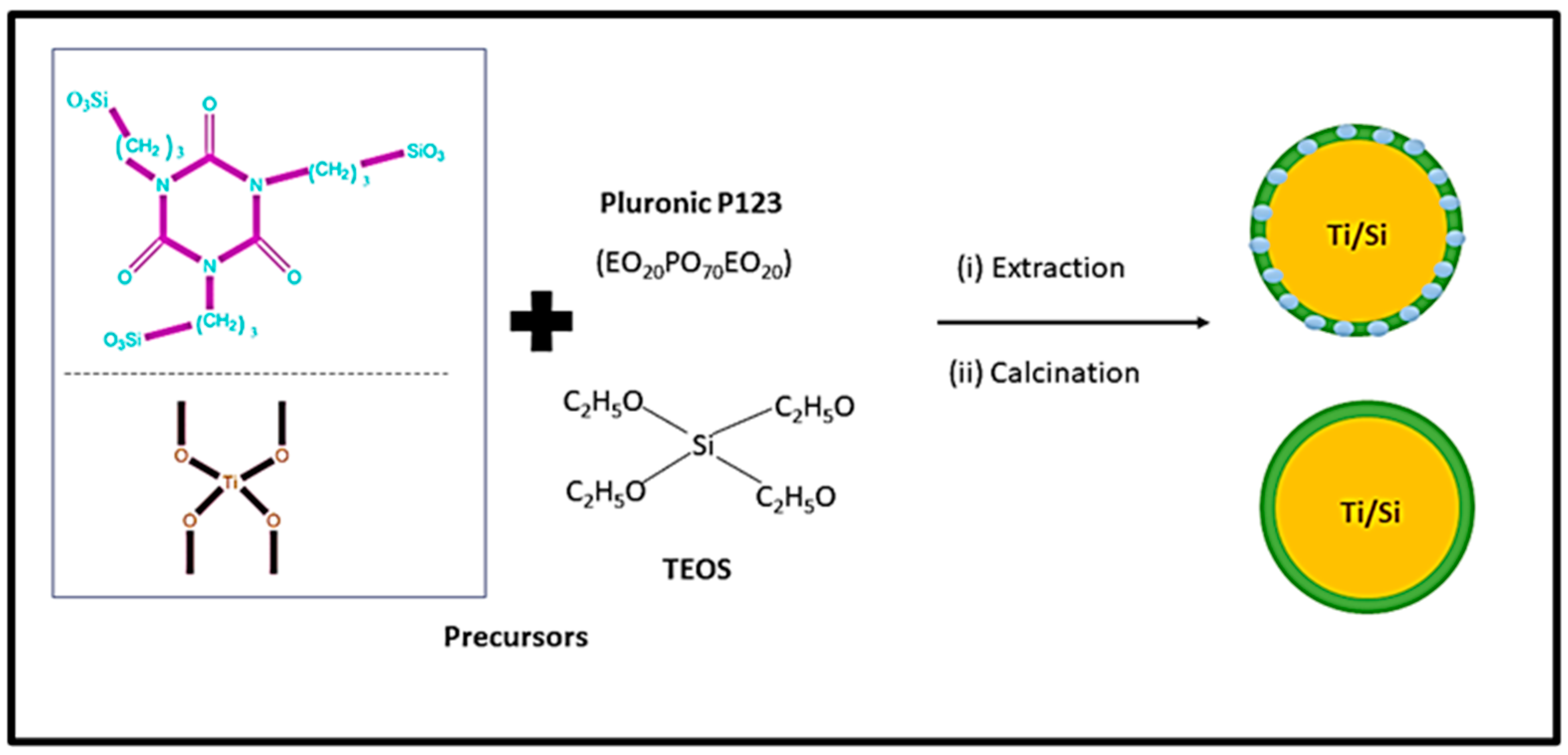
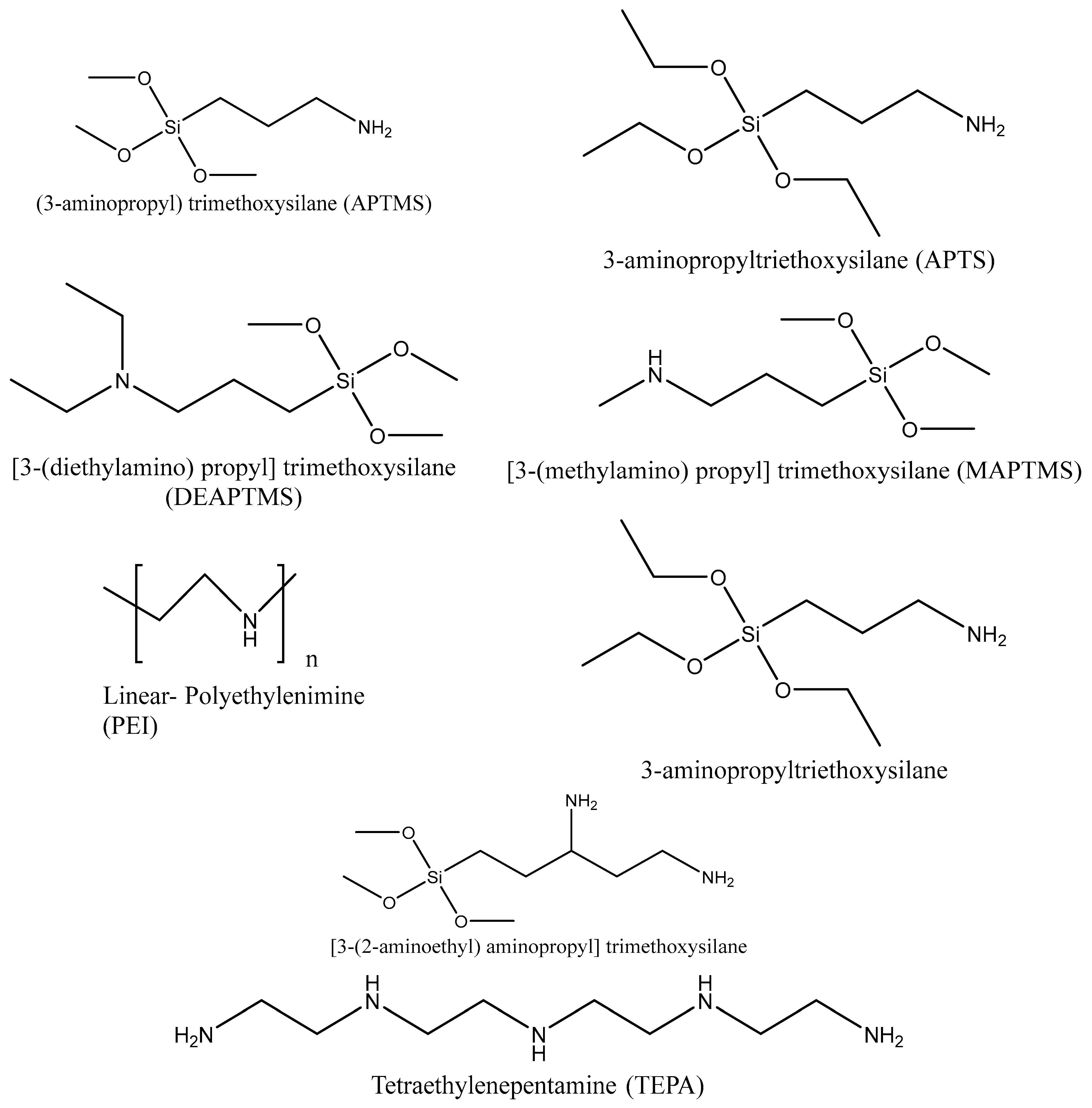
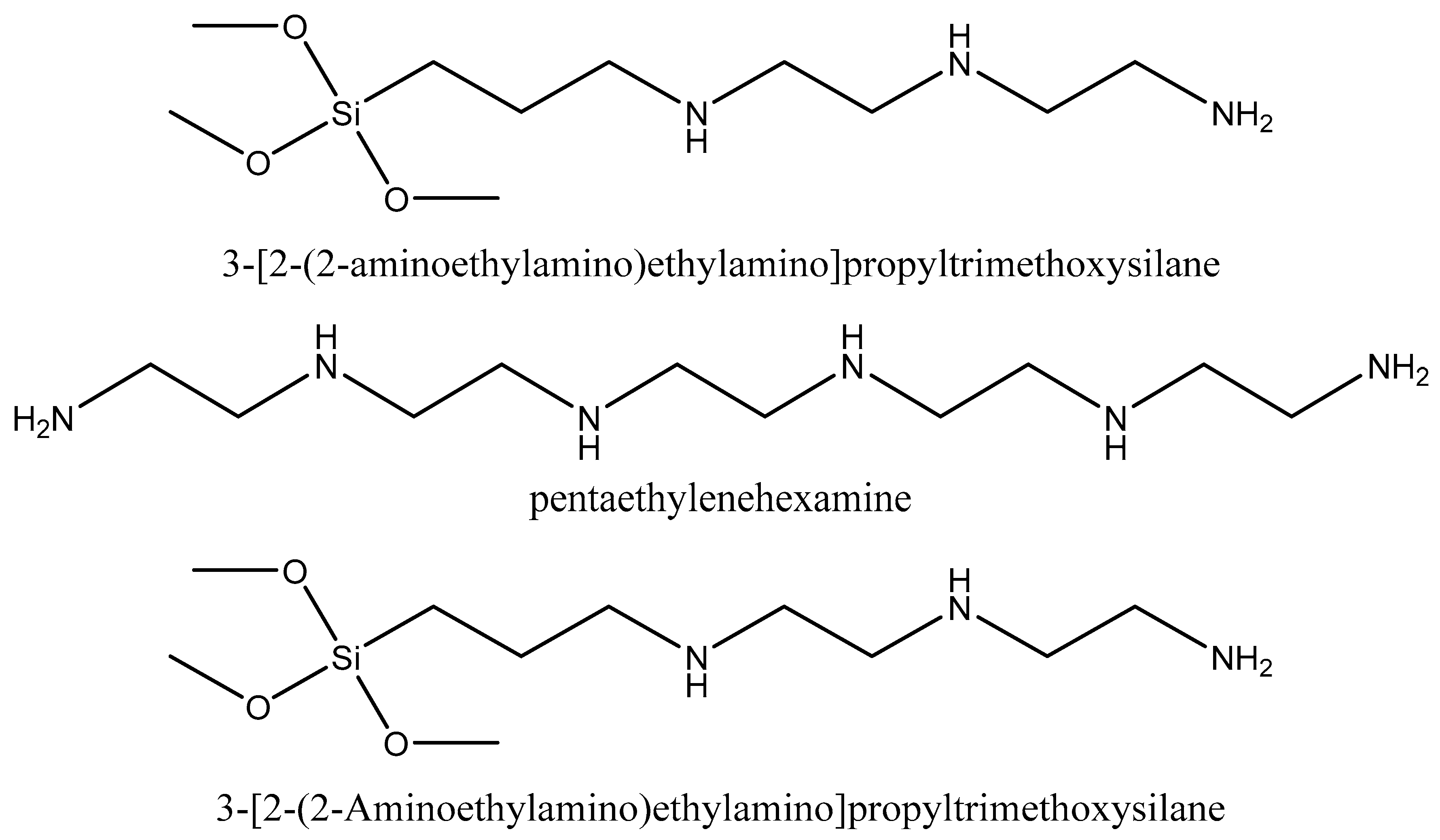
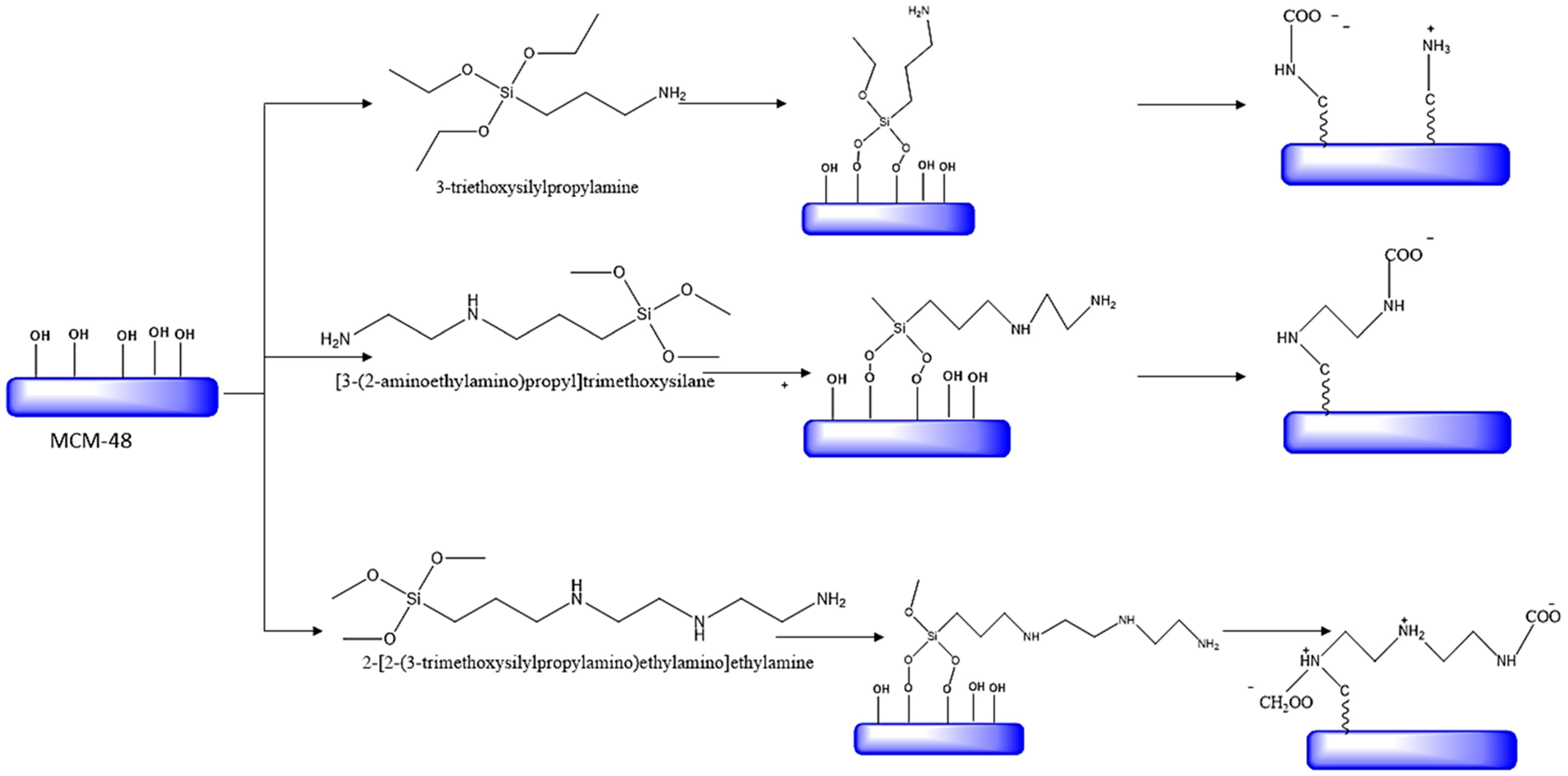
| Type of Approach | Details |
|---|---|
| Improve energy efficiency and promote energy conservation |
|
| Increase in usage of low carbon or clean fuels such as natural gas, hydrogen or nuclear power; Substitution for Power generation |
|
| Deploy renewable energy |
|
| CO2 capture and storage |
|
| Technology | Types | Examples | Efficiency (%) | Advantages | Disadvantages | Ref. |
|---|---|---|---|---|---|---|
| Absorption | Chemical | Amines Caustics | >90 |
|
| [21,22] |
| Physical | Selexol Rectisol fluorinated solvents | |||||
| Adsorption | Chemical | Metal Oxides Si based materials | >85 |
|
| [6,21] |
| Physical | Carbons Zeolites Si based materials | |||||
| Membrane-based technologies | Organic Cellulose derivatives Polyamides | Polyphenyleneoxide, Polydimethylsiloxane | >80 |
|
| [6,21] |
| Inorganic | Metallic Ceramics | |||||
| Cryogenic distillation |
|
| [6,21,23] |
| Material Types | Examples | Advantages | Disadvantages |
|---|---|---|---|
| Pours silica materials | M41S SBA-n AMS |
|
|
| Zeolites | NaY 13X |
|
|
| Metal organic frameworks (MOFs) | M-MOF-74 IRMOF-6 USO-2-Ni Zn4O (BDC)3 (MOF-5) USO-1-Al (MIL-53) |
|
|
| Alkali-based dry adsorbents |
|
| |
| Metal oxides-based adsorbents | CaO, MgO |
|
|
| Chemisorption | Physisorption | |
|---|---|---|
| Description |
|
|
| Chemical Bonding |
|
|
| Advantages |
|
|
| Disadvantages |
|
|
| References | [55,56] | [25,57,58] |
| Porous SiO2 Material | Gas Mixture | Selectivity Value | Pressure (Bar) | Temperature (°C) | Reference |
|---|---|---|---|---|---|
| PEI-MCM-41 | CO2, N2 and H2 | 25.56 | 1 | 100 | [93] |
| SBA-15 | CO2/N2 | 123 | 1 | 25 | [154] |
| SBA-15 (calcination) | CO2/N2 | 55 | 1 | 25 | [154] |
| Mesoporous chitosan−SiO2 nanoparticles | - | 15.46 | 1 | 25 | [155] |
| hydrophobic microporous high-silica zeolites | CH4:N2 = 50%:50% | 36.5 | 1 | 25 | [156] |
| Hollow silica spherical particles (HSSP) | CO2/N2 | 8.5 | 4 | 25 | [157] |
| microporous silica xerogel | CO2/CH4 | 60 | 6 | 25 | [158] |
| Silica based xerogels | C2H4/C2H6 | 20 | 6 | 25 | [158] |
| Synthesis Method | Type of Silica-Based Sorbent | Amine Type | Regeneration Condition | Stability Performance | References | ||
|---|---|---|---|---|---|---|---|
| Temperature (°C) | Types of Gas Flow | No. of Cycles (Cyclic Runs) | Capacity Loss (%) | ||||
| Impregnated | MCM-41 | PEHA | 100 | N2 | 15 | Less than 1 | [159] |
| MCM-41 | TEPA + AMP | 100 | N2 for 60 min | 15 | 4.32 | [117] | |
| SBA-15 | PEI-linear | 100 | Ar | 12 | 13.5 | [160] | |
| SBA-15 | Acrylonitrile-modified TEPA | 100 | N2 | 12 | 1.1 | [161] | |
| HMS | PEI-linear | 75 | N2 for 100 min | 4 | 1.6 | [110] | |
| MCF | PEI-branched | 115 | Ar for 20 min | 10 | 32 | [162] | |
| MCF | PEI | 100 | H2 | 10 | 5 | [163] | |
| MCF | Guanidinylated poly(allylamine) | 120 | He | 5 | 17 | [52] | |
| Fumed silica | PEI-linear | 55 | N2 for 15 min | 180 | Stable | [164] | |
| MCM-41 | TEPA | 100 | N2 | 10 | 3.43 | [165] | |
| Silica fume | Diisopropanolamine | 50 | N2 | 10 | 7 | [166] | |
| Nano-SiO2 | PEI-branched | 120 | N2 | 30 | 10.5 | [167] | |
| Nano-SiO2 | PEI-branched | 120 | N2 | 30 | 19.4 | [168] | |
| Mesoporous-SiO2 | APTS | 120 | Air for 30 min | 11 | 4.3 | [169] | |
| Porous SiO2 | PEI | 100 | N2 for 30 min | 20 | 5 | [170] | |
| Silica aerogel | TEPA | 75 | Ar for 20 min | 10 | 3.9 | [171] | |
| Porous SiO2 | TEPA | 75 | He for 20 min | 10 | 2 | [172] | |
| SNT | PEI | 110 | N2 for 40 min | 10 | 3.3 | [132] | |
| KCC-1-SiO2 | TEPA | 110 | N2 | 21 | 1.2 | [173] | |
| Mesoporous multilamellar SiO2 | PEI | 110 | N2 | 10 | 3.7 | [174] | |
| Silica aerogel | TEPA | 80 | Ar for 30 min | 100 | 12 | [173] | |
| Mesoporous SiO2 | DEA | 90 | N2 | 10 | 12 | [169] | |
| Grafting | SBA-15 | AP | 90 | Vacuum | 10 | 1 | [175] |
| SBA-15 | DEAPTMS | 120 | N2 for 10 min | 100 | 7.2 | [176] | |
| MCM-48 | 2-[2-(3-trimethoxysilyl propylamino) ethylamino] ethylamine | - | N2 | 20 | Stable | [98] | |
| KIT-6 | APTES | 120 | He | 10 | Stable | [97] | |
| MCF | TRI | 150 | N2 for 30 min | 5 | 1.9 | [177] | |
| HMS | APTS | 110 | N2 for 180 min | 3 | Less than 1 | [178] | |
| MCM-41 | APTS | 105 | N2 for 90 min | 10 | Stable | [115] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amaraweera, S.M.; Gunathilake, C.A.; Gunawardene, O.H.P.; Dassanayake, R.S.; Cho, E.-B.; Du, Y. Carbon Capture Using Porous Silica Materials. Nanomaterials 2023, 13, 2050. https://doi.org/10.3390/nano13142050
Amaraweera SM, Gunathilake CA, Gunawardene OHP, Dassanayake RS, Cho E-B, Du Y. Carbon Capture Using Porous Silica Materials. Nanomaterials. 2023; 13(14):2050. https://doi.org/10.3390/nano13142050
Chicago/Turabian StyleAmaraweera, Sumedha M., Chamila A. Gunathilake, Oneesha H. P. Gunawardene, Rohan S. Dassanayake, Eun-Bum Cho, and Yanhai Du. 2023. "Carbon Capture Using Porous Silica Materials" Nanomaterials 13, no. 14: 2050. https://doi.org/10.3390/nano13142050








