Structure and Properties of Carboxylated Carbon Nanotubes@Expanded Graphite/Polyethersulfone Composite Bipolar Plates for PEM
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of a Network of MWCNTs Wrapped with an EG Surface
2.3. Preparation of Carboxylated Carbon Nanotubes Coated with Graphite
2.4. Preparation of Composite BPs
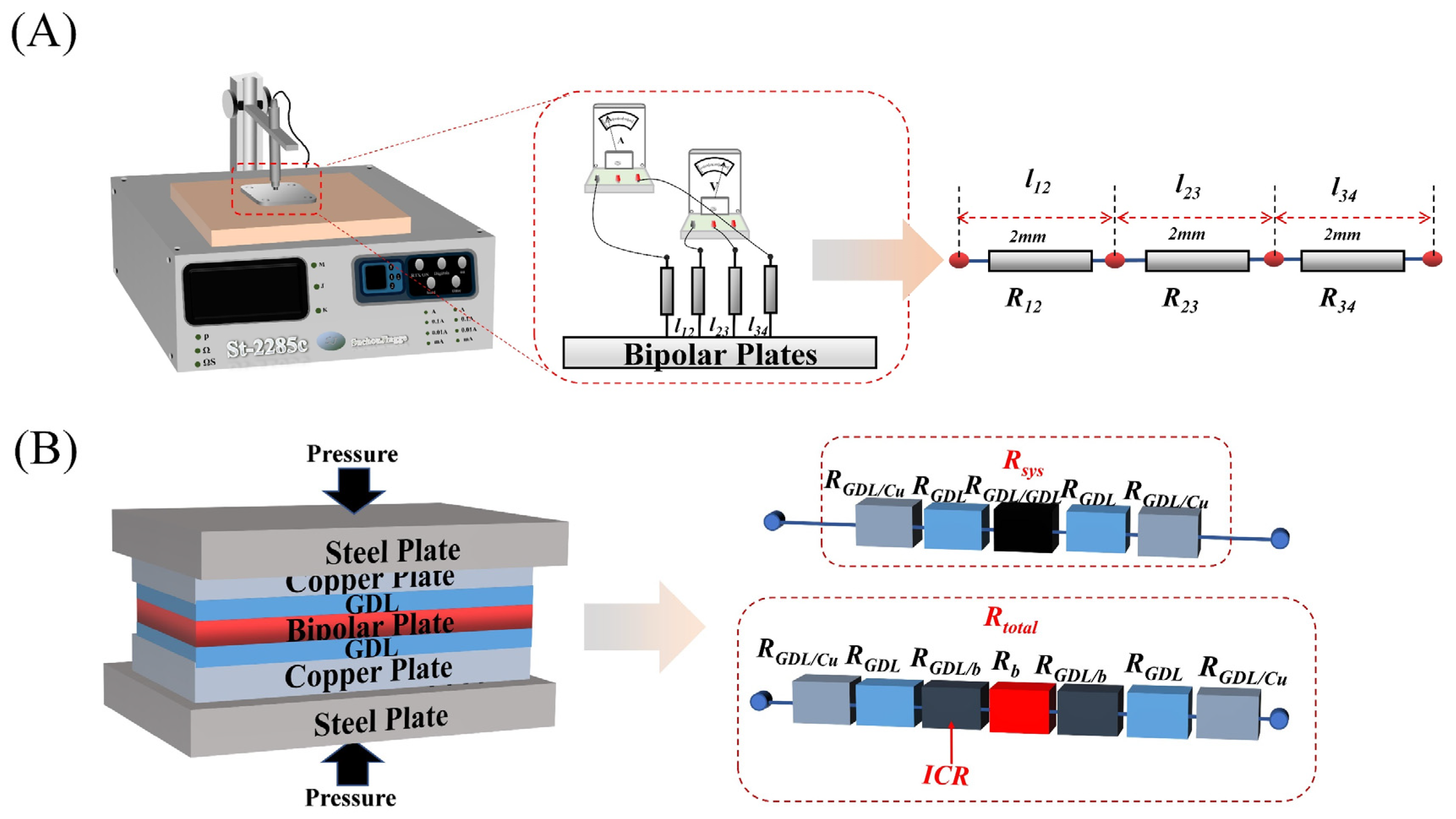
2.5. Characterization
3. Results and Discussion
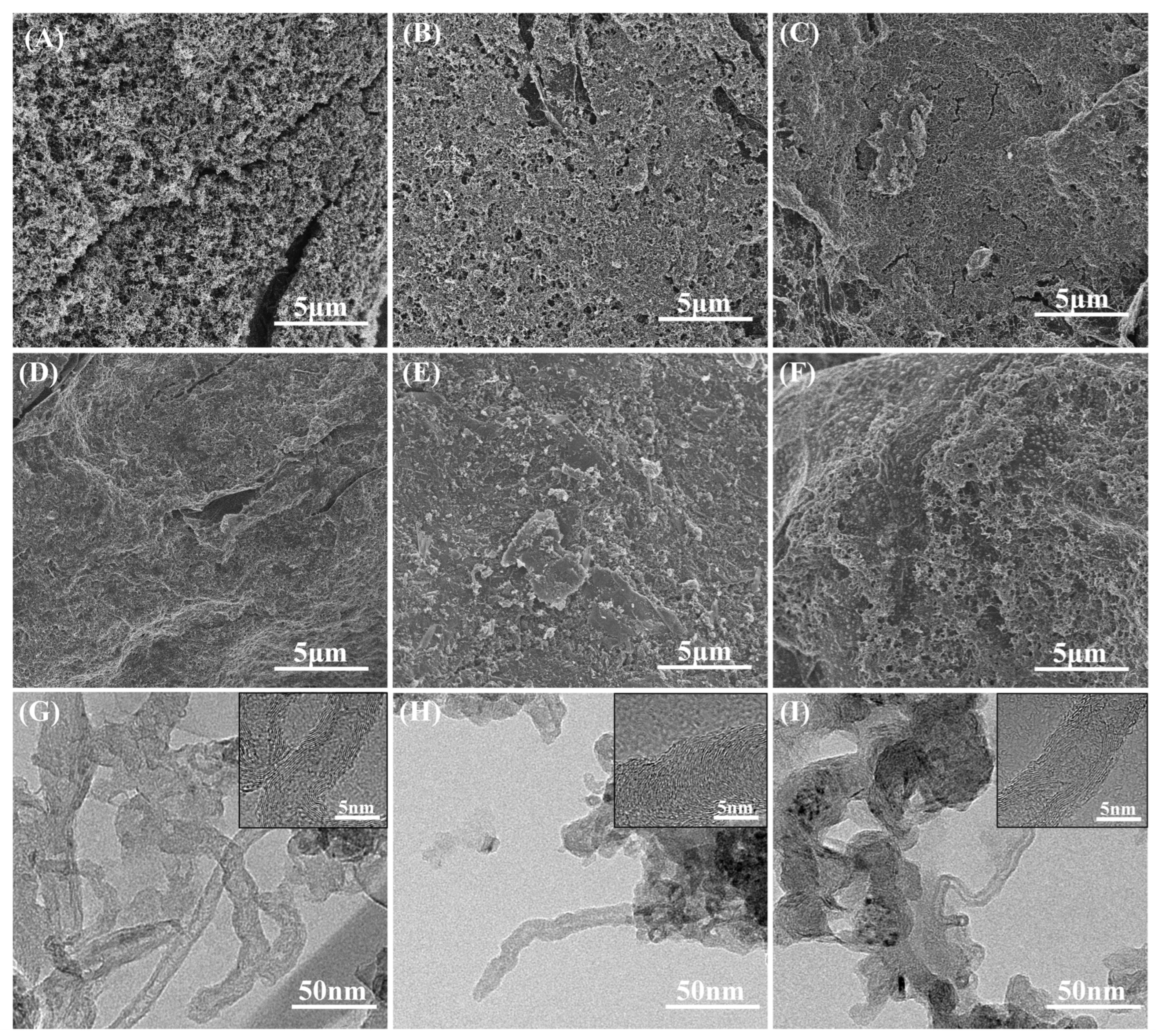
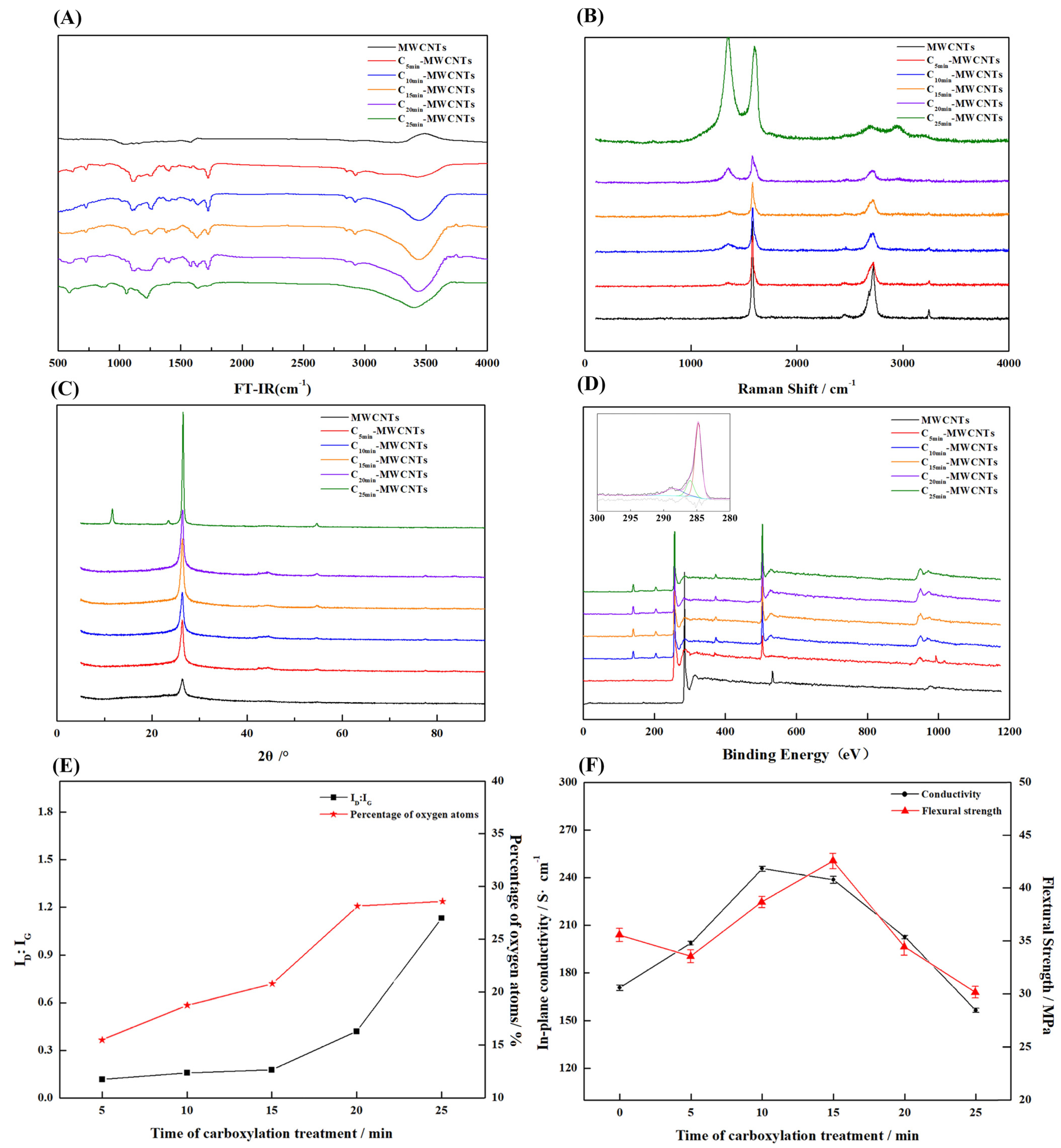
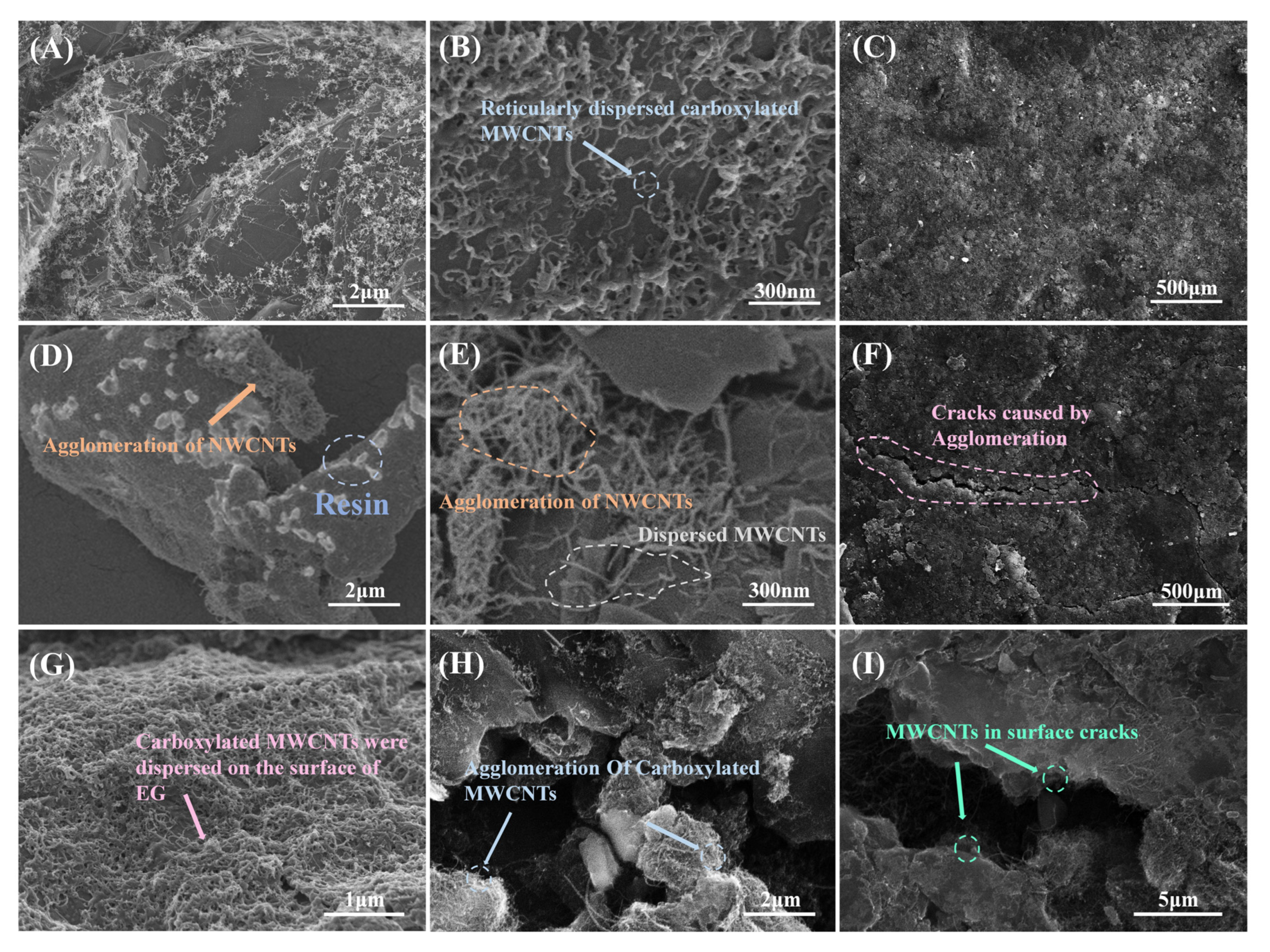
3.1. Effect of Carboxylation Time on the Performance of Layered MWCNTs and Composite BPs
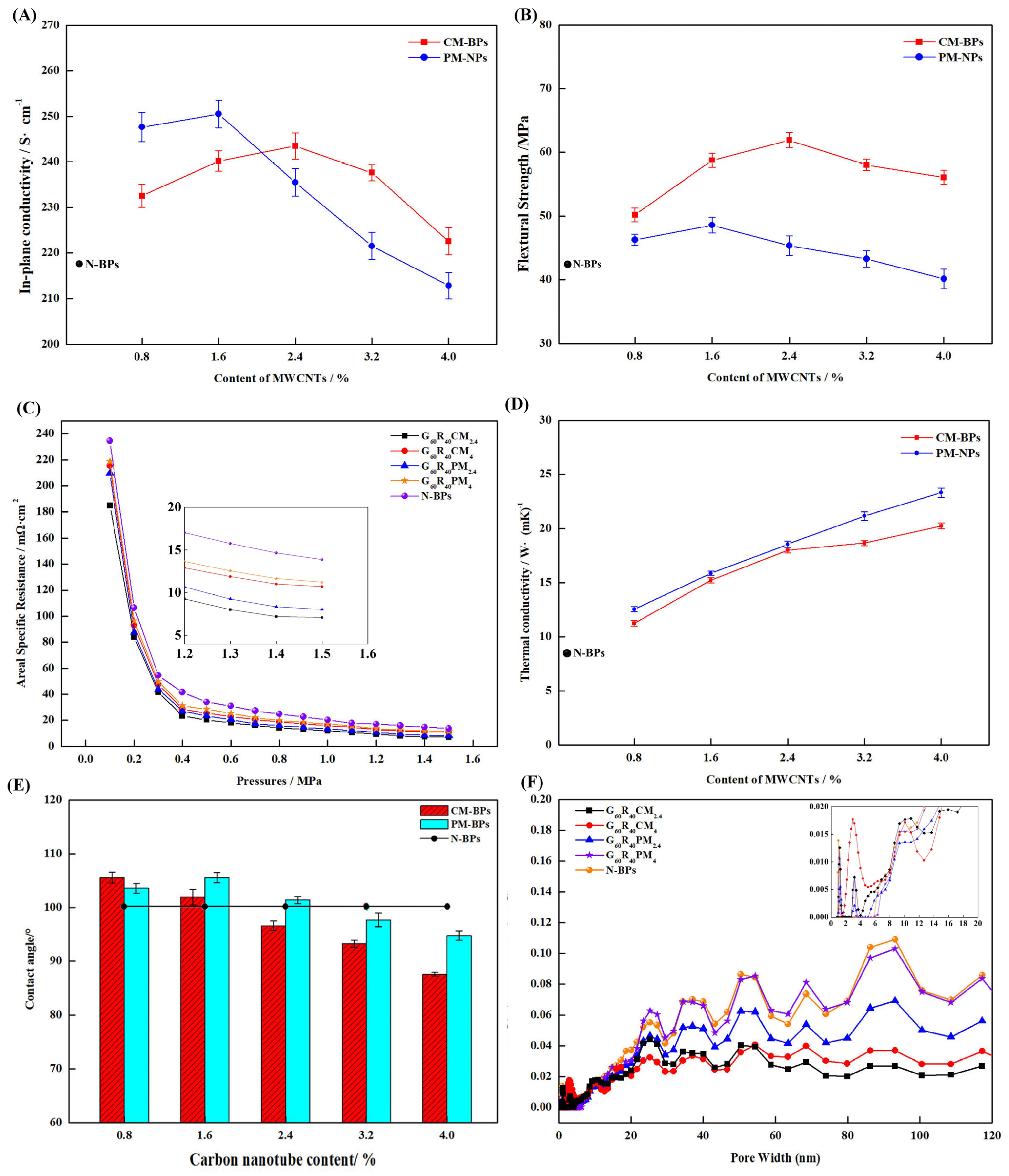
3.2. Effect of Carboxylated Carbon Tube Cladding on the Performance of BPs and Comparison with Pure MWNCTs
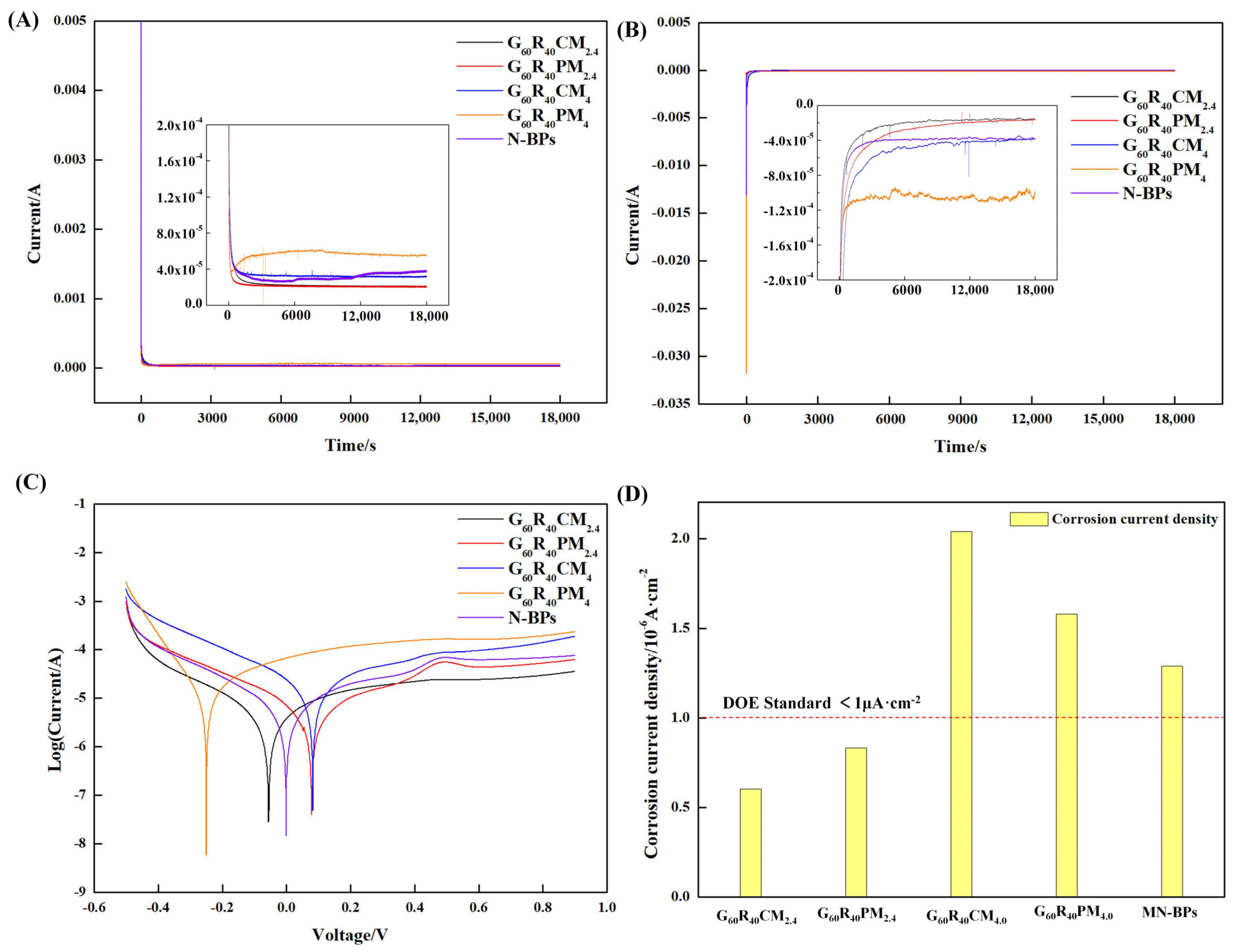
3.3. Effect of Carboxylated MWCNTs on the Corrosion Resistance of BP Performance and Comparison with Pure MWNCT
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharaf, O.Z.; Orhan, M.F. An overview of fuel cell technology: Fundamentals and applications. Renew. Sustain. Energy Rev. 2014, 32, 810–853. [Google Scholar] [CrossRef]
- Shahgaldi, S.; Hamelin, J. Improved carbon nanostructures as a novel catalyst support in the cathode side of PEMFC: A critical review. Carbon 2015, 94, 705–728. [Google Scholar] [CrossRef]
- Kraytsberg, A.; Ein-Eli, Y. Review of advanced materials for proton exchange membrane fuel cells. Energy Fuels 2014, 28, 7303–7330. [Google Scholar] [CrossRef]
- Pan, S.; Wen, Q.; Dan, X.; Li, Y.; Ning, F.; He, C.; Li, W.; Shen, M.; He, L.; Tian, B.; et al. Enhanced Triple-Phase Interface in PEMFC by Proton Conductor Absorption on the Pt Catalyst. Energy Mater. 2023, 6, 763–772. [Google Scholar] [CrossRef]
- Chu, T.; Tang, Q.; Wang, Q.; Wang, Y.; Du, H.; Guo, Y.; Li, B.; Yang, D.; Ming, P.; Zhang, C. Experimental study on the effect of flow channel parameters on the durability of PEMFC stack and analysis of hydrogen crossover mechanism. Energy 2023, 264, 126286. [Google Scholar] [CrossRef]
- Chauhan, S.; Ponkshe, S. A Review of Coated Metallic Bipolar Plates for Proton Exchange Membrane Fuel Cell (PEMFC). SAE Technical: Warrendale, PA, USA, 2023. [Google Scholar] [CrossRef]
- Liu, R.; Jia, Q.; Zhang, B.; Lai, Z.; Chen, L. Protective coatings for metal bipolar plates of fuel cells: A review. Int. J. Hydrog. Energy 2022, 47, 22915–22937. [Google Scholar] [CrossRef]
- Xiong, K.; Wu, W.; Wang, S.; Zhang, L. Modeling, design, materials and fabrication of bipolar plates for proton exchange membrane fuel cell: A review. Appl. Energy 2021, 301, 117443. [Google Scholar] [CrossRef]
- Mao, X.; Li, Y.; Hu, X.; Tian, R.; Yu, W. Expanded graphite (EG)/Ni@Melamine foam (MF)/EG sandwich-structured flexible bipolar plate with excellent electrical conductivity, mechanical properties, and gas permeability. Appl. Energy 2023, 338, 120929. [Google Scholar] [CrossRef]
- Antunes, R.A.; De Oliveira, M.C.; Ett, G.; Ett, V. Carbon materials in composite bipolar plates for polymer electrolyte membrane fuel cells: A review of the main challenges to improve electrical performance. J. Power Sources 2011, 196, 2945–2961. [Google Scholar] [CrossRef] [Green Version]
- Karimi, S.; Fraser, N.; Roberts, B.; Foulkes, F.R. A review of metallic bipolar plates for proton exchange membrane fuel cells: Materials and fabrication methods. Adv. Mater. Sci. Eng. 2012, 2012, 828070. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.O.; Kim, S.Y.; Kim, M. Improving the electrical performance of a carbon fiber reinforced polymer bipolar plate using a resin squeeze-out preprocess. Compos. Commun. 2022, 32, 101156. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, C.; Ling, C.Y.; Han, M.; Yong, R.Y.; Sun, D.; Chen, J. Review on current research of materials, fabrication and application for bipolar plate in proton exchange membrane fuel cell. Int. J. Hydrog. Energy 2020, 45, 29832–29847. [Google Scholar] [CrossRef]
- Al-Mufti, S.M.; Rizvi, S.J.A. Thermoset-Based Composite Bipolar Plates in Proton Exchange Membrane Fuel Cell: Recent Developments and Challenge. In Proton Exchange Membrane Fuel Cells: Electrochemical Methods and Computational Fluid Dynamics; Scrivener Publishing LLC.: Beverly, MA, USA, 2023; pp. 137–211. [Google Scholar] [CrossRef]
- Jeong, K.I.; Oh, J.; Song, S.A.; Lee, D.; Kim, S.S. A review of composite bipolar plates in proton exchange membrane fuel cells: Electrical properties and gas permeability. Compos. Struct. 2021, 262, 113617. [Google Scholar] [CrossRef]
- Hermann, A.; Chaudhuri, T.; Spagnol, P. Bipolar plates for PEM fuel cells: A review. Int. J. Hydrog. Energy 2005, 30, 22915–22937. [Google Scholar] [CrossRef]
- Yu, X.; Chang, H.; Luo, X.; Tu, Z. Experimental study on the dynamic performance of an air-cooled proton exchange membrane fuel cell stack with ultra-thin metal bipolar plate. Int. J. Hydrogen Energy 2022, 47, 36204–36215. [Google Scholar] [CrossRef]
- Wu, S.; Yang, W.; Yan, H.; Zuo, X.; Cao, Z.; Li, H.; Shi, M.; Chen, H. A review of modified metal bipolar plates for proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2021, 46, 8672–8701. [Google Scholar] [CrossRef]
- Fukutsuka, T.; Yamaguchi, T.; Miyano, S.I.; Matsuo, Y.; Sugie, Y.; Ogumi, Z. Carbon-coated stainless steel as PEFC bipolar plate material. J. Power Sources 2007, 174, 199–205. [Google Scholar] [CrossRef]
- Wind, J.; Späh, R.; Kaiser, W.; Böhm, G. Metallic bipolar plates for PEM fuel cells. J. Power Sources 2002, 150, 256–260. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, R.; Zeng, Y. Hydroforming rules and quality control parameters analysis for metal bipolar plate. Eng. Fail. Anal. 2022, 132, 105919. [Google Scholar] [CrossRef]
- Antunes, R.A.; Oliveira MC, L.; Ett, G.; Ett, V. Corrosion of metal bipolar plates for PEM fuel cells: A review. Int. J. Hydrog. Energy 2010, 35, 3632–3647. [Google Scholar] [CrossRef]
- Liu, G.; Hou, F.; Peng, S.; Wang, X.; Fang, B. Process and challenges of stainless steel based bipolar plates for proton exchange membrane fuel cells. Int. J. Miner. Metall. Mater. 2022, 33, 1099–1119. [Google Scholar] [CrossRef]
- Tan, Q.; Wang, Y. Preparation and properties of conductive Ti4O7 surface coating for Ti bipolar plates of proton exchange membrane fuel cells. J. Alloys Compd. 2022, 911, 165098. [Google Scholar] [CrossRef]
- Kakati, B.K.; Sathiyamoorthy, D.; Verma, A. Electrochemical and mechanical behavior of carbon composite bipolar plate for fuel cell. Int. J. Hydrog. Energy 2010, 35, 4185–4194. [Google Scholar] [CrossRef]
- Santana-Villamar, J.; Reyna, R.; Rigail-Cedeño, A.F.; Espinoza-Andaluz, M. Andaluz. Processing Methods of Epoxy/Graphite-Based Compounds for PEFC Bipolar Plates Using Different Secondary Fillers. J. Electrochem. Soc. 2021, 168, 064508. [Google Scholar] [CrossRef]
- Mathew, C.; Naina Mohamed, S.; Devanathan, L.S. A comprehensive review of current research on various materials used for developing composite bipolar plates in polymer electrolyte membrane fuel cells. Polym. Compos. 2022, 43, 4100–4114. [Google Scholar] [CrossRef]
- San FG, B.; Okur, O. The effect of compression molding parameters on the electrical and physical properties of polymer composite bipolar plates. Int. J. Hydrog. Energy 2017, 42, 23054–23069. [Google Scholar] [CrossRef]
- Rzeczkowski, P.; Krause, B.; Pötschke, P. Characterization of Highly Filled PP/Graphite Composites for Adhesive Joining in Fuel Cell Applications. Polymers 2019, 11, 462. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.S.; Kim, H.J.; Kim, S.G.; Ahn, S.H. Evaluation of graphite composite bipolar plate for PEM (proton exchange membrane) fuel cell: Electrical, mechanical, and molding properties. J. Mater. Process. Technol. 2007, 187–188, 425–442. [Google Scholar] [CrossRef]
- Dhakate, S.R.; Mathur, R.B.; Kakati, B.K.; Dhami, T.L. Properties of graphite-composite bipolar plate prepared by compression molding technique for PEM fuel cell. J. Mater. Process. Technol. 2007, 32, 4537–4543. [Google Scholar] [CrossRef]
- Hu, B.; He, G.; Chang, F.; Yang, H.; Cao, X.; Yin, X. Low filler and highly conductive composite bipolar plates with synergistic segregated structure for enhanced proton exchange membrane fuel cell performance. Energy 2022, 251, 123982. [Google Scholar] [CrossRef]
- Tariq, M.; Utkarsh Syed, N.A.; Behravesh, A.H.; Pop-Iliev, R.; Rizvi, G. Synergistic enrichment of electrically conductive polypropylene-graphite composites for fuel cell bipolar plates. Int. J. Energy Res. 2022, 46, 10955–10964. [Google Scholar] [CrossRef]
- Zheng, J.; Peng, Y.; Fan, R.; Chen, J.; Zhan, Z.; Yao, D.; Ming, P. Study on carbon matrix composite bipolar plates with balance of conductivity and flexural strength. Chin. Chem. Lett. 2023, 34, 107616. [Google Scholar] [CrossRef]
- Musse, D.; Lee, D. Parametric study of pulsed nanosecond laser interaction with carbon-nanotube composite bipolar plate for PEMFCs. Sci. Rep. 2023, 13, 2048. [Google Scholar] [CrossRef]
- Iwan, A.; Malinowski, M.; Pasciak, G. Polymer fuel cell components modified by graphene: Electrodes, electrolytes and bipolar plates. Renew. Sustain. Energy Rev. 2015, 49, 954–967. [Google Scholar] [CrossRef]
- Ramírez-Herrera, C.A.; Tellez-Cruz, M.M.; Pérez-González, J.; Solorza-Feria, O.; Flores-Vela, A.; Cabañas-Moreno, J.G. Enhanced mechanical properties and corrosion behavior of polypropylene/multi-walled carbon nanotubes/carbon nanofibers nanocomposites for application in bipolar plates of proton exchange membrane fuel cells. Int. J. Hydrog. Energy 2021, 46, 26110–26125. [Google Scholar] [CrossRef]
- Hu, B.; Chang, F.L.; Xiang, L.Y.; He, G.J.; Cao, X.W.; Yin, X.C. High performance polyvinylidene fluoride/graphite/multi-walled carbon nanotubes composite bipolar plate for PEMFC with segregated conductive networks. Int. J. Hydrog. Energy 2021, 46, 25666–25676. [Google Scholar] [CrossRef]
- Gul, S.; Abdullah, M.; Zafar, M.; Ali, I.; Ullah Khan, N.; Younas, M.; Rezakazemi, M. Development of hybrid composite by integrating functionalized multi-walled carbon nanotubes (f-MWCNTs) with glass fiber reinforced polyester composite. PLoS ONE 2022, 17, e0279647. [Google Scholar] [CrossRef]
- Escobar, M.; Moreno, M.S.; Candal, R.J.; Marchi, M.C.; Caso, A.; Polosecki, P.I.; Rubiolo, G.H.; Goyanes, S. Synthesis of carbon nanotubes by CVD: Effect of acetylene pressure on nanotubes characteristics. Appl. Surf. Sci. 2007, 254, 251–256. [Google Scholar] [CrossRef]
- Lee, G.W.; Kim, J.; Yoon, J.; Bae, J.S.; Shin, B.C.; Kim, I.S.; Oh, W.; Ree, M. Structural characterization of carboxylated multi-walled carbon nanotubes. Thin Solid Film. 2008, 516, 5781–5784. [Google Scholar] [CrossRef]
- Ghaee, A.; Zerafat, M.M.; Askari, P.; Sabbaghi, S.; Sadatnia, B. Fabrication of polyamide thin-film nanocomposite membranes with enhanced surface charge for nitrate ion removal from water resources. Environ. Technol. 2017, 38, 772–781. [Google Scholar] [CrossRef]
- Li, Z.; Guo, Z.; Yang, Y. Preparation and Appraisal of High-Modulus Resin-Based Composites Reinforced by Silica-Coated Multi-Walled Carbon Nanotubes. ChemistrySelect 2023, 8, e202203881. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, T. Lubricating performance of polytetrafluoroethylene hybrid fabric composites with removed thermosetting resin at cryogenic temperatures. J. Appl. Polym. Sci. 2023, 140, e53935. [Google Scholar] [CrossRef]
- Sharma, S.; Lee, J.; Dang, T.T.; Hur, S.H.; Choi, W.M.; Chung, J.S. Ultrathin freestanding PDA-Doped rGO/MWCNT composite paper for electromagnetic interference shielding applications. Chem. Eng. J. 2022, 430 Pt 2, 132808. [Google Scholar] [CrossRef]
- Ding, R.; He, X.; Hu, Y.; Yan, J.; Lu, J.; Shi, S.Q.; Han, G.; Cheng, W. Uniform grafting of amino-functionalized multi-walled carbon nanotubes on the surface of oxidized bamboo fiber to enhance the interfacial strength with epoxy resin. Appl. Surf. Sci. 2022, 603, 154480. [Google Scholar] [CrossRef]
- Li, C.; Dong, Y.; Yuan, X.; Qiu, Z.; Zhang, Y.; Yan, S.; Gao, X.; Zhu, B. Two-step method to realize continuous multi-wall carbon nanotube grafted on the fibers to improve the interface of carbon fibers/epoxy resin composites based on the Diels-Alder reaction. Carbon 2023, 212, 118131. [Google Scholar] [CrossRef]
- Wang, F.; Wang, J.; Fang, D.; Zhou, S.; Huang, J.; Zhao, G.; Liu, Y. Surface sizing introducing carbon nanotubes for interfacial bond strengthening of basalt fiber–reinforced polymer composites. Adv. Compos. Hybrid Mater. 2023, 6, 117. [Google Scholar] [CrossRef]
- Baek, K.; Shin, H.; Yoo, T.; Cho, M. Two-step multiscale homogenization for mechanical behaviour of polymeric nanocomposites with nanoparticulate agglomerations. Compos. Sci. Technol. 2019, 179, 97–105. [Google Scholar] [CrossRef]
- Shu, R.; Jiang, X.; Shao, Z.; Sun, D.; Zhu, D.; Luo, Z. Fabrication and mechanical properties of MWCNTs and graphene synergetically reinforced Cu–graphite matrix composites. Powder Technol. 2019, 349, 59–69. [Google Scholar] [CrossRef]
- Turaka, S.; Reddy, K.V.K.; Sahu, R.K.; Katiyar, J.K. Mechanical properties of MWCNTs and graphene nanoparticles modified glass fibre-reinforced polymer nanocomposite. Bull. Mater. Sci. 2021, 44, 194. [Google Scholar] [CrossRef]
- Rasana, N.; Jayanarayanan, K.; Deeraj, B.D.S.; Joseph, K. The thermal degradation and dynamic mechanical properties modeling of MWCNT/glass fiber multiscale filler reinforced polypropylene composites. Compos. Sci. Technol. 2019, 169, 249–259. [Google Scholar] [CrossRef]
- Garg, P.; Singh, B.P.; Kumar, G.; Gupta, T.; Pandey, I.; Seth, R.K.; Tandon, R.P.; Mathur, R.B. Effect of dispersion conditions on the mechanical properties of multi-walled carbon nanotubes based epoxy resin composites. Polym. Res. 2011, 18, 1397–1407. [Google Scholar] [CrossRef]
- Ma, L.; Liu, C.; Dou, R.; Yin, B. Significantly improved high dielectric MWCNTs filled PVDF/PS/HDPE composites via constructing double bi-continuous structure. Compos. Part B Eng. 2021, 224, 109158. [Google Scholar] [CrossRef]
- Park, J.G.; Cheng, Q.; Lu, J.; Bao, J.; Li, S.; Tian, Y.; Liang, Z.; Zhang, C.; Wang, B. Thermal conductivity of MWCNT/epoxy composites: The effects of length, alignment and functionalization. Carbon 2011, 50, 2083–2090. [Google Scholar] [CrossRef]
- Ivanov, E.; Kotsilkova, R.; Xia, H.; Chen, Y.; Donato, R.K.; Donato, K.; Godoy, A.P.; Di Maio, R.; Silvestre, C.; Cimmino, S.; et al. PLA/Graphene/MWCNT Composites with Improved Electrical and Thermal Properties Suitable for FDM 3D Printing Applications. Appl. Sci. 2019, 9, 1209. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Feng, Y.; Feng, W. Three-dimensional interconnected networks for thermally conductive polymer composites: Design, preparation, properties, and mechanisms. Mater. Sci. Eng. R Rep. 2020, 142, 100580. [Google Scholar] [CrossRef]
- Madadi, F.; Rezaeian, A.; Edris, H.; Zhiani, M. Influence of surface roughness and hydrophobicity of bipolar plates on PEM performance. Surf. Coat. Technol. 2020, 389, 125676. [Google Scholar] [CrossRef]
- Kahveci, E.E.; Taymaz, I. Experimental study on performance evaluation of PEM fuel cell by coating bipolar plate with materials having different contact angle. Fuel 2019, 253, 1274–1281. [Google Scholar] [CrossRef]
- Nair, S.T.; Bicy, K.; George, S.C.; Thomas, S. Effect of MWCNTs on the Wetting Behavior of PP/NR Blends. Macromol. Symp. 2021, 398, 2000214. [Google Scholar] [CrossRef]
- Seraji, A.A.; Aghvami-Panah, M.; Zadhoush, A.; Salimian, S.; Khoshnevis, H. Microstructural design and mechanical performance of epoxy/carbon nanotube fiber composite. J. Compos. Mater. 2022, 56, 3591–3602. [Google Scholar] [CrossRef]
- Rahimpour, A.; Jahanshahi, M.; Khalili, S.; Mollahosseini, A.; Zirepour, A.; Rajaeian, B. Novel functionalized carbon nanotubes for improving the surface properties and performance of polyethersulfone (PES) membrane. Desalination 2012, 286, 99–107. [Google Scholar] [CrossRef]
- Kumari, P.; Modi, A.; Bellare, J. Enhanced flux and antifouling property on municipal wastewater of polyethersulfone hollow fiber membranes by embedding carboxylated multi-walled carbon nanotubes and a vitamin E derivative. Sep. Purif. Technol. 2020, 235, 116199. [Google Scholar] [CrossRef]
- Anis, B.; El Fllah, H.; Ismail, T.; Fathallah, W.M.; Khalil AS, G.; Hemeda, O.M.; Badr, Y.A. Preparation, characterization, and thermal conductivity of polyvinyl-formaldehyde/MWCNTs foam: A low cost heat sink substrate. J. Mater. Res. Technol. 2020, 9, 2934–2945. [Google Scholar] [CrossRef]
- Zhou, W.; Wu, M.; Wang, Y.; Lu, P.; Wang, C.; Wei, W.; Miao, X. Effect of SiO2-MWCNTs on Mechanical and Tribological Properties of Acrylic Silicone Resin Coating. J. Inorg. Organomet. Polym. Mater. 2023, 33, 529–543. [Google Scholar] [CrossRef]
- Datsyuk, V.; Trotsenko, S.; Trakakis, G.; Boden, A.; Vyzas-Asimakopoulos, K.; Parthenios, J.; Galiotis, C.; Reich, S.; Papagelis, K.; Galiotis, C.; et al. Thermal properties enhancement of epoxy resins by incorporating polybenzimidazole nanofibers filled with graphene and carbon nanotubes as reinforcing material. Polym. Test. 2020, 82, 106317. [Google Scholar] [CrossRef]
- Liao, W.; Jiang, F.; Zhang, Y.; Zhou, X.; He, Z. Highly-conductive composite bipolar plate based on ternary carbon materials and its performance in redox flow batteries. Renew. Energy 2020, 152, 1310–1316. [Google Scholar] [CrossRef]
- Liao, W.; Zhang, Y.; Zhou, X.; Zhuang, M.; Guo, D.; Jiang, F.; Yu, Q. Low-Carbon-Content Composite Bipolar Plates: A Novel Design and Its Performance in Vanadium Redox Flow Batteries. ChemistrySelect 2019, 4, 2421. [Google Scholar] [CrossRef]
- Caglar, B.; Fischer, P.; Kauranen, P.; Karttunen, M.; Elsner, P. Development of carbon nanotube and graphite filled polyphenylene sulfide based bipolar plates for all-vanadium redox flow batteries. J. Power Sources 2014, 256, 88–95. [Google Scholar] [CrossRef]

| Materials | Carboxylation Treatment with H2SO4 and HNO3 Time (min) |
|---|---|
| MWCNTs | 0 |
| C5min-MWCNTs | 5 |
| C10min-MWCNTs | 10 |
| C15min-MWCNTs | 15 |
| C20min-MWCNTs | 20 |
| C25min-MWCNTs | 25 |
| Groups | Sample | EG wt% | PES wt% | Carboxylated MWCNTs wt% of EG + PES | Pure MWCNTs wt% of EG + PES |
|---|---|---|---|---|---|
| N-BPs | - | 60% | 40% | - | - |
| CM-BPs | G60R40CM0.8 | 60% | 40% | 0.8% | 0 |
| G60R40CM1.6 | 60% | 40% | 1.6% | 0 | |
| G60R40CM2.4 | 60% | 40% | 2.4% | 0 | |
| G60R40CM3.2 | 60% | 40% | 3.2% | 0 | |
| G60R40CM4 | 60% | 40% | 4% | 0 | |
| PM-BPs | G60R40PM0.8 | 60% | 40% | 0 | 0.8% |
| G60R40PM1.6 | 60% | 40% | 0 | 1.6% | |
| G60R40PM2.4 | 60% | 40% | 0 | 2.4% | |
| G60R40PM3.2 | 60% | 40% | 0 | 3.2% | |
| G60R40PM4 | 60% | 40% | 0 | 4% |
| Sample | Electrical Conductivity /S·cm−1 | Flexural Strength /MPa | Materials |
|---|---|---|---|
| N-BPs | 217.87 | 42.34- | PES, expanded graphite, MWCNTs |
| G60R40CM0.8 | 232.57 | 50.17 | |
| G60R40CM1.6 | 240.19 | 58.76 | |
| G60R40CM2.4 | 243.52 | 61.9 | |
| G60R40CM3.2 | 237.62 | 58.03 | |
| G60R40CM4 | 222.57 | 56.08 | |
| G60R40PM0.8 | 247.65 | 46.27 | |
| G60R40PM1.6 | 250.53 | 48.58 | |
| G60R40PM2.4 | 235.52 | 45.35 | |
| G60R40PM3.2 | 221.56 | 43.27 | |
| G60R40PM4 | 212.86 | 40.13 | |
| EG/Ni@MF/EG-EP [9] | 320 | 56 | Expanded graphite, (EG)/Ni@Melamine foam (MF) |
| G34CB6 [32] | 177.87 | 49.16 | Polyvinylidene fluoride, conductive carbon black, graphite |
| G82.5CB2 [33] | 25.7 | 34.8 | Polyethylene, graphite, carbon black |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Zhao, Y.; Pan, X.; Liu, M.; Qiu, S.; Xie, Z. Structure and Properties of Carboxylated Carbon Nanotubes@Expanded Graphite/Polyethersulfone Composite Bipolar Plates for PEM. Nanomaterials 2023, 13, 2055. https://doi.org/10.3390/nano13142055
Li W, Zhao Y, Pan X, Liu M, Qiu S, Xie Z. Structure and Properties of Carboxylated Carbon Nanotubes@Expanded Graphite/Polyethersulfone Composite Bipolar Plates for PEM. Nanomaterials. 2023; 13(14):2055. https://doi.org/10.3390/nano13142055
Chicago/Turabian StyleLi, Wenkai, Yixin Zhao, Xingchen Pan, Mingqi Liu, Shi Qiu, and Zhiyong Xie. 2023. "Structure and Properties of Carboxylated Carbon Nanotubes@Expanded Graphite/Polyethersulfone Composite Bipolar Plates for PEM" Nanomaterials 13, no. 14: 2055. https://doi.org/10.3390/nano13142055
APA StyleLi, W., Zhao, Y., Pan, X., Liu, M., Qiu, S., & Xie, Z. (2023). Structure and Properties of Carboxylated Carbon Nanotubes@Expanded Graphite/Polyethersulfone Composite Bipolar Plates for PEM. Nanomaterials, 13(14), 2055. https://doi.org/10.3390/nano13142055





