Boosting the Capacitive Performance of Supercapacitors by Hybridizing N, P-Codoped Carbon Polycrystalline with Mn3O4-Based Flexible Electrodes
Abstract
:1. Introduction
2. Materials and Methods
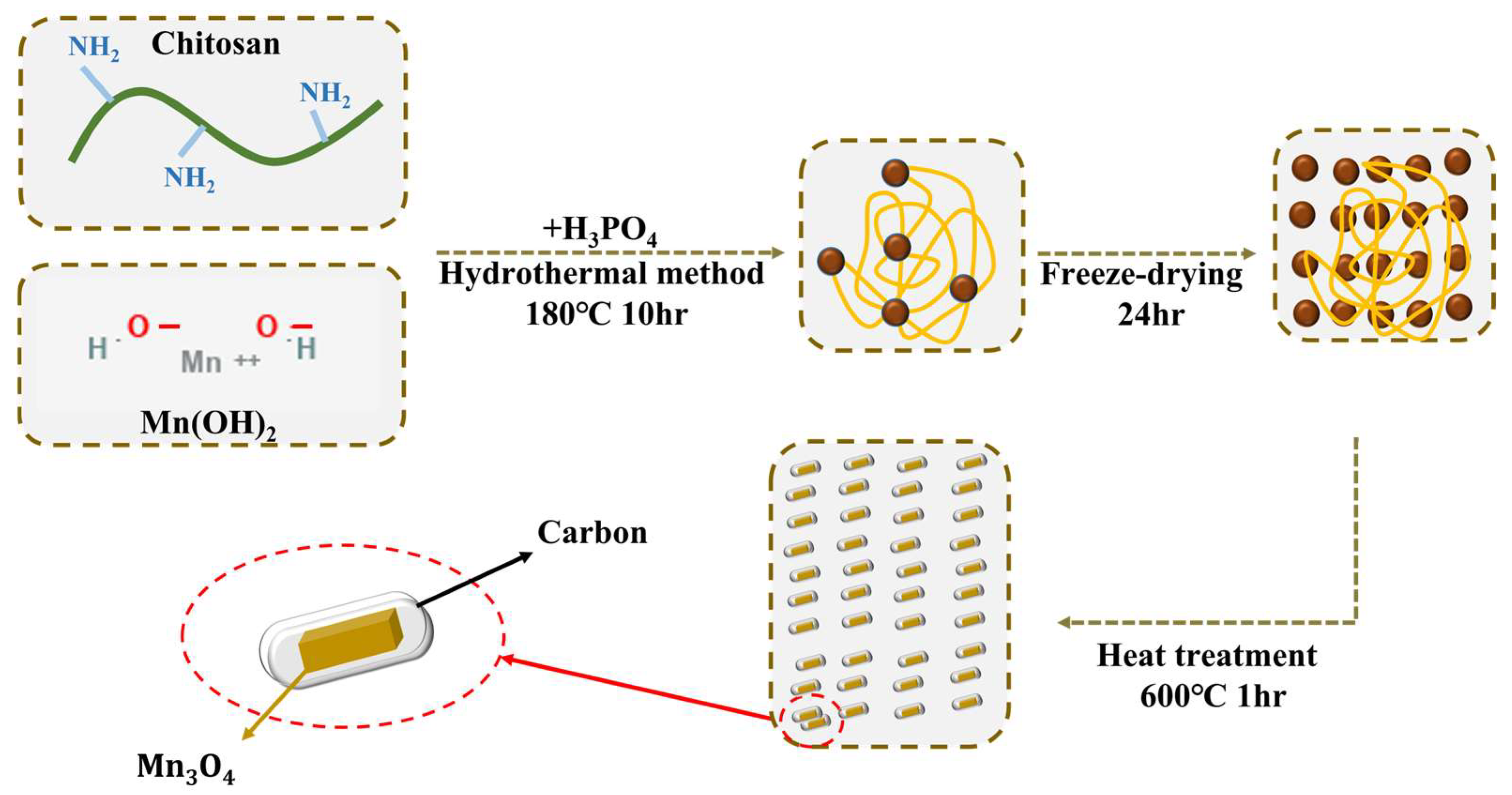
2.1. Synthesis of Mn3O4@NPC/CC Hybrid Electrode
2.2. Characterization
2.3. Electrochemical Properties of Mn3O4@NPC/CC Hybrid Electrode
3. Results
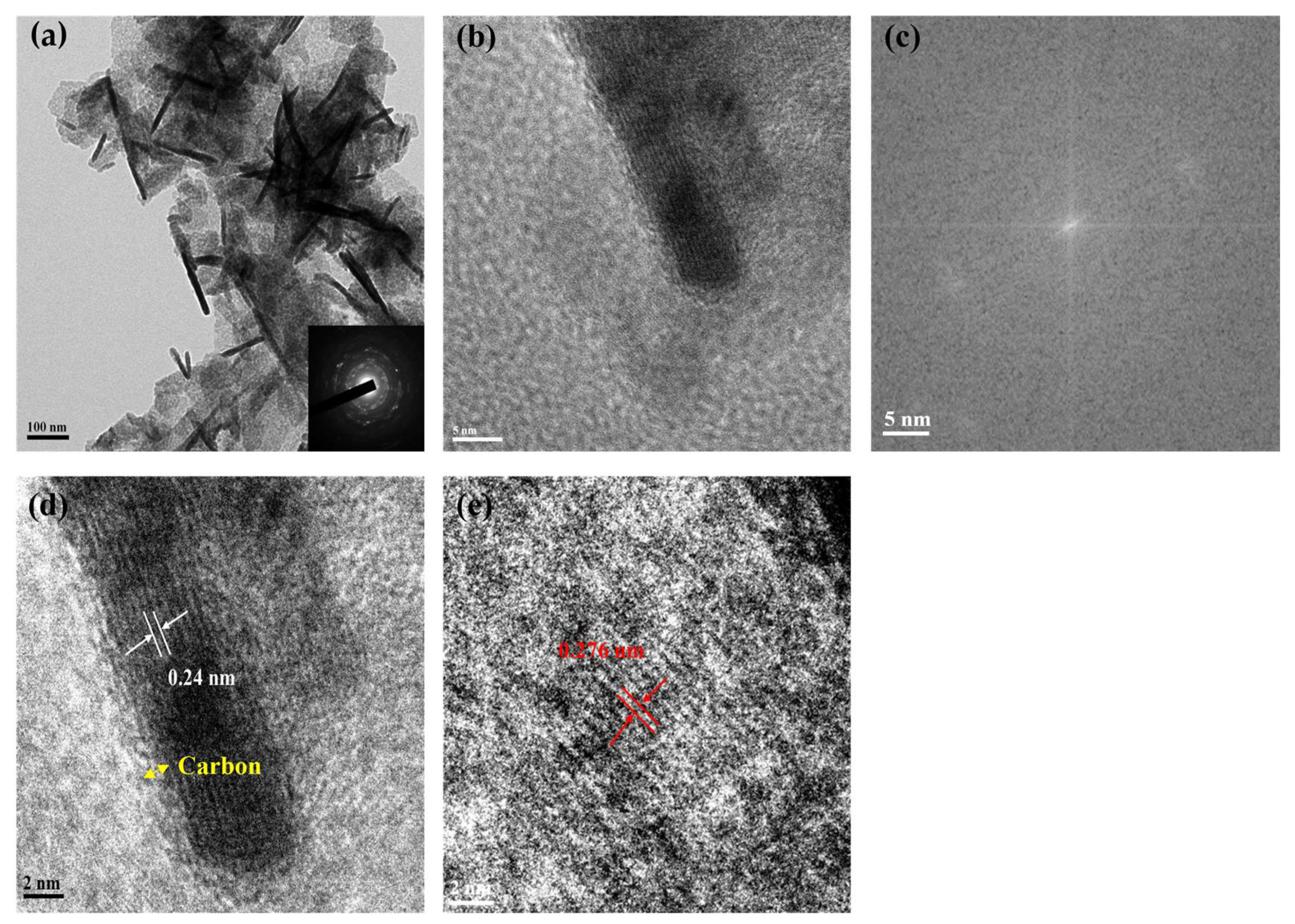
3.1. The Physical Properties of N, P-Doped Mn3O4 Material
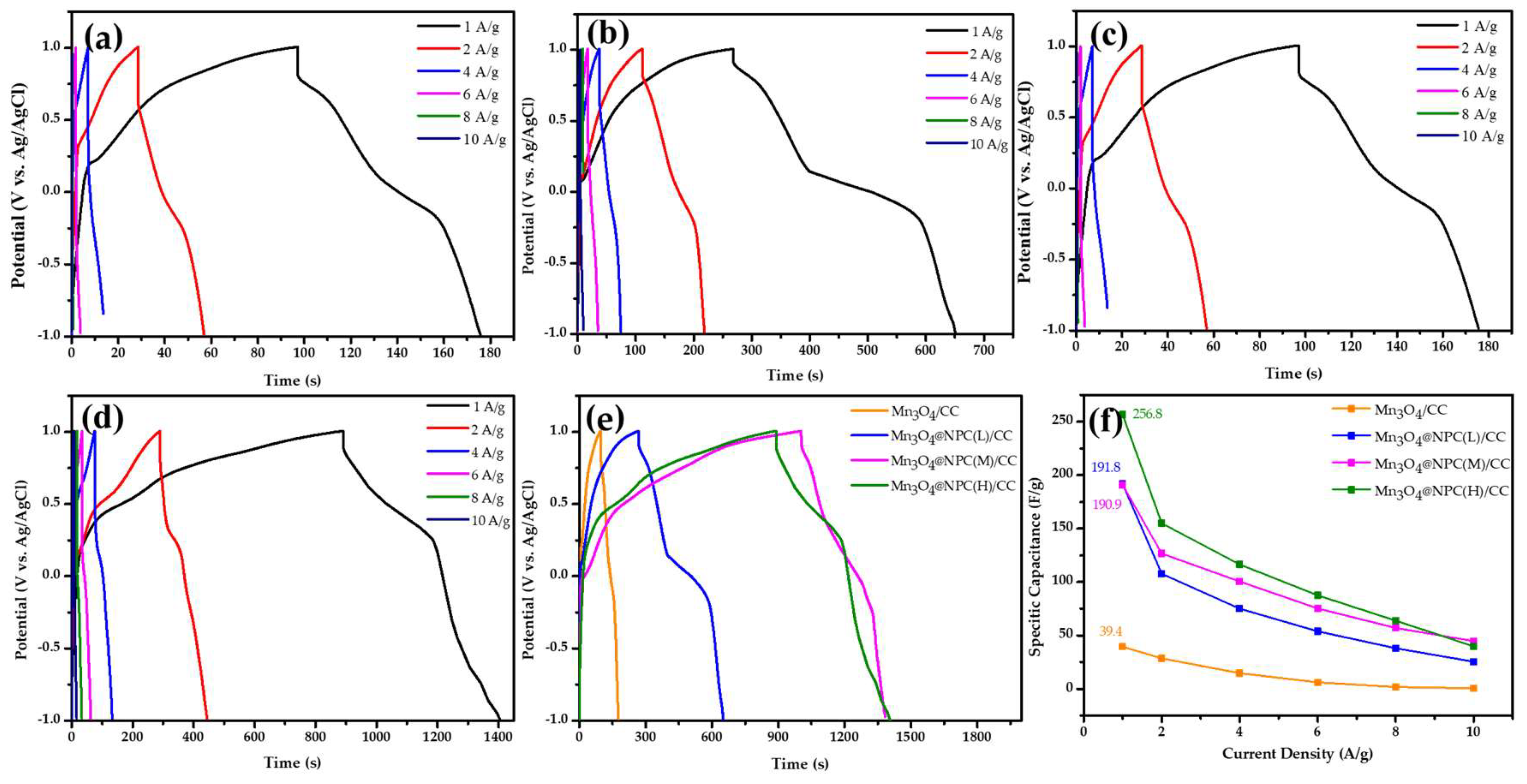
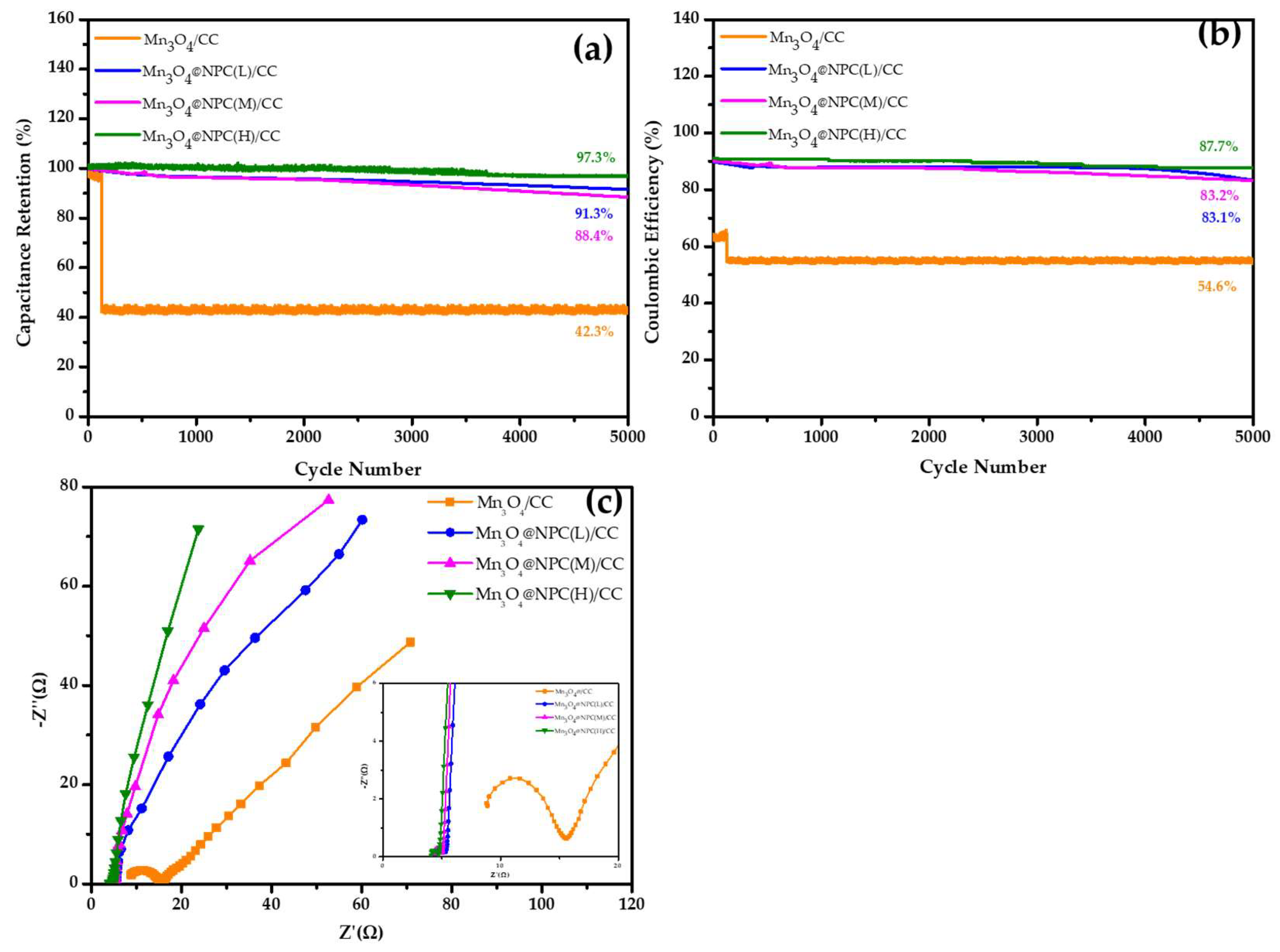
3.2. Electrochemical Properties of Mn3O4@NPC/CC Electrodes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Borenstein, A.; Strauss, V.; Kowal, M.D.; Yoonessi, M.; Muni, M.; Anderson, M.; Kaner, R.-B. Laser-reduced graphene-oxide/ferrocene: A 3-D redoxactive composite for supercapacitor electrodes. J. Mater. Chem. A 2018, 6, 20463–20472. [Google Scholar] [CrossRef]
- Chen, Y.M.; Yu, B.-Z.; Miao, Y.-Q.; Gao, F.; Jing, G.-Y.; Fan, H.-M. Pushing the cycling stability limit of hierarchical metal oxide core/shell nanoarrays pseudocapacitor electrodes by nanoscale interface optimization. Nanoscale 2018, 10, 14352–14358. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Fan, Z.; Sun, W.; Ning, G.; Wei, T.; Zhang, Q.; Zhang, R.; Zhi, L.; Wei, F. Advanced asymmetric supercapacitors based on Ni(OH)2/graphene and porous graphene electrodes with high energy density. Adv. Funct. Mater. 2012, 22, 2632–2641. [Google Scholar] [CrossRef]
- Fan, Z.; Yan, J.; Wei, T.; Zhi, L.; Ning, G.; Li, T.; Wei, F. Asymmetric supercapacitors based on graphene/MnO2 and activated carbon nanofiber electrodes with high power and energy density. Adv. Funct. Mater. 2011, 21, 2366–2375. [Google Scholar] [CrossRef]
- Khaligh, A.; Li, Z. Battery, ultracapacitor, fuel cell, and hybrid energy storage systems for electric, hybrid electric, fuel cell, and plug-in hybrid electric vehicles: State of the art. IEEE Trans. Veh. Technol. 2010, 59, 2806–2814. [Google Scholar] [CrossRef]
- Luan, Z.; Tian, Y.; Gai, L.; Jiang, H.; Guo, X.; Yang, Y. Environment-benign synthesis of rGO/MnOx nanocomposites with superior electrochemical performance for supercapacitors. J. Alloys Compd. 2017, 729, 9–18. [Google Scholar] [CrossRef]
- Rani, B.-J.; Ravina, M.; Ravi, G.; Ravichandran, S.; Ganesh, V.; Yuvakkumar, R. Synthesis and characterization of hausmannite (Mn3O4) nanostructures. Surf. Interfaces 2018, 11, 28–36. [Google Scholar] [CrossRef]
- Dubal, D.-P.; Dhawale, D.-S.; Salunkhe, R.-R.; Pawar, S.-M.; Lokhande, C.-D. A novel chemical synthesis and characterization of Mn3O4 thin films for supercapacitor application. Appl. Surf. Sci. 2010, 256, 4411–4416. [Google Scholar] [CrossRef]
- Ding, Y.; Dai, L.; Wang, R.; Wang, H.; Zhang, H.; Jiang, W.; Tang, J.; Zang, S.-Q. Bio-inspired Mn3O4@N, P-doped carbon cathode for 2.6 V flexible aqueous asymmetric supercapacitors. Chem. Eng. J. 2021, 407, 126874. [Google Scholar] [CrossRef]
- Sahoo, R.; Pham, D.-T.; Lee, T.-H.; Luu, T.-H.-T.; Seok, J.; Lee, Y.-H. Redox-driven route for widening voltage window in asymmetric supercapacitor. ACS Nano 2018, 12, 8494–8505. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Chen, X.; Su, F.; Lyu, X.; Miao, M. Sandwich-structured transition metal oxide/graphene/carbon nanotube composite yarn electrodes for flexible two-ply yarn supercapacitors. Ind. Eng. Chem. Res. 2020, 59, 5752–5759. [Google Scholar] [CrossRef]
- Yan, B.; Zheng, J.; Feng, L.; Zhang, Q.; Zhang, C.; Ding, Y.; Han, J.; Jiang, S.; He, S. Pore engineering: Structure-capacitance correlations for biomass derived porous carbon materials. Mater. Des. 2023, 229, 111904. [Google Scholar] [CrossRef]
- Zhao, W.; Yan, B.; Chen, D.; Chen, J.; Zhang, Q.; Jiang, L.; Lan, T.; Zhang, C.; Yang, W.; He, S. Free-standing carbon network with enhanced capacitive performance synthesized via green H2O2 activation. Colloids Surf. A Physicochem. Eng. Asp. 2023, 668, 131425. [Google Scholar] [CrossRef]
- Zhuang, R.; Dong, Y.; Li, D.; Liu, R.; Zhang, S.; Yu, Y.; Song, H.; Ma, J.; Liu, X.; Chen, X. Polyaniline-mediated coupling of Mn3O4 nanoparticles on activated carbon for high-performance asymmetric supercapacitors. J. Alloys Compd. 2021, 851, 156871. [Google Scholar] [CrossRef]
- Liu, L.; Feng, Y.; Wu, W. Recent progress in printed flexible solid-state supercapacitors for portable and wearable energy storage. J. Power Sources 2019, 410–411, 69–77. [Google Scholar] [CrossRef]
- Yang, J.; Wang, L.; Ma, Z.; Wei, M. In situ synthesis of Mn3O4 on Ni foam/graphene substrate as a newly self-supported electrode for high supercapacitive performance. J. Colloid Interface Sci. 2019, 534, 665–671. [Google Scholar] [CrossRef]
- Shahriary, L.; Athawale, A.-A. Graphene oxide synthesized by using modified Hummers approach. Int. J. Sustain. Energy 2014, 2, 58–63. [Google Scholar]
- Yang, W.-D.; Li, Y.-R.; Lee, Y.-C. Synthesis of r-GO/TiO2 composites via the UV assisted photocatalytic reduction of graphene oxide. Appl. Surf. Sci. 2016, 380, 249–256. [Google Scholar] [CrossRef]
- Jiang, M.; Liu, Z.; Hu, J.; Liu, Y.; Luo, Y.; Lai, X.; Xu, T. Facile electrodeposition of Mn3O4 nanoparticles on wood-derived porous carbon for high-performance asymmetric supercapacitor. Diam. Relat. Mater. 2021, 118, 108506. [Google Scholar] [CrossRef]
- Sun, L.; Song, G.; Sun, Y.; Fu, Q.; Pan, C. One-step construction of 3D N/P-codoped hierarchically porous carbon framework in-situ armored Mn3O4 nanoparticles for highperformance flexible supercapacitors. Electrochim. Acta 2020, 333, 135496. [Google Scholar] [CrossRef]
- Sun, L.; Fu, Q.; Pan, C. Mn3O4 embedded 3D multi-heteroatom codoped carbon sheets/carbon foams composites for high-performance flexible supercapacitors. J. Alloys Compd. 2020, 849, 156666. [Google Scholar] [CrossRef]
- Ye, Z.; Wang, F.; Jia, C.; Mu, K.; Yu, M.; Lv, Y.; Shao, Z. Nitrogen and oxygen-codoped carbon nanospheres for excellent specific capacitance and cyclic stability supercapacitor electrodes. Chem. Eng. J. 2017, 330, 1166–1173. [Google Scholar] [CrossRef]
- Wadekar, P.-H.; Khose, R.-V.; Pethsangave, D.-A.; Some, S. Waste-derived heteroatom-doped activated carbon/manganese dioxide trio-composite for supercapacitor applications. Energy Technol. 2020, 8, 1901402. [Google Scholar] [CrossRef]
- Hu, B.; Wang, Y.; Shang, X.; Xu, K.; Yang, J.; Huang, M.; Liu, J. Structure-tunable Mn3O4-Fe3O4@C hybrids for high-performance supercapacitor. J. Colloid Interface Sci. 2021, 581, 66–75. [Google Scholar] [CrossRef]
- Wang, Y.; Lai, W.; Wang, N.; Jiang, Z.; Wang, X.; Zou, P.; Lin, Z.; Fan, H.-J.; Kang, F.; Wong, C.-P. A reduced graphene oxide/mixed-valence manganese oxide composite electrode for tailorable and surface mountable supercapacitors with high capacitance and super-long life. Energy Environ. Sci. 2017, 10, 941–949. [Google Scholar] [CrossRef]
- Wu, Q.; Gao, M.; Cao, S.; Hu, J.; Huang, L.; Yu, S.; Ragauskas, A.J. Chitosan-based layered carbon materials prepared via ionic-liquid-assisted hydrothermal carbonization and their performance study. J. Taiwan Inst. Chem. Eng. 2019, 101, 231–243. [Google Scholar] [CrossRef]
- Wei, L.; Deng, W.; Li, S.; Wu, Z.; Cai, J.; Luo, J. Sandwich-like chitosan porous carbon Spheres/MXene composite with high specific capacitance and rate performance for supercapacitors. J. Bioresour. Bioprod. 2022, 7, 63–72. [Google Scholar] [CrossRef]
- Wang, H.; Deng, J.; Chen, Y.; Xu, F.; Wei, Z.; Wang, Y. Hydrothermal synthesis of manganese oxide encapsulated multiporous carbon nanofibers for supercapacitors. Nano Res. 2016, 9, 2672–2680. [Google Scholar] [CrossRef]
- Yang, W.-D.; Chou, Y.-R.; Kuo, C.-C.; Kang, Y.-M. Controlling the molar ratios of cation to anion of precursors for high performance capacitive properties of MnO2 hybridized carbon-based materials electrode. Batteries 2023, 9, 273. [Google Scholar] [CrossRef]
- Madhuri, S.; Chakra, C.-S.; Sadhana, K.; Divya, V. Sketchy synthesis of Mn3O4, Mn3O4/AC and Mn3O4/CNT composites for application of/in energy cache. Mater. Today 2022, 65, 2812–2818. [Google Scholar] [CrossRef]
- Guo, C.; Su, Y.; Xia, Y.; Yi, S.; Li, J. Construction of hierarchical yolk-shell structured Mn3O4@ NC as efficient sulfur hosts for Li–S batteries. Ceram. Int. 2022, 48, 6470–6476. [Google Scholar] [CrossRef]
- Rahman, M.-M.; Marwani, H.-M.; Algethami, F.-K.; Asiri, A.-M. Comparative performance of hydrazine sensors developed with Mn3O4/carbon-nanotubes, Mn3O4/graphene-oxides and Mn3O4/carbon-black nanocomposites. Mater. Express 2017, 7, 169–179. [Google Scholar] [CrossRef]
- Raj, A.-M.-E.; Victoria, S.G.; Jothy, V.-B.; Ravidhas, C.; Wollschläger, J.; Suendorf, M.; Neumann, M.; Jayachandran, M.; Sanjeeviraja, C. XRD and XPS characterization of mixed valence Mn3O4 hausmannite thin films prepared by chemical spray pyrolysis technique. Appl. Surf. Sci. 2010, 256, 2920–2926. [Google Scholar]
- Guo, L.; Ding, Y.; Qin, C.; Song, W.; Sun, S.; Fang, K.; Li, W.; Du, J.; Wang, F. Anchoring Mn3O4 nanoparticles onto nitrogen-doped porous carbon spheres derived from carboxymethyl chitosan as superior anodes for lithium-ion batteries. J. Alloys Compd. 2018, 735, 209–217. [Google Scholar] [CrossRef]
- Li, Z.; Lin, J.; Li, B.; Yu, C.; Wang, H.; Li, Q. Construction of heteroatom-doped and three-dimensional graphene materials for the applications in supercapacitors: A review. J. Energy Storage 2021, 44, 103437. [Google Scholar] [CrossRef]
- Jiang, H.; Zhou, C.; Yan, X.; Miao, J.; You, M.; Zhu, Y.; Li, Y.; Zhou, W.; Cheng, X. Effects of various electrolytes on the electrochemistry performance of Mn3O4/carbon cloth to ultra-flexible all-solid-state asymmetric supercapacitor. J. Energy Storage 2020, 32, 101898. [Google Scholar] [CrossRef]
- Li, Y.; Qu, J.; Gao, F.; Lv, S.; Shi, L.; He, C.; Sun, J. In situ fabrication of Mn3O4 decorated graphene oxide as a synergistic catalyst for degradation of methylene blue. Appl. Catal. B 2015, 162, 268–274. [Google Scholar] [CrossRef]
- Chen, H.; Zhou, W.; Zhu, D.; Liu, Z.; Feng, Z.; Li, J.; Chen, Y. Porous cube-like Mn3O4@ C as an advanced cathode for low-cost neutral zinc-ion battery. J. Alloys Compd. 2020, 813, 151812. [Google Scholar] [CrossRef]
- Qin, Y.; Jiang, Z.; Guo, L.; Huang, J.; Jiang, Z.J. Controlled thermal oxidation derived Mn3O4 encapsulated in nitrogen doped carbon as an anode for lithium/sodium ion batteries with enhanced performance. Chem. Eng. J. 2021, 406, 126894. [Google Scholar] [CrossRef]
- Tan, Q.; Li, X.; Zhang, B.; Chen, X.; Tian, Y.; Wan, H.; Zhang, L.; Miao, L.; Wang, C.; Gan, Y. Valence engineering via in situ carbon reduction on octahedron sites Mn3O4 for ultra-long cycle life aqueous Zn-ion battery. Adv. Energy Mater. 2020, 10, 2001050. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, K.; Hong, M.; Yang, Y.; Hu, N.; Su, Y.; Zhang, Y. Laser-induced MnO/Mn3O4/N-doped-graphene hybrid as binder-free anodes for lithium ion batteries. Chem. Eng. J. 2020, 385, 123720. [Google Scholar] [CrossRef]
- Chai, H.; Xu, J.; Han, J.; Su, Y.; Sun, Z.; Jia, D.; Zhou, W. Facile synthesis of Mn3O4-rGO hybrid materials for the high-performance electrocatalytic reduction of oxygen. J. Colloid Interface Sci. 2017, 488, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, T.; Golikand, A.-N.; Mashhadizadeh, M.-H.; Aghazadeh, M. High temperature and low current density synthesis of Mn3O4 porous nano spheres: Characterization and electrochemical properties. Curr. Appl. Phys. 2012, 12, 544–549. [Google Scholar] [CrossRef]
- Liu, K.; Zou, F.; Sun, Y.; Yu, Z.; Liu, X.; Zhou, L.; Xia, Y.; Vogt, B.-D.; Zhu, Y. Self-assembled Mn3O4/C nanospheres as high-performance anode materials for lithium ion batteries. J. Power Sources 2018, 395, 92–97. [Google Scholar] [CrossRef]
- Wang, L.-H.; Ren, L.-L.; Qin, Y.-F.; Chen, J.; Chen, H.-Y.; Wang, K.; Li, Q. Preparation of Mn3O4 Nanoparticles via Precipitation in Presence of CTAB Molecules and Its Application as Anode Material for Lithium Ion Batteries. Int. J. Electrochem. Sci. 2022, 17, 220221. [Google Scholar] [CrossRef]
- Miankushki, H.-N.; Sedghi, A.; Baghshahi, S. A comparison of the Electrochemical Properties of graphene/Mn3O4 Composites fabricated by two Different Methods. Int. J. Electrochem. Sci 2018, 13, 2462–2473. [Google Scholar] [CrossRef]
- Sun, D.; He, L.; Lai, Y.; Lian, J.; Sun, J.; Xie, A.; Lin, B. Structure and Electrochemical Properties of Mn3O4 Nanocrystal-Coated Porous Carbon Microfiber Derived from Cotton. Materials 2018, 11, 1987. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.-J.; Chen, S.; Jiang, C.; Wang, N.; Yang, P.; Liu, M.; Cheng, Y. Assembly of flower-like Mn3O4/NiCo-LDH@ carbon nanotube nanocomposites on Ni foam for binder-free capacitor electrode. J. Alloys Compd. 2023, 930, 167466. [Google Scholar] [CrossRef]
- Beknalkar, S.-A.; Teli, A.-M.; Harale, N.-S.; Shin, J.-C.; Patil, P.-S. Construction of IrO2@ Mn3O4 core-shell heterostructured nanocomposites for high performance symmetric supercapacitor device. J. Alloys Compd. 2021, 887, 161328. [Google Scholar] [CrossRef]
- Zhao, K.; Lyu, K.; Liu, S.; Gan, Q.; He, Z.; Zhou, Z. Ordered porous Mn3O4@ N-doped carbon/graphene hybrids derived from metal–organic frameworks for supercapacitor electrodes. J. Mater. Sci. 2017, 52, 446–457. [Google Scholar] [CrossRef]
- Shunmugapriya, B.; Vijayakumar, T. H2O2-assisted structural transformation of Mn3O4 nanoparticles to nanorods for supercapacitor applications. J. Mater. Sci. Mater. 2022, 33, 9334–9346. [Google Scholar] [CrossRef]
- Feng, X.; Huang, Y.; Li, C.; Xiao, Y.; Chen, X.; Gao, X.; Chen, C. Construction of carnations-like Mn3O4@NiCo2O4@NiO hierarchical nanostructures for high-performance supercapacitors. Electrochim. Acta 2019, 308, 142–149. [Google Scholar] [CrossRef]
- Liu, C.; Sun, H.; Qian, J.; Chen, Z.; Chen, F.; Liu, S.; Lv, Y.; Lu, X.; Chen, A. Ultrafine Mn3O4/CeO2 nanorods grown on reduced graphene oxide sheets as high-performance supercapacitor electrodes. J. Alloys Compd. 2017, 722, 54–59. [Google Scholar] [CrossRef]
- Kumaresan, L.; Harshini, K.-S.; Amir, H.; Shanmugavelayutham, G.; Viswanathan, C. Single-step synthesis of Mn3N2, MnxON and Mn3O4 nanoparticles by thermal plasma arc discharge technique and their comparative study as electrode material for supercapacitor application. J. Alloys Compd. 2023, 942, 169121. [Google Scholar] [CrossRef]





| Mn3O4@NPC(L) | Mn3O4@NPC(M) | Mn3O4@NPC (H) | |
|---|---|---|---|
| Surface Area (m2/g) | 49.83 | 48.18 | 86.16 |
| Micropore Area (m2/g) | 33.42 | 29.07 | 52.46 |
| Pore Volume (cm3/g) | 0.134 | 0.246 | 0.179 |
| Pore Size (nm) | 17.95 | 14.93 | 8.31 |
| Material | Electrolyte | Current Density (A/g) | Capacitance Retention (%) | Specific Capacitance (F/g) | Ref. |
| NPCM/Mn3O4 | 6 M KOH | 0.5 | 99 (5000 cycle) | 384 | [20] |
| Mn3O4-Fe3O4@C | 1 M Na2SO4 | 1 | 95 (1000 cycle) | 178 | [24] |
| Mn3O4-MC | 2 M KOH | 1 | 93.7 (1000 cycle) | 236.7 | [26] |
| Cr-doped Mn3O4 | 1 M Na2SO4 | 1 | 70 (1000 cycle) | 209 | [53] |
| Mn3O4 NPs | 1 M KOH | 5 | 84.2 (5000 cycle) | 209 | [54] |
| Mn3O4@NPC(H)/CC | 1 M Na2SO4 | 1 | 97.3 (5000 cycle) | 256.8 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, Y.-M.; Yang, W.-D. Boosting the Capacitive Performance of Supercapacitors by Hybridizing N, P-Codoped Carbon Polycrystalline with Mn3O4-Based Flexible Electrodes. Nanomaterials 2023, 13, 2060. https://doi.org/10.3390/nano13142060
Kang Y-M, Yang W-D. Boosting the Capacitive Performance of Supercapacitors by Hybridizing N, P-Codoped Carbon Polycrystalline with Mn3O4-Based Flexible Electrodes. Nanomaterials. 2023; 13(14):2060. https://doi.org/10.3390/nano13142060
Chicago/Turabian StyleKang, Yu-Min, and Wein-Duo Yang. 2023. "Boosting the Capacitive Performance of Supercapacitors by Hybridizing N, P-Codoped Carbon Polycrystalline with Mn3O4-Based Flexible Electrodes" Nanomaterials 13, no. 14: 2060. https://doi.org/10.3390/nano13142060




