Preparation and Characterization of Metal–Organic Framework Coatings for Improving Protein Crystallization Screening
Abstract
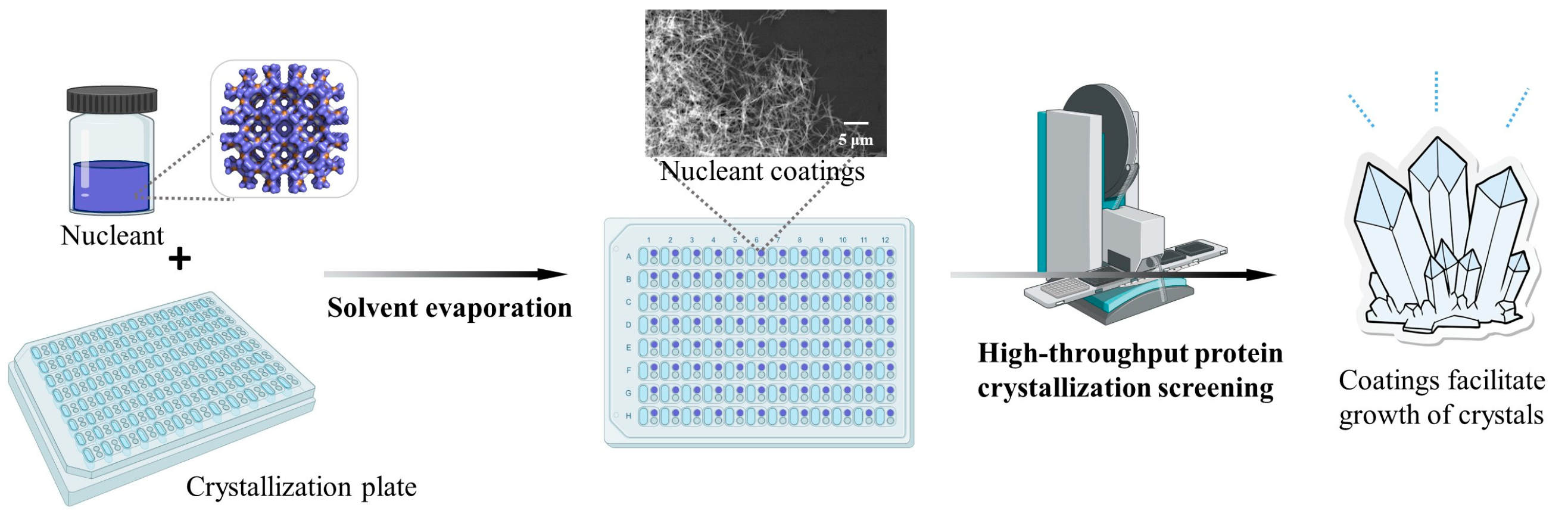
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of HKUST-1 Suspension
2.3. Preparation and Characterization of HKUST-1 Coatings
2.4. Crystallization Experiment
3. Results and Discussion
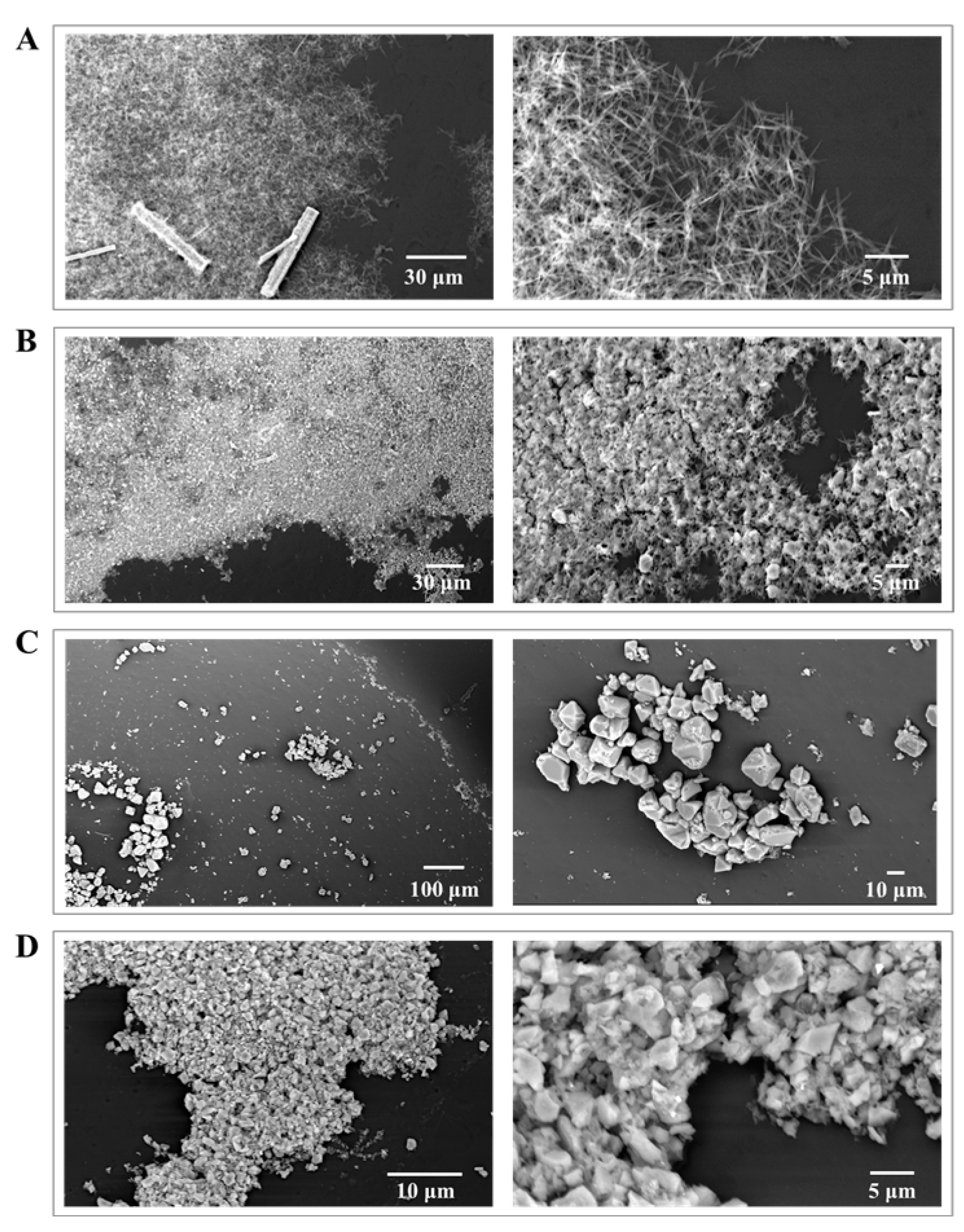
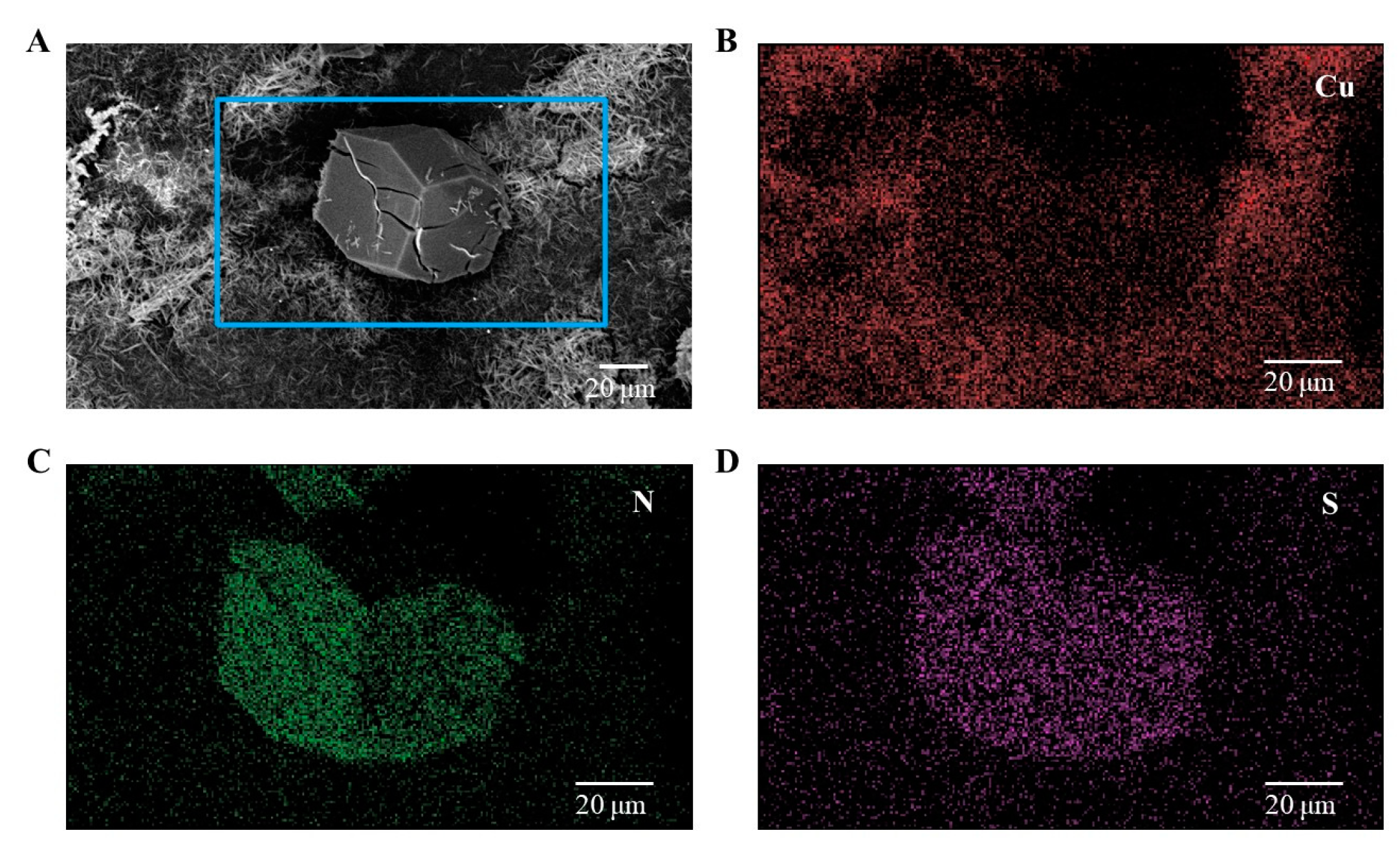
3.1. Preparation and Characterization of HKUST-1 Coatings
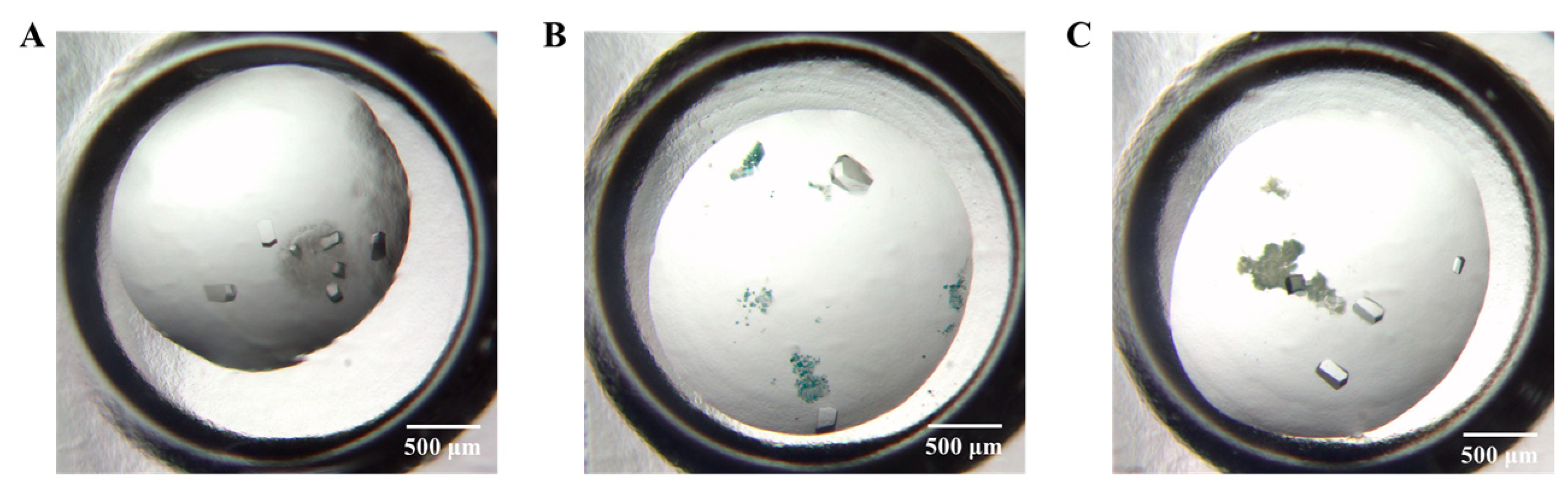
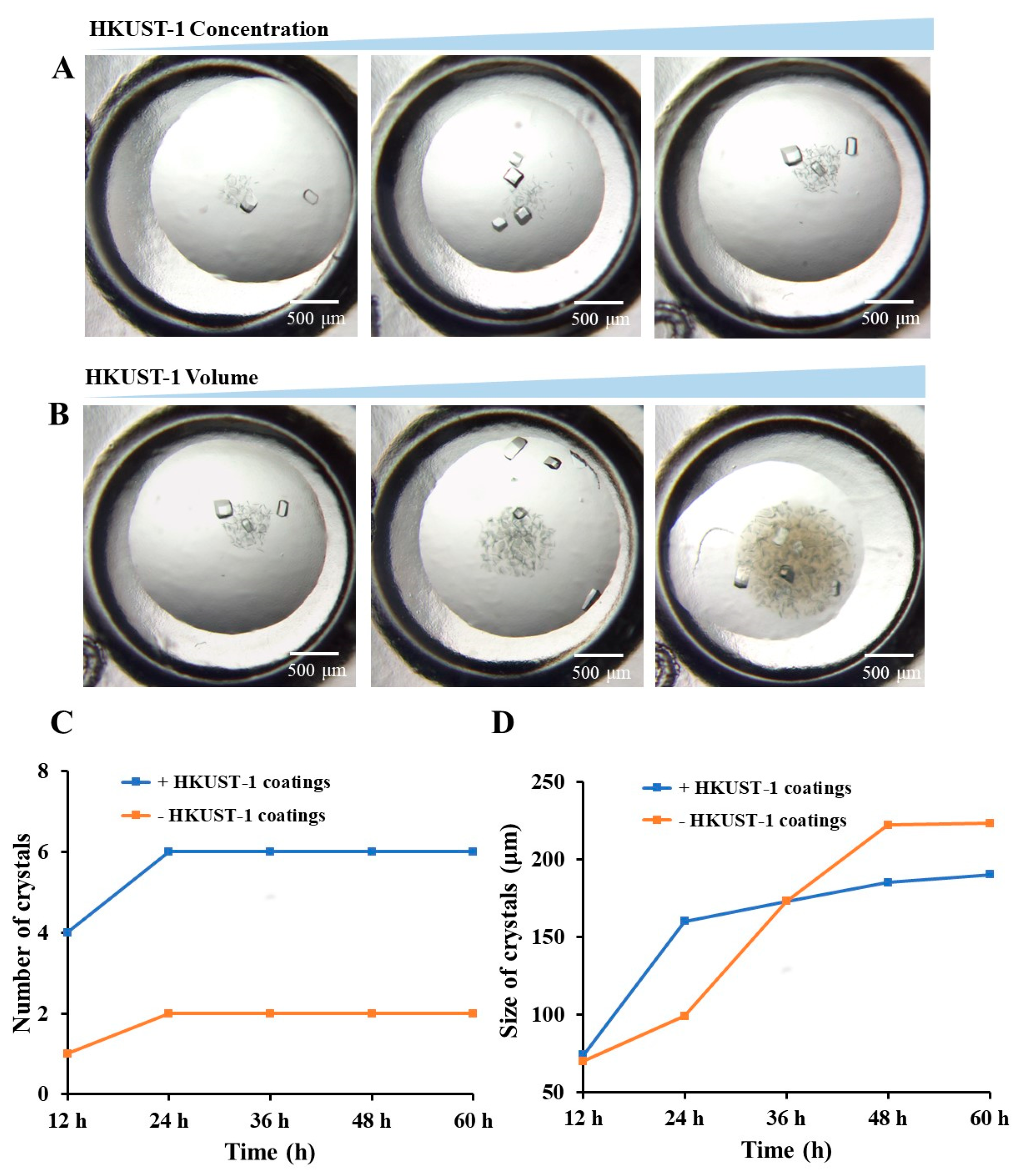
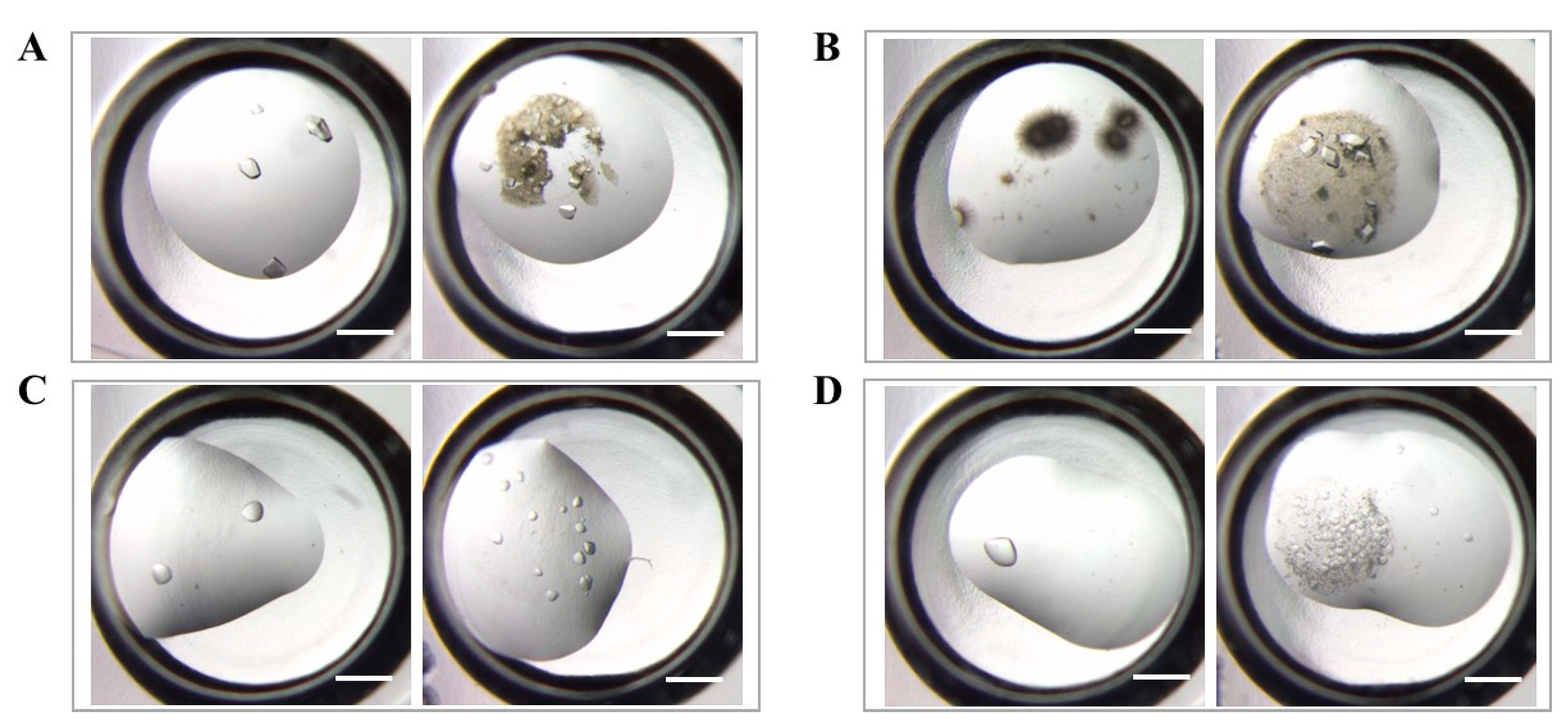
3.2. Effectiveness of Different HKUST-1 Coatings on Protein Crystallization
3.3. The Application of HKUST-1 Coatings for Crystals Screening Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barends, T.R.M.; Foucar, L.; Botha, S.; Doak, R.B.; Shoeman, R.L.; Nass, K.; Koglin, J.E.; Williams, G.J.; Boutet, S.; Messerschmidt, M.; et al. De novo protein crystal structure determination from X-ray free-electron laser data. Nature 2014, 505, 244–247. [Google Scholar] [CrossRef] [PubMed]
- Zatsepin, N.A.; Li, C.; Colasurd, P.; Nannenga, B.L. The complementarity of serial femtosecond crystallography and MicroED for structure determination from microcrystals. Curr. Opin. Struct. Biol. 2019, 58, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Kleywegt, G.J.; Jones, T.A. Databases in protein Crystallography. Acta Crystallogr. D Biol. Crystallogr. 1998, 54, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, E.P.; Beis, K.; Cameron, A.D.; Iwata, S. Overcoming the challenges of membrane protein crystallography. Curr. Opin. Struct. Biol. 2008, 18, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Grey, J.L.; Thompson, D.H. Challenges and opportunities for new protein crystallization strategies in structure-based drug design. Expert Opin. Drug Discov. 2010, 5, 1039–1045. [Google Scholar] [CrossRef] [PubMed]
- Gorrec, F. The MORPHEUS protein crystallization screen. J. Appl. Cryst. 2009, 42, 1035–1042. [Google Scholar] [CrossRef]
- Lau, B.T.C.; Baitz, C.A.; Dong, X.P.; Hansen, C.L. A complete microfluidic screening platform for rational protein crystallization. J. Am. Chem. Soc. 2007, 129, 454–455. [Google Scholar] [CrossRef]
- DeLucas, L.J.; Hamrick, D.; Cosenza, L.; Nagy, L.; McCombs, D.; Bray, T.; Chait, A.; Stoops, B.; Belgovskiy, A.; Wilson, W.W.; et al. Protein crystallization: Virtual screening and optimization. Prog. Biophys. Mol. Biol. 2005, 88, 285–309. [Google Scholar] [CrossRef]
- Van Alstine, J.M.; Malmsten, M.; Long, M.M.; Johnson, V.K.; DeLucas, L.J. Polymer coatings for improved protein crystal growth. Colloids Surf. 1999, 14, 197–211. [Google Scholar] [CrossRef]
- Wang, Q.J.; Zhao, G.; Zhang, C.Y. Cyclodextrin and its derivatives enhance protein crystallization by grafted on crystallization plates. J. Cryst. Growth 2020, 536, 125591. [Google Scholar] [CrossRef]
- Gao, A.T.; Wu, Q.; Wang, D.H.; Ha, Y.; Chen, Z.J.; Yang, P. A superhydrophobic surface templated by protein self-assembly and emerging application toward protein crystallization. Adv. Mater. 2016, 28, 579–587. [Google Scholar] [CrossRef]
- Ji, D.; Arnold, C.M.; Graupe, M.; Beadle, E.; Dunn, R.V.; Phan, M.N.; Villazana, R.J.; Benson, R.; Colorado, R.; Lee, T.R.; et al. Improved protein crystallization by vapor diffusion from drops in contact with transparent, self-assembled monolayers on gold-coated glass coverslips. J. Cryst. Growth 2000, 218, 390–398. [Google Scholar] [CrossRef]
- Pechkova, E.; Nicolini, C. Protein nucleation and crystallization by homologous protein thin film template. J. Cell. Biochem. 2002, 85, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Pechkova, E.; Nicolini, C. Atomic structure of a CK alpha human kinase by microfocus diffraction of extra-small microcrystals grown with nanobiofilm template. J. Cell. Biochem. 2004, 91, 1010–1020. [Google Scholar] [CrossRef] [PubMed]
- Pechkova, E.; Fiordoro, S.; Fontani, D.; Nicolini, C. Investigating crystal-growth mechanisms with and without LB template: Protein transfer from LB to crystal. Acta Crystallogr. D Biol. Crystallogr. 2005, 61, 809–812. [Google Scholar] [CrossRef] [PubMed]
- Saridakis, E.; Chayen, N.E. Towards a ‘universal’ nucleant for protein crystallization. Trends Biotechnol. 2009, 27, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Saridakis, E.; Chayen, N.E. Imprinted polymers assisting protein crystallization. Trends Biotechnol. 2013, 31, 515–520. [Google Scholar] [CrossRef]
- Khurshid, S.; Saridakis, E.; Govada, L.; Chayen, N.E. Porous nucleating agents for protein crystallization. Nat. Protoc. 2014, 9, 1621–1633. [Google Scholar] [CrossRef]
- Govada, L.; Leese, H.S.; Saridakis, E.; Kassen, S.; Chain, B.; Khurshid, S.; Menzel, R.; Hu, S.; Shaffer, M.S.P.; Chayen, N.E. Exploring carbon nanomaterial diversity for nucleation of protein crystals. Sci. Rep. 2016, 6, 20053. [Google Scholar] [CrossRef]
- Chen, W.; Park, S.J.; Kong, F.; Li, X.; Yang, H.; Heng, J.Y.Y. High protein-loading silica template for heterogeneous protein crystallization. Cryst. Growth Des. 2020, 20, 866–873. [Google Scholar] [CrossRef]
- Chui, S.S.Y.; Lo, S.M.F.; Charmant, J.P.H.; Orpen, A.G.; Williams, I.D. A chemically functionalizable nanoporous material [Cu3(TMA)2(H2O)3]n. Science 1999, 283, 1148–1150. [Google Scholar] [CrossRef]
- Ravera, F.; Santini, E.; Loglio, G.; Ferrari, M.; Liggieri, L. Effect of nanoparticles on the interfacial properties of liquid/liquid and liquid/air surface layers. J. Phys. Chem. B 2006, 110, 19543–19551. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Luo, M.; Dai, L.L. Influences of surfactant and nanoparticle assembly on effective interfacial tensions. Phys. Chem. Chem. Phys. 2008, 10, 2207–2213. [Google Scholar] [CrossRef]
- Silva, A.C.; Gonzalez-Mira, E.; Garcia, M.L.; Egea, M.A.; Fonseca, J.; Silva, R.; Santos, D.; Souto, E.B.; Ferreira, D. Preparation, characterization and biocompatibility studies on risperidone-loaded solid lipid nanoparticles (SLN): High pressure homogenization versus ultrasound. Colloids Surf. 2011, 86, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Shah, U.V.; Allenby, M.C.; Williams, D.R.; Heng, J.Y.Y. Crystallization of proteins at ultralow supersaturations using novel three-dimensional nanotemplates. Cryst. Growth Des. 2012, 12, 1772–1777. [Google Scholar] [CrossRef]
- Sleutel, M.; Van Driessche, A.E.S. Role of clusters in nonclassical nucleation and growth of protein crystals. Proc. Natl. Acad. Sci. USA 2014, 111, E546–E553. [Google Scholar] [CrossRef]
- Galkin, O.; Vekilov, P.G. Control of protein crystal nucleation around the metastable liquid-liquid phase boundary. Proc. Natl. Acad. Sci. USA 2000, 97, 6277–6281. [Google Scholar] [CrossRef] [PubMed]


| Protein | Concentration (mg/mL) | − Coatings | + Coatings | ||
|---|---|---|---|---|---|
| Total | New | Lost | |||
| Lysozyme | 6.5 | 2 | 15 | 13 | 0 |
| Concanavalin A | 4.5 | 11 | 13 | 7 | 5 |
| RNase A | 30 | 2 | 4 | 2 | 0 |
| Urease | 1 | 3 | 8 | 5 | 0 |
| Bovine serum albumin | 35 | 1 | 0 | 0 | 1 |
| Myoglobin | 5 | 0 | 1 | 1 | 0 |
| Papain | 1 | 0 | 2 | 2 | 0 |
| Lipase | 4 | 0 | 2 | 2 | 0 |
| Cytochrome C | 1.5 | 0 | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Q.; Zhang, Z.; Wang, L.; Xing, X.; Zhou, J.; Li, L. Preparation and Characterization of Metal–Organic Framework Coatings for Improving Protein Crystallization Screening. Nanomaterials 2023, 13, 2064. https://doi.org/10.3390/nano13142064
Yang Q, Zhang Z, Wang L, Xing X, Zhou J, Li L. Preparation and Characterization of Metal–Organic Framework Coatings for Improving Protein Crystallization Screening. Nanomaterials. 2023; 13(14):2064. https://doi.org/10.3390/nano13142064
Chicago/Turabian StyleYang, Qin, Zhenkun Zhang, Lin Wang, Xiwen Xing, Jiahai Zhou, and Long Li. 2023. "Preparation and Characterization of Metal–Organic Framework Coatings for Improving Protein Crystallization Screening" Nanomaterials 13, no. 14: 2064. https://doi.org/10.3390/nano13142064
APA StyleYang, Q., Zhang, Z., Wang, L., Xing, X., Zhou, J., & Li, L. (2023). Preparation and Characterization of Metal–Organic Framework Coatings for Improving Protein Crystallization Screening. Nanomaterials, 13(14), 2064. https://doi.org/10.3390/nano13142064






