Competitive Effects of Oxidation and Quantum Confinement on Modulation of the Photophysical Properties of Metallic-Phase Tungsten Dichalcogenide Quantum Dots
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Voiry, D.; Mohite, A.; Chhowalla, M. Phase engineering of transition metal dichalcogenides. Chem. Soc. Rev. 2015, 44, 2702–2712. [Google Scholar] [PubMed]
- Manzeli, S.; Ovchinnikov, D.; Pasquier, D.; Yazyev, O.V.; Kis, A. 2D transition metal dichalcogenides. Nat. Rev. Mater. 2017, 2, 17033. [Google Scholar]
- Kim, B.-H.; Jang, M.-H.; Yoon, H.; Kim, H.J.; Cho, Y.-H.; Jeon, S.; Song, S.-H. Metallic phase transition metal dichalcogenide quantum dots showing different optical charge excitation and decay pathways. NPG Asia Mater. 2021, 13, 41. [Google Scholar]
- Caigas, S.P.; Santiago, S.R.M.; Lin, T.-N.; Lin, C.-A.J.; Yuan, C.-T.; Shen, J.-L.; Lin, T.-Y. Origins of excitation-wavelength-dependent photoluminescence in WS2 quantum dots. Appl. Phys. Lett. 2018, 112, 092106. [Google Scholar]
- Tan, C.; Luo, Z.; Chaturvedi, A.; Cai, Y.; Du, Y.; Gong, Y.; Huang, Y.; Lai, Z.; Zhang, X.; Zheng, L. Preparation of high-percentage 1T-phase transition metal dichalcogenide nanodots for electrochemical hydrogen evolution. Adv. Mater. 2018, 30, 1705509. [Google Scholar]
- Sokolikova, M.S.; Sherrell, P.C.; Palczynski, P.; Bemmer, V.L.; Mattevi, C. Direct solution-phase synthesis of 1T’WSe2 nanosheets. Nat. Commun. 2019, 10, 712. [Google Scholar]
- Srivastava, R.R.; Mishra, H.; Singh, V.K.; Vikram, K.; Srivastava, R.K.; Srivastava, S.; Srivastava, A. pH dependent luminescence switching of tin disulfide quantum dots. J. Lumin. 2019, 213, 401–408. [Google Scholar]
- Takagahara, T.; Takeda, K. Theory of the quantum confinement effect on excitons in quantum dots of indirect-gap materials. Phys. Rev. B 1992, 46, 15578. [Google Scholar]
- Jin, S.H.; Kim, D.H.; Jun, G.H.; Hong, S.H.; Jeon, S. Tuning the photoluminescence of graphene quantum dots through the charge transfer effect of functional groups. ACS Nano 2013, 7, 1239–1245. [Google Scholar]
- Jin, H.; Ahn, M.; Jeong, S.; Han, J.H.; Yoo, D.; Son, D.H.; Cheon, J. Colloidal single-layer quantum dots with lateral confinement effects on 2D exciton. J. Am. Chem. Soc. 2016, 138, 13253–13259. [Google Scholar]
- Gan, Z.; Liu, L.; Wu, H.; Hao, Y.; Shan, Y.; Wu, X.; Chu, P.K. Quantum confinement effects across two-dimensional planes in MoS2 quantum dots. Appl. Phys. Lett. 2015, 106, 233113. [Google Scholar]
- Farzin, M.A.; Abdoos, H. A critical review on quantum dots: From synthesis toward applications in electrochemical biosensors for determination of disease-related biomolecules. Talanta 2021, 224, 121828. [Google Scholar] [PubMed]
- Azam, N.; Najabat Ali, M.; Javaid Khan, T. Carbon quantum dots for biomedical applications: Review and analysis. Front. Mater. 2021, 8, 700403. [Google Scholar]
- Mishra, H.; Umrao, S.; Singh, J.; Srivastava, R.K.; Ali, R.; Misra, A.; Srivastava, A. pH dependent optical switching and fluorescence modulation of molybdenum sulfide quantum dots. Adv. Opt. Mater. 2017, 5, 1601021. [Google Scholar]
- Thomas, A.; Jinesh, K.B. Excitons and Trions in MoS2 Quantum Dots: The Influence of the Dispersing Medium. ACS Omega 2022, 7, 6531–6538. [Google Scholar] [CrossRef]
- Loh, G.; Pandey, R.; Yap, Y.K.; Karna, S.P. MoS2 quantum dot: Effects of passivation, additional layer, and h-BN substrate on its stability and electronic properties. J. Phys. Chem. C 2015, 119, 1565–1574. [Google Scholar]
- Ding, X.; Peng, F.; Zhou, J.; Gong, W.; Slaven, G.; Loh, K.P.; Lim, C.T.; Leong, D.T. Defect engineered bioactive transition metals dichalcogenides quantum dots. Nat. Commun. 2019, 10, 41. [Google Scholar]
- Ren, K.; Sun, M.; Luo, Y.; Wang, S.; Yu, J.; Tang, W. First-principle study of electronic and optical properties of two-dimensional materials-based heterostructures based on transition metal dichalcogenides and boron phosphide. Appl. Surf. Sci. 2019, 476, 70–75. [Google Scholar]
- Vitukhnovsky, A.; Zvagelsky, R.; Kolymagin, D.; Pisarenko, A.; Chubich, D. Three-Dimensional Optical Lithography and Nanoscale Optical Connectors. Bull. Russ. Acad. Sci. Phys. 2020, 84, 760–765. [Google Scholar]
- Arzhanov, A.; Savostianov, A.; Magaryan, K.; Karimullin, K.; Naumov, A. Photonics of semiconductor quantum dots: Applied aspects. Photonics Russ 2022, 16, 96–112. [Google Scholar]
- Chang, J.; Register, L.F.; Banerjee, S.K. Ballistic performance comparison of monolayer transition metal dichalcogenide MX2 (M = Mo, W; X = S, Se, Te) metal-oxide-semiconductor field effect transistors. J. Appl. Phys. 2014, 115, 084506. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.; Bhagat, S.; Singh, J.; Singh, R.C.; Sharma, S. Excitation-dependent photoluminescence from WS2 nanostructures synthesized via top-down approach. J. Mater. Sci. 2017, 52, 11326–11336. [Google Scholar] [CrossRef]
- Ghorai, A.; Bayan, S.; Gogurla, N.; Midya, A.; Ray, S.K. Highly luminescent WS2 quantum dots/ZnO heterojunctions for light emitting devices. ACS Aappl. Mater. Interfaces 2017, 9, 558–565. [Google Scholar] [CrossRef]
- Kim, M.-J.; Jeon, S.-J.; Kang, T.W.; Ju, J.-M.; Yim, D.; Kim, H.-I.; Park, J.H.; Kim, J.-H. 2H-WS2 Quantum dots produced by modulating the dimension and phase of 1T-nanosheets for antibody-free optical sensing of neurotransmitters. ACS Appl. Mater. Interfaces 2017, 9, 12316–12323. [Google Scholar] [CrossRef]
- Ko, B.; Ahn, J.; Song, S.H. pH-Dependent Photophysical Properties of Metallic Phase MoSe2 Quantum Dots. Materials 2022, 15, 4945. [Google Scholar]
- Yang, H.; Zhao, Q.; Wang, X.; Wu, Y.; Su, Y.; Wei, W. Facile and highly selective sensing of hypochlorous acid in aqueous solution and living cells by using as-prepared WSe2 quantum dots. Sens. Actuators B Chem. 2021, 337, 129782. [Google Scholar] [CrossRef]
- Song, S.H.; Kim, B.H.; Choe, D.H.; Kim, J.; Kim, D.C.; Lee, D.J.; Kim, J.M.; Chang, K.J.; Jeon, S. Bandgap widening of phase quilted, 2D MoS2 by oxidative intercalation. Adv. Mater. 2015, 27, 3152–3158. [Google Scholar] [CrossRef]
- Park, K.H.; Yang, J.Y.; Jung, S.; Ko, B.M.; Song, G.; Hong, S.-J.; Kim, N.C.; Lee, D.; Song, S.H. Metallic phase transition metal dichalcogenide quantum dots as promising bio-imaging materials. Nanomaterials 2022, 12, 1645. [Google Scholar]
- Zhang, K.; Fu, L.; Zhang, W.; Pan, H.; Sun, Y.; Ge, C.; Du, Y.; Tang, N. Ultrasmall and monolayered tungsten dichalcogenide quantum dots with giant spin–valley coupling and purple luminescence. ACS Omega 2018, 3, 12188–12194. [Google Scholar] [CrossRef] [Green Version]
- Zhu, B.; Chen, X.; Cui, X. Exciton binding energy of monolayer WS2. Sci. Rep. 2015, 5, 9218. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.; Ghorannevis, Z.; Amara, K.K.; Pang, J.R.; Toh, M.; Zhang, X.; Kloc, C.; Tan, P.H.; Eda, G. Lattice dynamics in mono-and few-layer sheets of WS2 and WSe2. Nanoscale 2013, 5, 9677–9683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, K.-Q.; Ong, C.S.; Bange, S.; Faria Junior, P.E.; Peng, B.; Ziegler, J.D.; Zipfel, J.; Bäuml, C.; Paradiso, N.; Watanabe, K. Narrow-band high-lying excitons with negative-mass electrons in monolayer WSe2. Nat. Commun. 2021, 12, 5500. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Zan, W.; Chen, W.; Jiang, W.; Ding, X.; Li, B.L.; Mu, Y.; Wang, L.; Garaj, S.; Leong, D.T. Defect-Rich Molybdenum Sulfide Quantum Dots for Amplified Photoluminescence and Photonics-Driven Reactive Oxygen Species Generation. Adv. Mater. 2022, 34, 2200004. [Google Scholar] [CrossRef] [PubMed]
- Kośmider, K.; González, J.W.; Fernández-Rossier, J. Large spin splitting in the conduction band of transition metal dichalcogenide monolayers. Phys. Rev. B 2013, 88, 245436. [Google Scholar] [CrossRef] [Green Version]
- Hanbicki, A.; Currie, M.; Kioseoglou, G.; Friedman, A.; Jonker, B. Measurement of high exciton binding energy in the monolayer transition-metal dichalcogenides WS2 and WSe2. Solid State Commun. 2015, 203, 16–20. [Google Scholar] [CrossRef] [Green Version]
- Lin, L.; Xu, Y.; Zhang, S.; Ross, I.; Ong, A.; Allwood, D. Fabrication of luminescent monolayered tungsten dichalcogenides quantum dots with giant spin-valley coupling. ACS Nano 2013, 7, 8214–8223. [Google Scholar] [CrossRef]
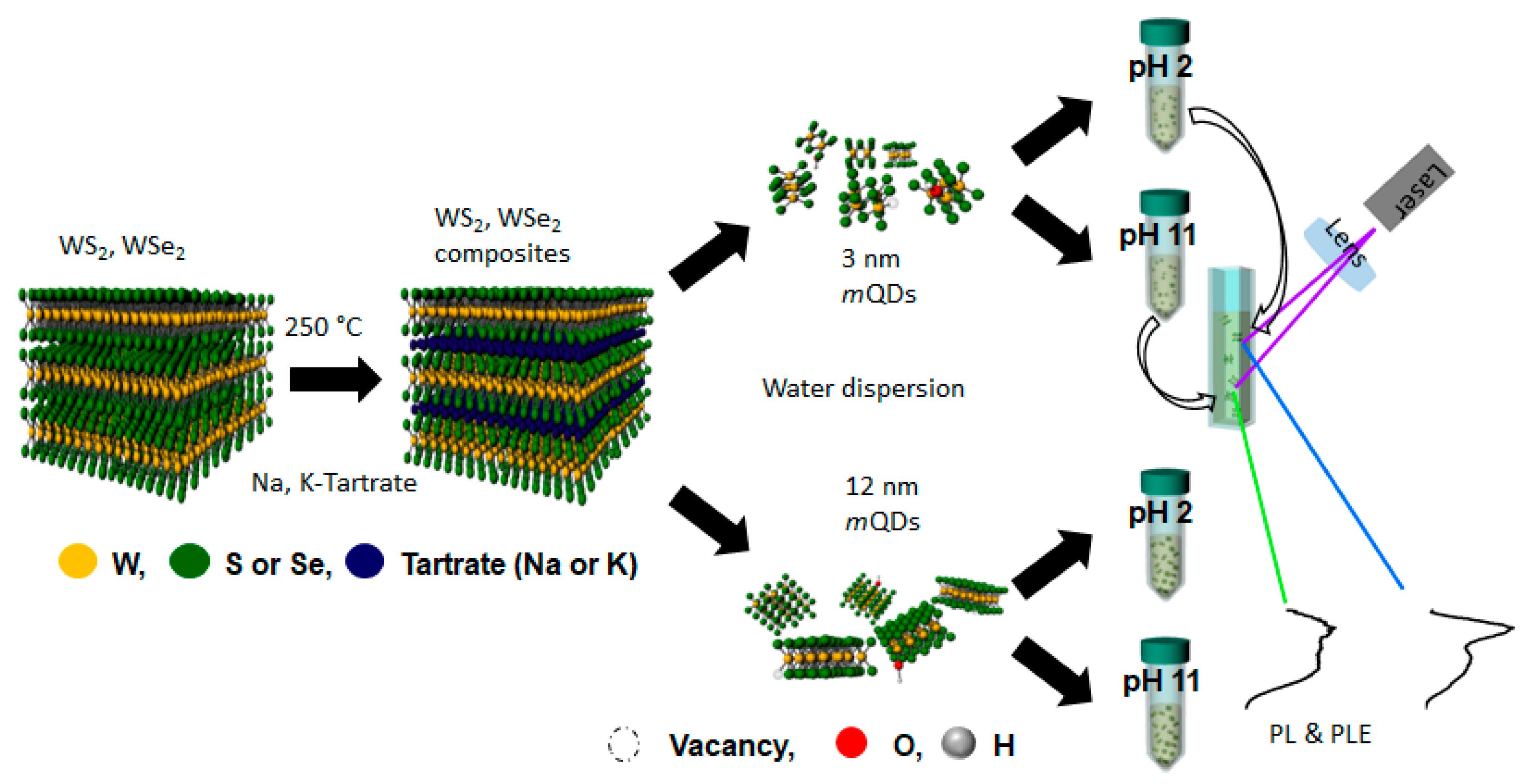
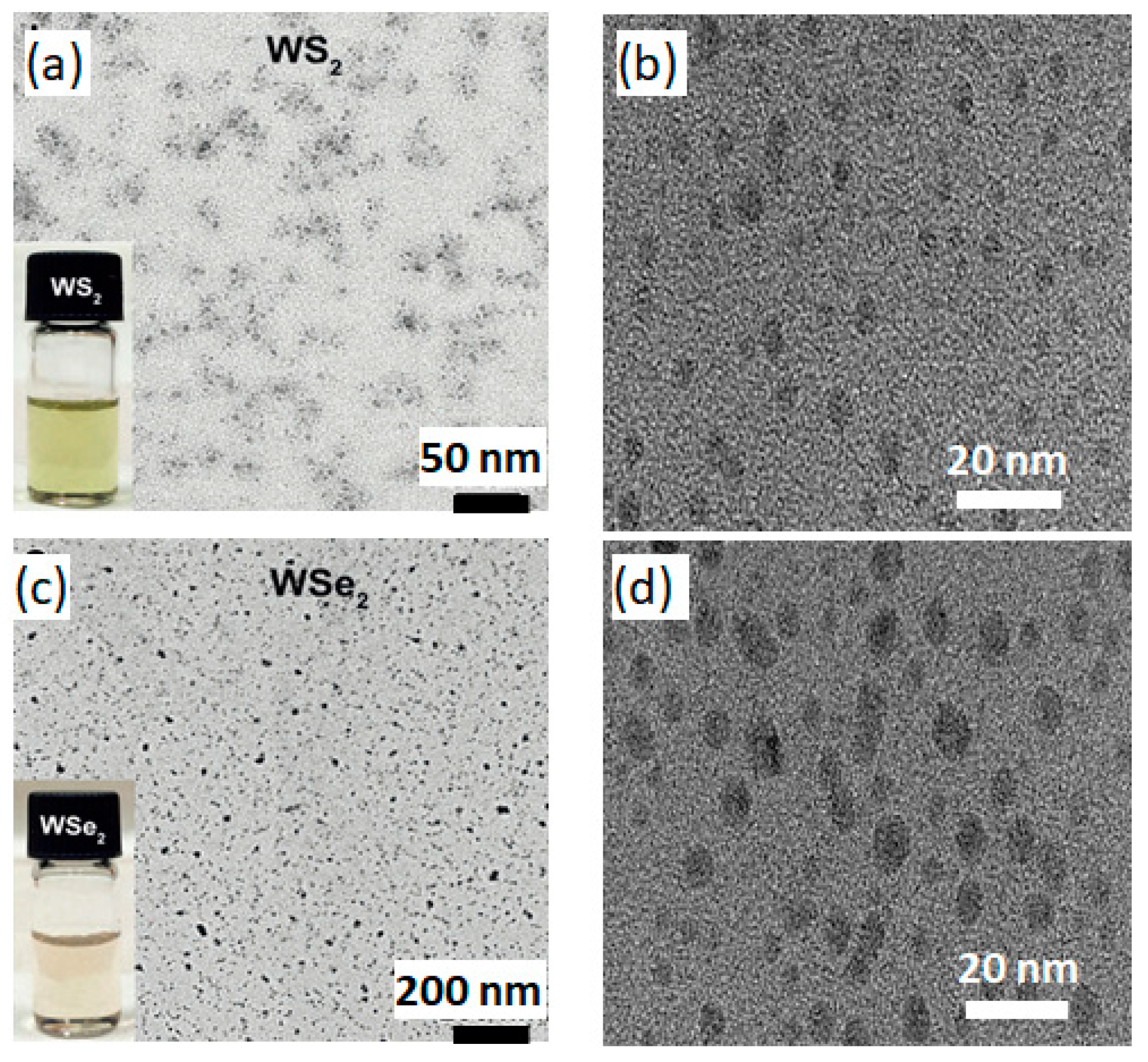

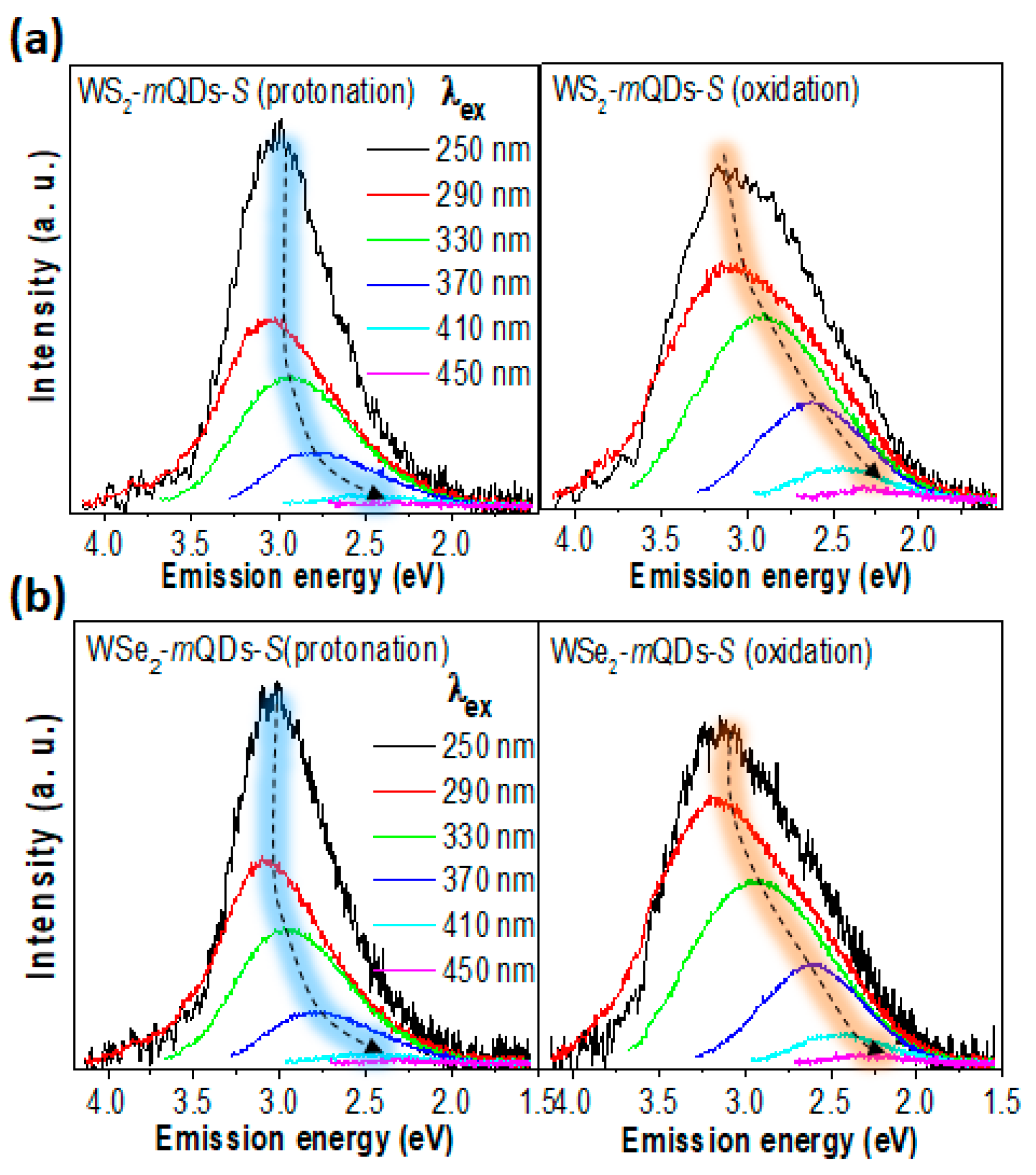
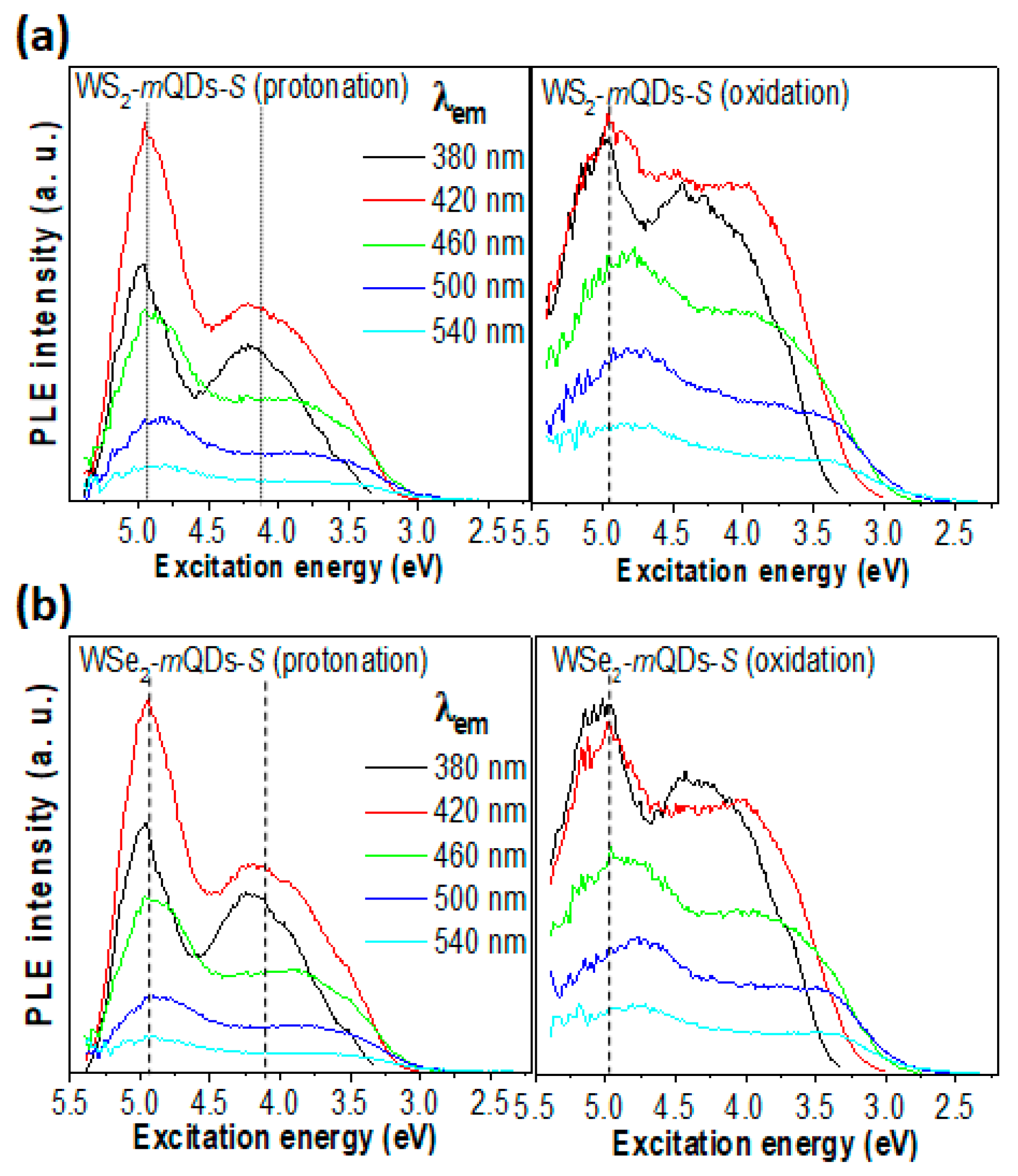
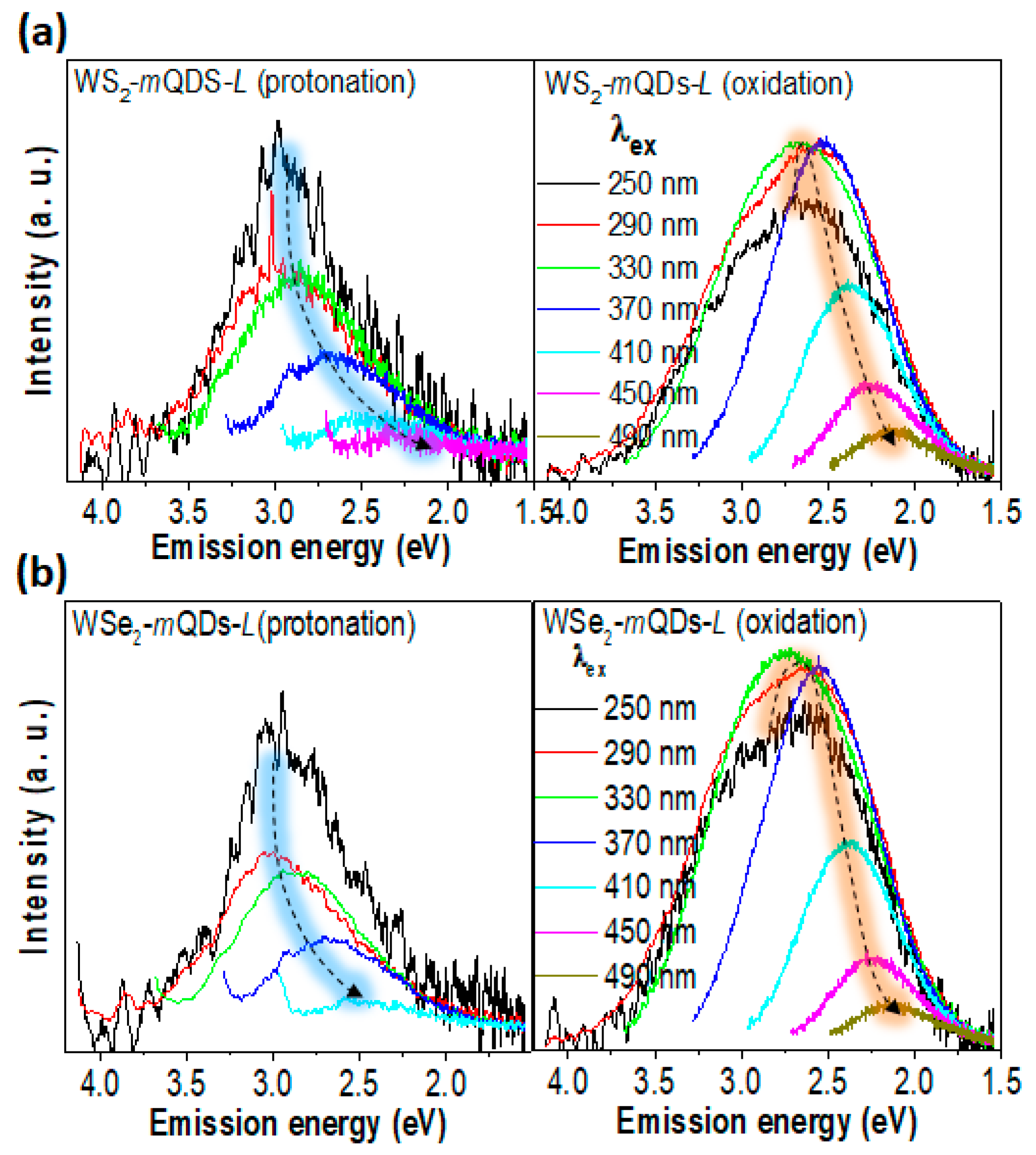
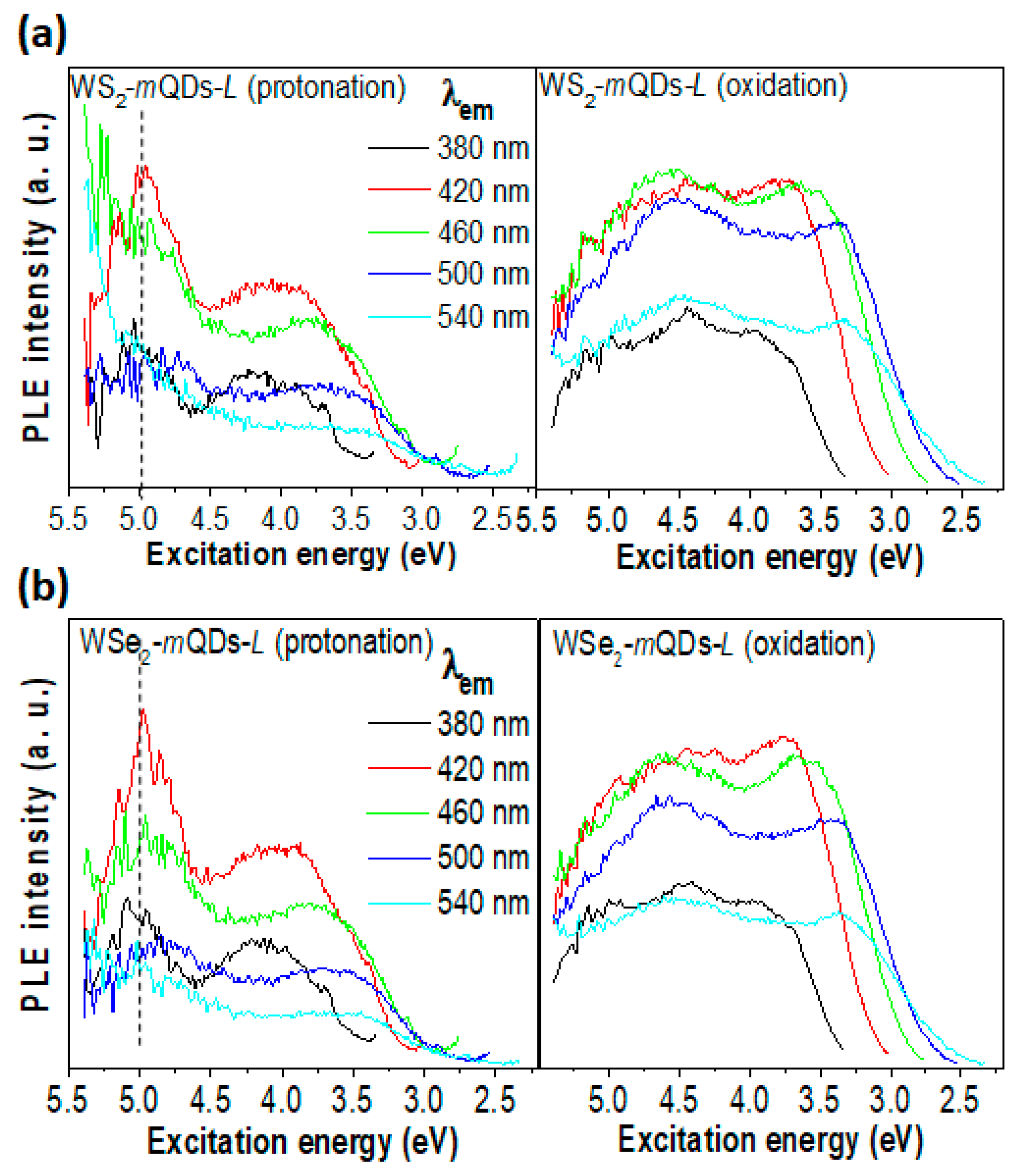
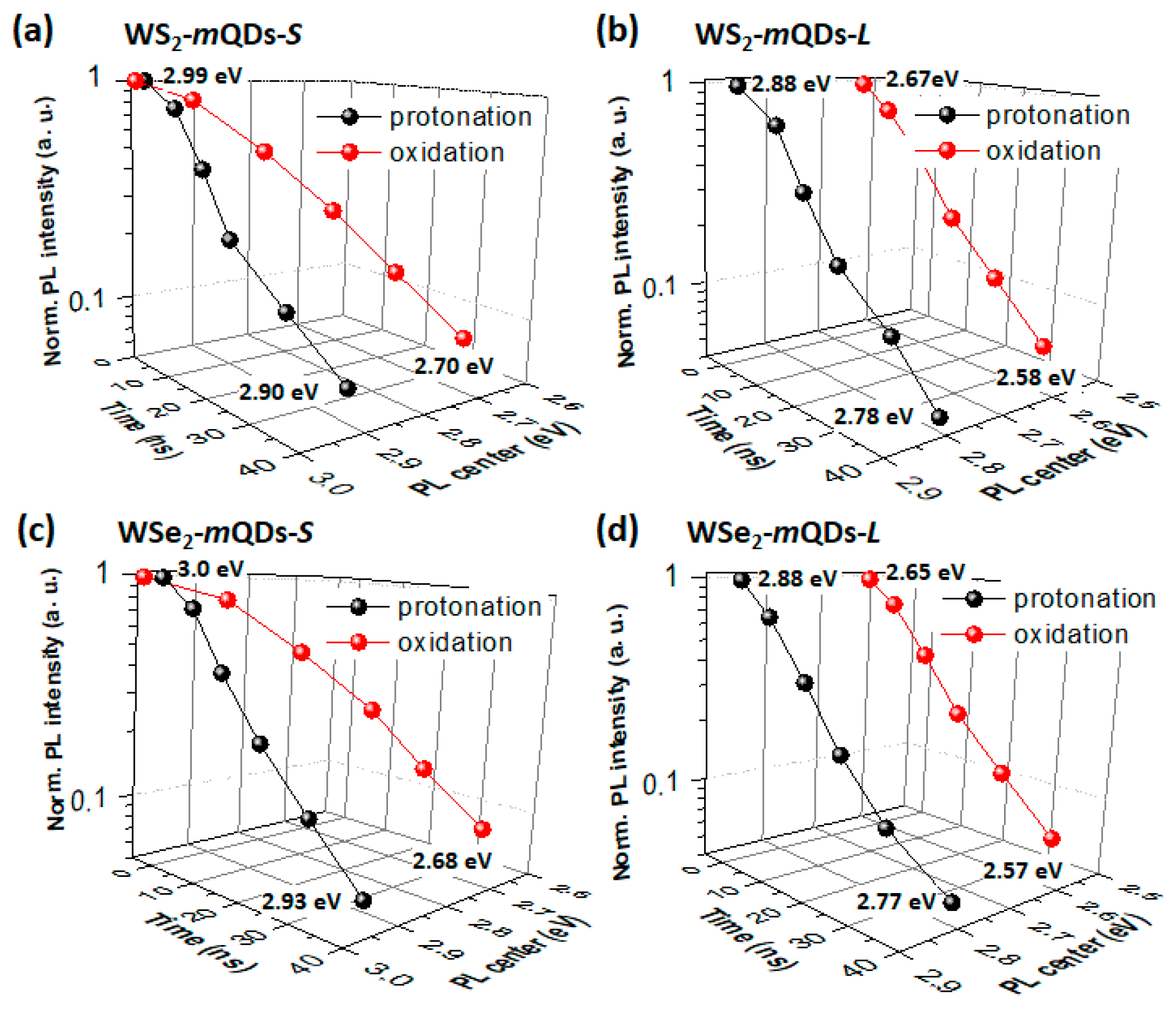

| Materials | pH | PL Peak (eV) | FWHM (eV) | QY (%) | Materials | pH | PL Peak (eV) | FWHM (eV) | QY (%) |
|---|---|---|---|---|---|---|---|---|---|
| WS2-mQDs-S | 2 | 2.95 | 0.78 | 2.8 | WSe2-mQDs-S | 2 | 2.98 | 0.78 | 3.3 |
| 11 | 2.92 | 0.95 | 4.9 | 11 | 2.93 | 0.97 | 4.1 | ||
| WS2-mQDs-L | 2 | 2.83 | 0.89 | Wse2-mQDs-L | 2 | 2.84 | 0.87 | ||
| 11 | 2.63 | 1.06 | 11 | 2.68 | 1.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, B.-H.; Yang, J.Y.; Park, K.H.; Lee, D.; Song, S.H. Competitive Effects of Oxidation and Quantum Confinement on Modulation of the Photophysical Properties of Metallic-Phase Tungsten Dichalcogenide Quantum Dots. Nanomaterials 2023, 13, 2075. https://doi.org/10.3390/nano13142075
Kim B-H, Yang JY, Park KH, Lee D, Song SH. Competitive Effects of Oxidation and Quantum Confinement on Modulation of the Photophysical Properties of Metallic-Phase Tungsten Dichalcogenide Quantum Dots. Nanomaterials. 2023; 13(14):2075. https://doi.org/10.3390/nano13142075
Chicago/Turabian StyleKim, Bo-Hyun, Jun Yong Yang, Kwang Hyun Park, DongJu Lee, and Sung Ho Song. 2023. "Competitive Effects of Oxidation and Quantum Confinement on Modulation of the Photophysical Properties of Metallic-Phase Tungsten Dichalcogenide Quantum Dots" Nanomaterials 13, no. 14: 2075. https://doi.org/10.3390/nano13142075





