Improvement of Supercapacitor Performance of In Situ Doped Laser-Induced Multilayer Graphene via NiO
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussions
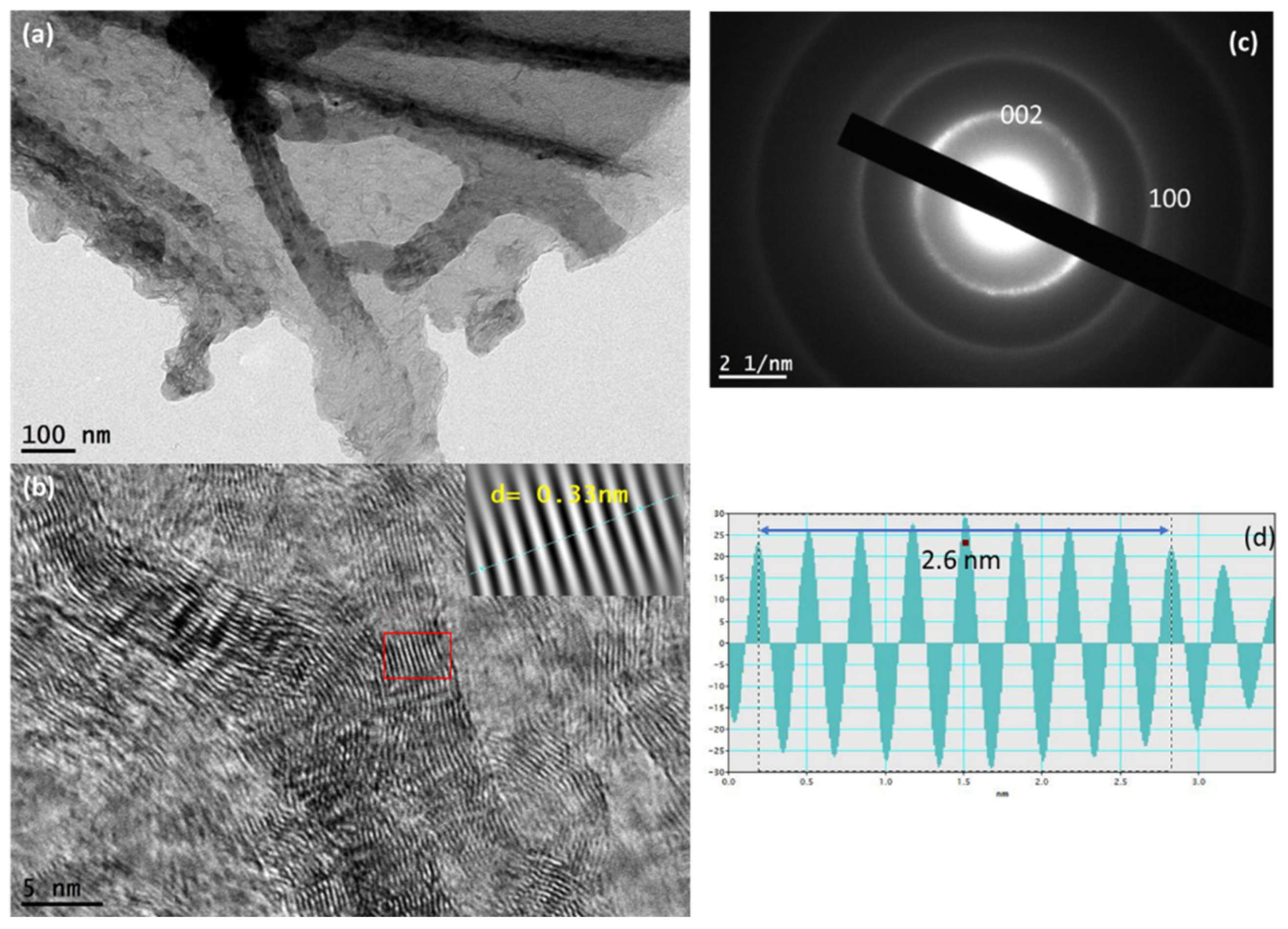
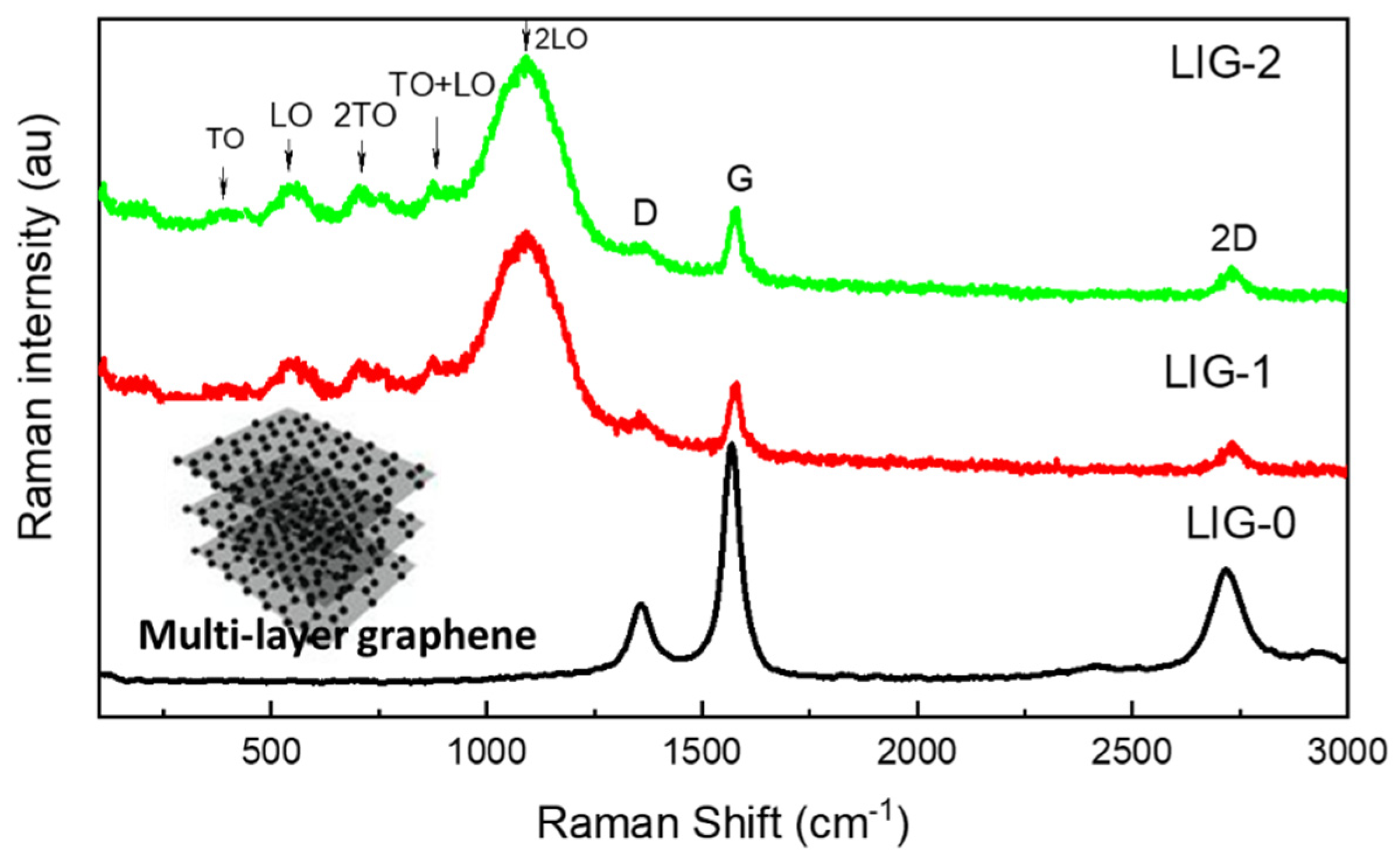
3.1. Structure Analysis
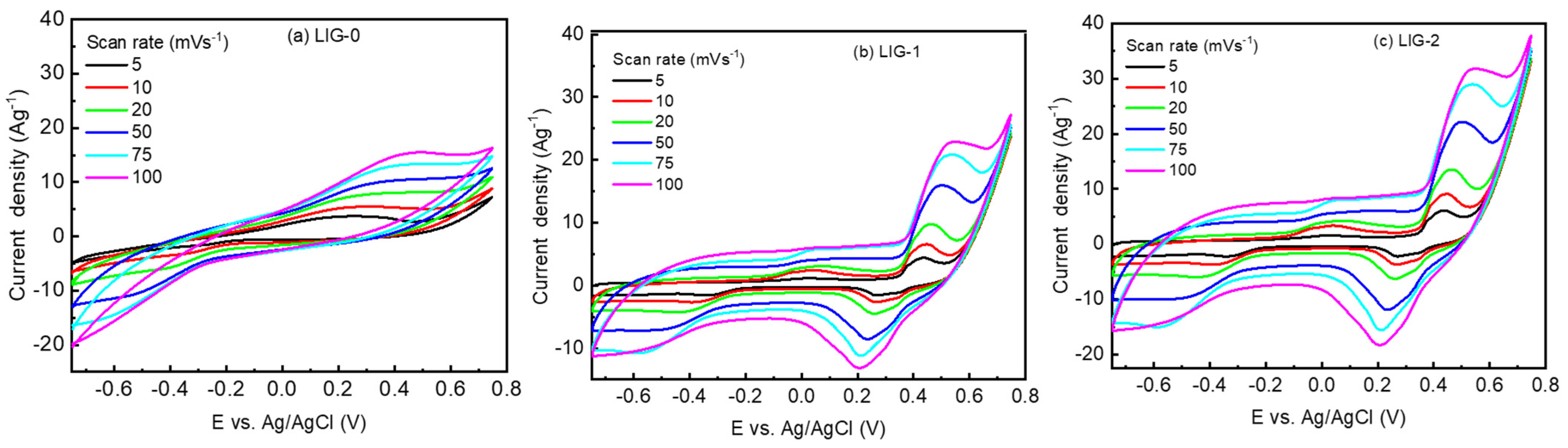
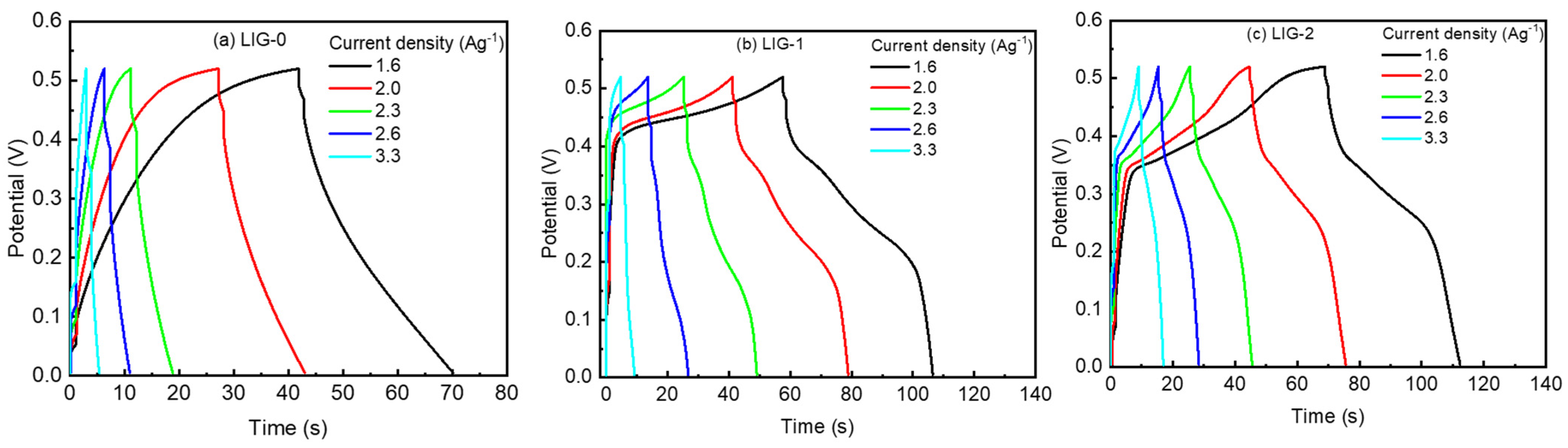
3.2. Electrochemical Characterizations
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jing, W.; Lai, C.H.; Wong, S.H.W.; Wong, M.L.D. Battery-supercapacitor hybrid energy storage system in standalone DC microgrids: Areview. IET Renew. Power Gener. 2017, 11, 461–469. [Google Scholar] [CrossRef]
- Sahin, M.E.; Blaabjerg, F. A Hybrid PV-Battery/Supercapacitor System and a Basic Active Power Control Proposal in MATLAB/Simulink. Energies 2020, 9, 129. [Google Scholar]
- Abdel Maksoud, M.I.A.; Fahim, R.A.; Shalan, A.E.; Abd Elkodous, M.; Olojede, S.O.; Osman, A.I.; Farrell, C.; Al-Muhtaseb, A.H.; Awed, A.S.; Ashour, A.H.; et al. Advanced Materials and Technologies for Supercapacitors Used in Energy Conversion and Storage: A Review; Springer International Publishing: New York, NY, USA, 2021. [Google Scholar] [CrossRef]
- Shaalan, N.M.; Ahmed, F.; Kumar, S.; Ahmad, M.M.; Al-Naim, A.F.; Hamad, D. Electrochemical Performance of Potassium Bromate Active Electrolyte for Laser-Induced KBr-Graphene Supercapacitor Electrodes. Inorganics 2023, 11, 109. [Google Scholar] [CrossRef]
- Shaalan, N.M.; Ahmed, F.; Rashad, M.; Kumar, S.; Saber, O.; Al-Naim, A.F.; Kotb, H.M.; Ezzeldien, M.; Mahmoud, A.Z. Ceramic Ti/TiO2/AuNP Film with 1-D Nanostructures for Selfstanding Supercapacitor Electrodes. Crystals 2022, 12, 791. [Google Scholar] [CrossRef]
- Lu, Q.-L.; Zhao, S.-X.; Chen, C.-K.; Wang, X.; Deng, Y.-F.; Nan, C.-W. A novel pseudocapacitance mechanism of elm seed-like mesoporous MoO3−x nanosheets as electrodes for supercapacitors. J. Mater. Chem. A 2016, 4, 14560–14566. [Google Scholar] [CrossRef]
- Allen, M.J.; Tung, V.C.; Kaner, R.B. Honeycomb Carbon: A Review of Graphene. Chem. Rev. 2010, 110, 132–145. [Google Scholar] [CrossRef]
- Ghuge, A.D.; Shirode, A.R.; Kadam, V.J. Graphene: A Comprehensive Review. Curr. Drug Targets 2017, 18, 724–733. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Deng, Y.; Hu, W.; Qiao, J.; Zhang, L.; Zhang, J. A review of electrolyte materials and compositions for electrochemical supercapacitors. Chem. Soc. Rev. 2015, 44, 7484–7539. [Google Scholar] [CrossRef]
- Forouzandeh, P.; Kumaravel, V.; Pillai, S.C. Electrode materials for supercapacitors: A review of recent advances. Catalysts 2020, 10, 969. [Google Scholar] [CrossRef]
- Singh, V.; Joung, D.; Zhai, L.; Das, S.; Khondaker, S.I.; Seal, S. Graphene based materials: Past, present and future. Prog. Mater. Sci. 2011, 56, 1178–1271. [Google Scholar] [CrossRef]
- Rodríguez-Estupiñán, P.; Miranda-Carvajal, I.; Campos, P.C.; Guerrero-Fajardo, C.A.; Giraldo, L.; Moreno-Piraján, J.C. Graphene-based materials: Analysis through calorimetric techniques. J. Therm. Anal. Calorim. 2022, 147, 9301–9351. [Google Scholar] [CrossRef]
- Yu, X.; Cheng, H.; Zhang, M.; Zhao, Y.; Qu, L.; Shi, G. Graphene-based smart materials. Nat. Rev. Mater. 2017, 2, 17046. [Google Scholar] [CrossRef]
- Komolov, A.S.; Zhukov, Y.M.; Lazneva, E.F.; Aleshin, A.N.; Pshenichnuk, S.A.; Gerasimova, N.B.; Panina, Y.A.; Zashikhin, G.D.; Baramygin, A.V. Thermally induced modification of the graphene oxide film on the tantalum surface. Mater. Des. 2017, 113, 319–325. [Google Scholar] [CrossRef]
- Lin, J.; Peng, Z.; Liu, Y.; Ruiz-Zepeda, F.; Ye, R.; Samuel, E.L.G.; Yacaman, M.J.; Yakobson, B.I.; Tour, J.M. Laser-induced porous graphene films from commercial polymers. Nat. Commun. 2014, 5, 5–12. [Google Scholar] [CrossRef] [Green Version]
- Stanford, M.G.; Yang, K.; Chyan, Y.; Kittrell, C.; Tour, J.M. Laser-Induced Graphene for Flexible and Embeddable Gas Sensors. ACS Nano 2019, 13, 3474–3482. [Google Scholar] [CrossRef]
- Ngidi, N.P.D.; Ollengo, M.A.; Nyamori, V.O. Effect of doping temperatures and nitrogen precursors on the physicochemical, optical, and electrical conductivity properties of nitrogen-doped reduced graphene oxide. Materials 2019, 12, 3376. [Google Scholar] [CrossRef] [Green Version]
- Rong, X.; Qiu, F.; Qin, J.; Zhao, H.; Yan, J.; Yang, D. A facile hydrothermal synthesis, adsorption kinetics and isotherms to Congo Red azo-dye from aqueous solution of NiO/graphene nanosheets adsorbent. J. Ind. Eng. Chem. 2014, 26, 354–363. [Google Scholar] [CrossRef]
- Shaalan, N.M.; Morsy, A.E.A.; Abdel-Rahim, M.A.; Rashad, M. Simple preparation of Ni/CuO nanocomposites with superior sensing activity toward the detection of methane gas. Appl. Phys. A Mater. Sci. Process. 2021, 127, 455. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Jorio, A.; Hofmann, M.; Dresselhaus, G.; Saito, R. Perspectives on carbon nanotubes and graphene Raman spectroscopy. Nano Lett. 2010, 10, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Faid, A.Y.; Oyarce, A.; Seland, F.; Sunde, S. Ni/NiO nanosheets for alkaline hydrogen evolution reaction: In situ electrochemical-Raman study. Electrochim. Acta J. 2020, 361, 137040. [Google Scholar] [CrossRef]
- Mironova-ulmane, N.; Kuzmin, A.; Sildos, I. Polarisation dependent Raman study of single-crystal nickel oxide. Cent. Eur. J. Phys. 2011, 9, 1096–1099. [Google Scholar] [CrossRef]
- Shaalan, N.M.; Ahmed, F.; Kumar, S.; Melaibari, A.; Hasan, P.M.Z.; Aljaafari, A. Monitoring Food Spoilage Based on a Defect-Induced Multiwall Carbon Nanotube Sensor at Room Temperature: Preventing Food Waste. ACS Omega 2020, 5, 30531–30537. [Google Scholar] [CrossRef]
- Lucchese, M.M.; Stavale, F.; Ferreira, E.H.M.; Vilani, C.; Moutinho, M.V.O.; Capaz, R.B.; Achete, C.A.; Jorio, A. Quantifying ion-induced defects and Raman relaxation length in graphene. Carbon 2010, 48, 1592–1597. [Google Scholar] [CrossRef]
- Ferrari, A.C. Raman spectroscopy of graphene and graphite: Disorder, electron–phonon coupling, doping and nonadiabatic effects. Solid State Commun. 2007, 143, 47–57. [Google Scholar] [CrossRef]
- Shaalan, N.M.; Saber, O.; Ahmed, F.; Aljaafari, A.; Kumar, S. Growth of defect-induced carbon nanotubes for low-temperature fruit monitoring sensor. Chemosensors 2021, 9, 131. [Google Scholar] [CrossRef]
- Stoller, M.D.; Park, S.; Zhu, Y.; An, J.; Ruoff, R.S. Graphene-Based Ultracapacitors. Nano Lett. 2008, 8, 3498–3502. [Google Scholar] [CrossRef] [PubMed]
- Vivekchand, S.R.C.; Rout, C.S.; Subrahmanyam, K.S.; Govindaraj, A.; Rao, C.N.R. Graphene-based electrochemical supercapacitors. J. Chem. Sci. 2008, 120, 9–13. [Google Scholar] [CrossRef]
- Seol, M.; Nam, I.; Ribeiro, E.L.; Segel, B.; Lee, D.; Palma, T.; Wu, H.; Mukherjee, D.; Khomami, B.; Hill, C.; et al. All-Printed In-Plane Supercapacitors by Sequential Additive Manufacturing Process. ACS Appl. Energy Mater. 2020, 3, 4965–4973. [Google Scholar] [CrossRef]
- Xu, K.; Ding, S.P.; Jow, T.R. Toward Reliable Values of Electrochemical Stability Limits for Electrolytes. J. Electrochem. Soc. 1999, 146, 4172–4178. [Google Scholar] [CrossRef]
- Hui, X.; Qian, L.; Harris, G.; Wang, T.; Che, J. Fast fabrication of NiO@graphene composites for supercapacitor electrodes: Combination of reduction and deposition. Mater. Des. 2016, 109, 242–250. [Google Scholar] [CrossRef] [Green Version]
- Lu, S.; Hummel, M.; Kang, S.; Pathak, R.; He, W.; Qi, X.; Gu, Z. Density Functional Theory Investigation of the NiO@Graphene Composite as a Urea Oxidation Catalyst in the Alkaline Electrolyte. ACS Omega 2021, 6, 14648–14654. [Google Scholar] [CrossRef]
- Bin, J.; Hsia, B.; Yoo, J.; Hyun, S.; Carraro, C.; Maboudian, R.; Grigoropoulos, C.P. Facile fabrication of flexible all solid-state micro-supercapacitor by direct laser writing of porous carbon in polyimide. Carbon 2014, 83, 144–151. [Google Scholar] [CrossRef]
- Liu, C.; Liang, H.; Wu, D.; Lu, X.; Wang, Q. Graphene-Based Supercapacitors: Direct Semiconductor Laser Writing of Few-Layer Graphene Polyhedra Networks for Flexible Solid-State Supercapacitor (Adv. Electron. Mater. 7/2018). Adv. Electron. Mater. 2018, 4, 1870034. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Mo, X.; Law, W.-C.; Chan, K.C. 3D printed graphene/nickel electrodes for high areal capacitance electrochemical storage. J. Mater. Chem. A 2019, 7, 4055–4062. [Google Scholar] [CrossRef]
- Zhou, C.; Hong, M.; Yang, Y.; Yang, C.; Hu, N.; Zhang, L.; Yang, Z. Laser-induced bi-metal sulfide/graphene nanoribbon hybrid frameworks for high-performance all-in-one fiber supercapacitors. J. Power Sources 2019, 438, 227044. [Google Scholar] [CrossRef]
- Khandelwal, M.; Nguyen, A.P.; Van Tran, C.; Bin In, J. Simple fabrication of Co3O4 nanoparticles on N-doped laser-induced graphene for high-performance supercapacitors. RSC Adv. 2021, 11, 38547–38554. [Google Scholar] [CrossRef]
- Cho, E.; Chang-Jian, C.; Syu, W.; Tseng, H.; Lee, K. Applied Surface Science PEDOT-modi fi ed laser-scribed graphene fi lms as bginder—And metallic current collector—Free electrodes for large-sized supercapacitors. Appl. Surf. Sci. 2020, 518, 146193. [Google Scholar] [CrossRef]





| Sample | Rs (Ω) | Rct (Ω) |
|---|---|---|
| LIG-0 | 3 | 5 |
| LIG-1 | 10 | 18 |
| LIG-2 | 13 | 22 |
| Active Material | Capacitance | Energy | Power | Ref. |
|---|---|---|---|---|
| LIG | 800 µF/cm2 at 10 mV/s | -- | -- | [33] |
| LIG | 34 mF/cm2 at 0.1 mA/cm2 | 1.0 mWh/cm3 | 11 mW/cm3 | [34] |
| LIG | 995 mF/cm2 at 1 mA/cm2 | 55.9 µWh/cm2 | 9.39 mW/cm2 | [35] |
| LIG+MoS2+MnS | 58.3 mF/m2 at 50 mA/cm2 | 7 µWh/cm2 | 49.9 µW/cm2 | [36] |
| NiO/Co3O4/LIG | 29.5 mF/cm2 at 0.05 mA/cm2 | -- | -- | [37] |
| LIG + PEDOT | 115.2 F/g at 0.5 A/g | -- | -- | [38] |
| NiO-doped LIG | 174 F/g at 5 mV/s | 5.6 Wh/kg | 429 W/kg | Present |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaalan, N.M.; Kumar, S.; Ahmed, F.; Arshi, N.; Dalela, S.; Chae, K.H. Improvement of Supercapacitor Performance of In Situ Doped Laser-Induced Multilayer Graphene via NiO. Nanomaterials 2023, 13, 2081. https://doi.org/10.3390/nano13142081
Shaalan NM, Kumar S, Ahmed F, Arshi N, Dalela S, Chae KH. Improvement of Supercapacitor Performance of In Situ Doped Laser-Induced Multilayer Graphene via NiO. Nanomaterials. 2023; 13(14):2081. https://doi.org/10.3390/nano13142081
Chicago/Turabian StyleShaalan, Nagih M., Shalendra Kumar, Faheem Ahmed, Nishat Arshi, Saurabh Dalela, and Keun Hwa Chae. 2023. "Improvement of Supercapacitor Performance of In Situ Doped Laser-Induced Multilayer Graphene via NiO" Nanomaterials 13, no. 14: 2081. https://doi.org/10.3390/nano13142081








