Particle Size and Rheology of Silica Particle Networks at the Air–Water Interface
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Particle Surface Characterization
2.3. Microstructure of Particle Networks at the Air–Water Interface
2.4. Interfacial Shear Rheology
2.5. Interfacial Dilational Rheology
2.6. Estimation of the Interparticle Interaction Energy
2.7. Statistical Analysis
3. Results and Discussion
3.1. Particle Wettability and Surface Charge
3.2. Langmuir Trough Studies
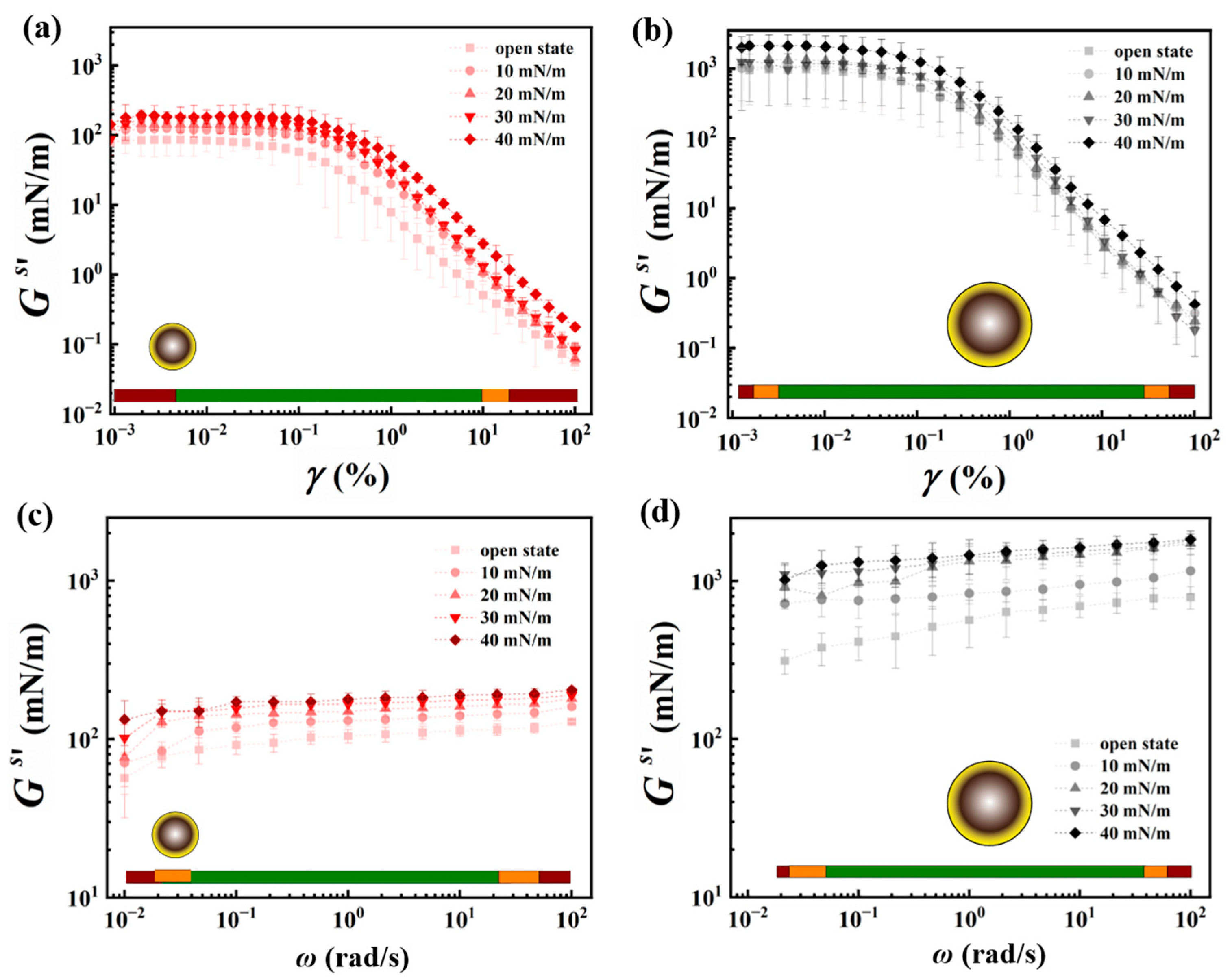
3.3. Shear Rheology of the Interfacial Particle Networks
3.4. Dilational Rheology of Interfacial Particle Networks
3.5. Analysis of Interparticle Interactions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Forth, J.; Kim, P.Y.; Xie, G.; Liu, X.; Helms, B.A.; Russell, T.P. Building Reconfigurable Devices Using Complex Liquid–Fluid Interfaces. Adv. Mater. 2019, 31, 1806370. [Google Scholar] [CrossRef]
- Pochan, D.; Scherman, O. Introduction: Molecular Self-Assembly. Chem. Rev. 2021, 121, 13699–13700. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, Y.; Liu, Y.; Liang, T.; Tian, Y.; Sakthivel, T.; Peng, S.; Kim, S.Y.; Dai, Z. Macroporous SnO2/MoS2 Inverse Opal Hierarchitecture for Highly Efficient Trace NO2 Gas Sensing. Chem. Commun. 2023, 59, 2931–2934. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.B.; Kim, B.; Chen, Y.; Yoo, S.J.; Cho, Y.; Jiang, P. Elevated Surface Plasmon Resonance Sensing Sensitivity of Au-Covered Silica Sphere Monolayer Prepared by Langmuir–Blodgett Coating. J. Ind. Eng. Chem. 2021, 99, 179–186. [Google Scholar] [CrossRef]
- Somorjai, G.A.; Tao, F.; Park, J.Y. The Nanoscience Revolution: Merging of Colloid Science, Catalysis and Nanoelectronics. Top. Catal. 2008, 47, 1–14. [Google Scholar] [CrossRef]
- Ching, H.; Mohraz, A. Bijel Rheology Reveals a 2D Colloidal Glass Wrapped in 3D. Soft Matter 2022, 18, 4227–4238. [Google Scholar] [CrossRef]
- Li, H.; Chen, X.; Shen, D.; Wu, F.; Pleixats, R.; Pan, J. Functionalized Silica Nanoparticles: Classification, Synthetic Approaches and Recent Advances in Adsorption Applications. Nanoscale 2021, 13, 15998–16016. [Google Scholar] [CrossRef]
- Berton-Carabin, C.C.; Schroën, K. Pickering Emulsions for Food Applications: Background, Trends, and Challenges. Annu. Rev. Food Sci. Technol. 2015, 6, 263–297. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez Ortiz, D.; Pochat-Bohatier, C.; Cambedouzou, J.; Bechelany, M.; Miele, P. Current Trends in Pickering Emulsions: Particle Morphology and Applications. Engineering 2020, 6, 468–482. [Google Scholar] [CrossRef]
- Venkataramani, D.; Tsulaia, A.; Amin, S. Fundamentals and Applications of Particle Stabilized Emulsions in Cosmetic Formulations. Adv. Colloid Interface Sci. 2020, 283, 102234. [Google Scholar] [CrossRef]
- Maestro, A.; Guzmán, E. Colloids at Fluid Interfaces. Processes 2019, 7, 942. [Google Scholar] [CrossRef] [Green Version]
- Correia, E.L.; Brown, N.; Razavi, S. Janus Particles at Fluid Interfaces: Stability and Interfacial Rheology. Nanomaterials 2021, 11, 374. [Google Scholar] [CrossRef] [PubMed]
- Noskov, B.A.; Bykov, A.G. Dilational Rheology of Monolayers of Nano- and Micropaticles at the Liquid-Fluid Interfaces. Curr. Opin. Colloid Interface Sci. 2018, 37, 1–12. [Google Scholar] [CrossRef]
- Kralchevsky, P.A.; Nagayama, K. Capillary Interactions between Particles Bound to Interfaces, Liquid Films and Biomembranes. Adv. Colloid Interface Sci. 2000, 85, 145–192. [Google Scholar] [CrossRef]
- Yang, Y.; Fang, Z.; Chen, X.; Zhang, W.; Xie, Y.; Chen, Y.; Liu, Z.; Yuan, W. An Overview of Pickering Emulsions: Solid-Particle Materials, Classification, Morphology, and Applications. Front. Pharmacol. 2017, 8, 287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.; Park, J.H. Chemically Robust Antifog Nanocoating through Multilayer Deposition of Silica Composite Nanofilms. ACS Appl. Mater. Interfaces 2020, 12, 42109–42118. [Google Scholar] [CrossRef]
- Chen, H.; Feng, P.; Du, Y.; Jiang, J.; Sun, W. The Effect of Superhydrophobic Nano-Silica Particles on the Transport and Mechanical Properties of Hardened Cement Pastes. Constr. Build. Mater. 2018, 182, 620–628. [Google Scholar] [CrossRef]
- Binks, B.P.; Lumsdon, S.O. Effects of Oil Type and Aqueous Phase Composition on Oil–Water Mixtures Containing Particles of Intermediate Hydrophobicity. Phys. Chem. Chem. Phys. 2000, 2, 2959–2967. [Google Scholar] [CrossRef]
- Van Hooghten, R.; Blair, V.E.; Vananroye, A.; Schofield, A.B.; Vermant, J.; Thijssen, J.H.J. Interfacial Rheology of Sterically Stabilized Colloids at Liquid Interfaces and Its Effect on the Stability of Pickering Emulsions. Langmuir 2017, 33, 4107–4118. [Google Scholar] [CrossRef] [Green Version]
- Katepalli, H.; John, V.T.; Tripathi, A.; Bose, A. Microstructure and Rheology of Particle Stabilized Emulsions: Effects of Particle Shape and Inter-Particle Interactions. J. Colloid Interface Sci. 2017, 485, 11–17. [Google Scholar] [CrossRef]
- Czakaj, A.; Chatzigiannakis, E.; Vermant, J.; Krzan, M.; Warszyński, P. The Influence of the Surface Chemistry of Cellulose Nanocrystals on Ethyl Lauroyl Arginate Foam Stability. Polymers 2022, 14, 5402. [Google Scholar] [CrossRef] [PubMed]
- Brown, N.; de la Pena, A.; Razavi, S. Interfacial Rheology Insights: Particle Texture and Pickering Foam Stability. J. Phys. Condens. Matter 2023, 35, 384002. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Sun, H.Q.; Zhang, L.; Li, Z.Q.; Luo, L.; Zhao, S. Interfacial Dilational Rheology Related to Enhance Oil Recovery. Soft Matter 2011, 7, 7601–7611. [Google Scholar] [CrossRef]
- Rühs, P.A.; Böni, L.; Fuller, G.G.; Inglis, R.F.; Fischer, P. In-Situ Quantification of the Interfacial Rheological Response of Bacterial Biofilms to Environmental Stimuli. PLoS ONE 2013, 8, 78524. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Ao, F.; Ge, X.; Shen, W. Food-Grade Pickering Emulsions: Preparation, Stabilization and Applications. Molecules 2020, 25, 3202. [Google Scholar] [CrossRef] [PubMed]
- Farrokhpay, S. The Importance of Rheology in Mineral Flotation: A Review. Miner. Eng. 2012, 36–38, 272–278. [Google Scholar] [CrossRef]
- Chaturvedi, K.R.; Sharma, T. In-Situ Formulation of Pickering CO2 Foam for Enhanced Oil Recovery and Improved Carbon Storage in Sandstone Formation. Chem. Eng. Sci. 2021, 235, 116484. [Google Scholar] [CrossRef]
- Cardoso, C.E.D.; Almeida, J.C.; Lopes, C.B.; Trindade, T.; Vale, C.; Pereira, E. Recovery of Rare Earth Elements by Carbon-Based Nanomaterials—A Review. Nanomaterials 2019, 9, 814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, R.; Ferri, J.K.; Javadi, A.; Krägel, J.; Mucic, N.; Wüstneck, R. Rheology of Interfacial Layers. Colloid Polym. Sci. 2010, 288, 937–950. [Google Scholar] [CrossRef]
- Leser, M.E.; Acquistapace, S.; Cagna, A.; Makievski, A.V.; Miller, R. Limits of Oscillation Frequencies in Drop and Bubble Shape Tensiometry. Colloids Surfaces A Physicochem. Eng. Asp. 2005, 261, 25–28. [Google Scholar] [CrossRef]
- Barman, S.; Christopher, G.F. Role of Capillarity and Microstructure on Interfacial Viscoelasticity of Particle Laden Interfaces. J. Rheol. 2015, 60, 35. [Google Scholar] [CrossRef]
- Zang, D.Y.; Rio, E.; Langevin, D.; Wei, B.; Binks, B.P. Viscoelastic Properties of Silica Nanoparticle Monolayers at the Air-Water Interface. Eur. Phys. J. E 2010, 31, 125–134. [Google Scholar] [CrossRef]
- Zang, D.Y.; Rio, E.; Delon, G.; Langevin, D.; Wei, B.; Binks, B.P. Influence of the Contact Angle of Silica Nanoparticles at the Air–Water Interface on the Mechanical Properties of the Layers Composed of These Particles. Mol. Phys. 2011, 109, 1057–1066. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Bournival, G.; Ata, S. Effect of Polydispersity on the Behaviour of the Particle-Laden Interface. Colloids Surfaces A Physicochem. Eng. Asp. 2020, 607, 125494. [Google Scholar] [CrossRef]
- Beltramo, P.J.; Gupta, M.; Alicke, A.; Liascukiene, I.; Gunes, D.Z.; Baroud, C.N.; Vermant, J. Arresting Dissolution by Interfacial Rheology Design. Proc. Natl. Acad. Sci. USA 2017, 114, 10373–10378. [Google Scholar] [CrossRef] [PubMed]
- Bertsch, P.; Diener, M.; Adamcik, J.; Scheuble, N.; Geue, T.; Mezzenga, R.; Fischer, P. Adsorption and Interfacial Layer Structure of Unmodified Nanocrystalline Cellulose at Air/Water Interfaces. Langmuir 2018, 34, 15195–15202. [Google Scholar] [CrossRef]
- van den Berg, M.E.H.; Kuster, S.; Windhab, E.J.; Sagis, L.M.C.; Fischer, P. Nonlinear Shear and Dilatational Rheology of Viscoelastic Interfacial Layers of Cellulose Nanocrystals. Phys. Fluids 2018, 30, 072103. [Google Scholar] [CrossRef]
- Correia, E.L.; Winter, H.H.; Razavi, S. Two-Dimensional Glass Transition–like Behavior of Janus Particle–Laden Interface. Rheol. Acta 2023, 62, 239–251. [Google Scholar] [CrossRef]
- Vu, T.V.; Razavi, S.; Papavassiliou, D.V. Effect of Janus Particles and Non-Ionic Surfactants on the Collapse of the Oil-Water Interface under Compression. J. Colloid Interface Sci. 2022, 609, 158–169. [Google Scholar] [CrossRef]
- Razavi, S.; Lin, B.; Lee, K.Y.C.; Tu, R.S.; Kretzschmar, I. Impact of Surface Amphiphilicity on the Interfacial Behavior of Janus Particle Layers under Compression. Langmuir 2019, 35, 15813–15824. [Google Scholar] [CrossRef]
- Safouane, M.; Langevin, D.; Binks, B.P. Effect of Particle Hydrophobicity on the Properties of Silica Particle Layers at the Air-Water Interface. Langmuir 2007, 23, 11546–11553. [Google Scholar] [CrossRef] [PubMed]
- Razavi, S.; Cao, K.D.; Lin, B.; Lee, K.Y.C.; Tu, R.S.; Kretzschmar, I. Collapse of Particle-Laden Interfaces under Compression: Buckling vs Particle Expulsion. Langmuir 2015, 31, 7764–7775. [Google Scholar] [CrossRef] [PubMed]
- Bykov, A.G.; Loglio, G.; Miller, R.; Noskov, B.A. Dilational Surface Elasticity of Monolayers of Charged Polystyrene Nano- and Microparticles at Liquid/Fluid Interfaces. Colloids Surfaces A Physicochem. Eng. Asp. 2015, 485, 42–48. [Google Scholar] [CrossRef]
- Guzmán, E.; Abelenda-Núñez, I.; Maestro, A.; Ortega, F.; Santamaria, A.; Rubio, R.G. Particle-Laden Fluid/Fluid Interfaces: Physico-Chemical Foundations. J. Phys. Condens. Matter 2021, 33, 333001. [Google Scholar] [CrossRef] [PubMed]
- Horozov, T.S.; Aveyard, R.; Clint, J.H.; Binks, B.P. Order-Disorder Transition in Monolayers of Modified Monodisperse Silica Particles at the Octane-Water Interface. Langmuir 2003, 19, 2822–2829. [Google Scholar] [CrossRef]
- Vogel, N.; De Viguerie, L.; Jonas, U.; Weiss, C.K.; Landfester, K. Wafer-Scale Fabrication of Ordered Binary Colloidal Monolayers with Adjustable Stoichiometries. Adv. Funct. Mater. 2011, 21, 3064–3073. [Google Scholar] [CrossRef]
- Trevenen, S.; Rahman, M.A.; Hamilton, H.S.C.; Ribbe, A.E.; Bradley, L.C.; Beltramo, P.J. Nanoscale Porosity in Microellipsoids Cloaks Interparticle Capillary Attraction at Fluid Interfaces. ACS Nano 2023, 17, 11892–11904. [Google Scholar] [CrossRef]
- Bykov, A.G.; Noskov, B.A.; Loglio, G.; Lyadinskaya, V.V.; Miller, R. Dilational Surface Elasticity of Spread Monolayers of Polystyrene Microparticles. Soft Matter 2014, 10, 6499–6505. [Google Scholar] [CrossRef]
- Pieranski, P. Two-Dimensional Interfacial Colloidal Crystals. Phys. Rev. Lett. 1980, 45, 569. [Google Scholar] [CrossRef]
- Ghezzi, F.; Earnshaw, J.C.; Finnis, M.; McCluney, M. Pattern Formation in Colloidal Monolayers at the Air-Water Interface. J. Colloid Interface Sci. 2001, 238, 433–446. [Google Scholar] [CrossRef]
- Kralchevsky, P.A.; Danov, K.D.; Petkov, P.V. Soft Electrostatic Repulsion in Particle Monolayers at Liquid Interfaces: Surface Pressure and Effect of Aggregation. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2016, 374, 20150130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiao, Y.; Ma, X.; Liu, Z.; Manno, M.A.; Keim, N.C.; Cheng, X. Tuning the Rheology and Microstructure of Particle-Laden Fluid Interfaces with Janus Particles. J. Colloid Interface Sci. 2022, 618, 241–247. [Google Scholar] [CrossRef]
- Dani, A.; Yeganeh, M.; Maldarelli, C. Hydrodynamic Interactions between Charged and Uncharged Brownian Colloids at a Fluid-Fluid Interface. J. Colloid Interface Sci. 2022, 628, 931–945. [Google Scholar] [CrossRef] [PubMed]
- Martínez, G.; Merinero, M.; Pérez-Aranda, M.; Pérez-Soriano, E.; Ortiz, T.; Villamor, E.; Begines, B.; Alcudia, A. Environmental Impact of Nanoparticles’ Application as an Emerging Technology: A Review. Materials 2020, 14, 166. [Google Scholar] [CrossRef]
- Barthel, H. Surface Interactions of Dimethylsiloxy Group-Modified Fumed Silica. Colloids Surfaces A Physicochem. Eng. Asp. 1995, 101, 217–226. [Google Scholar] [CrossRef]
- Brandriss, S.; Margel, S. Synthesis and Characterization of Self-Assembled Hydrophobic Monolayer Coatings on Silica Colloids. Langmuir 1993, 9, 1232–1240. [Google Scholar] [CrossRef]
- Garbin, V. Collapse Mechanisms and Extreme Deformation of Particle-Laden Interfaces. Curr. Opin. Colloid Interface Sci. 2019, 39, 202–211. [Google Scholar] [CrossRef] [Green Version]
- Tô, K.I.; Nagel, S.R. Rifts in Rafts. Soft Matter 2023, 19, 905–912. [Google Scholar] [CrossRef]
- Kim, K.H.; Kim, B.Q.; Kim, J.Q.; Choi, S.Q. New Collapse Mechanism of Colloidal Particle Monolayers via Depletion Pressure: Formation of Large-Area Particle Multilayers at the Air-Water Interface. J. Phys. Chem. C 2019, 123, 27862–27867. [Google Scholar] [CrossRef]
- Neilson, J.; Avery, M.P.; Derby, B. Tiled Monolayer Films of 2D Molybdenum Disulfide Nanoflakes Assembled at Liquid/Liquid Interfaces. ACS Appl. Mater. Interfaces 2020, 12, 25125–25134. [Google Scholar] [CrossRef]
- Vandebril, S.; Franck, A.; Fuller, G.G.; Moldenaers, P.; Vermant, J. A Double Wall-Ring Geometry for Interfacial Shear Rheometry. Rheol. Acta 2010, 49, 131–144. [Google Scholar] [CrossRef]
- Renggli, D.; Alicke, A.; Ewoldt, R.H.; Vermant, J. Operating Windows for Oscillatory Interfacial Shear Rheology. J. Rheol. 2020, 64, 141–160. [Google Scholar] [CrossRef] [Green Version]
- Razavi, S.; Hernandez, L.M.; Read, A.; Vargas, W.L.; Kretzschmar, I. Surface Tension Anomaly Observed for Chemically-Modified Janus Particles at the Air/Water Interface. J. Colloid Interface Sci. 2020, 558, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Berry, J.D.; Neeson, M.J.; Dagastine, R.R.; Chan, D.Y.C.; Tabor, R.F. Measurement of Surface and Interfacial Tension Using Pendant Drop Tensiometry. J. Colloid Interface Sci. 2015, 454, 226–237. [Google Scholar] [CrossRef] [PubMed]
- Stillinger, F.H. Interfacial Solutions of the Poisson-Boltzmann Equation. J. Chem. Phys. 2004, 35, 1584. [Google Scholar] [CrossRef] [Green Version]
- Muntz, I.; Waggett, F.; Hunter, M.; Schofield, A.B.; Bartlett, P.; Marenduzzo, D.; Thijssen, J.H.J. Interaction between Nearly Hard Colloidal Spheres at an Oil-Water Interface. Phys. Rev. Res. 2020, 2, 023388. [Google Scholar] [CrossRef]
- Oettel, M.; Dietrich, S. Colloidal Interactions at Fluid Interfaces. Langmuir 2008, 24, 1425–1441. [Google Scholar] [CrossRef]
- Israelachvili, J.N. Intermolecular and Surface Forces, 3rd ed.; Academic Press: Cambridge, MA, USA, 2011; ISBN 9780123919274. [Google Scholar]
- Williams, D.F.; Berg, J.C. The Aggregation of Colloidal Particles at the Air—Water Interface. J. Colloid Interface Sci. 1992, 152, 218–229. [Google Scholar] [CrossRef] [Green Version]
- Stamou, D.; Duschl, C.; Johannsmann, D. Long-Range Attraction between Colloidal Spheres at the Air-Water Interface: The Consequence of an Irregular Meniscus. Phys. Rev. E 2000, 62, 5263. [Google Scholar] [CrossRef]
- Arzt, E.; Quan, H.; McMeeking, R.M.; Hensel, R. Functional Surface Microstructures Inspired by Nature—From Adhesion and Wetting Principles to Sustainable New Devices. Prog. Mater. Sci. 2021, 120, 100823. [Google Scholar] [CrossRef]
- Munshi, A.M.; Singh, V.N.; Kumar, M.; Singh, J.P. Effect of Nanoparticle Size on Sessile Droplet Contact Angle. J. Appl. Phys. 2008, 103, 084315. [Google Scholar] [CrossRef]
- Park, I.W.; Ribe, J.M.; Fernandino, M.; Dorao, C.A. The Criterion of the Cassie–Baxter and Wenzel Wetting Modes and the Effect of Elastic Substrates on It. Adv. Mater. Interfaces 2023, 10, 2202439. [Google Scholar] [CrossRef]
- Ji, T.; Ma, C.; Brisbin, L.; Mu, L.; Robertson, C.G.; Dong, Y.; Zhu, J. Organosilane Grafted Silica: Quantitative Correlation of Microscopic Surface Characters and Macroscopic Surface Properties. Appl. Surf. Sci. 2017, 399, 565–572. [Google Scholar] [CrossRef] [Green Version]
- Horozov, T.S.; Binks, B.P.; Aveyard, R.; Clint, J.H. Effect of Particle Hydrophobicity on the Formation and Collapse of Fumed Silica Particle Monolayers at the Oil–Water Interface. Colloids Surfaces A Physicochem. Eng. Asp. 2006, 282–283, 377–386. [Google Scholar] [CrossRef]
- Vella, D.; Aussillous, P.; Mahadevan, L. Elasticity of an Interfacial Particle Raft. Europhys. Lett. 2004, 68, 212. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.; Da, C.; Chen, J.; Johnston, K.P. Ultrastable N2/Water Foams Stabilized by Dilute Nanoparticles and a Surfactant at High Salinity and High Pressure. Langmuir 2021, 38, 5392–5403. [Google Scholar] [CrossRef]
- Lide, R.D. Handbook of Chemistry and Physics, 73rd ed.; CRC Press: Boca Raton, FL, USA, 1992. [Google Scholar]
- Tadmor, R.; Hernández-Zapata, E.; Chen, N.; Pincus, P.; Israelachvili, J.N. Debye Length and Double-Layer Forces in Polyelectrolyte Solutions. Macromolecules 2002, 35, 2380–2388. [Google Scholar] [CrossRef]
- Drazin, P.G.; Reid, W.H. Hydrodynamic Stability; Cambridge University Press: Cambridge, UK, 2004. [Google Scholar] [CrossRef]
- Matos, C.; De Castro, B.; Gameiro, P.; Lima, J.L.F.C.; Reis, S. Zeta-Potential Measurements as a Tool to Quantify the Effect of Charged Drugs on the Surface Potential of Egg Phosphatidylcholine Liposomes. Langmuir 2004, 20, 369–377. [Google Scholar] [CrossRef]
- Horozov, T.S.; Aveyard, R.; Binks, B.P.; Clint, J.H. Structure and Stability of Silica Particle Monolayers at Horizontal and Vertical Octane-Water Interfaces. Langmuir 2005, 21, 7405–7412. [Google Scholar] [CrossRef]







| Particle Attribute ⇒ | (mV) | (μC/cm2) |
|---|---|---|
| Particle Type ⇓ | ||
| Untreated 250 nm | −43 ± 3 | 0.074 ± 0.010 |
| Treated 250 nm | −33 ± 3 | 0.062 ± 0.007 |
| Untreated 1000 nm | −48 ± 2 | 0.051 ± 0.003 |
| Treated 1000 nm | −32 ± 3 | 0.041 ± 0.003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thakur, S.; Razavi, S. Particle Size and Rheology of Silica Particle Networks at the Air–Water Interface. Nanomaterials 2023, 13, 2114. https://doi.org/10.3390/nano13142114
Thakur S, Razavi S. Particle Size and Rheology of Silica Particle Networks at the Air–Water Interface. Nanomaterials. 2023; 13(14):2114. https://doi.org/10.3390/nano13142114
Chicago/Turabian StyleThakur, Siddharth, and Sepideh Razavi. 2023. "Particle Size and Rheology of Silica Particle Networks at the Air–Water Interface" Nanomaterials 13, no. 14: 2114. https://doi.org/10.3390/nano13142114