The Thermophysical and Physicochemical Properties of the Aqueous Dispersion of Graphene Oxide Dual-Beam Thermal Lens Spectrometry
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Graphene Oxide Dispersions
2.2. Thermal-Lens SPECTROMETER
2.3. Thermal-Lens Measurements
2.4. Other Instruments and Their Operation Parameters
3. Results
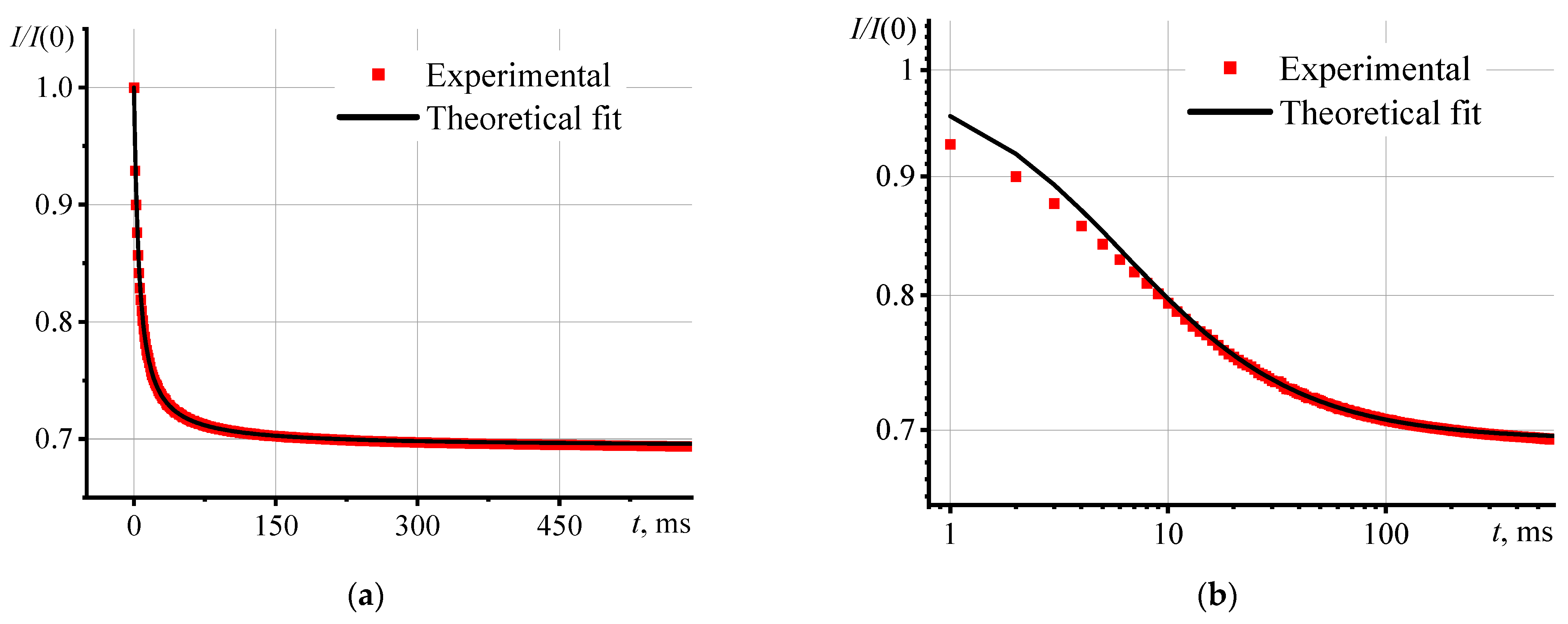
3.1. Verification of Correct Operation of the Spectrometer
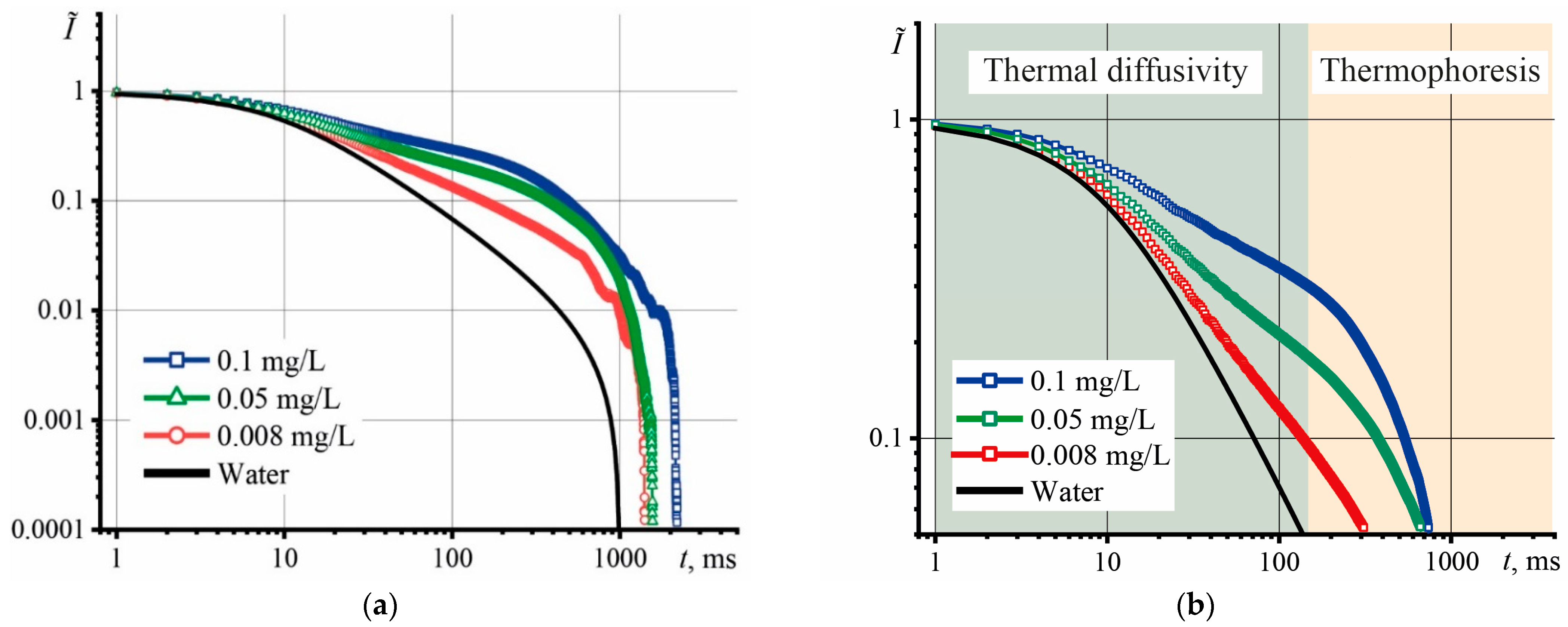
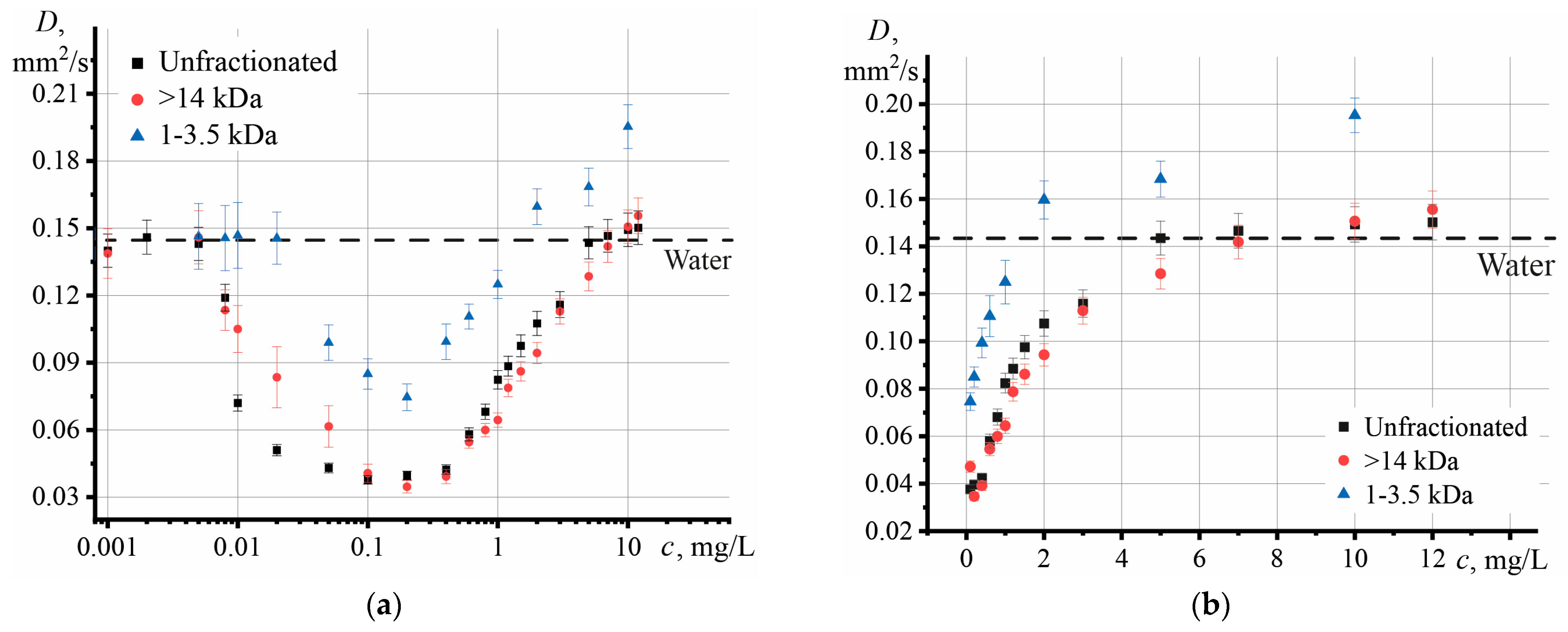
3.2. Effect of Graphene Oxide Concentration and Size on the Thermophysical Properties of Aqueous Dispersions
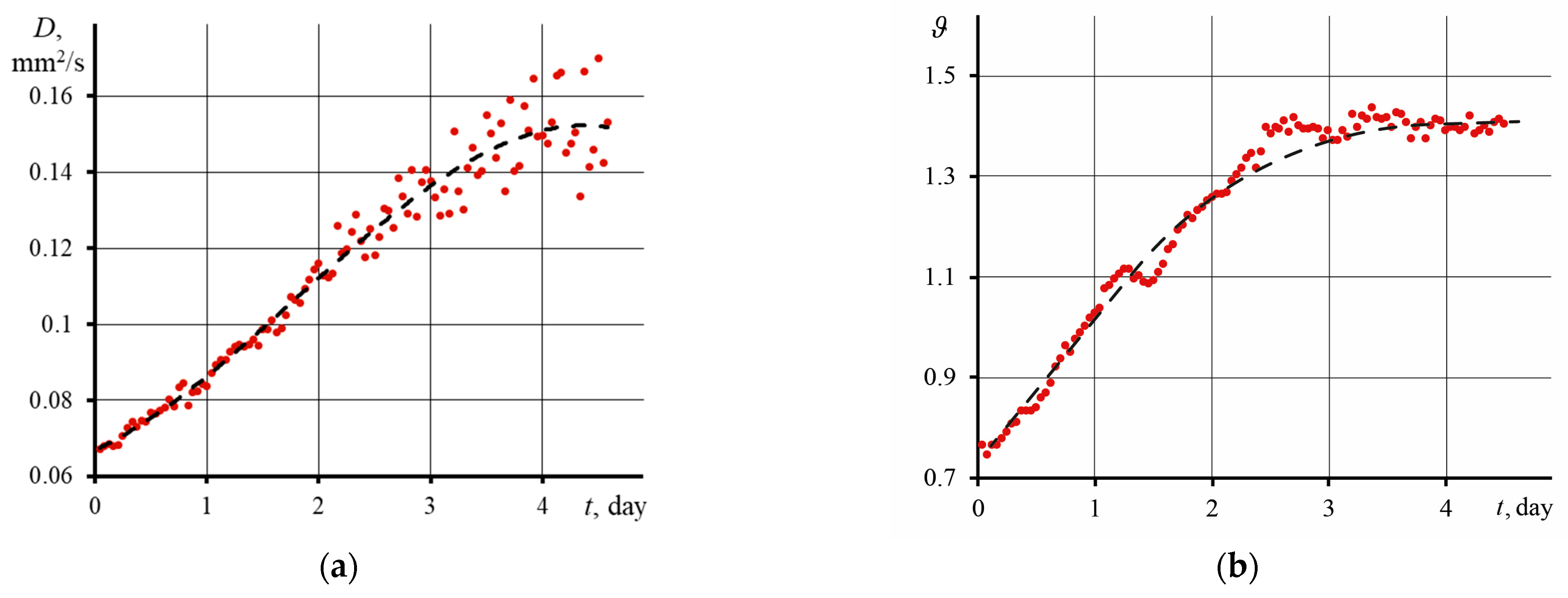
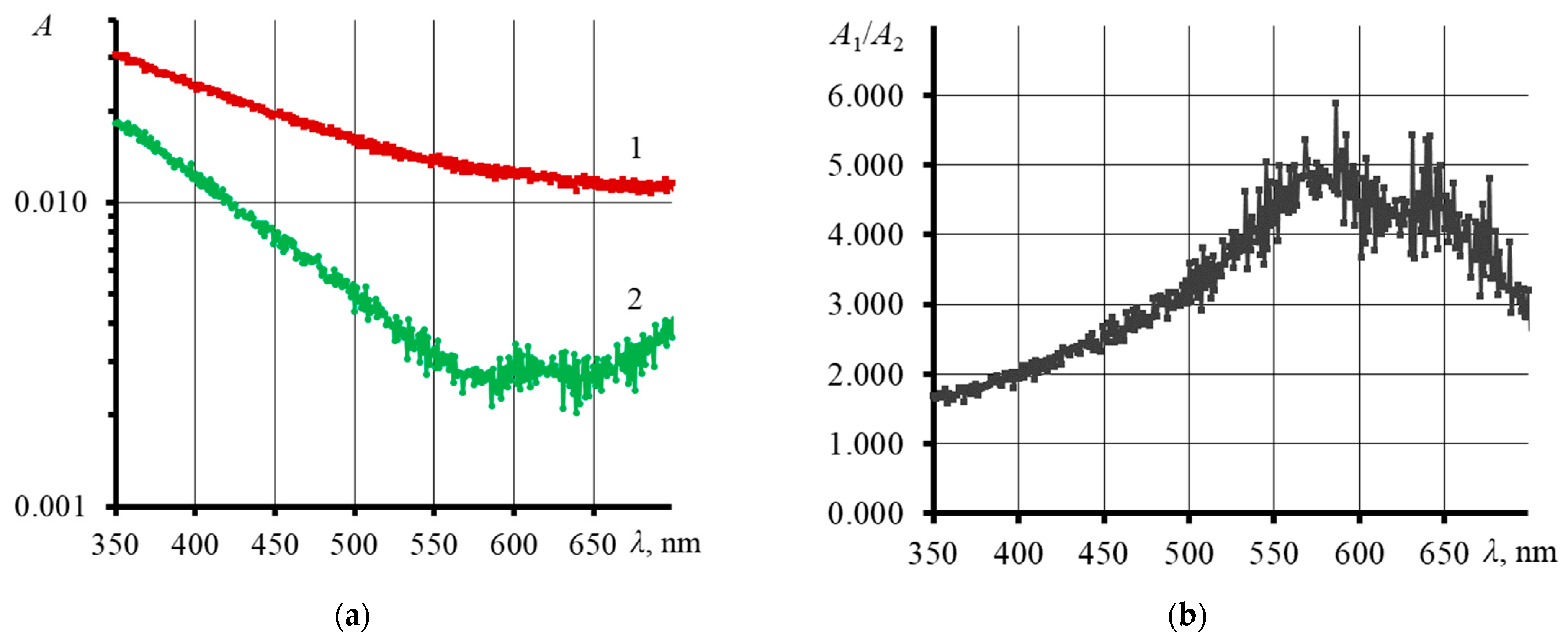
3.3. Influence of an Excitation Laser on the Physicochemical and Thermal Properties of GO
4. Discussion
4.1. Effect of Graphene Oxide Concentration and Size on the Thermophysical Properties of Aqueous Dispersions
- (1)
- The largest particles contribute the most to the decrease in thermal diffusivity;
- (2)
- The contribution of particles with different molar masses to the increase in thermal diffusivity, which was observed at c > 0.1 mg/L, is comparable with each other.
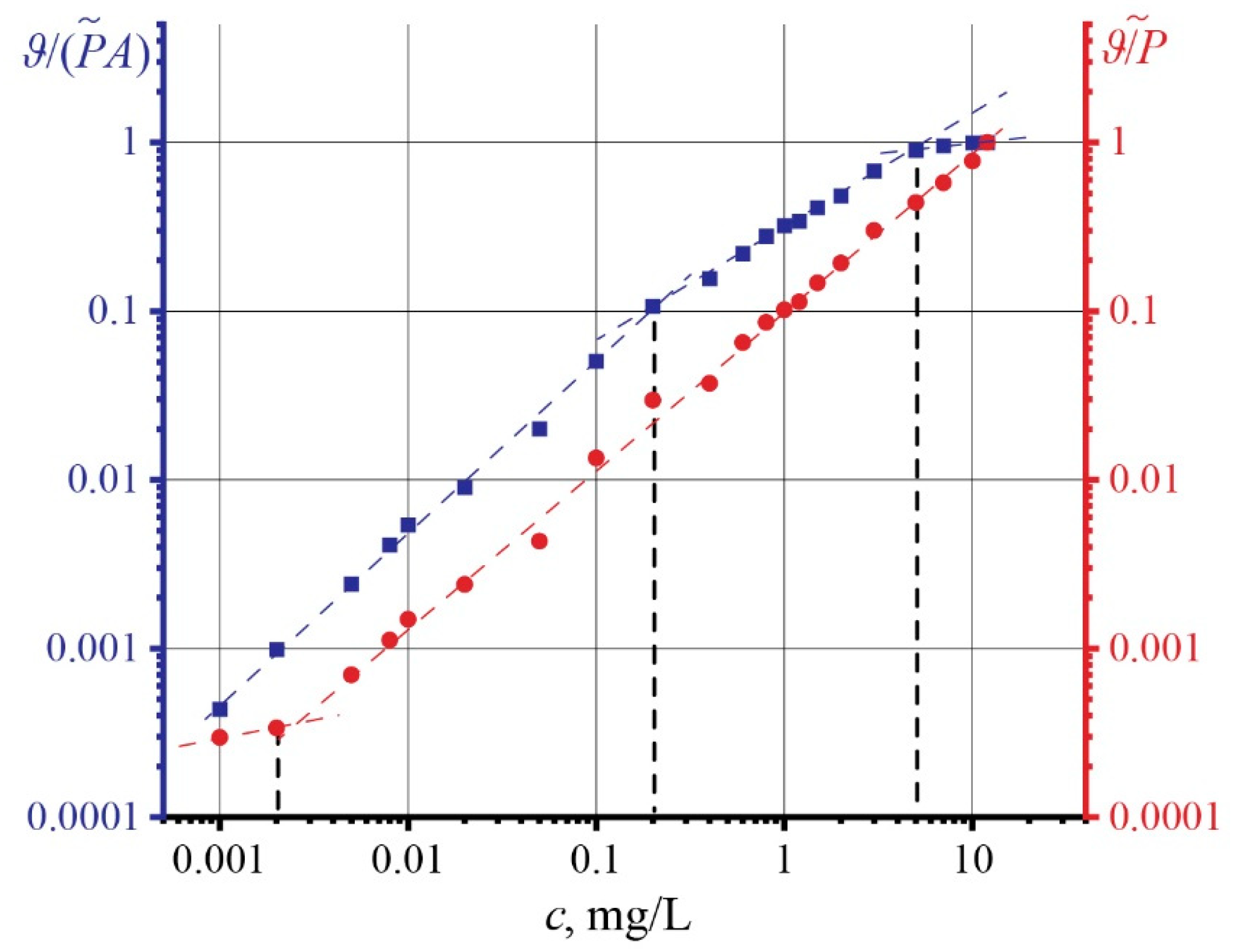
4.2. Influence of High Optical Absorption on the Reliability of Finding the Thermal Diffusivity
4.3. Influence of Laser Radiation on Physicochemical and Thermal Properties of Graphene Oxide Dispersion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dinker, A.; Agarwal, M.; Agarwal, G.D. Heat storage materials, geometry and applications: A review. J. Energy Inst. 2017, 90, 1–11. [Google Scholar] [CrossRef]
- Sarbu, I.; Sebarchievici, C. A Comprehensive Review of Thermal Energy Storage. Sustainability 2018, 10, 191. [Google Scholar] [CrossRef]
- Sarbu, I.; Dorca, A. Review on heat transfer analysis in thermal energy storage using latent heat storage systems and phase change materials. Int. J. Energy Res. 2018, 43, 29–64. [Google Scholar] [CrossRef]
- Qiu, L.; Ouyang, Y.; Li, F. Experimental techniques overview. In Micro and Nano Thermal Transport; Qiu, L., Feng, Y., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 19–45. [Google Scholar]
- Souza, R.R.; Faustino, V.; Gonçalves, I.M.; Moita, A.S.; Bañobre-López, M.; Lima, R. A Review of the Advances and Challenges in Measuring the Thermal Conductivity of Nanofluids. Nanomaterials 2022, 12, 2526. [Google Scholar] [CrossRef]
- Elarem, R.; Alqahtani, T.; Mellouli, S.; Askri, F.; Edacherian, A.; Vineet, T.; Badruddin, I.A.; Abdelmajid, J. A comprehensive review of heat transfer intensification methods for latent heat storage units. Energy Storage 2020, 3, e127. [Google Scholar] [CrossRef]
- Gupta, M.; Singh, V.; Kumar, R.; Said, Z. A review on thermophysical properties of nanofluids and heat transfer applications. Renew. Sustain. Energy Rev. 2017, 74, 638–670. [Google Scholar] [CrossRef]
- Mamat, H.; Ramadan, M. Nanofluids: Thermal Conductivity and Applications. In Encyclopedia of Smart Materials; Olabi, A.-G., Ed.; Elsevier: Oxford, UK, 2022; pp. 288–296. [Google Scholar]
- Muneeshwaran, M.; Srinivasan, G.; Muthukumar, P.; Wang, C.-C. Role of hybrid-nanofluid in heat transfer enhancement—A review. Int. Commun. Heat Mass Transf. 2021, 125, 105341. [Google Scholar] [CrossRef]
- Moreira, T.A.; Moreira, D.C.; Ribatski, G. Nanofluids for heat transfer applications: A review. J. Braz. Soc. Mech. Sci. Eng. 2018, 40, 303. [Google Scholar] [CrossRef]
- Choi, S.U.S.; Eastman, J. Enhancing Thermal Conductivity of Fluids with Nanoparticles. In Proceedings of the ASME International Mechanical Engineering Congress & Exposition, San Francisco, CA, USA, 12–17 November 1995; Volume 66. [Google Scholar]
- Zamiri, R.; Azmi, B.Z.; Shahriari, E.; Naghavi, K.; Saion, E.; Rizwan, Z.; Husin, M.S. Thermal diffusivity measurement of silver nanofluid by thermal lens technique. J. Laser Appl. 2011, 23, 042002. [Google Scholar] [CrossRef]
- Zamiri, R.; Azmi, B.Z.; Shahril Husin, M.; Zamiri, G.; Ahangar, H.A.; Rizwan, Z. Thermal diffusivity measurement of copper nanofluid using pulsed laser thermal lens technique. J. Eur. Opt. Soc. Rapid Publ. 2012, 7, 12022. [Google Scholar] [CrossRef]
- Joseph, S.A.; Hari, M.; Mathew, S.; Sharma, G.; Soumya; Hadiya, V.M.; Radhakrishnan, P.; Nampoori, V.P.N. Thermal diffusivity of rhodamine 6G incorporated in silver nanofluid measured using mode-matched thermal lens technique. Opt. Commun. 2010, 283, 313–317. [Google Scholar] [CrossRef]
- Shahriari, E.; Moradi, M.; Raeisi, M. An experimental study of thermal diffusivity of Au nanoparticles: Effects of concentration particle size. J. Theor. Appl. Phys. 2016, 10, 259–263. [Google Scholar] [CrossRef]
- Mathew, S.; Francis, F.; Joseph, S.A.; Kala, M.S. Enhanced thermal diffusivity of water based ZnO nanoflower/rGO nanofluid using the dual-beam thermal lens technique. Nano-Struct. Nano-Objects 2021, 28, 100784. [Google Scholar] [CrossRef]
- Francis, F.; Anila, E.I.; Joseph, S.A. Dependence of thermal diffusivity on nanoparticle shape deduced through thermal lens technique taking ZnO nanoparticles and nanorods as inclusions in homogeneous dye solution. Optik 2020, 219, 165210. [Google Scholar] [CrossRef]
- Lenart, V.M.; Astrath, N.G.C.; Turchiello, R.F.; Goya, G.F.; Gómez, S.L. Thermal diffusivity of ferrofluids as a function of particle size determined using the mode-mismatched dual-beam thermal lens technique. J. Appl. Phys. 2018, 123, 085107. [Google Scholar] [CrossRef]
- Ramya, M.; Nideep, T.K.; Nampoori, V.P.N.; Kailasnath, M. Particle size and concentration effect on thermal diffusivity of water-based ZnO nanofluid using the dual-beam thermal lens technique. Appl. Phys. B 2019, 125, 181. [Google Scholar] [CrossRef]
- Sebastian, R.; Swapna, M.S.; Raj, V.; Hari, M.; Sankararaman, S. Thermal diffusivity control in titanium dioxide nanofluid through phase tuning. Mater. Res. Express 2018, 5, 075001. [Google Scholar] [CrossRef]
- Nideep, T.K.; Ramya, M.; Nampoori, V.P.N.; Kailasnath, M. The size dependent thermal diffusivity of water soluble CdTe quantum dots using dual beam thermal lens spectroscopy. Phys. E Low-Dimens. Syst. Nanostruct. 2020, 116, 113724. [Google Scholar] [CrossRef]
- Sánchez Ramírez, J.F.; Arvizu Amador, S.F.; Jiménez Pérez, J.L.; Bautista Hernández, A.; Delgado Macuil, R.J.; Díaz Reyes, J.; Chigo Anota, E. Decreased thermal diffusivity in fluids containing InP nanocrystals. J. Therm. Anal. Calorim. 2015, 120, 1563–1571. [Google Scholar] [CrossRef]
- Swapna, M.N.S.; Korte, D.; Sankararaman, S.I. Solid-Volume-Fraction Retained Tailoring of Thermal Diffusivity of Multiwalled Carbon Nanotube Nanofluid: A Photothermal Investigation. Phys. Status Solidi A 2023, 220, 2200797. [Google Scholar] [CrossRef]
- Sindhu Swapna, M.N.; Raj, V.; Cabrera, H.; Sankararaman, S.I. Thermal Lensing of Multi-walled Carbon Nanotube Solutions as Heat Transfer Nanofluids. ACS Appl. Nano Mater. 2021, 4, 3416–3425. [Google Scholar] [CrossRef]
- Cabrera, H.; Mendoza, D.; Benítez, J.L.; Bautista Flores, C.; Alvarado, S.; Marín, E. Thermal diffusivity of few-layers graphene measured by an all-optical method. J. Phys. D 2015, 48, 465501. [Google Scholar] [CrossRef]
- Vijesh, K.R.; Sony, U.; Ramya, M.; Mathew, S.; Nampoori, V.P.N.; Thomas, S. Concentration dependent variation of thermal diffusivity in highly fluorescent carbon dots using dual beam thermal lens technique. Int. J. Therm. Sci. 2018, 126, 137–142. [Google Scholar] [CrossRef]
- Jiménez-Pérez, J.L.; López-Gamboa, G.; Sánchez-Ramírez, J.F.; Correa-Pacheco, Z.N.; Netzahual-Lopantzi, A.; Cruz-Orea, A. Thermal Diffusivity Dependence with Highly Concentrated Graphene Oxide/Water Nanofluids by Mode-Mismatched Dual-Beam Thermal Lens Technique. Int. J. Thermophys. 2021, 42, 107. [Google Scholar] [CrossRef]
- Swapna, M.S.; Raj, V.; Sankararaman, S. Allotropic transformation instigated thermal diffusivity of soot nanofluid: Thermal lens study. Phys. Fluids 2019, 31, 117106. [Google Scholar] [CrossRef]
- Awais, M.; Bhuiyan, A.A.; Salehin, S.; Ehsan, M.M.; Khan, B.; Rahman, M.H. Synthesis, heat transport mechanisms and thermophysical properties of nanofluids: A critical overview. Int. J. Thermofluids 2021, 10, 100086. [Google Scholar] [CrossRef]
- Trong Tam, N.; Viet Phuong, N.; Hong Khoi, P.; Ngoc Minh, P.; Afrand, M.; Van Trinh, P.; Hung Thang, B.; Żyła, G.; Estellé, P. Carbon Nanomaterial-Based Nanofluids for Direct Thermal Solar Absorption. Nanomaterials 2020, 10, 1199. [Google Scholar] [CrossRef] [PubMed]
- Fritea, L.; Banica, F.; Costea, T.O.; Moldovan, L.; Dobjanschi, L.; Muresan, M.; Cavalu, S. Metal Nanoparticles and Carbon-Based Nanomaterials for Improved Performances of Electrochemical (Bio)Sensors with Biomedical Applications. Materials 2021, 14, 6319. [Google Scholar] [CrossRef]
- Varshney, K. Carbon Nanotubes: A Review on Synthesis, Properties and Applications. Int. J. Eng. Res. Gen. Sci. 2014, 2, 660. [Google Scholar]
- Liu, W.; Speranza, G. Functionalization of Carbon Nanomaterials for Biomedical Applications. C—J. Carbon Res. 2019, 5, 72. [Google Scholar] [CrossRef]
- Balandin, A.A. Thermal properties of graphene and nanostructured carbon materials. Nat. Mater. 2011, 10, 569–581. [Google Scholar] [CrossRef]
- Apmann, K.; Fulmer, R.; Soto, A.; Vafaei, S. Thermal Conductivity and Viscosity: Review and Optimization of Effects of Nanoparticles. Materials 2021, 14, 1291. [Google Scholar] [CrossRef]
- Ebadi-Dehaghani, H.; Nazempour, M. Thermal Conductivity of Nanoparticles Filled Polymers. In Smart Nanoparticles Technology; Abbass, H., Ed.; IntechOpen: Rijeka, Croatia, 2012; Chapter 23. [Google Scholar]
- Idris, A.O.; Akanji, S.P.; Orimolade, B.O.; Olorundare, F.O.; Azizi, S.; Mamba, B.; Maaza, M. Using Nanomaterials as Excellent Immobilisation Layer for Biosensor Design. Biosensors 2023, 13, 192. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Dkhar, D.S.; Chandra, P.; Azad, U.P. Nanobiosensors Design Using 2D Materials: Implementation in Infectious and Fatal Disease Diagnosis. Biosensors 2023, 13, 166. [Google Scholar] [CrossRef] [PubMed]
- Joshi, D.J.; Koduru, J.R.; Malek, N.I.; Hussain, C.M.; Kailasa, S.K. Surface modifications and analytical applications of graphene oxide: A review. TrAC Trends Anal. Chem. 2021, 144, 116448. [Google Scholar] [CrossRef]
- Karbalaei Akbari, M.; Siraj Lopa, N.; Shahriari, M.; Najafzadehkhoee, A.; Galusek, D.; Zhuiykov, S. Functional Two-Dimensional Materials for Bioelectronic Neural Interfacing. J. Funct. Biomater. 2023, 14, 35. [Google Scholar] [CrossRef] [PubMed]
- Dabrowski, B.; Zuchowska, A.; Brzozka, Z. Graphene oxide internalization into mammalian cells—A review. Colloids Surf. B 2023, 221, 112998. [Google Scholar] [CrossRef] [PubMed]
- Itoo, A.M.; Vemula, S.L.; Gupta, M.T.; Giram, M.V.; Kumar, S.A.; Ghosh, B.; Biswas, S. Multifunctional graphene oxide nanoparticles for drug delivery in cancer. J. Control. Release 2022, 350, 26–59. [Google Scholar] [CrossRef]
- Deshwal, N.; Singh, M.B.; Bahadur, I.; Kaushik, N.; Kaushik, N.K.; Singh, P.; Kumari, K. A review on recent advancements on removal of harmful metal/metal ions using graphene oxide: Experimental and theoretical approaches. Sci. Total Environ. 2023, 858, 159672. [Google Scholar] [CrossRef]
- Singh, S.; Naik, T.S.S.K.; Shehata, N.; Aguilar-Marcelino, L.; Dhokne, K.; Lonare, S.; Chauhan, V.; Kumar, A.; Singh, J.; Ramamurthy, P.C.; et al. Novel insights into graphene oxide-based adsorbents for remediation of hazardous pollutants from aqueous solutions: A comprehensive review. J. Mol. Liq. 2023, 369, 120821. [Google Scholar] [CrossRef]
- Joel, E.F.; Lujanienė, G. Progress in Graphene Oxide Hybrids for Environmental Applications. Environments 2022, 9, 153. [Google Scholar] [CrossRef]
- Rashi. Exploring the methods of synthesis, functionalization, and characterization of graphene and graphene oxide for supercapacitor applications. Ceram. Int. 2023, 49, 40–47. [Google Scholar] [CrossRef]
- Smith, A.T.; LaChance, A.M.; Zeng, S.; Liu, B.; Sun, L. Synthesis, properties, and applications of graphene oxide/reduced graphene oxide and their nanocomposites. Nano Mater. Sci. 2019, 1, 31–47. [Google Scholar] [CrossRef]
- Anwar, A.; Liu, X.; Zhang, L. Nano-cementitious composites modified with Graphene Oxide—A review. Thin-Walled Struct. 2023, 183, 110326. [Google Scholar] [CrossRef]
- Zhao, W.; Chen, Y.; Liu, Z.; Wang, L.; Li, X. Effects of surface-modified coal-bearing metakaolin and graphene oxide on the properties of cement mortar. Constr. Build. Mater. 2023, 372, 130796. [Google Scholar] [CrossRef]
- Jena, G.; Philip, J. A review on recent advances in graphene oxide-based composite coatings for anticorrosion applications. Prog. Org. Coat. 2022, 173, 107208. [Google Scholar] [CrossRef]
- Li, F.-F. Comprehensive Review of Recent Research Advances on Flame-Retardant Coatings for Building Materials: Chemical Ingredients, Micromorphology, and Processing Techniques. Molecules 2023, 28, 1842. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Lin, J.; Liang, Z.; Yao, R.; Wu, W.; Fang, Z.; Zou, W.; Wu, Z.; Ning, H.; Peng, J. Graphene oxide as a promising nanofiller for polymer composite. Surf. Interfaces 2023, 37, 102747. [Google Scholar] [CrossRef]
- Ye, M.; Zhang, Z.; Zhao, Y.; Qu, L. Graphene Platforms for Smart Energy Generation and Storage. Joule 2018, 2, 245–268. [Google Scholar] [CrossRef]
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior thermal conductivity of single-layer graphene. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Bobbo, S.; Buonomo, B.; Manca, O.; Vigna, S.; Fedele, L. Analysis of the Parameters Required to Properly Define Nanofluids for Heat Transfer Applications. Fluids 2021, 6, 65. [Google Scholar] [CrossRef]
- Das, P.K. A review based on the effect and mechanism of thermal conductivity of normal nanofluids and hybrid nanofluids. J. Mol. Liq. 2017, 240, 420–446. [Google Scholar] [CrossRef]
- Khabibullin, V.R.; Usoltseva, L.O.; Mikheev, I.V.; Proskurnin, M.A. Thermal Diffusivity of Aqueous Dispersions of Silicon Oxide Nanoparticles by Dual-Beam Thermal Lens Spectrometry. Nanomaterials 2023, 13, 1006. [Google Scholar] [CrossRef] [PubMed]
- Thakur, S.N. Photoacoustic and photothermal spectroscopy. In Photoacoustic and Photothermal Spectroscopy; Thakur, S.N., Rai, V.N., Singh, J.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 1–19. [Google Scholar]
- Shimizu, H.; Chen, C.; Tsuyama, Y.; Tsukahara, T.; Kitamori, T. Photothermal spectroscopy and micro/nanofluidics. J. Appl. Phys. 2022, 132, 060902. [Google Scholar] [CrossRef]
- Proskurnin, M.A.; Khabibullin, V.R.; Usoltseva, L.O.; Vyrko, E.A.; Mikheev, I.V.; Volkov, D.S. Photothermal and optoacoustic spectroscopy: State of the art and prospects. Phys. Uspekhi 2022, 65, 270–312. [Google Scholar] [CrossRef]
- Lopes, C.S.; Lenart, V.M.; Turchiello, R.F.; Gómez, S.L. Determination of the Thermal Diffusivity of Plasmonic Nanofluids Containing PVP-Coated Ag Nanoparticles Using Mode-Mismatched Dual-Beam Thermal Lens Technique. Adv. Condens. Matter Phys. 2018, 2018, 3052793. [Google Scholar] [CrossRef]
- Mikheev, I.V.; Usoltseva, L.O.; Ivshukov, D.A.; Volkov, D.S.; Korobov, M.V.; Proskurnin, M.A. Approach to the Assessment of Size-Dependent Thermal Properties of Disperse Solutions: Time-Resolved Photothermal Lensing of Aqueous Pristine Fullerenes C60 and C70. J. Phys. Chem. C 2016, 120, 28270–28287. [Google Scholar] [CrossRef]
- Usoltseva, L.O.; Korobov, M.V.; Proskurnin, M.A. Photothermal spectroscopy: A promising tool for nanofluids. J. Appl. Phys. 2020, 128, 190901. [Google Scholar] [CrossRef]
- Netzahual-Lopantzi, Á.; Sánchez-Ramírez, J.F.; Jiménez-Pérez, J.L.; Cornejo-Monroy, D.; López-Gamboa, G.; Correa-Pacheco, Z.N. Study of the thermal diffusivity of nanofluids containing SiO2 decorated with Au nanoparticles by thermal lens spectroscopy. Appl. Phys. A 2019, 125, 588. [Google Scholar] [CrossRef]
- Gutierrez Fuentes, R.; Pescador Rojas, J.A.; Jiménez-Pérez, J.L.; Sanchez Ramirez, J.F.; Cruz-Orea, A.; Mendoza-Alvarez, J.G. Study of thermal diffusivity of nanofluids with bimetallic nanoparticles with Au(core)/Ag(shell) structure. Appl. Surf. Sci. 2008, 255, 781–783. [Google Scholar] [CrossRef]
- Augustine, A.K.; Mathew, S.; Girijavallabhan, C.P.; Radhakrishnan, P.; Nampoori, V.P.N.; Kailasnath, M. Size dependent variation of thermal diffusivity of CdSe nanoparticles based nanofluid using laser induced mode-matched thermal lens technique. J. Opt. 2014, 44, 85–91. [Google Scholar] [CrossRef]
- Franko, M.; Tran, C.D. Thermal Lens Spectroscopy. In Encyclopedia of Analytical Chemistry; Meyers, R.A., Ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2010. [Google Scholar] [CrossRef]
- Khabibullin, V.R.; Franko, M.; Proskurnin, M.A. Accuracy of Measurements of Thermophysical Parameters by Dual-Beam Thermal-Lens Spectrometry. Nanomaterials 2023, 13, 430. [Google Scholar] [CrossRef] [PubMed]
- Mikheev, I.V.; Byvsheva, S.M.; Sozarukova, M.M.; Kottsov, S.Y.; Proskurnina, E.V.; Proskurnin, M.A. High-Throughput Preparation of Uncontaminated Graphene-Oxide Aqueous Dispersions with Antioxidant Properties by Semi-Automated Diffusion Dialysis. Nanomaterials 2022, 12, 4159. [Google Scholar] [CrossRef]
- Matsumoto, I.; Sekiya, R.; Haino, T. A protocol for size separation of nanographenes. RSC Adv. 2019, 9, 33843–33846. [Google Scholar] [CrossRef]
- Bonanni, A.; Ambrosi, A.; Chua, C.K.; Pumera, M. Oxidation Debris in Graphene Oxide Is Responsible for Its Inherent Electroactivity. ACS Nano 2014, 8, 4197–4204. [Google Scholar] [CrossRef] [PubMed]
- Dovichi, N.J.; Bialkowski, S.E. Thermo-Optical Spectrophotometries in Analytical Chemistry. Crit. Rev. Anal. Chem. 1987, 17, 357–423. [Google Scholar] [CrossRef]
- Constantino, R.; Lenzi, G.G.; Franco, M.G.; Lenzi, E.K.; Bento, A.C.; Astrath, N.G.C.; Malacarne, L.C.; Baesso, M.L. Thermal Lens Temperature Scanning technique for evaluation of oxidative stability and time of transesterification during biodiesel synthesis. Fuel 2017, 202, 78–84. [Google Scholar] [CrossRef]
- Deus, W.B.; Ventura, M.; Silva, J.R.; Andrade, L.H.C.; Catunda, T.; Lima, S.M. Monitoring of the ester production by near-near infrared thermal lens spectroscopy. Fuel 2019, 253, 1090–1096. [Google Scholar] [CrossRef]
- Muhmood, T.; Xia, M.; Lei, W.; Wang, F.; Mahmood, A. Fe-ZrO2 imbedded graphene like carbon nitride for acarbose (ACB) photo-degradation intermediate study. Adv. Powder Technol. 2018, 29, 3233–3240. [Google Scholar] [CrossRef]
- Dimiev, A.M.; Alemany, L.B.; Tour, J.M. Graphene Oxide. Origin of Acidity, Its Instability in Water, and a New Dynamic Structural Model. ACS Nano 2013, 7, 576–588. [Google Scholar] [CrossRef]
- Ratova, D.-M.V.; Mikheev, I.V.; Chermashentsev, G.R.; Maslakov, K.I.; Kottsov, S.Y.; Stolbov, D.N.; Maksimov, S.V.; Sozarukova, M.M.; Proskurnina, E.V.; Proskurnin, M.A. Green and Sustainable Ultrasound-Assisted Anodic Electrochemical Preparation of Graphene Oxide Dispersions and Their Antioxidant Properties. Molecules 2023, 28, 3238. [Google Scholar] [CrossRef] [PubMed]
- Angayarkanni, S.A.; Philip, J. Review on thermal properties of nanofluids: Recent developments. Adv. Colloid Interface Sci. 2015, 225, 146–176. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.-P.; Wang, B.-X.; Peng, X.-F.; Du, X.-Z.; Yang, Y.-P. On the Specific Heat Capacity of CuO Nanofluid. Adv. Mech. Eng. 2015, 2, 172085. [Google Scholar] [CrossRef]
- Ganvir, R.B.; Walke, P.V.; Kriplani, V.M. Heat transfer characteristics in nanofluid—A review. Renew. Sustain. Energy Rev. 2017, 75, 451–460. [Google Scholar] [CrossRef]
- Simpson, S.; Schelfhout, A.; Golden, C.; Vafaei, S. Nanofluid Thermal Conductivity and Effective Parameters. Appl. Sci. 2019, 9, 87. [Google Scholar] [CrossRef]
- Maldovan, M. Sound and heat revolutions in phononics. Nature 2013, 503, 209–217. [Google Scholar] [CrossRef]
- Li, A.; Zhang, C.; Zhang, Y.-F. Thermal Conductivity of Graphene-Polymer Composites: Mechanisms, Properties, and Applications. Polymers 2017, 9, 437. [Google Scholar] [CrossRef]
- de Freitas Cabral, A.J.; Furtado, C.A.; Fantini, C.; Alcantara, P., Jr. Thermal Diffusivity of Multi-Walled Carbon Nanotubes Dispersed in Oleic Acid. J. Nano Res. 2012, 21, 125–130. [Google Scholar] [CrossRef]
- Leal, N.N.S.; Brandão-Silva, A.C.; Fantini, C.; Hickmann, J.M.; Fonseca, E.J.S.; Alencar, M.A.R.C. Thermo-optical response of colloidal metallic and semiconducting single-walled carbon nanotubes. Opt. Laser Technol. 2019, 116, 315–321. [Google Scholar] [CrossRef]
- Usoltseva, L.O.; Volkov, D.S.; Avramenko, N.V.; Korobov, M.V.; Proskurnin, M.A. Nanodiamond aqueous dispersions as potential nanofluids: The determination of properties by thermal lensing and other techniques. Nanosyst. Phys. Chem. Math. 2018, 9, 17–20. [Google Scholar] [CrossRef]
- Esfahani, M.R.; Languri, E.M.; Nunna, M.R. Effect of particle size and viscosity on thermal conductivity enhancement of graphene oxide nanofluid. Int. Commun. Heat Mass Transf. 2016, 76, 308–315. [Google Scholar] [CrossRef]
- Hajjar, Z.; Rashidi, A.M.; Ghozatloo, A. Enhanced thermal conductivities of graphene oxide nanofluids. Int. Commun. Heat Mass Transf. 2014, 57, 128–131. [Google Scholar] [CrossRef]
- Mei, X.; Sha, X.; Jing, D.; Ma, L. Thermal Conductivity and Rheology of Graphene Oxide Nanofluids and a Modified Predication Model. Appl. Sci. 2022, 12, 3567. [Google Scholar] [CrossRef]
- Cham Saard, W.; Fawcett, D.; Fung, C.C.; Chapman, P.; Rattan, S.; Poinern, G.E.J. Synthesis, characterisation and thermo-physical properties of highly stable graphene oxide-based aqueous nanofluids for potential low-temperature direct absorption solar applications. Sci. Rep. 2021, 11, 16549. [Google Scholar] [CrossRef] [PubMed]
- Mathew, R.M.; Zachariah, E.S.; Jose, J.; Thomas, T.; John, J.; Titus, T.; Unni, N.G.; Mathew, S.; Mujeeb, A.; Thomas, V. Synthesis, characterization and evaluation of tunable thermal diffusivity of phosphorus-doped carbon nanodot. Appl. Phys. A 2020, 126, 828. [Google Scholar] [CrossRef]
- Joseph, M.; Anugop, B.; Vijesh, K.R.; Balan, V.; Nampoori, V.P.N.; Kailasnath, M. Morphology and concentration-dependent thermal diffusivity of biofunctionalized zinc oxide nanostructures using dual-beam thermal lens technique. Mater. Lett. 2022, 323, 132599. [Google Scholar] [CrossRef]
- Pradeep Kumar, V.; Radhakrishnan, P.; Mujeeb, A. Thermal Diffusivity Measurement of PVP Capped Nickel Oxide Nanoparticles by Dual Beam Thermal Lens Technique. In Proceedings of the ICOL-2019, Dehradun, India, 19–22 October 2019; Springer: Singapore, 2021; pp. 841–843. [Google Scholar]
- Shahriari, E.; Varnamkhasti, M.G.; Zamiri, R. Characterization of thermal diffusivity and optical properties of Ag nanoparticles. Optik 2015, 126, 2104–2107. [Google Scholar] [CrossRef]
- Zamiri, R.; Zakaria, A.; Shahriari, E.; Mat Yunus, W.M.; Naghavi, K.; Saion, E.; Mahdi, M.A.; Husin, M. Dependence of thermal diffusivity on particle size in Au nano-fluid. Optoelectron. Adv. Mater. Rapid. Commun. 2010, 4, 1551–1553. [Google Scholar]
- Philip, J.; Shima, P.D. Thermal properties of nanofluids. Adv. Colloid Interface Sci. 2012, 183–184, 30–45. [Google Scholar] [CrossRef]
- Shen, J.; Lowe, R.D.; Snook, R.D. A model for cw laser induced mode-mismatched dual-beam thermal lens spectrometry. Chem. Phys. 1992, 165, 385–396. [Google Scholar] [CrossRef]
- Rodriguez, L.G.; Iza, P.; Paz, J.L. Study of dependence between thermal diffusivity and sample concentration measured by means of frequency-resolved thermal lens experiment. J. Nonlinear Opt. Phys. Mater. 2016, 25, 1650022. [Google Scholar] [CrossRef]
- Mu, H.; Yu, W.; Yuan, J.; Lin, S.; Zhang, G. Interface and surface engineering of black phosphorus: A review for optoelectronic and photonic applications. Mater. Futures 2022, 1, 012301. [Google Scholar] [CrossRef]
- Gómez-Mancebo, M.B.; Fernández-Martínez, R.; Ruiz-Perona, A.; Rubio, V.; Bastante, P.; García-Pérez, F.; Borlaf, F.; Sánchez, M.; Hamada, A.; Velasco, A.; et al. Comparison of Thermal and Laser-Reduced Graphene Oxide Production for Energy Storage Applications. Nanomaterials 2023, 13, 1391. [Google Scholar] [CrossRef] [PubMed]
- Gallegos-Perez, W.R.; Reynosa-Martinez, A.C.; Soto-Ortiz, C.; Angelica Alvarez-Lemus, M.; Barroso-Flores, J.; Garcia Montalvo, V.; Lopez-Honorato, E. Effect of UV radiation on the structure of graphene oxide in water and its impact on cytotoxicity and As(III) adsorption. Chemosphere 2020, 249, 126160. [Google Scholar] [CrossRef] [PubMed]
- Konkena, B.; Vasudevan, S. Engineering a Water-Dispersible, Conducting, Photoreduced Graphene Oxide. J. Phys. Chem. C 2015, 119, 6356–6362. [Google Scholar] [CrossRef]
- Huang, L.; Liu, Y.; Ji, L.-C.; Xie, Y.-Q.; Wang, T.; Shi, W.-Z. Pulsed laser assisted reduction of graphene oxide. Carbon 2011, 49, 2431–2436. [Google Scholar] [CrossRef]
- de Lima, B.S.; Bernardi, M.I.B.; Mastelaro, V.R. Wavelength effect of ns-pulsed radiation on the reduction of graphene oxide. Appl. Surf. Sci. 2020, 506, 144808. [Google Scholar] [CrossRef]
- Bai, H.; Jiang, W.; Kotchey, G.P.; Saidi, W.A.; Bythell, B.J.; Jarvis, J.M.; Marshall, A.G.; Robinson, R.A.; Star, A. Insight into the Mechanism of Graphene Oxide Degradation via the Photo-Fenton Reaction. J. Phys. Chem. C Nanomater. Interfaces 2014, 118, 10519–10529. [Google Scholar] [CrossRef]
- Chen, J.; Li, L. Thermal Conductivity of Graphene Oxide: A Molecular Dynamics Study. JETP Lett. 2020, 112, 117–121. [Google Scholar] [CrossRef]
- Chen, J.; Li, L. Effect of oxidation degree on the thermal properties of graphene oxide. J. Mater. Res. Technol. 2020, 9, 13740–13748. [Google Scholar] [CrossRef]
- Pattarith, K.; Areerob, Y. Fabrication of Ag nanoparticles adhered on RGO based on both electrodes in dye-sensitized solar cells (DSSCs). Renew. Wind Water Solar 2020, 7, 1. [Google Scholar] [CrossRef]
- Faria, A.F.; Perreault, F.; Elimelech, M. Elucidating the Role of Oxidative Debris in the Antimicrobial Properties of Graphene Oxide. ACS Appl. Nano Mater. 2018, 1, 1164–1174. [Google Scholar] [CrossRef]
- Valmonte, Z.; Baker, Z.; Loor, J.; Sarkar, A. Concurrent Reduction and Stabilization of Graphene Oxide Dispersion by Silk-Inspired Polymer. ACS Appl. Polym. Mater. 2023, 5, 7. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khabibullin, V.R.; Ratova, D.-M.V.; Stolbov, D.N.; Mikheev, I.V.; Proskurnin, M.A. The Thermophysical and Physicochemical Properties of the Aqueous Dispersion of Graphene Oxide Dual-Beam Thermal Lens Spectrometry. Nanomaterials 2023, 13, 2126. https://doi.org/10.3390/nano13142126
Khabibullin VR, Ratova D-MV, Stolbov DN, Mikheev IV, Proskurnin MA. The Thermophysical and Physicochemical Properties of the Aqueous Dispersion of Graphene Oxide Dual-Beam Thermal Lens Spectrometry. Nanomaterials. 2023; 13(14):2126. https://doi.org/10.3390/nano13142126
Chicago/Turabian StyleKhabibullin, Vladislav R., Daria-Maria V. Ratova, Dmitrii N. Stolbov, Ivan V. Mikheev, and Mikhail A. Proskurnin. 2023. "The Thermophysical and Physicochemical Properties of the Aqueous Dispersion of Graphene Oxide Dual-Beam Thermal Lens Spectrometry" Nanomaterials 13, no. 14: 2126. https://doi.org/10.3390/nano13142126
APA StyleKhabibullin, V. R., Ratova, D.-M. V., Stolbov, D. N., Mikheev, I. V., & Proskurnin, M. A. (2023). The Thermophysical and Physicochemical Properties of the Aqueous Dispersion of Graphene Oxide Dual-Beam Thermal Lens Spectrometry. Nanomaterials, 13(14), 2126. https://doi.org/10.3390/nano13142126





