Iridium-Based Nanohybrids: Synthesis, Characterization, Optical Limiting, and Nonlinear Optical Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Ir-Based NP Solutions
2.3. Materials Characterization
2.4. Z-Scan Measurements
2.5. Optical Limiting
3. Results and Discussion
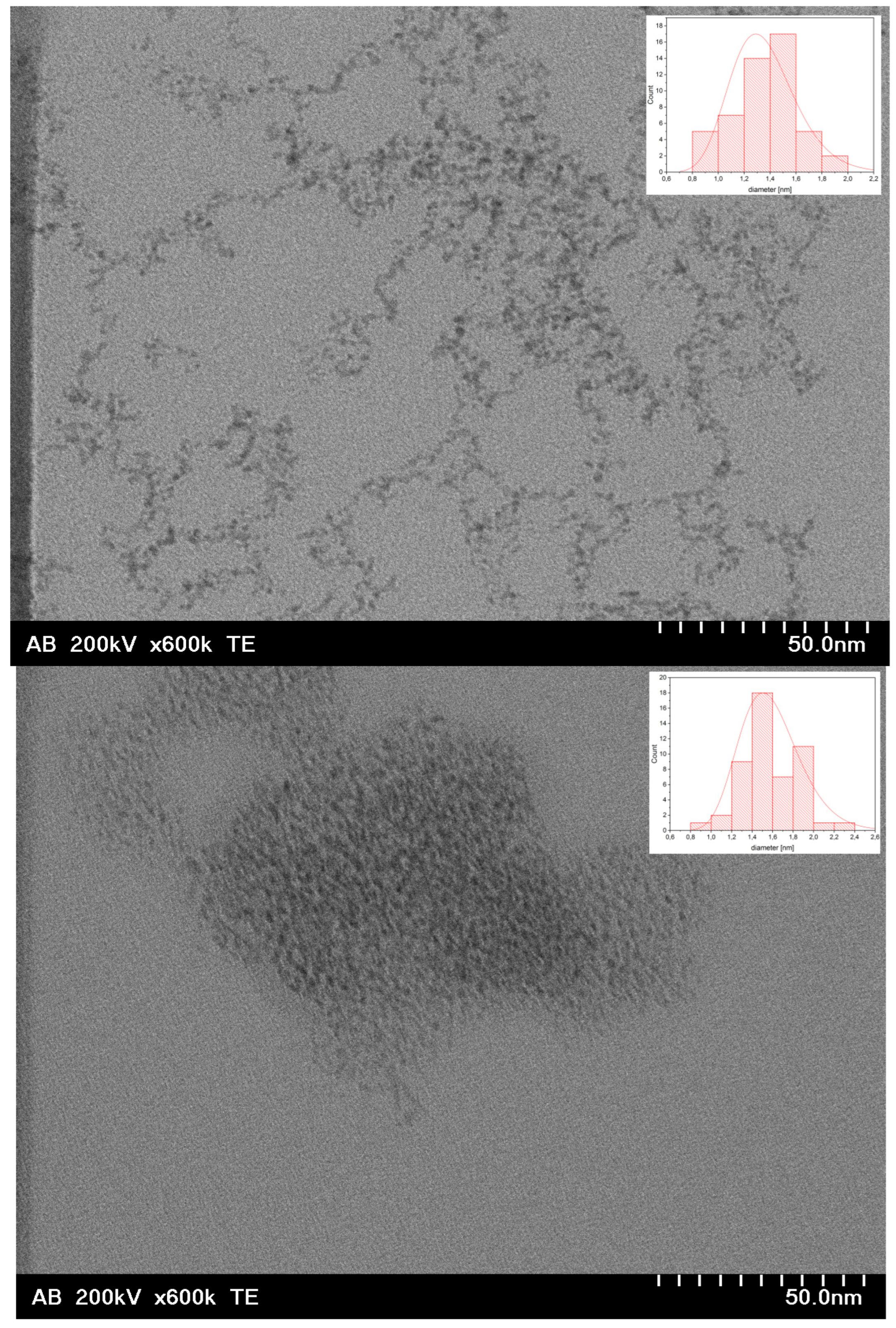
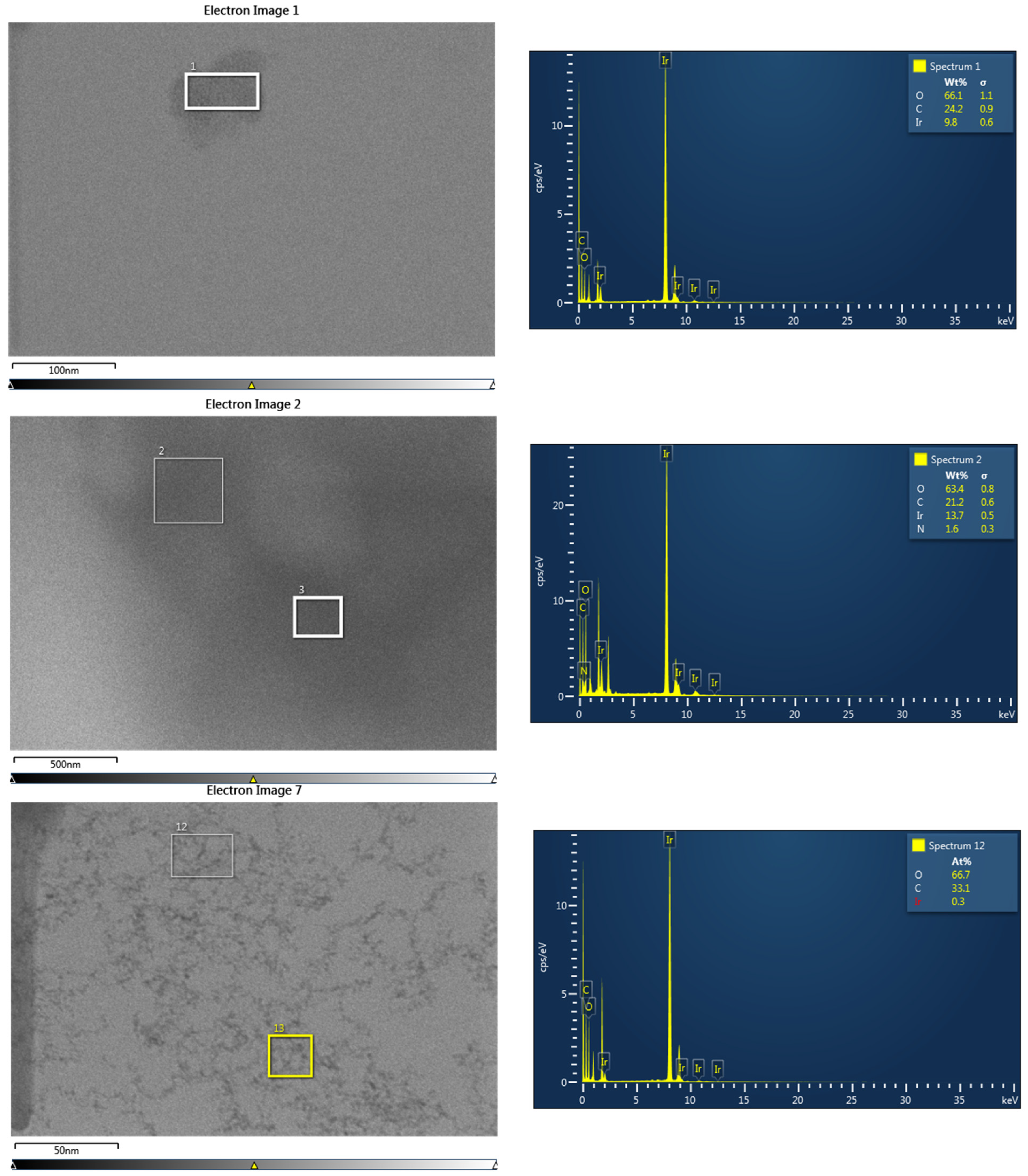
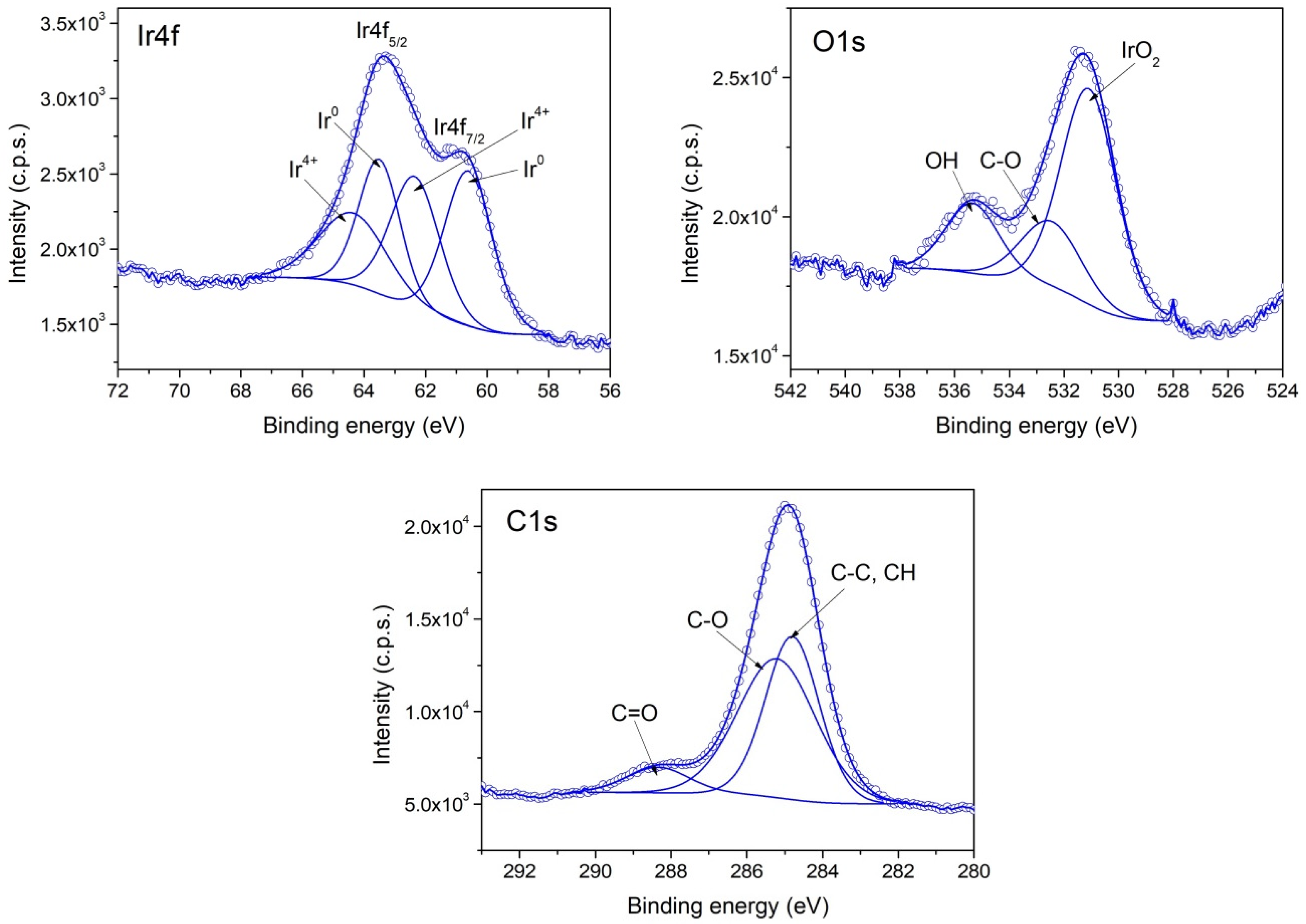
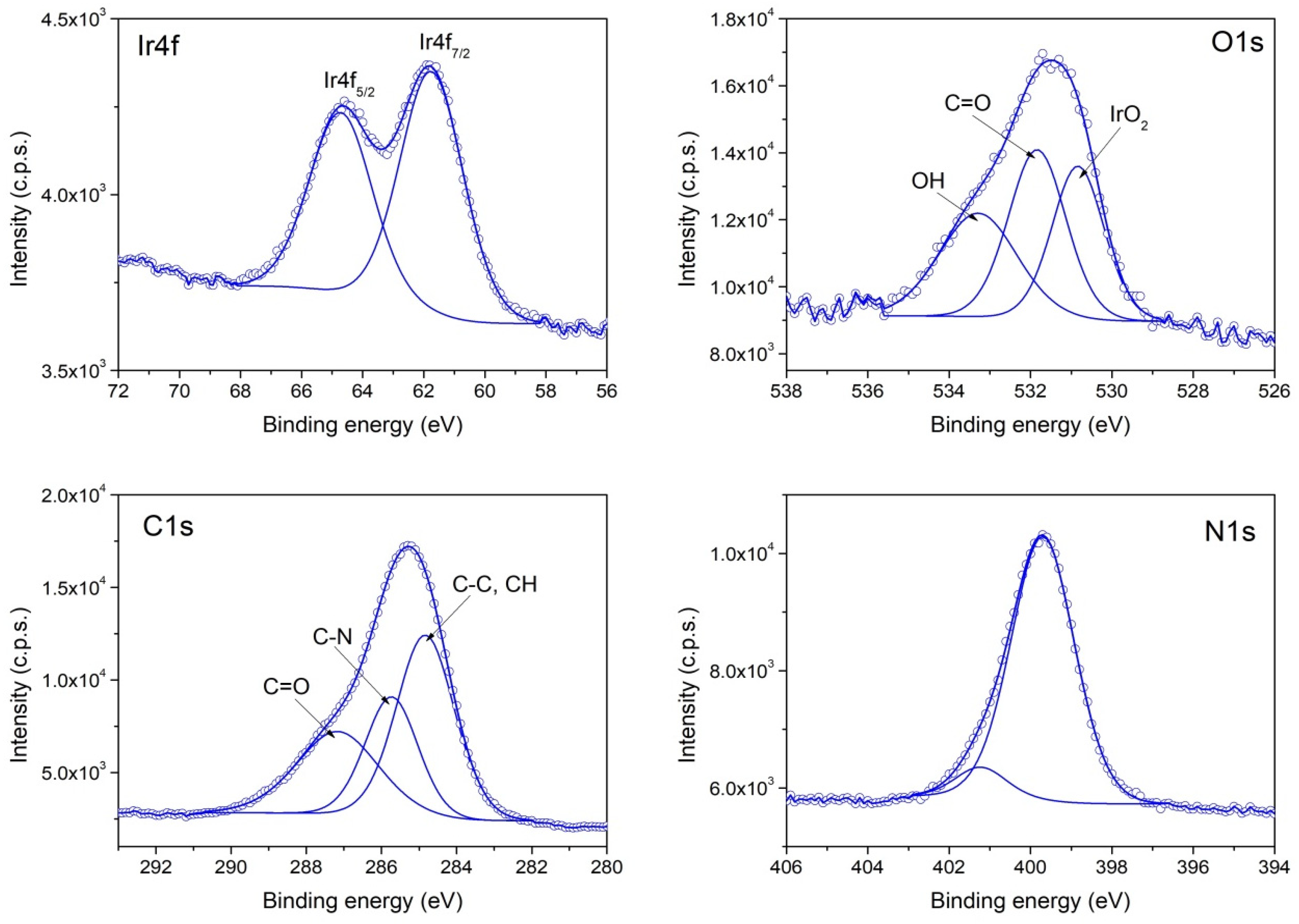
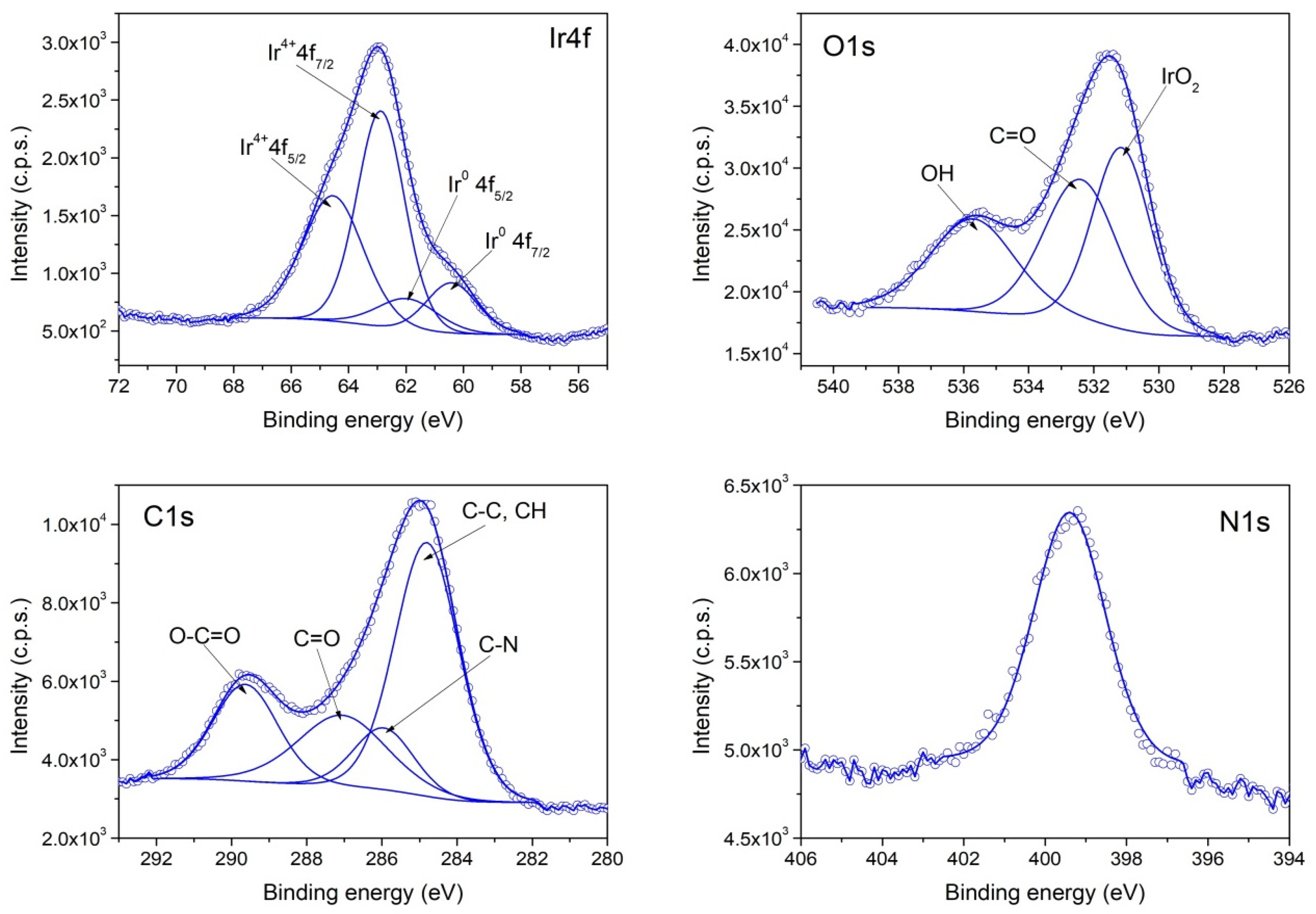
3.1. Chemical Composition and Morphology
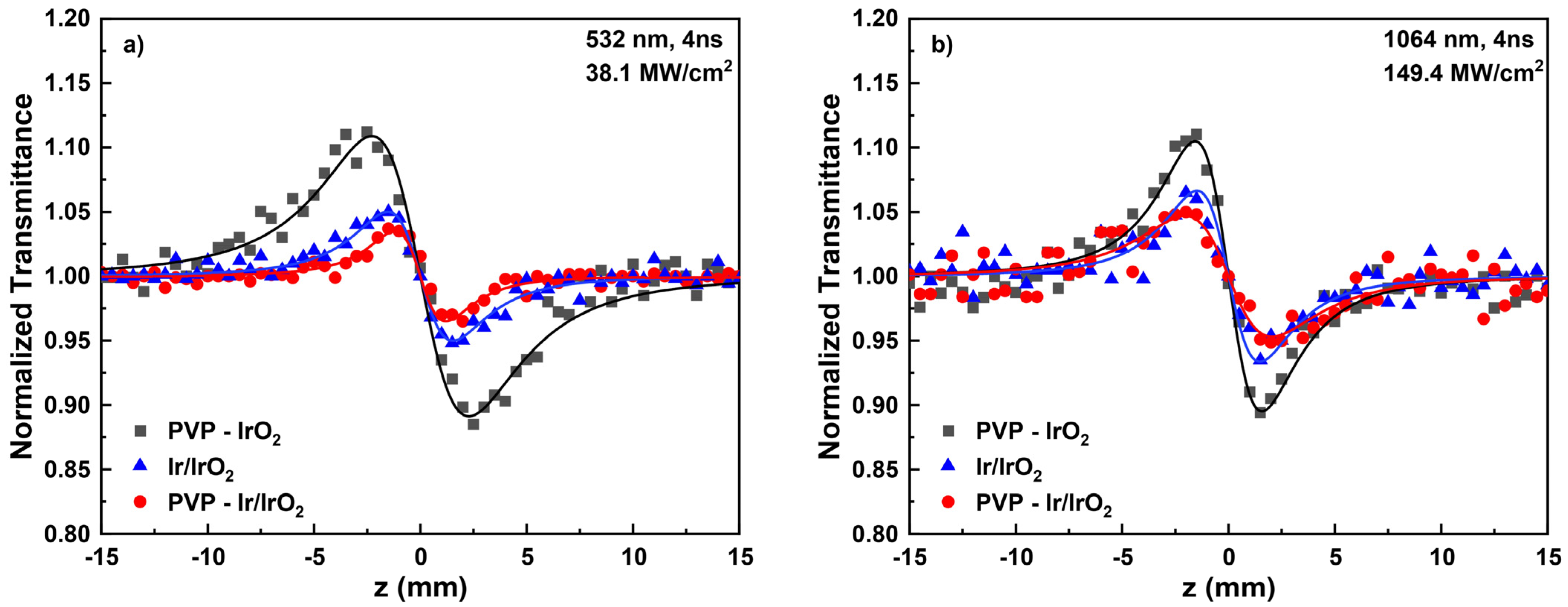
3.2. NLO Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rodrigues, T.S.; Da Silva, A.G.M.; Camargo, P.H.C. Nanocatalysis by Noble Metal Nanoparticles: Controlled Synthesis for the Optimization and Understanding of Activities. J. Mater. Chem. A 2019, 7, 5857–5874. [Google Scholar] [CrossRef] [Green Version]
- Jin, R. The Impacts of Nanotechnology on Catalysis by Precious Metal Nanoparticles. Nanotechnol. Rev. 2012, 1, 31–56. [Google Scholar] [CrossRef]
- Krajczewski, J.; Ambroziak, R.; Kudelski, A. Formation and Selected Catalytic Properties of Ruthenium, Rhodium, Osmium and Iridium Nanoparticles. RSC Adv. 2022, 12, 2123–2144. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, T.; Yang, J.-H. Precious Metal Nanomaterial-Modified Electrochemical Sensors for Nitrite Detection. Ionics 2022, 28, 2041–2064. [Google Scholar] [CrossRef]
- Doria, G.; Conde, J.; Veigas, B.; Giestas, L.; Almeida, C.; Assunção, M.; Rosa, J.; Baptista, P.V. Noble Metal Nanoparticles for Biosensing Applications. Sensors 2012, 12, 1657–1687. [Google Scholar] [CrossRef]
- Arvizo, R.R.; Bhattacharyya, S.; Kudgus, R.A.; Giri, K.; Bhattacharya, R.; Mukherjee, P. Intrinsic Therapeutic Applications of Noble Metal Nanoparticles: Past, Present and Future. Chem. Soc. Rev. 2012, 41, 2943. [Google Scholar] [CrossRef] [Green Version]
- Azharuddin, M.; Zhu, G.H.; Das, D.; Ozgur, E.; Uzun, L.; Turner, A.P.F.; Patra, H.K. A Repertoire of Biomedical Applications of Noble Metal Nanoparticles. Chem. Commun. 2019, 55, 6964–6996. [Google Scholar] [CrossRef]
- Li, N.; Ma, J.; Zhang, Y.; Zhang, L.; Jiao, T. Recent Developments in Functional Nanocomposite Photocatalysts for Wastewater Treatment: A Review. Adv. Sustain. Syst. 2022, 6, 2200106. [Google Scholar] [CrossRef]
- Jeong, S.-H.; Choi, H.; Kim, J.Y.; Lee, T.-W. Silver-Based Nanoparticles for Surface Plasmon Resonance in Organic Optoelectronics. Part. Part. Syst. Charact. 2015, 32, 164–175. [Google Scholar] [CrossRef]
- Wei, W.; Bai, F.; Fan, H. Oriented Gold Nanorod Arrays: Self-Assembly and Optoelectronic Applications. Angew. Chem. Int. Ed. 2019, 58, 11956–11966. [Google Scholar] [CrossRef]
- Quinson, J. Iridium and IrOx Nanoparticles: An Overview and Review of Syntheses and Applications. Adv. Colloid Interface Sci. 2022, 303, 102643. [Google Scholar] [CrossRef]
- Handwerk, D.R.; Shipman, P.D.; Whitehead, C.B.; Özkar, S.; Finke, R.G. Particle Size Distributions via Mechanism-Enabled Population Balance Modeling. J. Phys. Chem. C 2020, 124, 4852–4880. [Google Scholar] [CrossRef]
- Özkar, S.; Finke, R.G. Dust Effects on Nucleation Kinetics and Nanoparticle Product Size Distributions: Illustrative Case Study of a Prototype Ir(0)n Transition-Metal Nanoparticle Formation System. Langmuir 2017, 33, 6550–6562. [Google Scholar] [CrossRef] [Green Version]
- Cui, M.-L.; Chen, Y.-S.; Xie, Q.-F.; Yang, D.-P.; Han, M.-Y. Synthesis, Properties and Applications of Noble Metal Iridium Nanomaterials. Coord. Chem. Rev. 2019, 387, 450–462. [Google Scholar] [CrossRef]
- Goel, A.; Rani, N. Effect of PVP, PVA and POLE Surfactants on the Size of Iridium Nanoparticles. Open J. Inorg. Chem. 2012, 2, 67–73. [Google Scholar] [CrossRef] [Green Version]
- Mévellec, V.; Roucoux, A.; Ramirez, E.; Philippot, K.; Chaudret, B. Surfactant-Stabilized Aqueous Iridium(0) Colloidal Suspension: An Efficient Reusable Catalyst for Hydrogenation of Arenes in Biphasic Media. Adv. Synth. Catal. 2004, 346, 72–76. [Google Scholar] [CrossRef]
- Bizzotto, F.; Quinson, J.; Zana, A.; Kirkensgaard, J.J.K.; Dworzak, A.; Oezaslan, M.; Arenz, M. Ir Nanoparticles with Ultrahigh Dispersion as Oxygen Evolution Reaction (OER) Catalysts: Synthesis and Activity Benchmarking. Catal. Sci. Technol. 2019, 9, 6345–6356. [Google Scholar] [CrossRef] [Green Version]
- Quinson, J.; Kacenauskaite, L.; Schröder, J.; Simonsen, S.B.; Theil Kuhn, L.; Vosch, T.; Arenz, M. UV-Induced Syntheses of Surfactant-Free Precious Metal Nanoparticles in Alkaline Methanol and Ethanol. Nanoscale Adv. 2020, 2, 2288–2292. [Google Scholar] [CrossRef]
- Singh, S.K.; Xu, Q. Bimetallic Nickel-Iridium Nanocatalysts for Hydrogen Generation by Decomposition of Hydrous Hydrazine. Chem. Commun. 2010, 46, 6545. [Google Scholar] [CrossRef]
- Chen, Z.; Duan, X.; Wei, W.; Wang, S.; Ni, B.-J. Iridium-Based Nanomaterials for Electrochemical Water Splitting. Nano Energy 2020, 78, 105270. [Google Scholar] [CrossRef]
- Schick, L.; Sanchis, R.; González-Alfaro, V.; Agouram, S.; López, J.M.; Torrente-Murciano, L.; García, T.; Solsona, B. Size-Activity Relationship of Iridium Particles Supported on Silica for the Total Oxidation of Volatile Organic Compounds (VOCs). J. Chem. Eng. 2019, 366, 100–111. [Google Scholar] [CrossRef]
- Kurbanoglu, S.; Rivas, L.; Ozkan, S.A.; Merkoçi, A. Electrochemically Reduced Graphene and Iridium Oxide Nanoparticles for Inhibition-Based Angiotensin-Converting Enzyme Inhibitor Detection. Biosens. Bioelectron. 2017, 88, 122–129. [Google Scholar] [CrossRef]
- Zhen, W.; Liu, Y.; Lin, L.; Bai, J.; Jia, X.; Tian, H.; Jiang, X. BSA-IrO2: Catalase-like Nanoparticles with High Photothermal Conversion Efficiency and a High X-Ray Absorption Coefficient for Anti-Inflammation and Antitumor Theranostics. Angew. Chem. Int. Ed. 2018, 57, 10309–10313. [Google Scholar] [CrossRef]
- Ito, S.; Abe, Y.; Kawamura, M.; Kim, K.H. Electrochromic Properties of Iridium Oxide Thin Films Prepared by Reactive Sputtering in O2 or H2O Atmosphere. J. Vac. Sci. Technol. B Nanotechnol. Microelectron. 2015, 33, 041204. [Google Scholar] [CrossRef]
- Hari, M.; Mathew, S.; Nithyaja, B.; Joseph, S.A.; Nampoori, V.P.N.; Radhakrishnan, P. Saturable and Reverse Saturable Absorption in Aqueous Silver Nanoparticles at Off-Resonant Wavelength. Opt. Quant. Electron. 2012, 43, 49–58. [Google Scholar] [CrossRef]
- Papagiannouli, I.; Aloukos, P.; Rioux, D.; Meunier, M.; Couris, S. Effect of the Composition on the Nonlinear Optical Response of Aux Ag1–x Nano-Alloys. J. Phys. Chem. C 2015, 119, 6861–6872. [Google Scholar] [CrossRef]
- Chehrghani, A.; Torkamany, M.J. Nonlinear Optical Properties of Laser Synthesized Pt Nanoparticles: Saturable and Reverse Saturable Absorption. Laser Phys. 2014, 24, 015901. [Google Scholar] [CrossRef]
- Papagiannouli, I.; Potamianos, D.; Krasia-Christoforou, T.; Couris, S. Third-Order Optical Nonlinearities of PVP/Pd Nanohybrids. Opt. Mater. 2017, 72, 226–232. [Google Scholar] [CrossRef]
- Alani, I.A.M.; Ahmad, B.A.; Ahmed, M.H.M.; Latiff, A.A.; Al-Masoodi, A.H.H.; Lokman, M.Q.; Harun, S.W. Nanosecond Mode-Locked Erbium Doped Fiber Laser Based on Zinc Oxide Thin Film Saturable Absorber. Indian J. Phys. 2019, 93, 93–99. [Google Scholar] [CrossRef]
- Wang, J.; Xie, L.; Wang, Y.; Lan, Y.; Wu, P.; Lv, J.; Zhang, G.; Miao, Z.; Cheng, G. High-Damage Vanadium Pentoxide Film Saturable Absorber for Sub-Nanosecond Nd:YAG Lasers. Infrared Phys. Technol. 2023, 129, 104580. [Google Scholar] [CrossRef]
- Chatzikyriakos, G.; Iliopoulos, K.; Bakandritsos, A.; Couris, S. Nonlinear Optical Properties of Aqueous Dispersions of Ferromagnetic γ-Fe2O3 Nanoparticles. Chem. Phys. Lett. 2010, 493, 314–318. [Google Scholar] [CrossRef]
- Stavrou, M.; Papaparaskeva, G.; Stathis, A.; Stylianou, A.; Turcu, R.; Krasia-Christoforou, T.; Couris, S. Synthesis, Characterization and Nonlinear Optical Response of Polyelectrolyte-Stabilized Copper Hydroxide and Copper Oxide Colloidal Nanohybrids. Opt. Mater. 2021, 119, 111329. [Google Scholar] [CrossRef]
- Htwe, Z.M.; Zhang, Y.-D.; Yao, C.-B.; Li, H.; Li, H.-Y.; Yuan, P. Investigation of Third Order Nonlinear Optical Properties of Undoped and Indium Doped Zinc Oxide (InZnO) Thin Films by Nanosecond Z-Scan Technique. Opt. Mater. 2016, 52, 6–13. [Google Scholar] [CrossRef]
- Krishna Mariserla, B.M.; Alee, K.S.; Kasthuri, S.; Gawas, P.; Rao, D.N.; Nutalapati, V. Broadband Optical Power Limiting with the Decoration of TiO2 Nanoparticles on Graphene Oxide. Opt. Mater. 2020, 109, 110366. [Google Scholar] [CrossRef]
- Menon, P.S.; Kunjumon, J.; Bansal, M.; Nair, S.S.; Beryl, C.; Vinitha, G.; Maity, T.; Abraham, P.M.; Sajan, D.; Philip, R. Role of Surface Defects in the Third Order Nonlinear Optical Properties of Pristine NiO and Cr Doped NiO Nanostructures. Ceram. Int. 2023, 49, 5815–5827. [Google Scholar] [CrossRef]
- Muller, O.; Gibot, P. Optical Limiting Properties of Templated Cr2O3 and WO3 Nanoparticles. Opt. Mater. 2019, 95, 109220. [Google Scholar] [CrossRef]
- Molli, M.; Pradhan, P.; Dutta, D.; Jayaraman, A.; Bhat Kademane, A.; Muthukumar, V.S.; Kamisetti, V.; Philip, R. Study of Nonlinear Optical Absorption Properties of Sb2Se3 Nanoparticles in the Nanosecond and Femtosecond Excitation Regime. Appl. Phys. A 2016, 122, 549. [Google Scholar] [CrossRef]
- Jia, W.; Douglas, E.P.; Guo, F.; Sun, W. Optical Limiting of Semiconductor Nanoparticles for Nanosecond Laser Pulses. Appl. Phys. Lett. 2004, 85, 6326–6328. [Google Scholar] [CrossRef]
- Peng, Q.; Juzeniene, A.; Chen, J.; Svaasand, L.O.; Warloe, T.; Giercksky, K.-E.; Moan, J. Lasers in Medicine. Rep. Prog. Phys. 2008, 71, 056701. [Google Scholar] [CrossRef]
- Dutta Majumdar, J.; Manna, I. Laser Material Processing. Int. Mater. Rev. 2011, 56, 341–388. [Google Scholar] [CrossRef]
- Coffey, V. High-Energy Lasers: New Advances in Defense Applications. Opt. Photonics News 2014, 25, 28–35. [Google Scholar] [CrossRef]
- Koch, T.L.; Koren, U. Semiconductor Lasers for Coherent Optical Fiber Communications. J. Light. Technol. 1990, 8, 274–293. [Google Scholar] [CrossRef]
- Gao, Y.; Kong, D. Nonlinear Optical Response of Noble Metal Nanoparticles. In Laser Technology and its Applications; Ma, Y., Ed.; IntechOpen: London, UK, 2019; ISBN 978-1-78984-917-2. [Google Scholar]
- Olesiak-Banska, J.; Waszkielewicz, M.; Obstarczyk, P.; Samoc, M. Two-Photon Absorption and Photoluminescence of Colloidal Gold Nanoparticles and Nanoclusters. Chem. Soc. Rev. 2019, 48, 4087–4117. [Google Scholar] [CrossRef]
- Abrinaei, F.; Molahasani, N. Effects of Mn Doping on the Structural, Linear, and Nonlinear Optical Properties of ZnO Nanoparticles. J. Opt. Soc. Am. B 2018, 35, 2015–2022. [Google Scholar] [CrossRef]
- Koczkur, K.M.; Mourdikoudis, S.; Polavarapu, L.; Skrabalak, S.E. Polyvinylpyrrolidone (PVP) in Nanoparticle Synthesis. Dalton Trans. 2015, 44, 17883–17905. [Google Scholar] [CrossRef] [Green Version]
- Sheik-bahae, M.; Said, A.A.; Van Stryland, E.W. High-Sensitivity, Single-Beam n2 Measurements. Opt. Lett. 1989, 14, 955–957. [Google Scholar] [CrossRef] [Green Version]
- Swierk, J.R.; Méndez-Hernández, D.D.; McCool, N.S.; Liddell, P.; Terazono, Y.; Pahk, I.; Tomlin, J.J.; Oster, N.V.; Moore, T.A.; Moore, A.L.; et al. Metal-Free Organic Sensitizers for Use in Water-Splitting Dye-Sensitized Photoelectrochemical Cells. Proc. Natl. Acad. Sci. USA 2015, 112, 1681–1686. [Google Scholar] [CrossRef]
- Brown, A.L.; Winter, H.; Goforth, A.M.; Sahay, G.; Sun, C. Facile Synthesis of Ligand-Free Iridium Nanoparticles and Their In Vitro Biocompatibility. Nanoscale Res. Lett. 2018, 13, 208. [Google Scholar] [CrossRef] [Green Version]
- Finkelstein-Shapiro, D.; Fournier, M.; Méndez-Hernández, D.D.; Guo, C.; Calatayud, M.; Moore, T.A.; Moore, A.L.; Gust, D.; Yarger, J.L. Understanding Iridium Oxide Nanoparticle Surface Sites by Their Interaction with Catechol. Phys. Chem. Chem. Phys. 2017, 19, 16151–16158. [Google Scholar] [CrossRef]
- Böhm, D.; Beetz, M.; Schuster, M.; Peters, K.; Hufnagel, A.G.; Döblinger, M.; Böller, B.; Bein, T.; Fattakhova-Rohlfing, D. Efficient OER Catalyst with Low Ir Volume Density Obtained by Homogeneous Deposition of Iridium Oxide Nanoparticles on Macroporous Antimony-Doped Tin Oxide Support. Adv. Funct. Mater. 2020, 30, 1906670. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Kim, J.Y.; Lee, S.Y.; Hong, J.A.; Kim, N.; Baik, J.; Hwang, Y.J. Comparative Study of Catalytic Activities among Transition Metal-Doped IrO2 Nanoparticles. Sci. Rep. 2018, 8, 16777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christodoulides, D.N.; Khoo, I.C.; Salamo, G.J.; Stegeman, G.I.; Van Stryland, E.W. Nonlinear Refraction and Absorption: Mechanisms and Magnitudes. Adv. Opt. Photonics 2010, 2, 60–200. [Google Scholar] [CrossRef]
- Sutherland, R.L.; McLean, D.G.; Kirkpatrick, S. Handbook of Nonlinear Optics; Marcel Dekker, Inc.: New York, NY, USA, 2003. [Google Scholar]
- Said, A.A.; Sheik-Bahae, M.; Hagan, D.J.; Wei, T.H.; Wang, J.; Young, J.; Van Stryland, E.W. Determination of Bound-Electronic and Free-Carrier Nonlinearities in ZnSe, GaAs, CdTe, and ZnTe. J. Opt. Soc. Am. B 1992, 9, 405–414. [Google Scholar] [CrossRef]
- Tauc, J. Optical Properties and Electronic Structure of Amorphous Ge and Si. Mater. Res. Bull. 1968, 3, 37–46. [Google Scholar] [CrossRef]





| Sample | Excitation Conditions | α0 (cm−1) | β × 10−11 (m/W) | Imχ(3) × 10−13 (esu) | Imχ(3)/α0 × 10−13 (esu cm) | αs (%) | Isat (MW/cm2) | Ref. |
|---|---|---|---|---|---|---|---|---|
| PVP-IrO2 | 4 ns, | 1.77 | −115.6 | −53.0 | −30.6 | 4 | 80 | this work |
| 532 nm | ||||||||
| Ag | 7 ns, | ~9.2 | −2562 | −1178.0 | 128 | N/A | 32.1 | [25] |
| 532 nm | ||||||||
| Au | 4 ns, | ~0.9 | −17.6 | −8.3 | 9.2 | N/A | 230 | [26] |
| 532 nm | ||||||||
| Pt | 10 ns, | 0.91 | −3 | −8.53 | 9.4 | N/A | 18 | [27] |
| 532 nm | ||||||||
| Pd | 4 ns, 532 nm 4 ns, 1064 nm | 7.5 | −54 | −32.5 | 4.3 | N/A | N/A | [28] |
| 6.8 | −5.9 | −6.7 | 0.99 | N/A | N/A | |||
| ZnO | 400 ns, | N/A | N/A | N/A | N/A | 5 | 60 | [29] |
| 1560 nm | ||||||||
| V2O5 | 0.7 ns, | N/A | N/A | N/A | N/A | 21.8 | 0.33 | [30] |
| 1064 nm | ||||||||
| γ-Fe2O3 | 4 ns, | ~5.1 | N/A | −2.2 | 0.43 | N/A | N/A | [31] |
| 532 nm | ||||||||
| 4 ns, | ~1.15 | N/A | −1.5 | 1.3 | N/A | N/A | ||
| 1064 nm | ||||||||
| Cu(OH)2 | 4 ns, | 1 | −97.3 | −44.7 | −44.7 | N/A | N/A | [32] |
| 532 nm | ||||||||
| Cu(OH)2/CuO | 4 ns, | 1 | −594 | −273 | −273 | N/A | N/A | |
| 532 nm |
| Sample | Excitation Conditions | α0 (cm−1) | β × 10−11 (m/W) | Imχ(3) × 10−13 (esu) | Imχ(3)/α0 × 10−13 (esu cm) | OLon (J/cm2) | Ref. |
|---|---|---|---|---|---|---|---|
| PVP-Ir/IrO2 | 4 ns, 532 nm | 1.8 | 13.8 | 6.3 | 3.5 | 0.38 | this work |
| Ir/IrO2 | 1.79 | 24.2 | 11.1 | 6.2 | 0.18 | ||
| ZnO | 6 ns, 532 nm | 2.7 × 105 | 4.86 × 10−4 | 2.6 × 10−3 | 0.96 × 10−8 | N/A | [33] |
| InZnO | 1.8 × 105 | 5.58 × 10−4 | 3.4 × 10−3 | 1.9 × 10−8 | N/A | ||
| TiO2 | 6 ns, 532 nm | ~6.9 | 395 | 181.6 | 26.3 | ~1 | [34] |
| NiO | 5 ns, 532 nm | N/A | 3.5–31 | 1.9–16.5 | N/A | ~0.8–1 | [35] |
| Cr2O3 | 4 ns, 1064 | 0.31 | 3.17 | 3 | 9.7 | ~0.8 | [36] |
| WO3 | 0.25 | 2.51 | 2.4 | 9.6 | ~1.9 | ||
| Sb2Se3 | 15 ns, 532 nm | 50 | 2 | [36] | |||
| CdS | 4.1 ns, 532 nm | 1.37 | N/A | N/A | N/A | 1.4 | [38] |
| Ag2S | 0.73 | N/A | N/A | N/A | 0.6 |
| λ (nm) | Samples | C (mmol/L) | α0 (cm−1) | β × 10−11 (m/W) | γ′ × 10−18 (m2/W) | χ(3) × 10−13 (esu) | χ(3)/α0 × 10−13 (esu cm) |
|---|---|---|---|---|---|---|---|
| 532 nm | PVP-IrO2 | 4.5 | 1.77 | −115.6 ± 18.0 | −89.8 ± 16.0 | 113.6 ± 20.0 | 65.3 ± 11.0 |
| 0.6 | 0.24 | −18.2 ± 2.0 | −11.1 ± 2.0 | 15.0 ± 2.0 | 62.5 ± 10.0 | ||
| PVP-Ir/IrO2 | [Ir]: 0.9; [IrO2]: 3.6 | 2.92 | 21.0 ± 2.0 | −30.5 ± 3.0 | 35.6 ± 4.0 | 12.2 ± 1.0 | |
| [Ir]: 0.5; [IrO2]: 2.1 | 1.8 | 13.8 ± 2.0 | −20.0 ± 2.0 | 23.3 ± 3.0 | 12.9 ± 1.0 | ||
| Ir/IrO2 | [Ir]: 2.2; [IrO2]: 2.2 | 3.15 | 47.7 ± 6.0 | 51.9 ± 6.0 | 61.4 ± 7.0 | 19.4 ± 2.0 | |
| [Ir]: 1.2; [IrO2]: 1.2 | 1.79 | 24.2 ± 2.0 | −28.8 ± 2.0 | 34.2 ± 3.0 | 19.1 ± 2.0 | ||
| 1064 nm | PVP-IrO2 | 4.5 | 0.39 | - | −28 ± 4 | 31.9 ± 5.0 | 82.9 ± 12.0 |
| 2.5 | 0.22 | - | −14.3 ± 1.0 | 16.3 ± 1.0 | 75.8 ± 7.0 | ||
| PVP-Ir/IrO2 | [Ir]: 0.9; [IrO2]: 3.6 | 0.52 | - | −10.2 ± 1.0 | 11.3 ± 1.0 | 21.6 ± 2.0 | |
| [Ir]: 0.6; [IrO2]: 2.6 | 0.38 | - | −7.8 ± 0.4 | 8.7 ± 0.4 | 22.6 ± 1.0 | ||
| Ir/IrO2 | [Ir]: 2.2; [IrO2]: 2.2 | 0.71 | - | −19.8 ± 2.0 | 21.9 ± 3.0 | 30.9 ± 4.0 | |
| [Ir]: 1.3; [IrO2]: 1.3 | 0.43 | - | −13.3 ± 2.0 | 14.7 ± 2.0 | 34.0 ± 4.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chazapis, N.; Stavrou, M.; Papaparaskeva, G.; Bunge, A.; Turcu, R.; Krasia-Christoforou, T.; Couris, S. Iridium-Based Nanohybrids: Synthesis, Characterization, Optical Limiting, and Nonlinear Optical Properties. Nanomaterials 2023, 13, 2131. https://doi.org/10.3390/nano13142131
Chazapis N, Stavrou M, Papaparaskeva G, Bunge A, Turcu R, Krasia-Christoforou T, Couris S. Iridium-Based Nanohybrids: Synthesis, Characterization, Optical Limiting, and Nonlinear Optical Properties. Nanomaterials. 2023; 13(14):2131. https://doi.org/10.3390/nano13142131
Chicago/Turabian StyleChazapis, Nikolaos, Michalis Stavrou, Georgia Papaparaskeva, Alexander Bunge, Rodica Turcu, Theodora Krasia-Christoforou, and Stelios Couris. 2023. "Iridium-Based Nanohybrids: Synthesis, Characterization, Optical Limiting, and Nonlinear Optical Properties" Nanomaterials 13, no. 14: 2131. https://doi.org/10.3390/nano13142131
APA StyleChazapis, N., Stavrou, M., Papaparaskeva, G., Bunge, A., Turcu, R., Krasia-Christoforou, T., & Couris, S. (2023). Iridium-Based Nanohybrids: Synthesis, Characterization, Optical Limiting, and Nonlinear Optical Properties. Nanomaterials, 13(14), 2131. https://doi.org/10.3390/nano13142131










