Structures, Electronic, and Magnetic Properties of CoKn (n = 2–12) Clusters: A Particle Swarm Optimization Prediction Jointed with First-Principles Investigation
Abstract
:1. Introduction
2. Computational Details
3. Results and Discussion
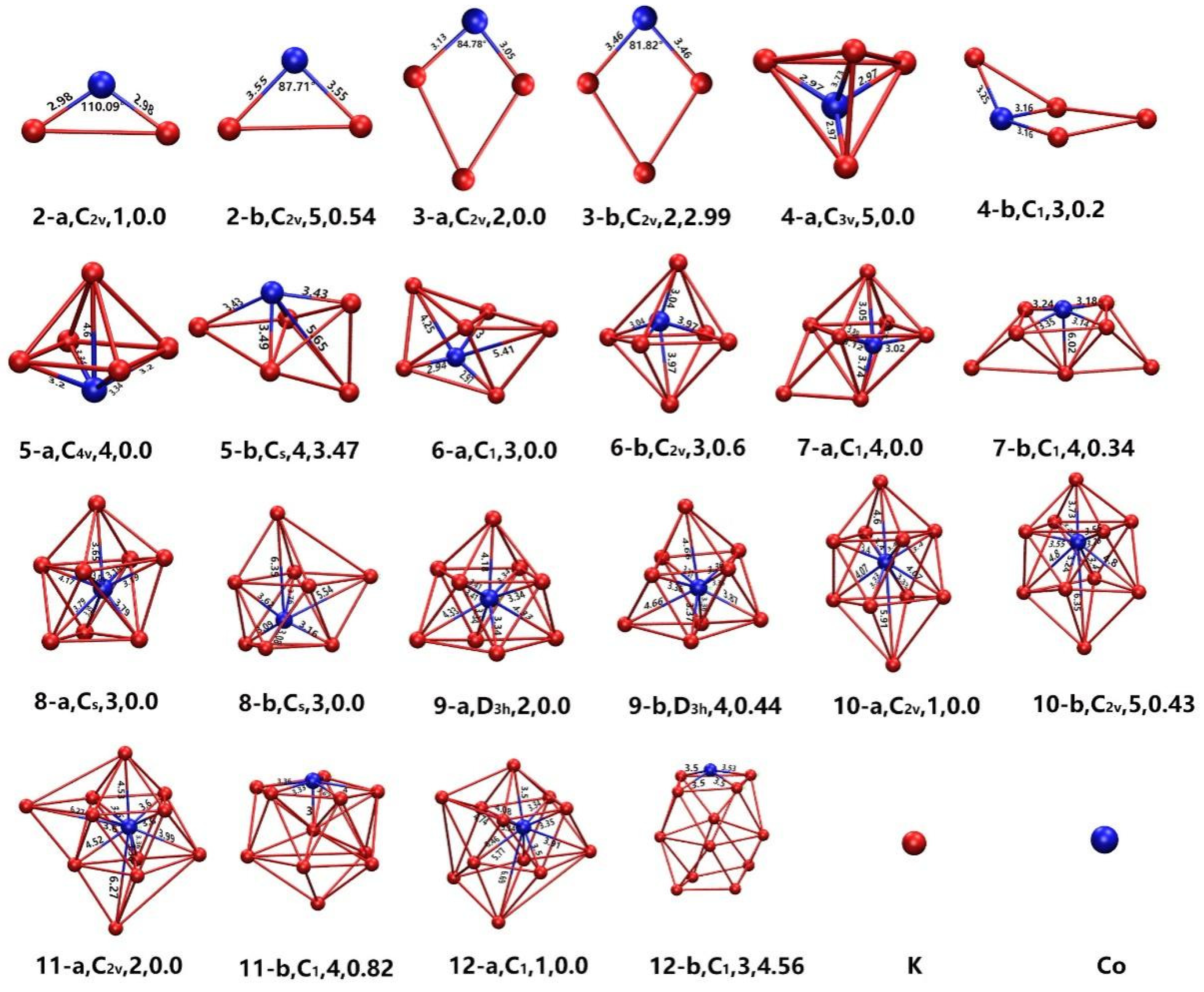
3.1. The Geometries of Lowest-Energy Clusters
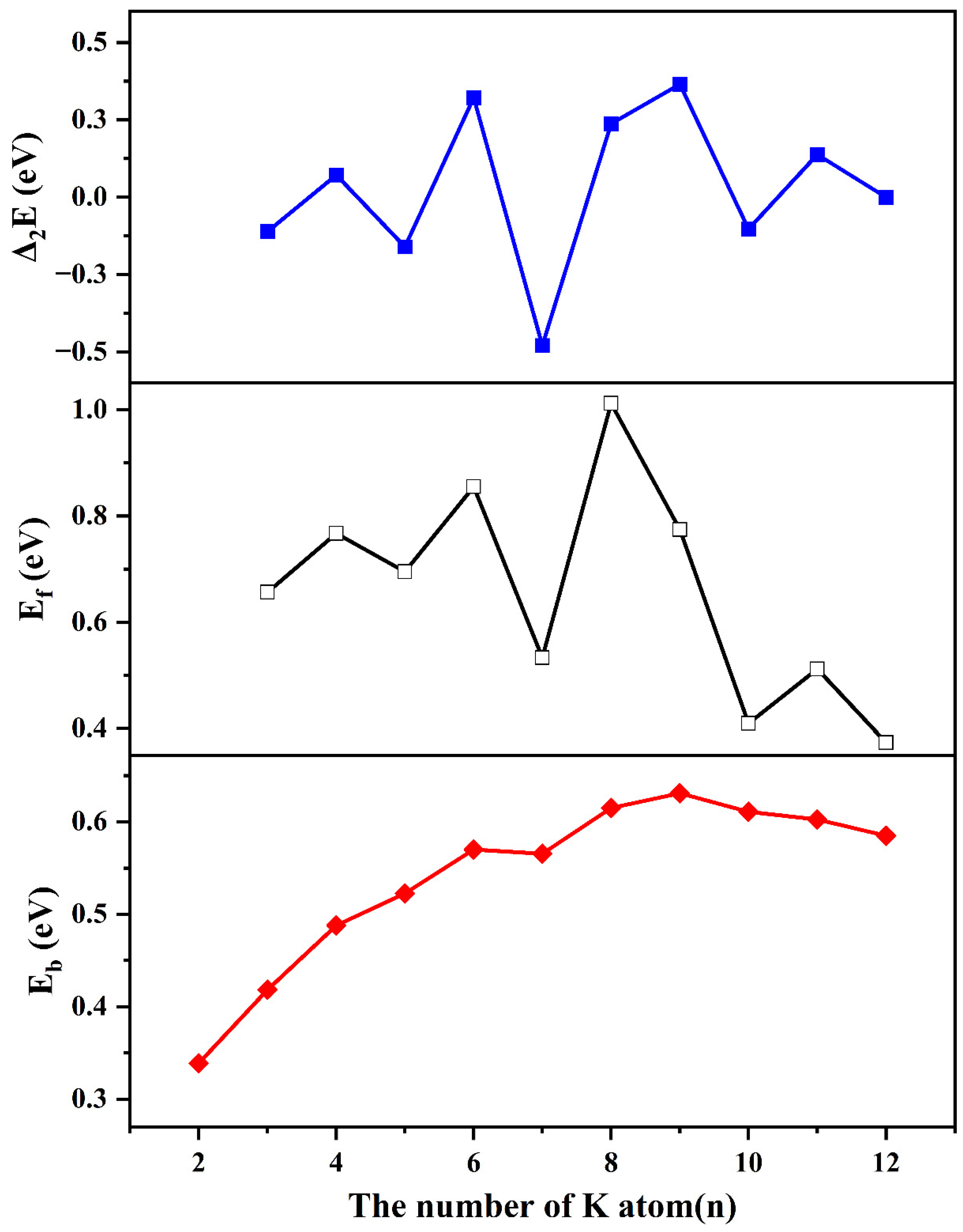
3.2. Relative Stability
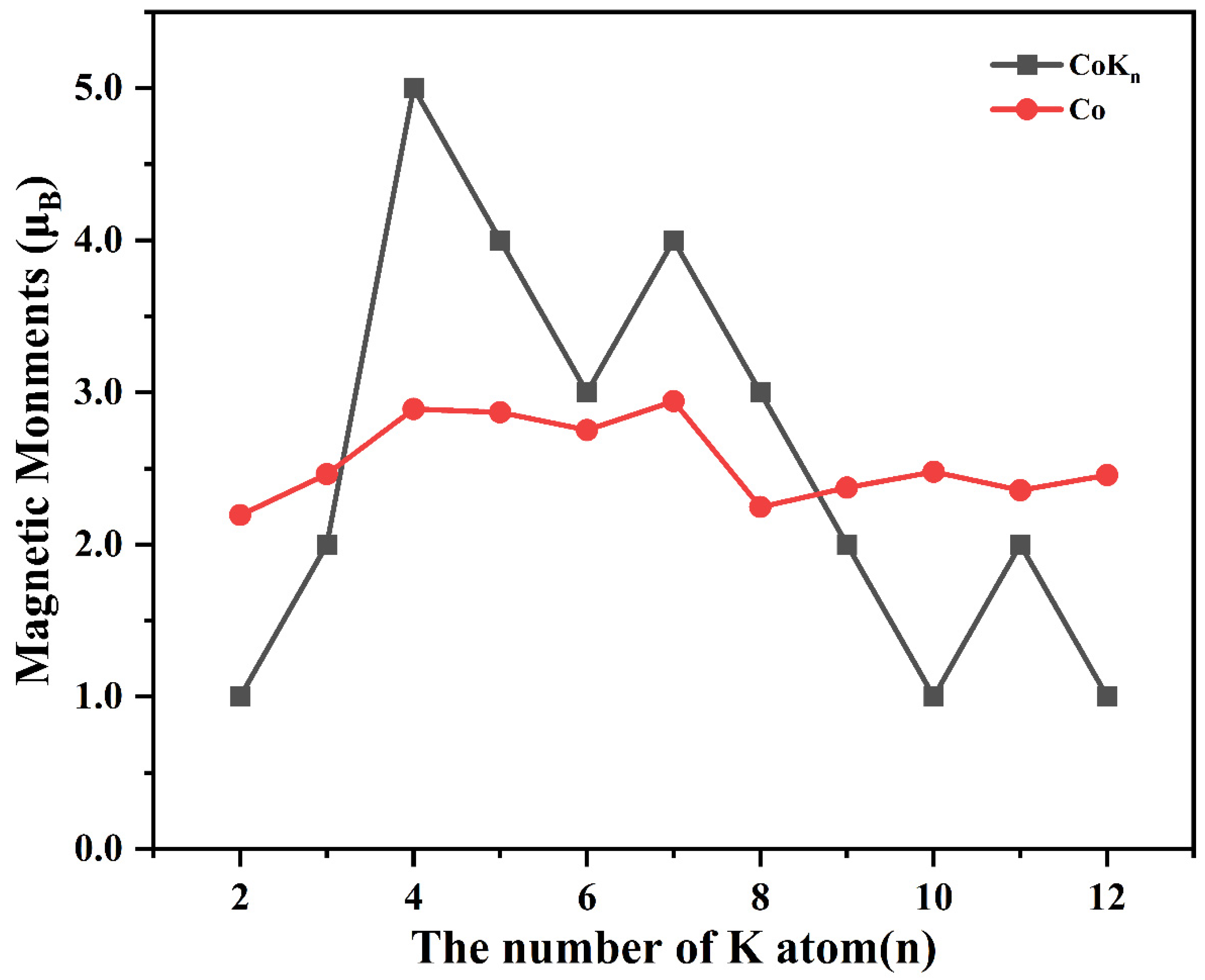
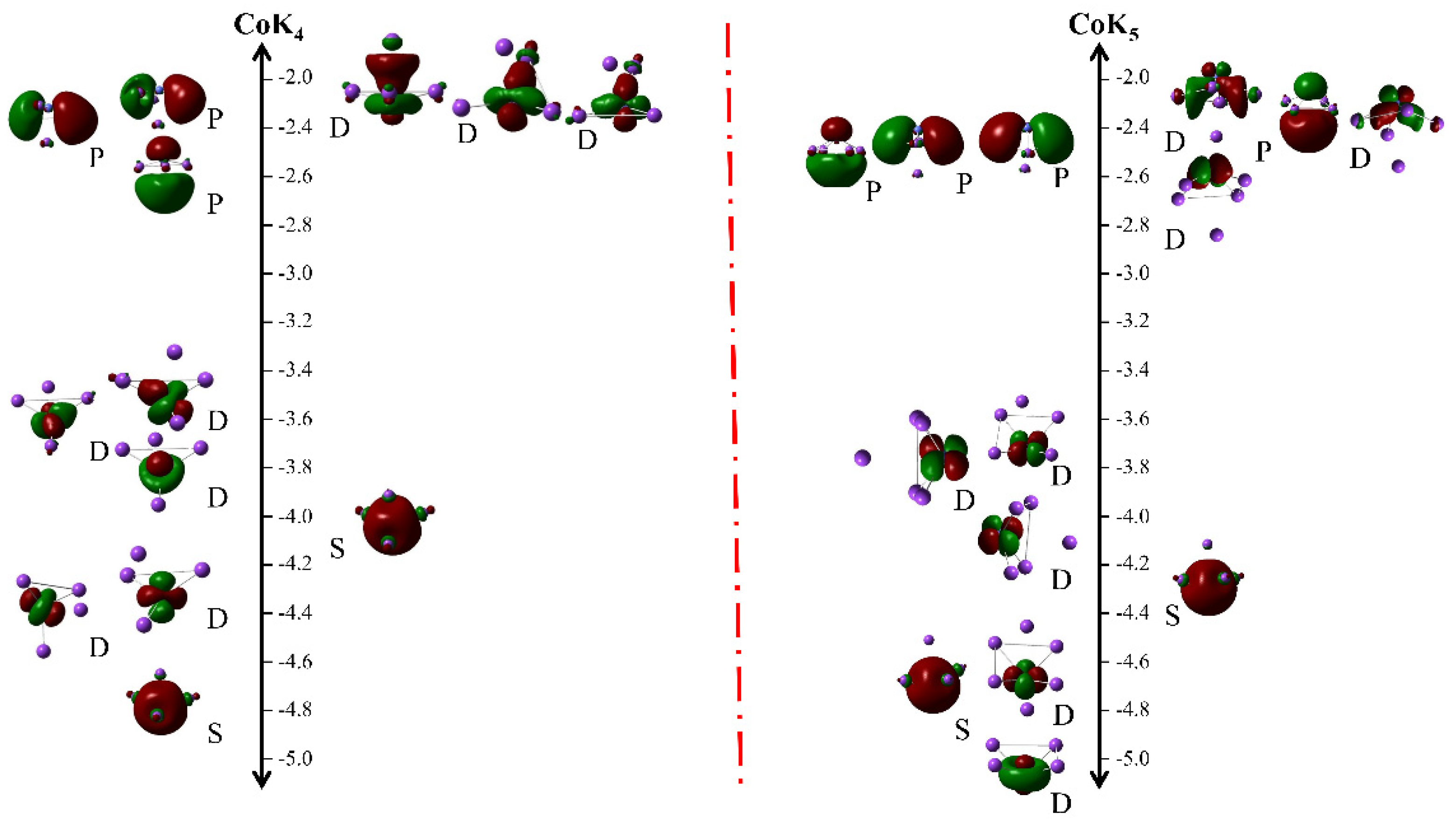
3.3. Magnetic Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cheng, N.; Stambula, S.; Wang, D.; Banis, M.N.; Liu, J.; Riese, A.; Xiao, B.; Li, R.; Sham, T.K.; Liu, L.M.; et al. Platinum single-atom and cluster catalysis of the hydrogen evolution reaction. Nat. Commun. 2016, 7, 13638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tofanelli, M.A.; Ackerson, C.J. Superatom electron configuration predicts thermal stability of Au25(SR)18 nanoclusters. J. Am. Chem. Soc. 2012, 134, 16937–16940. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Du, Q.; Zhou, S.; Kumar, V. Endohedrally doped cage clusters. Chem. Rev. 2020, 120, 9021–9163. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.J.; Huang, X.M.; Jin, P.; Chen, Z.F. Magnetic properties of atomic clusters and endohedral metallofullerenes. Coord. Chem. Rev. 2015, 289, 315–340. [Google Scholar] [CrossRef]
- Kibsgaard, J.; Jaramillo, T.F.; Besenbacher, F. Building an appropriate active-site motif into a hydrogen-evolution catalyst with thiomolybdate [Mo3S13]2− clusters. Nat. Chem. 2014, 6, 248–253. [Google Scholar] [CrossRef] [Green Version]
- Hu, Q.; Gao, K.; Wang, X.; Zheng, H.; Cao, J.; Mi, L.; Huo, Q.; Yang, H.; Liu, J.; He, C. Subnanometric Ru clusters with upshifted D band center improve performance for alkaline hydrogen evolution reaction. Nat. Commun. 2022, 13, 3958. [Google Scholar] [CrossRef]
- Luo, Z.; Castleman, A.W., Jr.; Khanna, S.N. Reactivity of metal clusters. Chem. Rev. 2016, 116, 14456–14492. [Google Scholar] [CrossRef]
- Chakraborty, I.; Pradeep, T. Atomically precise clusters of noble metals: Emerging link between atoms and nanoparticles. Chem. Rev. 2017, 117, 8208–8271. [Google Scholar] [CrossRef]
- Wu, X.; Liao, R.; Liang, X.; Sai, L.; Liu, Y.; Yang, G.; Zhao, J. Pivotal role of the B12-core in the structural evolution of B52–B64 clusters. Nanoscale 2023, 15, 10430–10436. [Google Scholar] [CrossRef]
- Yang, H.; Hu, Y.-F.; Yuan, Y.-Q.; Ding, J.-J. Probing the structural evolution and stabilities of CsBn0/− (n = 2–12) clusters. Phys. B Condens. Matter 2023, 652, 414628. [Google Scholar] [CrossRef]
- Xie, Q.; Pan, M.; Wang, Z.; Si, W.; Zhang, R.; Shu, Y.; Sun, G.; Jing, Q.; Shen, Y.; Uyama, H. Enhancing the oxygen reduction activity by constructing nanocluster-scaled Fe2O3/Cu interfaces. Nanoscale 2023, 15, 4388–4396. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-G.; Cui, Y.-Q.; Tian, H.; Shen, Z.-G.; Shao, Q.-Q.; Ding, Y.-L.; Ren, B.-Z. Theoretical investigations on the structures and electronic and optical properties of neutral and anionic M2-doped B24 clusters (M = Li, Na, and K). New J. Chem. 2023, 47, 6612–6620. [Google Scholar] [CrossRef]
- Ge, G.X.; Han, Y.; Wan, J.G.; Zhao, J.J.; Wang, G.H. First-principles prediction of magnetic superatoms in 4d-transition-metal-doped magnesium clusters. J. Chem. Phys. 2013, 139, 174309. [Google Scholar] [CrossRef]
- Li, H.S.; Wei, D.; Zhao, X.; Ren, X.; Zhang, D.; Ju, W. Thermal stability of Ag13− clusters studied by ab initio molecular dynamics simulations. J. Phys. Chem. A 2020, 124, 4325–4332. [Google Scholar] [CrossRef]
- Liu, J.T.; Duan, H.M. Molecular dynamics simulation of structures and melting behaviours of iridium clusters with different potentials. Acta Phys. Sin. 2009, 58, 4826–4834. [Google Scholar]
- Lv, J.; Wang, Y.; Zhu, L.; Ma, Y. Particle-swarm structure prediction on clusters. J. Chem. Phys. 2012, 137, 084104. [Google Scholar] [CrossRef] [PubMed]
- Lyakhov, A.O.; Oganov, A.R.; Stokes, H.T.; Zhu, Q. New developments in evolutionary structure prediction algorithm USPEX. Comput. Phys. Commun. 2013, 184, 1172–1182. [Google Scholar] [CrossRef]
- Jorgensen, M.S.; Groves, M.N.; Hammer, B. Combining evolutionary algorithms with clustering toward rational global structure optimization at the atomic scale. J. Chem. Theory Comput. 2017, 13, 1486–1493. [Google Scholar] [CrossRef] [Green Version]
- Fronzi, M.; Amos, R.D.; Kobayashi, R.; Matsumura, N.; Watanabe, K.; Morizawa, R.K. Evaluation of machine learning interatomic potentials for the properties of gold nanoparticles. Nanomaterials 2022, 12, 3891. [Google Scholar] [CrossRef]
- Tong, Q.; Xue, L.; Lv, J.; Wang, Y.; Ma, Y. Accelerating CALYPSO structure prediction by data-driven learning of a potential energy surface. Faraday Discuss. 2018, 211, 31–43. [Google Scholar] [CrossRef] [Green Version]
- Ceriotti, M. Unsupervised machine learning in atomistic simulations, between predictions and understanding. J. Chem. Phys. 2019, 150, 150901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barroso, J.; Pan, S.; Merino, G. Structural transformations in boron clusters induced by metal doping. Chem. Soc. Rev. 2022, 51, 1098–1123. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, V.; Sen, P. Electronic and magnetic properties of 3d transition metal-doped strontium clusters: Prospective magnetic superatoms. Chem. Phys. 2013, 417, 37–44. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, J.; Feng, X.; Zhang, H.; Zhao, L.; Luo, Y.; Cao, W. Magnetic superatoms in VLin (n = 1–13) clusters: A first-principles prediction. J. Phys. Chem. A 2013, 117, 13025–13036. [Google Scholar] [CrossRef]
- Lu, S.-J.; Wu, L.-S.; Lin, F. Structural, bonding, and superhalogen properties of Au4X−/04 (X = F, Cl, Br, and I) clusters. Theor. Chem. Acc. 2019, 138, 51. [Google Scholar] [CrossRef]
- Wang, J.; Bai, J.; Jellinek, J.; Zeng, X.C. Gold-coated transition-metal anion [Mn13@Au20]− with ultrahigh magnetic moment. J. Am. Chem. Soc. 2007, 129, 4110. [Google Scholar] [CrossRef]
- Zhao, G.F.; Zhang, J.; Jing, Q.; Luo, Y.H.; Wang, Y.X. A density functional study of YnAl (n = 1–14) clusters. J. Chem. Phys. 2007, 127, 234312. [Google Scholar] [CrossRef]
- Aireti, M.; Jiang, Y.; Cao, H.; Duan, H.; Cui, X.; Jing, Q.; Xue, X. The cage-like structure enhanced magnetic moment in ScKn (n = 2–12) clusters: A first-principles jointed particle swarm optimization investigation. Int. J. Quantum Chem. 2021, 121, e26654. [Google Scholar] [CrossRef]
- Poudel, M.B.; Kim, A.A.; Lohani, P.C.; Yoo, D.J.; Kim, H.J. Assembling zinc cobalt hydroxide/ternary sulfides hetereostructure and iron oxide nanorods on three-dimensional hollow porous carbon nanofiber as high energy density hybrid supercapacitor. J. Energy Storage 2023, 60, 106713. [Google Scholar] [CrossRef]
- Poudel, M.B.; Logeshwaran, N.; Kim, A.R.; Karthikeyan, S.C.; Vijayapradeep, S.; Yoo, D.J. Integrated core-shell assembly of Ni3S2 nanowires and CoMoP nanosheets as highly efficient bifunctional electrocatalysts for overall water splitting. J. Alloys Compd. 2023, 960, 170678. [Google Scholar] [CrossRef]
- Jing, Q.; Tian, F.Y.; Wang, Y.X. No quenching of magnetic moment for the GenCo (n = 1–13) clusters: First-principles calculations. J. Chem. Phys. 2008, 128, 124319. [Google Scholar] [CrossRef] [PubMed]
- Jing, Q.; Cao, H.B.; Ge, G.X.; Wang, Y.X.; Yan, H.X.; Zhang, Z.Y.; Liu, Y.H. Giant magnetic moment of the core-shell Co13@Mn20 clusters: First-principles calculations. J. Comput. Chem. 2011, 32, 2474. [Google Scholar] [CrossRef] [PubMed]
- Hao, A.; Xue, H.; Jia, J. Geometries, stabilities, and magnetic properties of Co2Bn (n = 1–10) clusters. J. Mol. Model. 2019, 25, 27. [Google Scholar] [CrossRef]
- Song, Z. First-principle investigation on growth patterns and properties of cobalt-doped lithium nanoclusters. J. Mol. Model. 2016, 22, 133. [Google Scholar] [CrossRef]
- Wang, Y.; Lv, J.; Zhu, L.; Ma, Y. Crystal structure prediction via particle-swarm optimization. Phys. Rev. B 2010, 82, 094116. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Lv, J.; Zhu, L.; Ma, Y. CALYPSO: A method for crystal structure prediction. Comput. Phys. Commun. 2012, 183, 2063–2070. [Google Scholar] [CrossRef] [Green Version]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmuller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B Condens. Matter 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Sun, W.G.; Wang, J.J.; Lu, C.; Xia, X.X.; Kuang, X.Y.; Hermann, A. Evolution of the structural and electronic properties of medium-sized sodium clusters: A honeycomb-like Na20 Cluster. Inorg. Chem. 2017, 56, 1241–1248. [Google Scholar] [CrossRef] [Green Version]
- Lu, C.; Li, Q.; Ma, Y.; Chen, C. Extraordinary indentation strain stiffening produces superhard tungsten nitrides. Phys. Rev. Lett. 2017, 119, 115503. [Google Scholar] [CrossRef] [Green Version]
- Lu, C.; Amsler, M.; Chen, C. Unraveling the structure and bonding evolution of the newly discovered iron oxide FeO2. Phys. Rev. B 2018, 98, 054102. [Google Scholar] [CrossRef] [Green Version]
- Tang, X.; Sun, W.G.; Gu, Y.T.; Lu, C.; Kou, L.Z.; Chen, C.F. CoB6 monolayer: A robust two-dimensional ferromagnet. Phys. Rev. B 2019, 99, 045445. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Cao, H.; Cui, X.; Duan, H.; Jing, Q.; Wang, Q. The geometry, electronic and magnetic properties of VLin (n = 2–13) clusters using the first-principles and PSO method. Mol. Phys. 2020, 118, e1791990. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [Green Version]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Revision E.01; Gaussian Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B Condens. Matter 1988, 37, 785–789. [Google Scholar] [CrossRef] [Green Version]
- Krishnan, R.; Binkley, J.S.; Seeger, R.; Pople, J.A. Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J. Chem. Phys. 1980, 72, 650–654. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F.W. Calculation of Molecular Orbital Composition. Acta Chim. Sin. 2011, 69, 2393–2406. [Google Scholar]
- Lu, T.; Chen, F.-W. Meaning and Functional Form of the Electron Localization Function. Acta Phys.-Chim. Sin. 2011, 27, 2786–2792. [Google Scholar]
- Li, L.; Cui, X.-H.; Cao, H.-B.; Jiang, Y.; Duan, H.-M.; Jing, Q.; Liu, J.; Wang, Q. Structural evolution and magnetic properties of ScLin (n = 2–13) clusters: A PSO and DFT investigation. Chin. Phys. B 2020, 29, 077101. [Google Scholar] [CrossRef]

| Cluster | NEC(Co) | m(CoKn) | m(Co) | m(K) | q(Co) | R(Co-K) |
|---|---|---|---|---|---|---|
| CoK2 | 4s1.743d7.454p0.31 | 1 | 2.1937 | −1.1937 | −0.4264 | 2.98 |
| CoK3 | 4s1.833d7.434p0.18 | 2 | 2.4643 | −0.5186 | −0.2175 | 4.08 |
| CoK4 | 4s1.683d7.514p1.16 | 5 | 2.8923 | 2.1077 | −0.8717 | 2.91 |
| CoK5 | 4s1.83d7.44p1.06 | 4 | 2.8704 | 1.1418 | −0.2659 | 3.54 |
| CoK6 | 4s1.733d7.564p1.2 | 3 | 2.7535 | 0.2465 | −0.8875 | 3.69 |
| CoK7 | 4s1.713d7.484p2.02 | 4 | 2.9430 | 1.0570 | −0.9731 | 3.63 |
| CoK8 | 4s1.673d7.434p2.64 | 3 | 2.2473 | 0.7501 | −0.7206 | 3.53 |
| CoK9 | 4s1.913d7.394p2.68 | 2 | 2.3750 | −0.3750 | −0.4794 | 3.65 |
| CoK10 | 4s1.843d7.44p2.53 | 1 | 2.4785 | −1.4785 | −0.4925 | 3.89 |
| CoK11 | 4s1.793d7.424p2.44 | 2 | 2.3572 | −0.3572 | −0.4844 | 4.16 |
| CoK12 | 4s1.783d7.414p2.46 | 1 | 2.4580 | −1.4673 | −0.5966 | 4.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Y.; Aireti, M.; Leng, X.; Ji, X.; Liu, J.; Cui, X.; Duan, H.; Jing, Q.; Cao, H. Structures, Electronic, and Magnetic Properties of CoKn (n = 2–12) Clusters: A Particle Swarm Optimization Prediction Jointed with First-Principles Investigation. Nanomaterials 2023, 13, 2155. https://doi.org/10.3390/nano13152155
Jiang Y, Aireti M, Leng X, Ji X, Liu J, Cui X, Duan H, Jing Q, Cao H. Structures, Electronic, and Magnetic Properties of CoKn (n = 2–12) Clusters: A Particle Swarm Optimization Prediction Jointed with First-Principles Investigation. Nanomaterials. 2023; 13(15):2155. https://doi.org/10.3390/nano13152155
Chicago/Turabian StyleJiang, Yi, Maidina Aireti, Xudong Leng, Xu Ji, Jing Liu, Xiuhua Cui, Haiming Duan, Qun Jing, and Haibin Cao. 2023. "Structures, Electronic, and Magnetic Properties of CoKn (n = 2–12) Clusters: A Particle Swarm Optimization Prediction Jointed with First-Principles Investigation" Nanomaterials 13, no. 15: 2155. https://doi.org/10.3390/nano13152155






