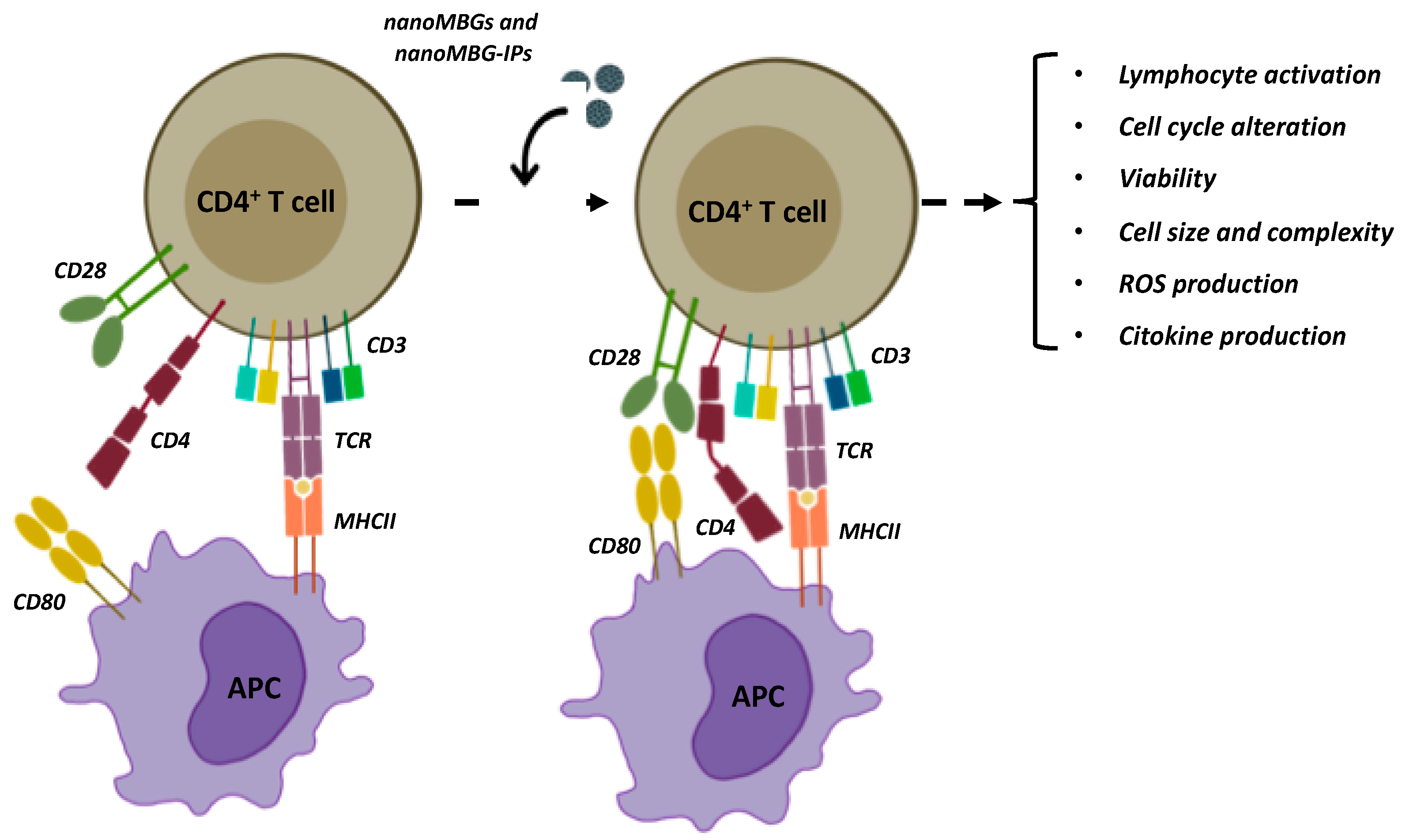
Osteoimmune Properties of Mesoporous Bioactive Nanospheres: A Study on T Helper Lymphocytes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Mesoporous Bioactive Nanoparticles
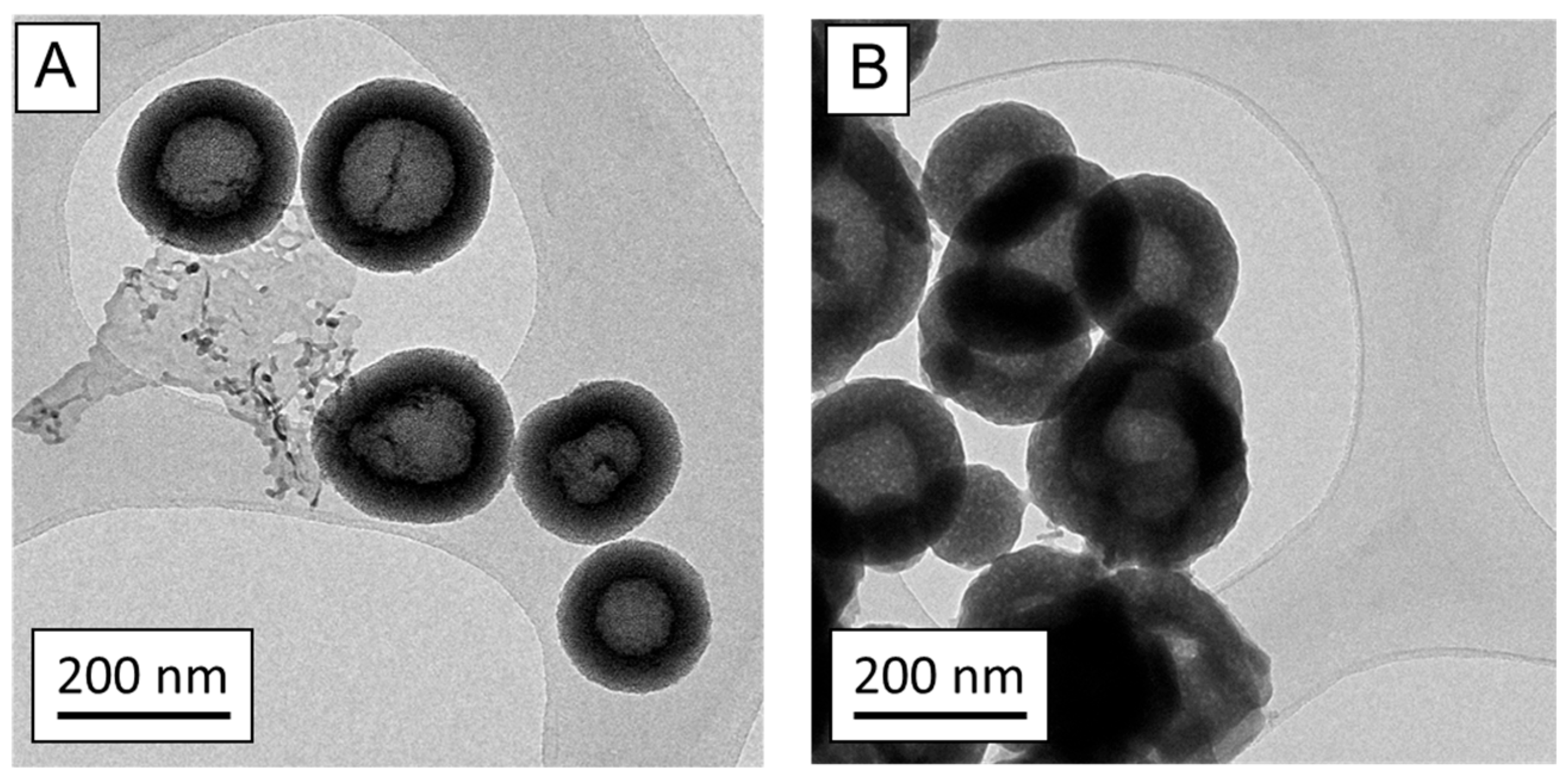
2.2. NanoMBGs Characterization
2.3. Culture of CD4+ Th2 SR.D10 Lymphocyte Cell Line
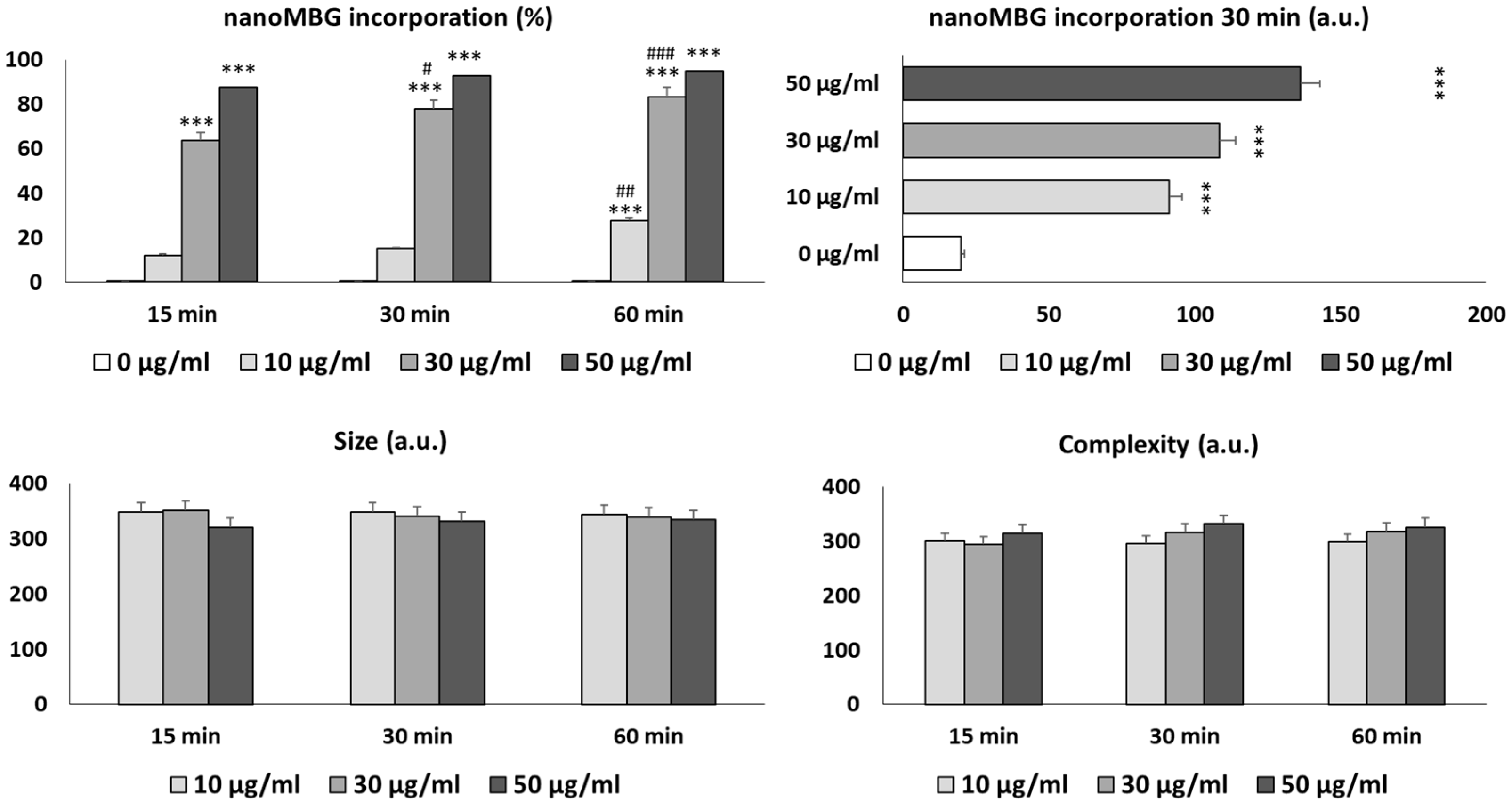
2.4. Treatment and Activation Assays of SR.D10 Lymphocytes with nanoMBGs and nanoMBG-IPs
2.5. Effects of nanoMBGs on Cell Cycle Phases of SR.D10 Lymphocytes
2.6. Cell Viability Studies of CD4+ Th2 SR.D10 Lymphocytes Evaluated using Flow Cytometry
2.7. Effects of nanoMBGs on the Size and Intracellular Complexity of SR.D10 Lymphocytes
2.8. Intracellular Content of Reactive Oxygen Species (ROS) in SR.D10 Lymphocytes
2.9. Mechanisms for the Incorporation of nanoMBGs by CD4+ Th2 SR.D10 Lymphocytes
2.10. Detection of Interleukins 4 (IL-4) and 10 (IL-10)
2.11. Data Analysis and Statistics
3. Results and Discussion
3.1. Characterization of nanoMBGs and nanoMBG-IPs
3.2. Uptake of nanoMBGs by Th2 CD4+ SR.D10 Lymphocytes
3.3. Endocytic Strategies for the Uptake of nanoMBGs by Th2 CD4+ SR.D10 Lymphocytes
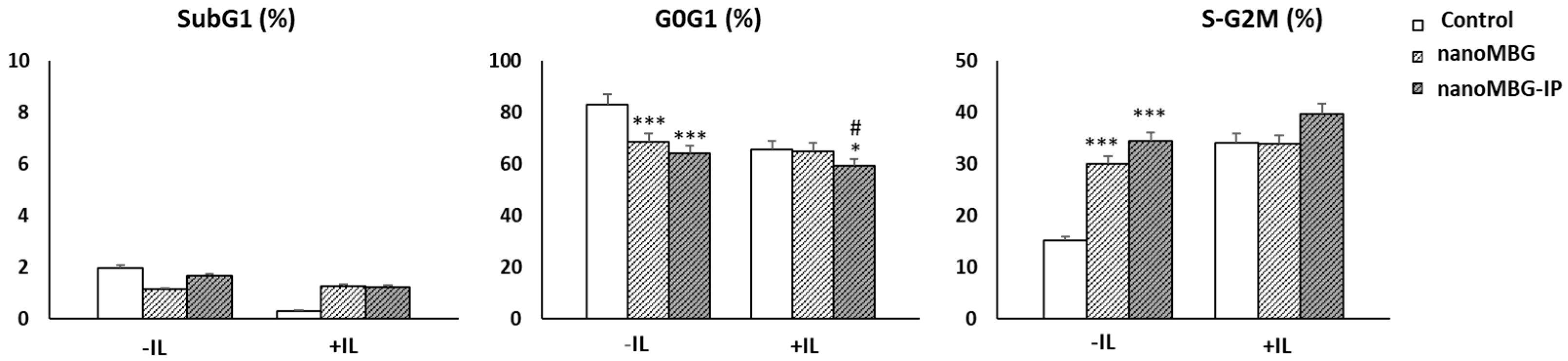
3.4. Impact of Nanoparticles (MBGs and MBG-IPs) on Cell-Cycle Phases of CD4+ Th2 Memory Cells
3.5. Proliferation of CD4+ Th2 SR.D10 Lymphocytes after Treatment with nanoMBGs and nanoMBG-IPs in Basal and Stimulated Conditions
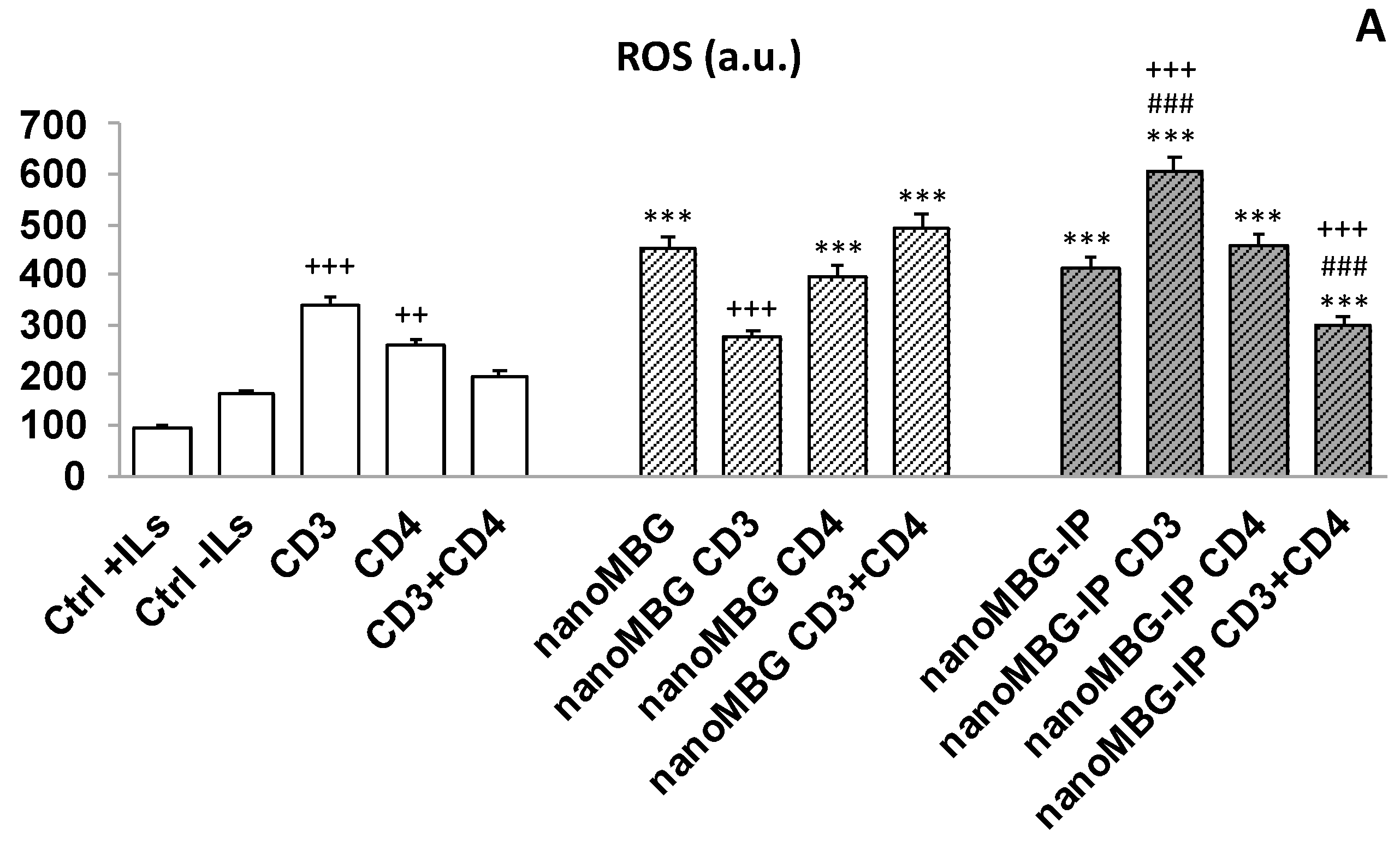
3.6. Effects of nanoMBGs and nanoMBG-IPs on Cell Viability and Intracellular Reactive Oxygen Species (ROS) Content in CD4+ Th2 SR.D10 Lymphocytes in Basal and Stimulated Conditions
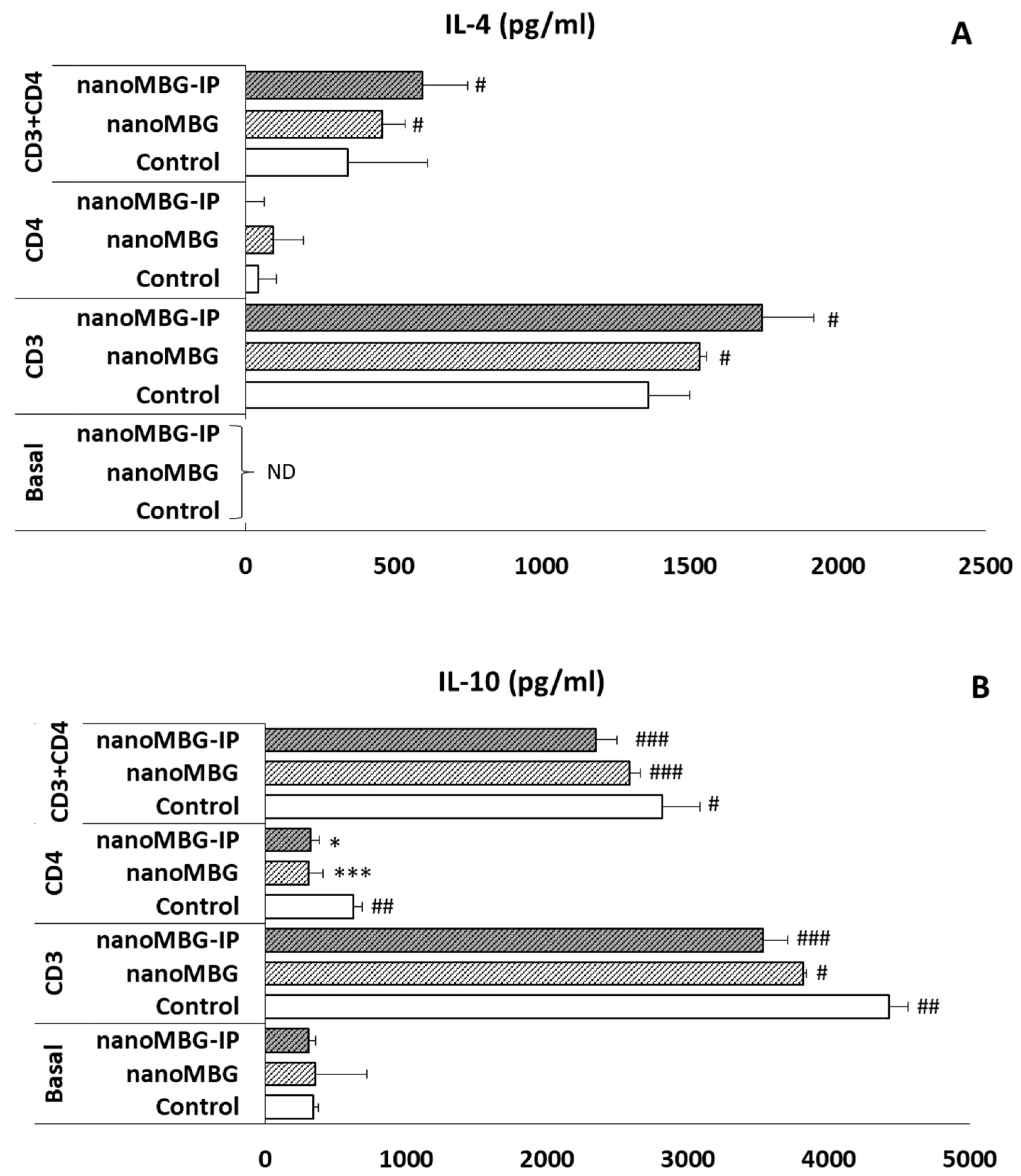
3.7. Effects of nanoMBGs and nanoMBG-IPs on Interleukin-4 (IL-4) and Interleukin-10 (IL-10) Secretion by CD4+ Th2 SR.D10 Lymphocytes in Basal and Stimulated Conditions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arcos, D.; Portolés, M.T. Mesoporous bioactive nanoparticles for bone tissue applications. Int. J. Mol. Sci. 2023, 24, 3249. [Google Scholar] [CrossRef]
- Burdușel, A.-C.; Gherasim, O.; Andronescu, E.; Grumezescu, A.M.; Ficai, A. Inorganic Nanoparticles in Bone Healing Applications. Pharmaceutics 2022, 14, 770. [Google Scholar] [CrossRef] [PubMed]
- Mora-Raimundo, P.; Lozano, D.; Benito, M.; Mulero, F.; Manzano, M.; Vallet-Regí, M. Osteoporosis Remission and New Bone Formation with Mesoporous Silica Nanoparticles. Adv. Sci. 2021, 8, e2101107. [Google Scholar] [CrossRef] [PubMed]
- Arcos, D.; Gómez-Cerezo, N.; Saiz-Pardo, M.; de Pablo, D.; Ortega, L.; Enciso, S.; Fernández-Tomé, B.; Díaz-Güemes, I.; Sánchez-Margallo, F.M.; Casarrubios, L.; et al. Injectable mesoporous bioactive nanoparticles regenerate bone tissue under osteoporosis conditions. Acta Biomater. 2022, 151, 501. [Google Scholar] [CrossRef]
- Yan, X.; Yu, C.; Zhou, X.; Tang, J.; Zhao, D. Highly Ordered Mesoporous Bioactive Glasses with Superior In Vitro Bone-Forming Bioactivities. Angew. Chem. Int. Ed. 2004, 43, 5980–5984. [Google Scholar] [CrossRef]
- Zheng, K.; Boccaccini, A.R. Sol-gel processing of bioactive glass nanoparticles: A review. Adv. Colloid Interface Sci. 2017, 249, 363–373. [Google Scholar] [CrossRef]
- Vichery, C.; Nedelec, J.-M. Bioactive Glass Nanoparticles: From Synthesis to Materials Design for Biomedical Applications. Materials 2016, 9, 288. [Google Scholar] [CrossRef] [Green Version]
- Casarrubios, L.; Polo-Montalvo, A.; Serrano, M.C.; Feito, M.J.; Vallet-Regí, M.; Arcos, D.; Portolés, M.T. Effects of Ipriflavone-Loaded Mesoporous Nanospheres on the Differentiation of Endothelial Progenitor Cells and Their Modulation by Macrophages. Nanomaterials 2021, 11, 1102. [Google Scholar] [CrossRef]
- Guder, C.; Gravius, S.; Burger, C.; Wirtz, D.C.; Schildberg, F.A. Osteoimmunology: A Current Update of the Interplay Between Bone and the Immune System. Front. Immunol. 2020, 11, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arron, J.R.; Choi, Y. Bone versus immune system. Nature 2000, 408, 535–536. [Google Scholar] [CrossRef]
- Tsukasaki, M.; Takayanagi, H. Osteoimmunology: Evolving concepts in bone–immune interactions in health and disease. Nat. Rev. Immunol. 2019, 19, 626–642. [Google Scholar] [CrossRef]
- Collins, F.L.; Stone, M.D.; Turton, J.; McCabe, L.R.; Wang, E.C.Y.; Williams, A.S. Oestrogen-deficiency induces bone loss by modulating CD14+ monocyte and CD4+ T cell DR3 expression and serum TL1A levels. BMC Musculoskelet. Disord. 2019, 20, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Dang, K.; Huai, Y.; Qian, A. Osteoimmunology: The Regulatory Roles of T Lymphocytes in Osteoporosis. Front. Endocrinol. 2020, 11, 465. [Google Scholar] [CrossRef] [PubMed]
- Takayanagi, H. Osteoimmunology in 2014: Two-faced immunology-from osteogenesis to bone resorption. Nat. Rev. Rheumatol. 2015, 11, 74. [Google Scholar] [CrossRef] [PubMed]
- Fischer, V.; Haffner-Luntzer, M. Interaction between bone and immune cells: Implications for postmenopausal osteoporosis. Semin. Cell Dev. Biol. 2021, 123, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Cai, D.; Gao, W.; He, R.; Li, Y.; Zhou, Y.; Klein, T.; Xiao, L.; Xiao, Y. Osteoimmunomodulatroy nanoparticles for bone regeneration. Nanomaterials 2023, 13, 692. [Google Scholar] [CrossRef]
- Cui, Y.; Li, H.; Li, Y.; Mao, L. Novel insights into nanomaterials for immunomodulatoty bone regeneration. Nanoscale Adv. 2022, 4, 334. [Google Scholar] [CrossRef]
- Casarrubios, L.; Gómez-Cerezo, N.; Feito, M.J.; Vallet-Regí, M.; Arcos, D.; Portolés, M.T. Incorporation and effects of mesoporous SiO2-CaO nanospheres loaded with ipriflavone on osteoblast/osteoclast cocultures. Eur. J. Pharm. Biopharm. 2018, 133, 258–268. [Google Scholar] [CrossRef] [Green Version]
- Ye, X.; Jiang, H.; Wang, Y.; Ji, Y.; Jiang, X. A correlative studies between osteoporosis and blood cell composition: Implications for auxiliary diagnosis of osteoporosis. Medicine 2020, 99, e20864. [Google Scholar] [CrossRef]
- Chakravarti, A.; Marceau, A.-A.; Flamand, L.; Poubelle, P.E. Normal human primary CD4+ T lymphocytes synthesize and release functional osteoprotegerin in vitro. Lab. Investig. 2008, 88, 171–184. [Google Scholar] [CrossRef] [Green Version]
- Kaye, J.; Porcelli, S.; Tite, J.; Jones, B.; Janeway, C.A. Both a monoclonal antibody and antisera specific for determinants unique to individual cloned helper T cell lines can substitute for antigen and antigen-presenting cells in the activation of T cells. J. Exp. Med. 1983, 158, 836–856. [Google Scholar] [CrossRef]
- Yagi, J.; Baron, J.; Buxser, S.; Janeway, J.C.A. Bacterial proteins that mediate the association of a defined subset of T cell receptor:CD4 complexes with class II MHC. J. Immunol. 1990, 144, 892–901. [Google Scholar] [CrossRef] [PubMed]
- Portolés, P.; Rojo, J.; Golby, A.; Bonneville, M.; Gromkowski, S.; Greenbaum, L.; Janeway, C.A.; Murphy, D.B.; Bottomly, K. Monoclonal antibodies to murine CD3e define distinct epitopes, one of which may interact with CD4 during T cell activation. J. Immunol. 1989, 142, 4169. [Google Scholar] [CrossRef]
- Casarrubios, L.; Gómez-Cerezo, N.; Feito, M.J.; Vallet-Regí, M.; Arcos, D.; Portolés, M.T. Ipriflavone-Loaded Mesoporous Nanospheres with Potential Applications for Periodontal Treatment. Nanomaterials 2020, 10, 2573. [Google Scholar] [CrossRef]
- Diez-Orejas, R.; Casarrubios, L.; Feito, M.J.; Rojo, J.M.; Vallet-Regí, M.; Arcos, D.; Portolés, M.T. Effects of mesoporous SiO2-CaO nanospheres on the murine peritoneal macrophages/Candida albicans interface. Int. Immunopharmacol. 2021, 94, 107457. [Google Scholar] [CrossRef] [PubMed]
- Montes-Casado, M.; Sanvicente, A.; Casarrubios, L.; Feito, M.J.; Rojo, J.M.; Vallet-Regí, M.; Arcos, D.; Portolés, P.; Portolés, M.T. An Immunological Approach to the Biocompatibility of Mesoporous SiO2-CaO Nanospheres. Int. J. Mol. Sci. 2020, 21, 8291. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Oh, N. Endocytosis and exocytosis of nanoparticles in mammalian cells. Int. J. Nanomed. 2014, 9, 51–63. [Google Scholar] [CrossRef] [Green Version]
- Mäger, I.; Langel, K.; Lehto, T.; Eiríksdóttir, E.; Langel, Ü. The role of endocytosis on the uptake kinetics of luciferin-conjugated cell-penetrating peptides. Biochim. Biophys. Acta–Biomembr. 2012, 1818, 502–511. [Google Scholar] [CrossRef] [Green Version]
- Linares, J.; Matesanz, M.C.; Vila, M.; Feito, M.J.; Gonçalves, G.; Vallet-Regí, M.; Marques, P.A.A.P.; Portolés, M.T. Endocytic Mechanisms of Graphene Oxide Nanosheets in Osteoblasts, Hepatocytes and Macrophages. ACS Appl. Mater. Interfaces 2014, 6, 13697–13706. [Google Scholar] [CrossRef]
- Schulz, W.L.; Haj, A.K.; Schiff, L.A. Reovirus Uses Multiple Endocytic Pathways for Cell Entry. J. Virol. 2012, 86, 12665–12675. [Google Scholar] [CrossRef] [Green Version]
- Tran, C.; Stary, C.M.; Schilling, J.M.; Bentley, B.; Patel, H.H.; Roth, D.M. Role of caveolin-3 in lymphocyte activation. Life Sci. 2015, 121, 35–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, J.; Xu, X.; Rasheed, T.K.; Yoder, A.; Yu, D.; Liang, H.; Yi, F.; Hawley, T.; Jin, T.; Ling, B.; et al. Genistein interferes with SDF-1- and HIV-mediated actin dynamics and inhibits HIV infection of resting CD4 T cells. Retrovirology 2013, 10, 62. [Google Scholar] [CrossRef] [Green Version]
- Caetano, M.S.; Vieira-De-Abreu, A.; Teixeira, L.K.; Werneck, M.B.; Barcinski, M.A.; Viola, J.P. NFATC2 transcription factor regulates cell cycle progression during lymphocyte activation: Evidence of its involvement in the control of cyclin gene expression. FASEB J. 2002, 16, 1940–1942. [Google Scholar] [CrossRef]
- Duthoo, E.; Vral, A.; Baeyens, A. An updated view into the cell cycle kinetics of human T lymphocytes and the impact of irradiation. Sci. Rep. 2022, 12, 1–12. [Google Scholar] [CrossRef]
- Feito, M.J.; Cicuéndez, M.; Casarrubios, L.; Diez-Orejas, R.; Fateixa, S.; Silva, D.; Barroca, N.; Marques, P.A.A.P.; Portolés, M.T. Effects of Graphene Oxide and Reduced Graphene Oxide Nanostructures on CD4+ Th2 Lymphocytes. Int. J. Mol. Sci. 2022, 23, 10625. [Google Scholar] [CrossRef] [PubMed]
- Ojeda, G.; Ronda, M.; Ballester, S.; Díez-Orejas, R.; Feito, M.J.; García-Albert, L.; Rojo, J.M.; Portolés, P. A hyper reactive variant of a CD4+ T cell line is activated by syngeneic antigen presenting cells in the absence of antigen. Cell Immunol. 1995, 164, 265. [Google Scholar] [CrossRef] [PubMed]
- Rojo, J.M.; Saizawa, K.; Janeway, C.A. Physical association of CD4 and the T-cell receptor can be induced by anti-T-cell receptor antibodies. Proc. Natl. Acad. Sci. USA 1989, 86, 3311–3315. [Google Scholar] [CrossRef]
- Matesanz, M.-C.; Vila, M.; Feito, M.-J.; Linares, J.; Gonçalves, G.; Vallet-Regí, M.; Marques, P.A.A.P.; Portolés, M.-T. The effects of graphene oxide nanosheets localized on F-actin filaments on cell-cycle alterations. Biomaterials 2013, 34, 1562–1569. [Google Scholar] [CrossRef]
- Cicuéndez, M.; Casarrubios, L.; Barroca, N.; Silva, D.; Feito, M.J.; Diez-Orejas, R.; Marques, P.A.A.P.; Portolés, M.T. Benefits in the Macrophage Response Due to Graphene Oxide Reduction by Thermal Treatment. Int. J. Mol. Sci. 2021, 22, 6701. [Google Scholar] [CrossRef]
- Aslankoohi, N.; Mondal, D.; Rizkalla, A.S.; Mequanint, K. Bone Repair and Regenerative Biomaterials: Towards Recapitulating the Microenvironment. Polymers 2019, 11, 1437. [Google Scholar] [CrossRef] [Green Version]
- Dialynas, D.P.; Wilde, D.B.; Marrack, P.; Pierres, A.; Wall, K.A.; Havran, W.; Otten, G.; Loken, M.R.; Pierres, M.; Kappler, J.; et al. Characterization of the Murine Antigenic Determinant, Designated L3T4a, Recognized by Monoclonal Antibody GK 1.5: Expression of L3T4a by Functional T Cell Clones Appears to Correlate Primarily with Class II MHC Antigen-Reactivity. Immunol. Rev. 1983, 74, 29–56. [Google Scholar] [CrossRef]
- Na, H.; Cho, M.; Chung, Y. Regulation of Th2 Cell Immunity by Dendritic Cells. Immune Netw. 2016, 16, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Malissen, B.; Bongrand, P. Early T Cell Activation: Integrating Biochemical, Structural, and Biophysical Cues. Annu. Rev. Immunol. 2015, 33, 539–561. [Google Scholar] [CrossRef] [PubMed]
- Glassman, C.R.; Parrish, H.L.; Lee, M.S.; Kuhns, M.S. Reciprocal TCR-CD3 and CD4 Engagement of a Nucleating pMHCII Stabilizes a Functional Receptor Macrocomplex. Cell Rep. 2018, 22, 1263–1275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortega, N.; Rosas, C. Reactive oxygen species-associated cell signaling and its role in wound healing. J. Health Med. Sci. 2020, 6, 199. [Google Scholar]
- Plotnikov, A.; Zehorai, E.; Procaccia, S.; Seger, R. The MAPK cascades: Signaling components, nuclear roles and mechanisms of nuclear translocation. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2011, 1813, 1619–1633. [Google Scholar] [CrossRef] [Green Version]
- Callaway, D.A.; Jiang, J.X. Reactive oxygen species and oxidative stress in osteoclastogenesis, skeletal aging and bone diseases. J. Bone Miner. Metab. 2015, 33, 359–370. [Google Scholar] [CrossRef]
- Manolagas, S.C. From Estrogen-Centric to Aging and Oxidative Stress: A Revised Perspective of the Pathogenesis of Osteoporosis. Endocr. Rev. 2010, 31, 266–300. [Google Scholar] [CrossRef] [Green Version]
- Yassa, N.W.; Khalil, S.; Saleh, S.R.; Ghareeb, D.A.; El Demellawy, M.A.; El-Sayed, M.M. Ipriflavone and Ipriflavone loaded albumin nanoparticles reverse lipopolysaccharide induced neuroinflammation in rats. PLoS ONE 2020, 15, e0237929. [Google Scholar] [CrossRef]
- Chen, Y.; Li, J.; Shi, J.; Ning, D.; Feng, J.; Lin, W.; He, F.; Xie, Z. Ipriflavone suppresses NLRP3 inflammasome activation in host response to biomaterials and promotes early bone healing. J. Clin. Periodontol. 2022, 49, 814–827. [Google Scholar] [CrossRef]
- Saxena, Y.; Routh, S.; Mukhopadhaya, A. Immunoporosis: Role of Innate Immune Cells in Osteoporosis. Front. Immunol. 2021, 12, 687037. [Google Scholar] [CrossRef]
- Oishi, S.; Takano, R.; Tamura, S.; Tani, S.; Iwaizumi, M.; Hamaya, Y.; Takagaki, K.; Nagata, T.; Seto, S.; Horii, T.; et al. M2 polarization of murine peritoneal macrophages induces regulatory cytokine production and suppresses T-cell proliferation. Immunology 2016, 149, 320–328. [Google Scholar] [CrossRef] [Green Version]
- Wynn, T.A.; Vannella, K.M. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity 2016, 44, 450–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sica, A.; Mantovani, A. Macrophage plasticity and polarization: In vivo veritas. J. Clin. Investig. 2012, 122, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Ni, S.; Shan, F.; Geng, J. Interleukin-10 family members: Biology and role in the bone and joint diseases. Int. Immunopharmacol. 2022, 108, 108881. [Google Scholar] [CrossRef] [PubMed]
- Pestka, S.; Krause, C.D.; Sarkar, D.; Walter, M.R.; Shi, Y.; Fisher, P.B. Interleukin-10andRelatedCytokines andReceptors. Annu. Rev. Immunol. 2004, 22, 929–979. [Google Scholar] [CrossRef] [PubMed]
- Mangashetti, L.S.; Khapli, S.M.; Wani, M.R. IL-4 inhibits bone-resorbing activity of mature osteoclasts by affecting NF-kappa B and Ca2+ signaling. J. Immunol. 2005, 175, 917. [Google Scholar] [CrossRef] [Green Version]
- Moreno, J.L.; Kaczmarek, M.; Keegan, A.D.; Tondravi, M. IL-4 suppresses osteoclast development and mature osteoclast function by a STAT6-dependent mechanism: Irreversible inhibition of the differentiation program activated by RANKL. Blood 2003, 102, 1078–1086. [Google Scholar] [CrossRef] [Green Version]
- Pacifici, R. T cells: Critical bone regulators in health and disease. Bone 2010, 47, 461–471. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, R.K.; Dar, H.Y.; Mishra, P.K. Immunoporosis: Immunology of Osteoporosis—Role of T Cells. Front. Immunol. 2018, 9, 657. [Google Scholar] [CrossRef]
- Noben-Trauth, N.; Hu-Li, J.; Paul, W.E. IL-4 secreted from individual naive CD4+ T cells acts in an autocrine manner to induce Th2 differentiation. Eur. J. Immunol. 2002, 32, 1428–1433. [Google Scholar] [CrossRef] [PubMed]
- Kusaka, T.; Nakayama, M.; Nakamura, K.; Ishimiya, M.; Furusawa, E.; Ogasawara, K. Effect of Silica Particle Size on Macrophage Inflammatory Responses. PLoS ONE 2014, 9, e92634. [Google Scholar] [CrossRef] [PubMed]
- Lebre, F.; Sridharan, R.; Sawkins, M.J.; Kelly, D.J.; O’Brien, F.J.; Lavelle, E.C. The shape and size of hydroxyapatite particles dictate inflammatory responses following implantation. Sci. Rep. 2017, 7, 2922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
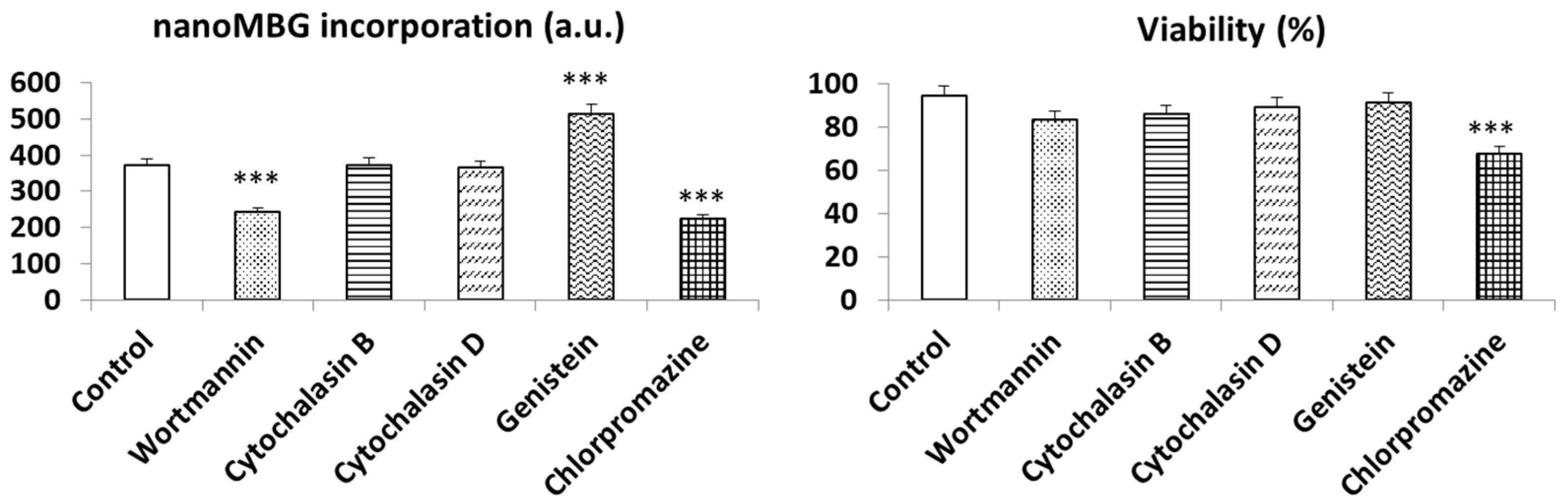


| Inhibitor | Concentration | Mechanisms | Target |
|---|---|---|---|
| Cytochalasin B | 20 µM | Macropinocytosis | Blockage of actin polymerization |
| Cytochalasin D | 4 µM | Macropinocytosis | Blockage of actin polymerization and other endocytosis routes |
| Chlorpromazine | 30 µM | Clathrin-mediated endocytosis | Alteration in the fluidity and permeability of the membrane and the assembly of clathrin-coat |
| Genistein | 3.7 µM | Non clathrin-mediated endocytosis | Blockage of caveolae dynamics and inhibition of Src tyrosine kinases |
| Phenylarsine oxide (PAO) | 3.7 µM | Clathrin-mediated endocytosis | Blockage of tyrosine phosphatases decreasing membrane fluidity |
| Wortmannin | 23 µM | Phagocytosis | Blockage of kinases (as PI3K) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casarrubios, L.; Cicuéndez, M.; Vallet-Regí, M.; Portolés, M.T.; Arcos, D.; Feito, M.J. Osteoimmune Properties of Mesoporous Bioactive Nanospheres: A Study on T Helper Lymphocytes. Nanomaterials 2023, 13, 2183. https://doi.org/10.3390/nano13152183
Casarrubios L, Cicuéndez M, Vallet-Regí M, Portolés MT, Arcos D, Feito MJ. Osteoimmune Properties of Mesoporous Bioactive Nanospheres: A Study on T Helper Lymphocytes. Nanomaterials. 2023; 13(15):2183. https://doi.org/10.3390/nano13152183
Chicago/Turabian StyleCasarrubios, Laura, Mónica Cicuéndez, María Vallet-Regí, María Teresa Portolés, Daniel Arcos, and María José Feito. 2023. "Osteoimmune Properties of Mesoporous Bioactive Nanospheres: A Study on T Helper Lymphocytes" Nanomaterials 13, no. 15: 2183. https://doi.org/10.3390/nano13152183






