Influence of Fe Doping on the Electrochemical Performance of a ZnO-Nanostructure-Based Electrode for Supercapacitors
Abstract
1. Introduction
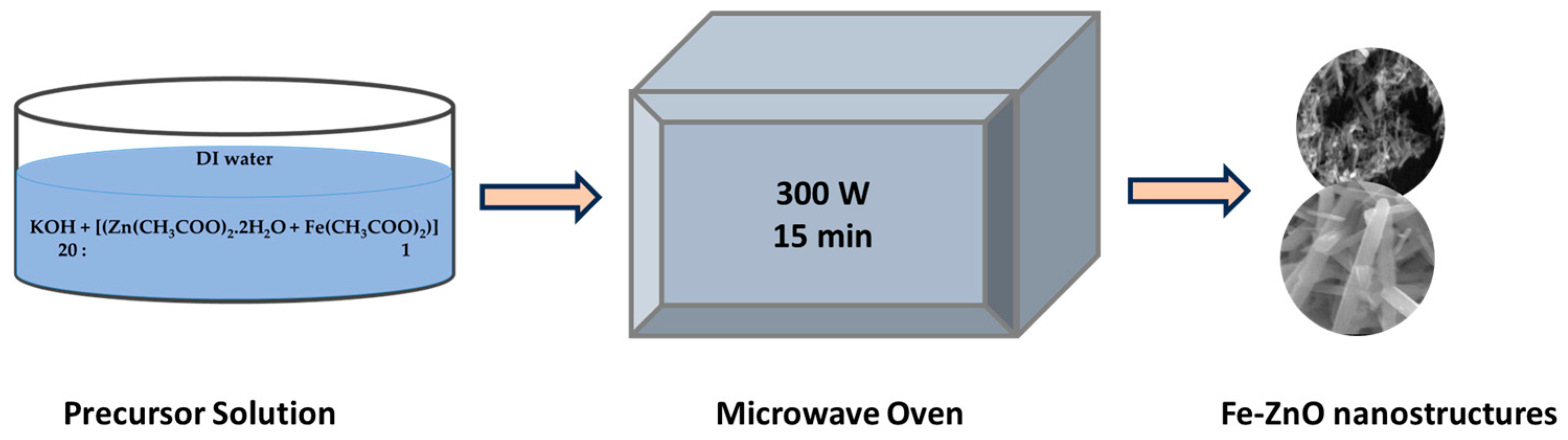
2. Experimental Section
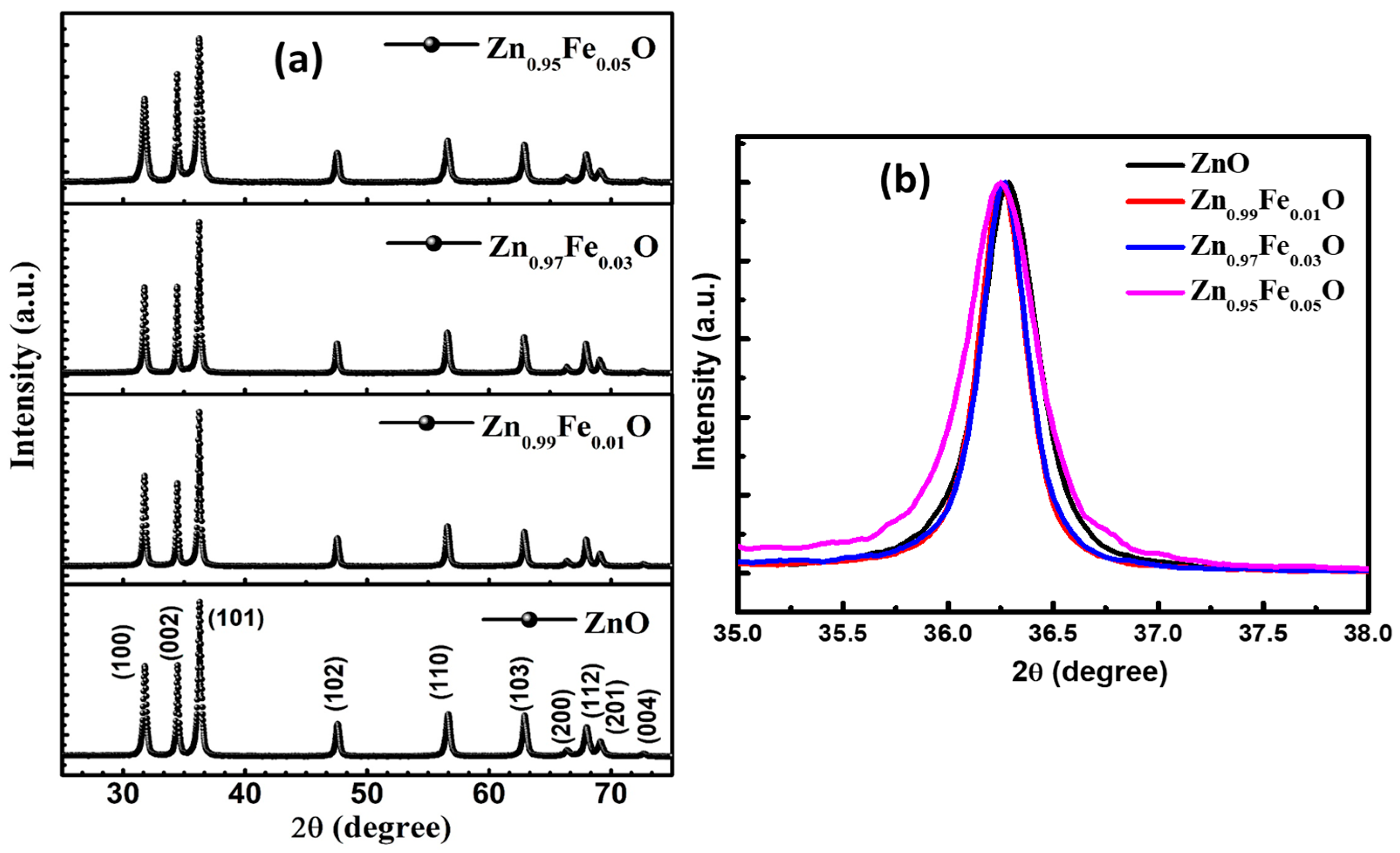
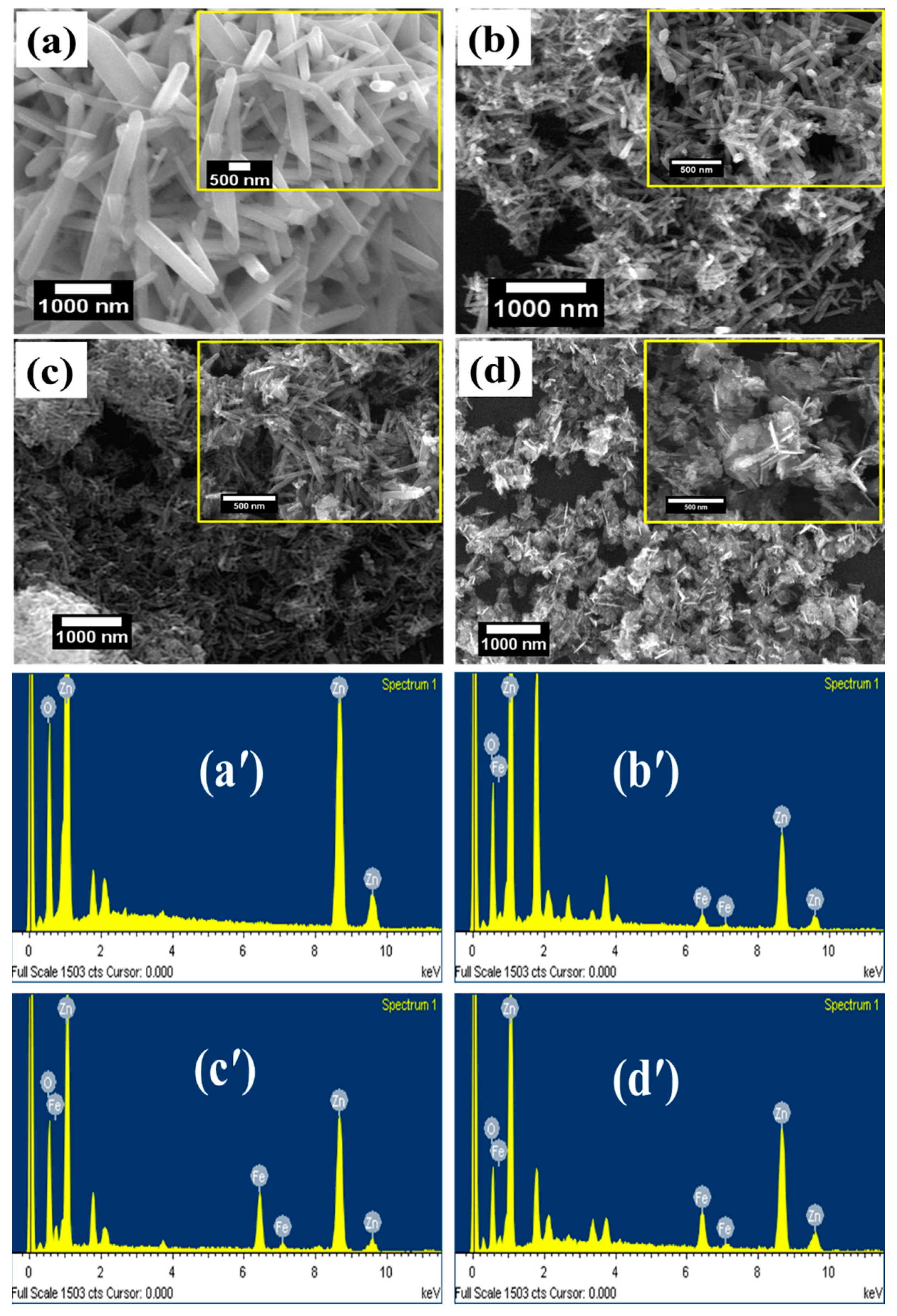
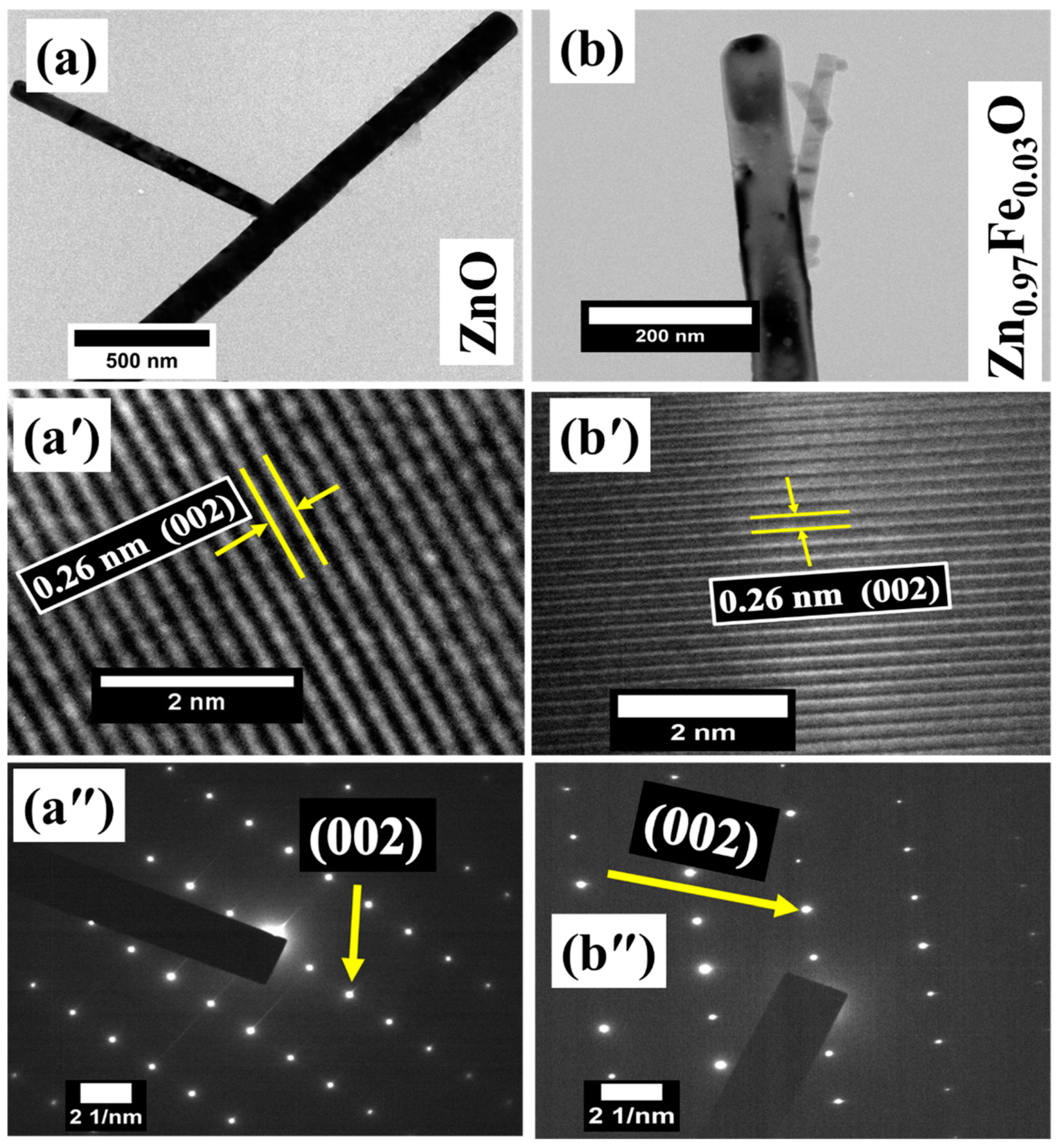
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, S.; Kim, Y.J.; Koo, B.H.; Gautam, S.; Chae, K.H.; Kumar, R.; Lee, C.G. Room temperature ferromagnetism in chemically synthesized ZnO rods. Mater. Lett. 2009, 63, 194–196. [Google Scholar] [CrossRef]
- Ahmed, F.; Arshi, N.; Anwar, M.S.; Danish, R.; Koo, B.H. Effect of Transition Metal (Co, Ni and Cu) doping on Lattice Volume, Band Gap, Morphology and Saturation Magnetization of ZnO Nanostructures. J. Korean Phys. Soc. 2013, 62, 1479–1484. [Google Scholar] [CrossRef]
- Consonni, V.; Briscoe, J.; Kärber, E.; Li, X.; Cossuet, T. ZnO nanowires for solar cells: A comprehensive review. Nanotechnology 2019, 30, 362001. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.; Kumar, S.; Arshi, N.; Anwar, M.S.; Heo, S.N.; Koo, B.H. Direct relationship between lattice volume, bandgap, morphology and magnetization of transition metals (Cr, Mn and Fe)-doped ZnO nanostructures. Acta Mater. 2012, 60, 5190–5196. [Google Scholar] [CrossRef]
- Kumar, S.; Kim, Y.J.; Koo, B.H.; Sharma, S.K.; Vargas, J.M.; Knobel, M.; Gautam, S.; Chae, K.H.; Kim, D.K.; Kim, Y.K.; et al. Structural and magnetic properties of chemically synthesized Fe doped ZnO. J. Appl. Phys. 2009, 105, 07C520. [Google Scholar] [CrossRef]
- Blinov, A.V.; Kachanov, M.D.; Gvozdenko, A.A.; Nagdalian, A.A.; Blinova, A.A.; Rekhman, Z.A.; Golik, A.B.; Vakalov, D.S.; Maglakelidze, D.G.; Nagapetova, A.G.; et al. Synthesis and Characterization of Zinc Oxide Nanoparticles Stabilized with Biopolymers for Application in Wound-Healing Mixed Gels. Gels 2023, 9, 57. [Google Scholar] [CrossRef]
- Umar, A.; Kim, S.H.; Kumar, R.; Al-Assiri, M.S.; Al-Salami, A.E.; Ibrahim, A.A.; Baskoutas, S. In-Doped ZnO Hexagonal Stepped Nanorods and Nanodisks as Potential Scaffold for Highly-Sensitive Phenyl Hydrazine Chemical Sensors. Materials 2017, 10, 1337. [Google Scholar] [CrossRef]
- Pimentel, A.; Samouco, A.; Nunes, D.; Araújo, A.; Martins, R.; Fortunato, E. Ultra-Fast Microwave Synthesis of ZnO Nanorods on Cellulose Substrates for UV Sensor Applications. Materials 2017, 10, 1308. [Google Scholar] [CrossRef]
- Deng, W.; Xu, Y.; Zhang, X.; Li, C.; Liu, Y.; Xiang, K.; Chen, H. (NH4)2Co2V10O28·16H2O/(NH4)2V10O25·8H2O Heterostructure as Cathode for High-Performance Aqueous Zn-Ion Batteries. J. Alloys Compd. 2022, 903, 163824. [Google Scholar] [CrossRef]
- Li, D.; Guo, H.; Jiang, S.; Zeng, G.; Zhou, W.; Li, Z. Microstructures and Electrochemical Performances of TiO2-Coated Mg–Zr Co-Doped NCM as a Cathode Material for Lithium-Ion Batteries with High Power and Long Circular Life. New J. Chem. 2021, 45, 19446–19455. [Google Scholar] [CrossRef]
- Zhou, W.; Zeng, G.; Jin, H.; Jiang, S.; Huang, M.; Zhang, C.; Chen, H. Bio-Template Synthesis of V2O3@Carbonized Dictyophora Composites for Advanced Aqueous Zinc-Ion Batteries. Molecules 2023, 28, 2147. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.-N.; Li, Y.-H.; Xu, D.-F.; Zhou, W.; Xiang, K.-X.; Chen, H. Three-Dimensional Hierarchically Porous Nitrogen-Doped Carbon from Water Hyacinth as Selenium Host for High-Performance Lithium–Selenium Batteries. Rare Met. 2022, 41, 3432–3445. [Google Scholar] [CrossRef]
- Sahu, J.; Kumar, S.; Ahmed, F.; Alvi, P.A.; Dalela, B.; Phase, D.M.; Gupta, M.; Dalela, S. Electrochemical and electronic structure properties of high-performance supercapacitor based on Nd-doped ZnO nanoparticles. J. Energy Storage 2023, 59, 106499. [Google Scholar] [CrossRef]
- Chang, S.-K.; Zainal, Z.; Tan, K.-B.; Yusof, N.A.; Wan Yusoff, W.M.D.; Prabaharan, S.R.S. Recent development in spinel cobaltites for supercapacitor application. Ceram. Int. 2015, 41, 1–14. [Google Scholar] [CrossRef]
- Selvakumar, M.; Krishna Bhat, D.; Manish Aggarwal, A.; Prahladh Iyer, S.; Sravani, G. Nano ZnO-activated carbon composite electrodes for supercapacitors. Phys. B Condens. Matter. 2010, 405, 2286–2289. [Google Scholar] [CrossRef]
- Lee, K.S.; Park, C.W.; Kim, J.D. Synthesis of ZnO/activated carbon with high surface area for supercapacitor electrodes. Colloids Surfaces A Physicochem. Eng. Asp. 2018, 555, 482–490. [Google Scholar] [CrossRef]
- Jayababu, N.; Kim, D. ZnO nanorods@conductive carbon black nanocomposite based flexible integrated system for energy conversion and storage through triboelectric nanogenerator and supercapacitor. Nano Energy 2021, 82, 105726. [Google Scholar] [CrossRef]
- Ogale, S.B. Dilute doping, defects, and ferromagnetism in metal oxide systems. Adv. Mater. 2010, 29, 3125–3155. [Google Scholar] [CrossRef]
- Malhotra, J.S.; Sharma, A.; Singh, A.K.; Kumar, S.; Rana, B.S.; Kumar, S. Investigations on Photocatalytic, Antimicrobial and Magnetic Properties of Sol–Gel-Synthesized Ga-Doped ZnO Nanoparticles. Int. J. Nanosci. 2018, 18, 1850014. [Google Scholar] [CrossRef]
- Agarwal, D.C.; Singh, U.B.; Gupta, S.; Singhal, R.; Kulriya, P.K.; Singh, F.; Tripathi, A.; Singh, J.; Joshi, U.S.; Avasthi, D.K. Enhanced room temperature ferromagnetism and green photoluminescence in Cu doped ZnO thin film synthesised by neutral beam sputtering. Sci. Rep. 2019, 9, 6675. [Google Scholar] [CrossRef]
- Lawes, G.; Risbud, A.S.; Ramirez, A.P.; Seshadri, R. Absence of ferromagnetism in Co and Mn substituted polycrystalline ZnO. Phys. Rev. B 2005, 71, 045201. [Google Scholar] [CrossRef]
- Liu, H.; Yang, J.; Zhang, Y.; Wang, Y.; Wei, M. Ferromagnetism and exchange bias in Fe-doped ZnO nanocrystals. Mater. Chem. Phys. 2008, 112, 1021–1023. [Google Scholar] [CrossRef]
- Mishra, A.K.; Das, D. Investigation on Fe-doped ZnO nanostructures prepared by a chemical route. Mater. Sci. Eng. B 2010, 171, 5–10. [Google Scholar] [CrossRef]
- Pal, B.; Sarkar, D.; Giri, P.K. Structural, optical, and magnetic properties of Ni doped ZnO nanoparticles: Correlation of magnetic moment with defect density. Appl. Surf. Sci. 2015, 356, 804–811. [Google Scholar] [CrossRef]
- Mohanty, S.; Ravi, S. Magnetic properties of Sn1 − xNixO2-based diluted magnetic semiconductors. Solid State Commun. 2010, 150, 1570–1574. [Google Scholar] [CrossRef]
- Maibam, B.; Baruah, S.; Kumar, S. Photoluminescence and intrinsic ferromagnetism of Fe doped zinc oxide. SN Appl. Sci. 2020, 2, 1712. [Google Scholar] [CrossRef]
- Gautam, S.; Kumar, S.; Thakur, P.; Chae, K.H.; Kumar, R.; Koo, B.H.; Lee, C.G. Electronic structure studies of Fe-doped ZnO nanorods by X-ray absorption fine structure. J. Phys. D Appl. Phys. 2009, 42, 175406. [Google Scholar] [CrossRef]
- Jang, D.M.; Kwak, I.H.; Kwon, E.L.; Jung, C.S.; Im, H.S.; Park, K.; Park, J. Transition-metal doping of oxide nanocrystals for enhanced catalytic oxygen evolution. J. Phys. Chem. C 2015, 119, 1921–1927. [Google Scholar] [CrossRef]
- Ahmad, I.; Akhtar, M.S.; Manzoor, M.F.; Wajid, M.; Noman, M.; Ahmed, E.; Ahmad, M.; Khan, W.Q.; Rana, A.M. Synthesis of yttrium and cerium doped ZnO nanoparticles as highly inexpensive and stable photocatalysts for hydrogen evolution. J. Rare Earths 2021, 39, 440–445. [Google Scholar] [CrossRef]
- Hui, A.; Ma, J.; Liu, J.; Bao, Y.; Zhang, J. Morphological evolution of Fe doped sea urchin-shaped ZnO nanoparticles with enhanced photocatalytic activity. J. Alloys Compd. 2017, 696, 639–647. [Google Scholar] [CrossRef]
- Rashid, A.R.; Abid, A.G.; Manzoor, S.; Mera, A.; Al-Muhimeed, T.I.; AlObaid, A.A.; Shah, S.N.; Ashiq, M.N.; Imran, M.; Najam-Ul-Haq, M. Inductive Effect in Mn-Doped ZnO Nanoribon Arrays Grown on Ni Foam: A Promising Key for Boosted Capacitive and High Specific Energy Supercapacitors. Ceram. Int. 2021, 47, 28338–28347. [Google Scholar] [CrossRef]
- Luo, Q.; Xu, P.; Qiu, Y.; Cheng, Z.; Chang, X.; Fan, H. Synthesis of ZnO tetrapods for high-performance supercapacitor applications. Mater. Lett. 2017, 198, 192–195. [Google Scholar] [CrossRef]
- Mordina, B.; Kumar, R.; Neeraj, N.S.; Srivastava, A.K.; Setua, D.K.; Sharma, A. Binder free high performance hybrid supercapacitor device based on nickel ferrite nanoparticles. J. Energy Storage 2020, 31, 101677. [Google Scholar] [CrossRef]
- Kumar, S.; Ahmed, F.; Ahmad, N.; Shaalan, N.M.; Kumar, R.; Alshoaibi, A.; Arshi, N.; Dalela, S.; Albossed, M.; Chae, K.H.; et al. Role of Cr Doping on the Structure, Electronic Structure, and Electrochemical Properties of BiFeO3 Nanoparticles. Materials 2022, 15, 4118. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.C.; Lyu, Y.R.; Fang, A.; Lee, G.J.; Karuppasamy, L.; Wu, J.J.; Lin, C.K.; Anandan, S.; Chen, C.Y. The design of ZnO nanorod arrays coated with MnOx for high electrochemical stability of a pseudocapacitor electrode. Nanomaterials 2020, 10, 475. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, H.; Pan, L.; Lu, T.; Sun, Z. Capacitive behavior of graphene–ZnO composite film for supercapacitors. J. Electroanal. Chem. 2009, 634, 68–71. [Google Scholar] [CrossRef]
- Jayalakshmi, M.; Palaniappa, M.; Balasubramanian, K. Single step solution combustion synthesis of ZnO/carbon composite and its electrochemical characterization for supercapacitor application. Int. J. Electrochem. Sci. 2008, 3, 96–103. [Google Scholar] [CrossRef]
- Lu, T.; Pan, L.; Li, H.; Zhu, G.; Lv, T.; Liu, X.; Sun, Z.; Chen, T.; Chua, D.H.C. Microwave-assisted synthesis of graphene–ZnO nanocomposite for electrochemical supercapacitors. J. Alloys Compd. 2011, 509, 5488–5492. [Google Scholar] [CrossRef]
- He, X.; Yoo, J.E.; Lee, M.H.; Bae, J. Morphology engineering of ZnO nanostructures for high performance supercapacitors: Enhanced electrochemistry of ZnO nanocones compared to ZnO nanowires. Nanotechnology 2017, 28, 245402. [Google Scholar] [CrossRef]
- Duan, G.; Zhang, H.; Zhang, C.; Jiang, S.; Hou, H. High Mass-Loading α-Fe2O3 Nanoparticles Anchored on Nitrogen-Doped Wood Carbon for High-Energy-Density Supercapacitor. Chin. Chem. Lett. 2023, 108283. [Google Scholar] [CrossRef]
- Guo, W.; Guo, X.; Yang, L.; Wang, T.; Zhang, M.; Duan, G.; Liu, X.; Li, Y. Synthetic Melanin Facilitates MnO Supercapacitors with High Specific Capacitance and Wide Operation Potential Window. Polymer 2021, 19, 235. [Google Scholar] [CrossRef]
- Yang, J.; Li, H.; He, S.; Du, H.; Liu, K.; Zhang, C.; Jiang, S. Facile Electrodeposition of NiCo2O4 Nanosheets on Porous Carbonized Wood for Wood-Derived Asymmetric Supercapacitors. Polymers 2022, 14, 2521. [Google Scholar] [CrossRef] [PubMed]
- Duan, G.; Zhao, L.; Chen, L.; Wang, F.; He, S.; Jiang, S.; Zhang, Q. ZnCl2 Regulated Flax-Based Porous Carbon Fibers for Supercapacitors with Good Cycling Stability. New J. Chem. 2021, 45, 22602–22609. [Google Scholar] [CrossRef]
- Wang, F.; Chen, L.; Li, H.; Duan, G.; He, S.; Zhang, L.; Zhang, G.; Zhou, Z.; Jiang, S. N-Doped Honeycomb-like Porous Carbon towards High-Performance Supercapacitor. Chin. Chem. Lett. 2020, 31, 1986–1990. [Google Scholar] [CrossRef]
- Li, H.; Cao, L.; Zhang, H.; Tian, Z.; Zhang, Q.; Yang, F.; Yang, H.; He, S.; Jiang, S. Intertwined Carbon Networks Derived from Polyimide/Cellulose Composite as Porous Electrode for Symmetrical Supercapacitor. J. Colloid Interface Sci. 2022, 609, 179–187. [Google Scholar] [CrossRef]
- Shin, H.J.; Abbas, S.; Kim, J.; Cho, J.; Ha, H.Y. Near-Perfect Suppression of Li Dendrite Growth by Novel Porous Hollow Carbon Fibers Embedded with ZnO Nanoparticles as Stable and Efficient Anode for Li Metal Batteries. Chem. Eng. J. 2023, 464, 142713. [Google Scholar] [CrossRef]
- Song, Y.; Chen, Y.; Wang, Z.; Zhao, W.; Qin, C.; Yu, H.; Wang, X.; Bakenov, Z.; Zhang, Y. Defective ZnOx@porous Carbon Nanofiber Network Inducing Dendrite-Free Zinc Plating as Zinc Metal Anode for High-Performance Aqueous Rechargeable Zn/Na4Mn9O18 Battery Based on Hybrid Electrolyte. J. Power Sources 2022, 518, 230761. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, Q.; Jiu, H.; Song, W.; Yang, J.; Li, X.; Wei, H.; Wang, C.; Li, X.; Zhao, J. High-Level Pyrrolic-N-Doped Carbon Coating Concave Hollow ZnO@C Dodecahedrons for High-Capacity Lithium-Ion Batteries. J. Alloys Compd. 2022, 915, 165353. [Google Scholar] [CrossRef]
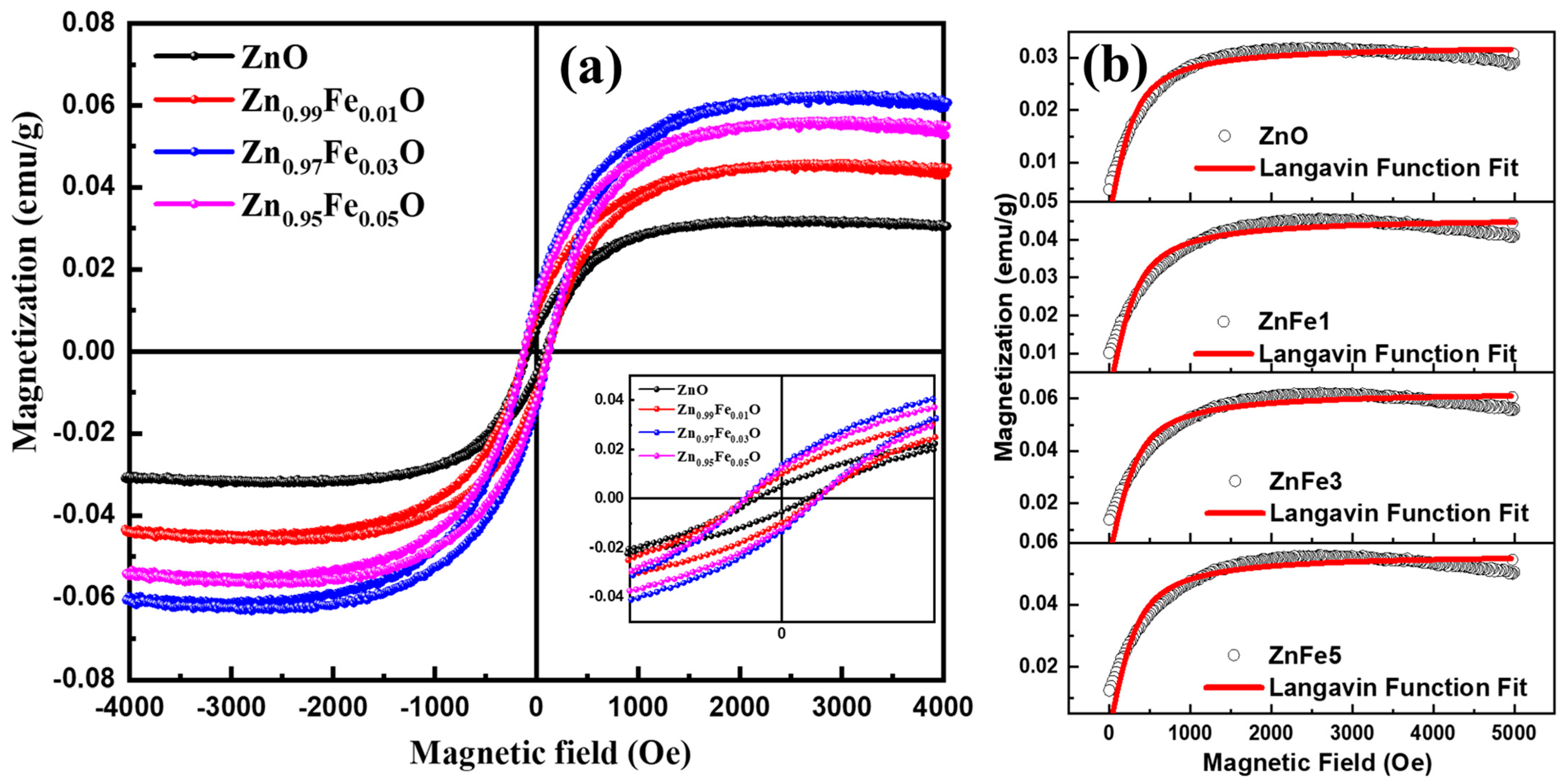



| Mo (emu/g) | meff/kBT | N (cm−3) | meff (emu) | |
|---|---|---|---|---|
| ZnO | 0.03247 | 0.00734 | 1.06799 × 1014 | 3.0403 × 10−16 |
| ZnFe1 | 0.04604 | 0.00678 | 1.6394 × 1014 | 2.8083 × 10−16 |
| ZnFe3 | 0.06289 | 0.00674 | 2.25269 × 1014 | 2.7918 × 10−16 |
| ZnFe5 | 0.0566 | 0.00674 | 2.02738 × 1014 | 2.7918 × 10−16 |
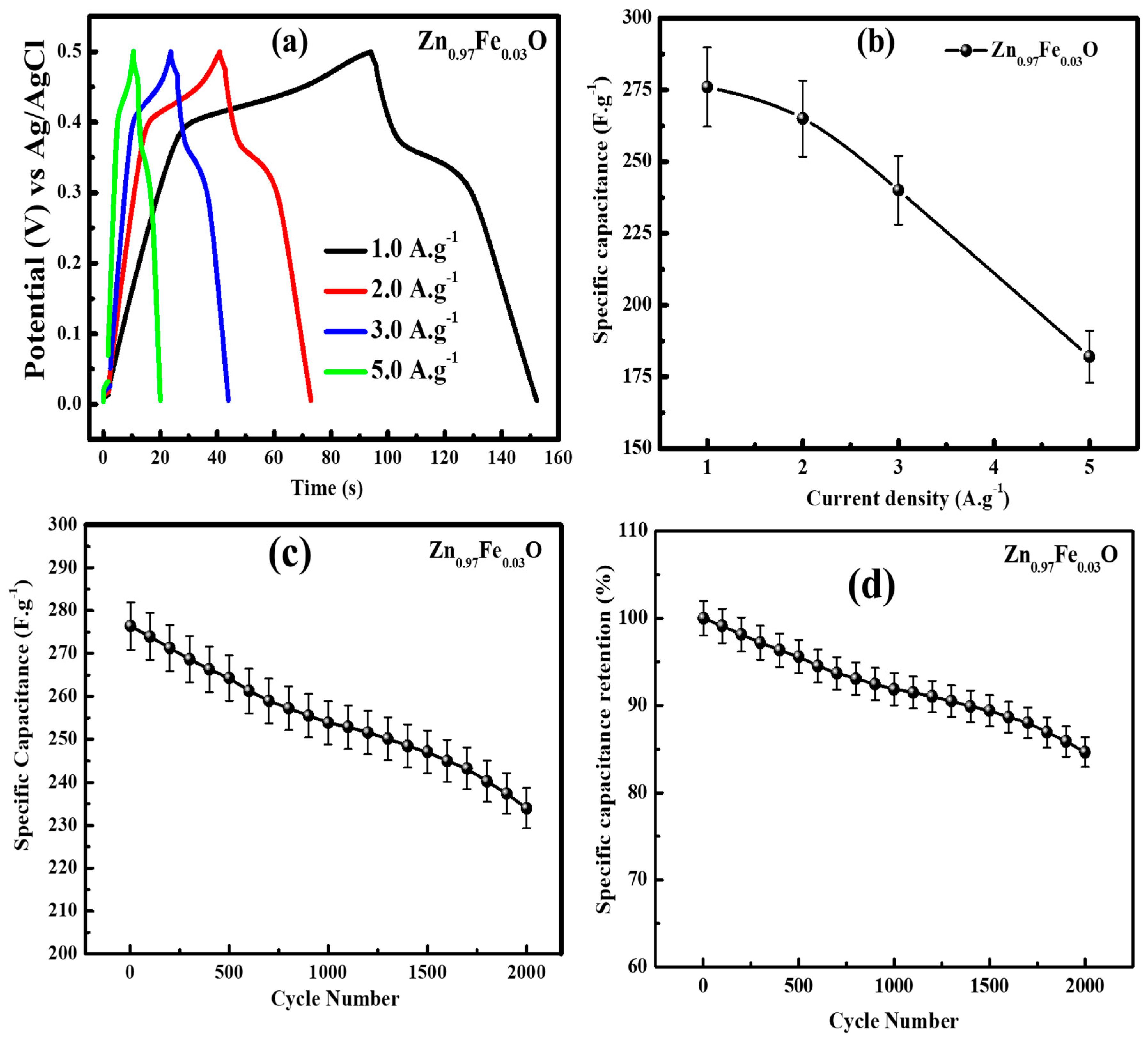
| S. No. | Material | Specific Capacitance (F/g) | Reference |
|---|---|---|---|
| 1 | Nd-doped ZnO | 154 | Sahu et al. [6] |
| 2 | ZnO Tetrapod | 160.4 | Luo et al. [22] |
| 3 | Graphene/ZnO | 146 | Lu et al. [28] |
| 4 | ZnO Nanowire | 191.5 | He et al. [31] |
| 5 | α-Fe2O3 Nanoparticles | 603 | Duan et al. [39] |
| 6 | MnO | 545 | Guo et al. [40] |
| 7 | NiCo2O4 Nanosheets | 1730 | Yang et al. [41] |
| 8 | ZnCl2 Porous Carbon Fibers | 105 | Duan et al. [42] |
| 9 | N-Doped Honeycomb-like Porous Carbon | 275 | Wang et al. [43] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, S.; Ahmed, F.; Shaalan, N.M.; Arshi, N.; Dalela, S.; Chae, K.H. Influence of Fe Doping on the Electrochemical Performance of a ZnO-Nanostructure-Based Electrode for Supercapacitors. Nanomaterials 2023, 13, 2222. https://doi.org/10.3390/nano13152222
Kumar S, Ahmed F, Shaalan NM, Arshi N, Dalela S, Chae KH. Influence of Fe Doping on the Electrochemical Performance of a ZnO-Nanostructure-Based Electrode for Supercapacitors. Nanomaterials. 2023; 13(15):2222. https://doi.org/10.3390/nano13152222
Chicago/Turabian StyleKumar, Shalendra, Faheem Ahmed, Nagih M. Shaalan, Nishat Arshi, Saurabh Dalela, and Keun Hwa Chae. 2023. "Influence of Fe Doping on the Electrochemical Performance of a ZnO-Nanostructure-Based Electrode for Supercapacitors" Nanomaterials 13, no. 15: 2222. https://doi.org/10.3390/nano13152222
APA StyleKumar, S., Ahmed, F., Shaalan, N. M., Arshi, N., Dalela, S., & Chae, K. H. (2023). Influence of Fe Doping on the Electrochemical Performance of a ZnO-Nanostructure-Based Electrode for Supercapacitors. Nanomaterials, 13(15), 2222. https://doi.org/10.3390/nano13152222











