Construction of Carbon Nanofiber-Wrapped SnO2 Hollow Nanospheres as Flexible Integrated Anode for Half/Full Li-Ion Batteries
Abstract
:1. Introduction
2. Experimental Section
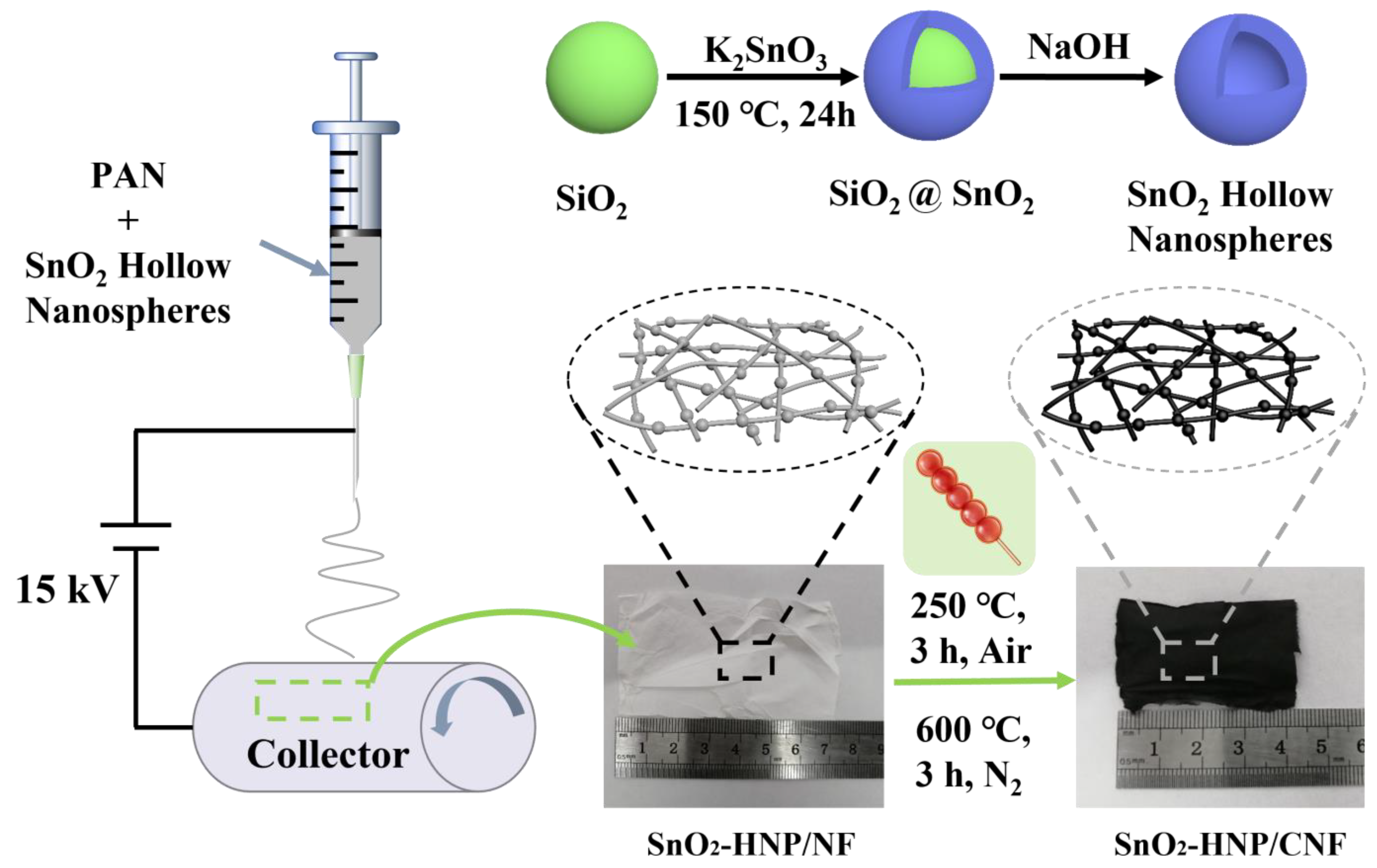
2.1. Synthesis of SiO2
2.2. Synthesis of SiO2@SnO2
2.3. Synthesis of SnO2-HNPs
2.4. Synthesis of SnO2-HNP/CNFs
2.5. Material Structure Characterization
2.6. Electrochemical Measurement
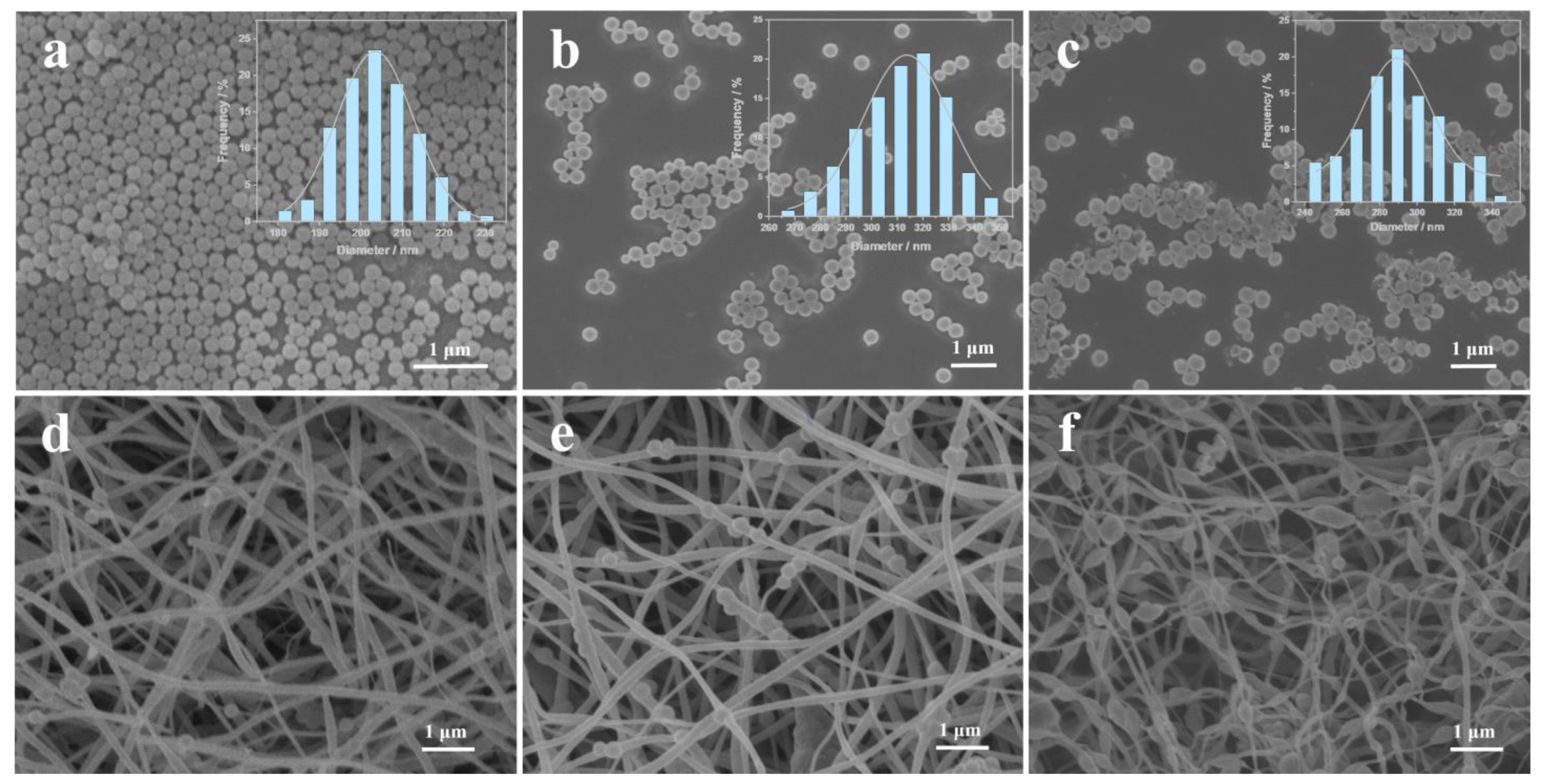
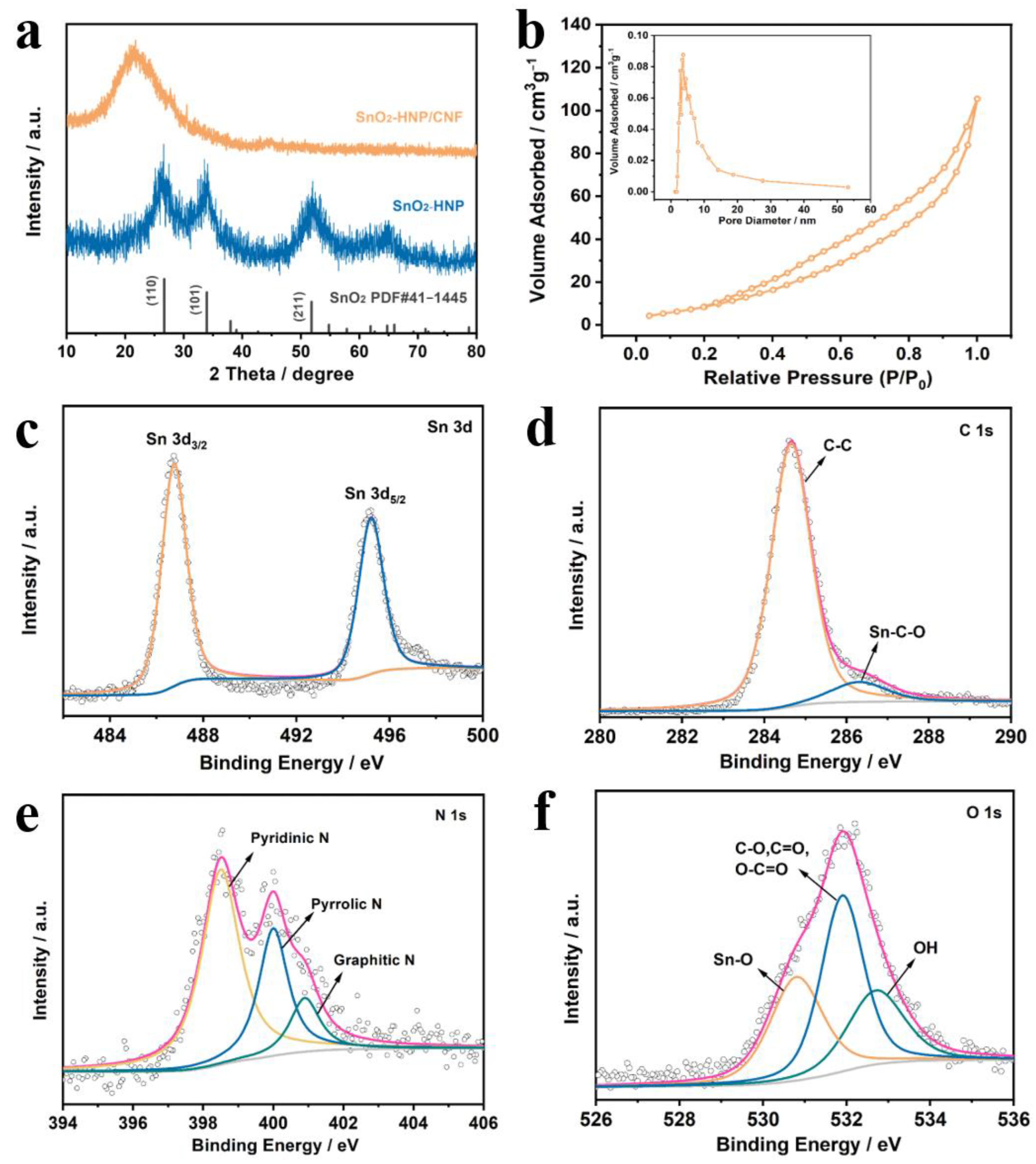
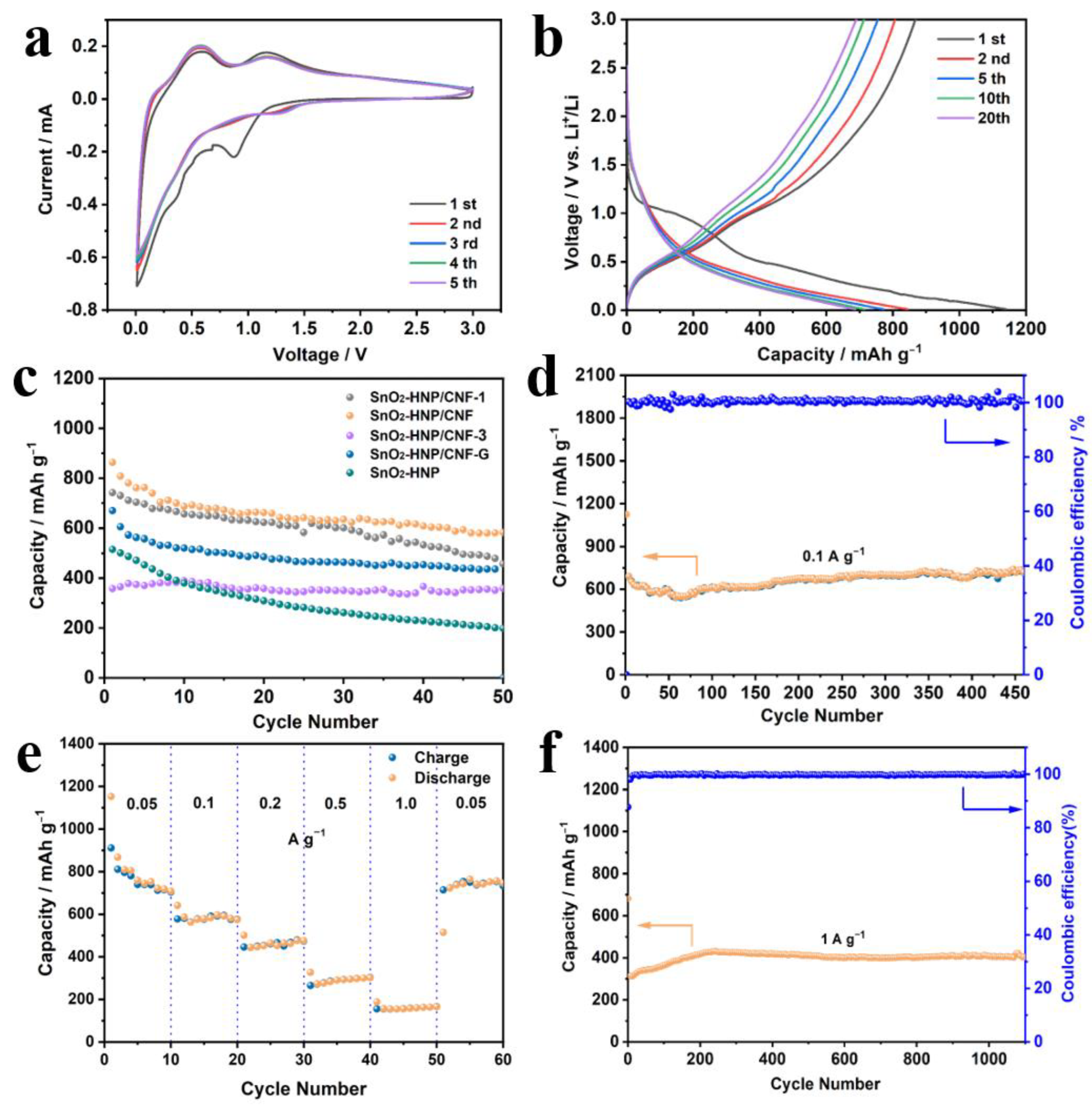
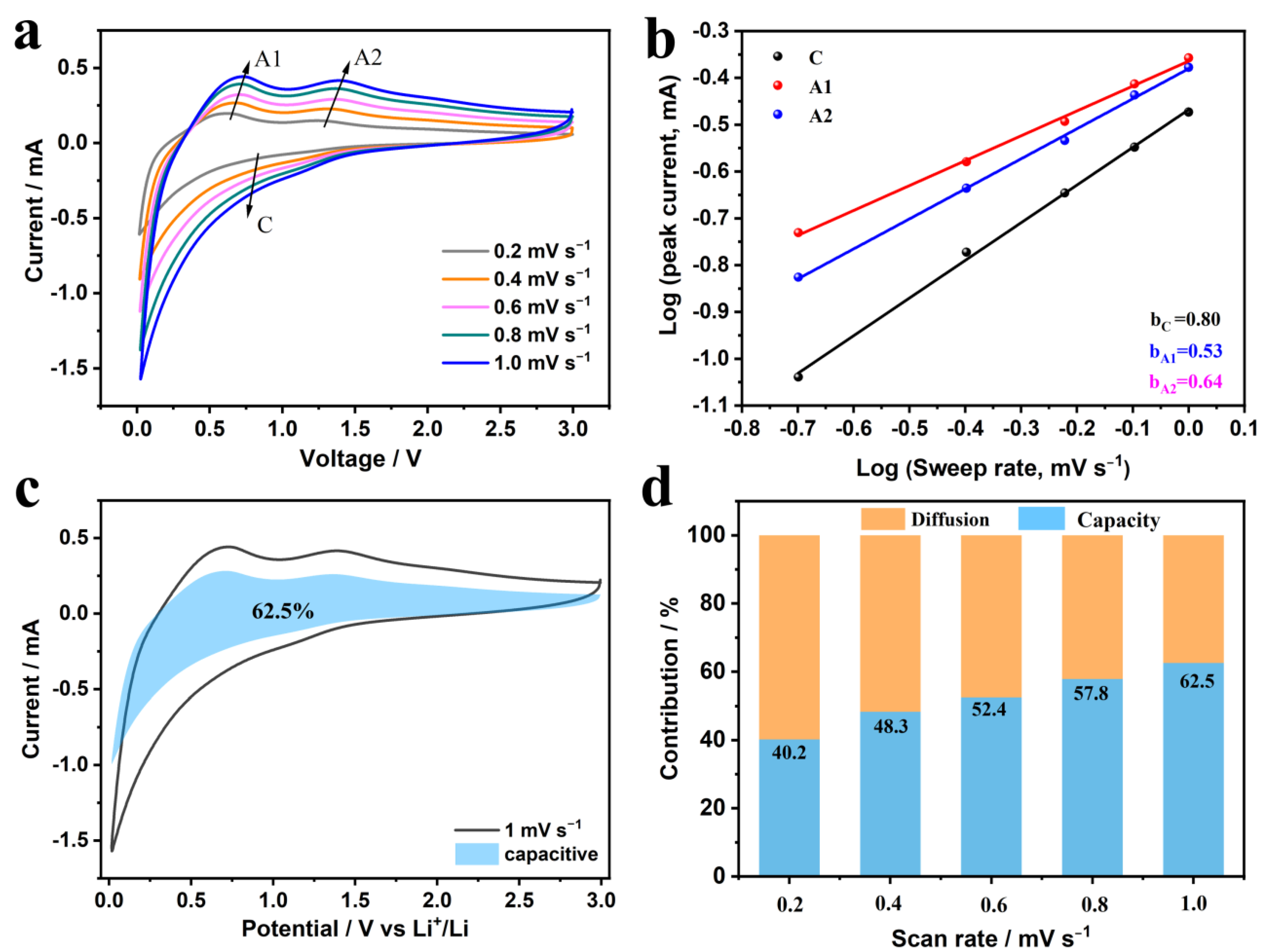
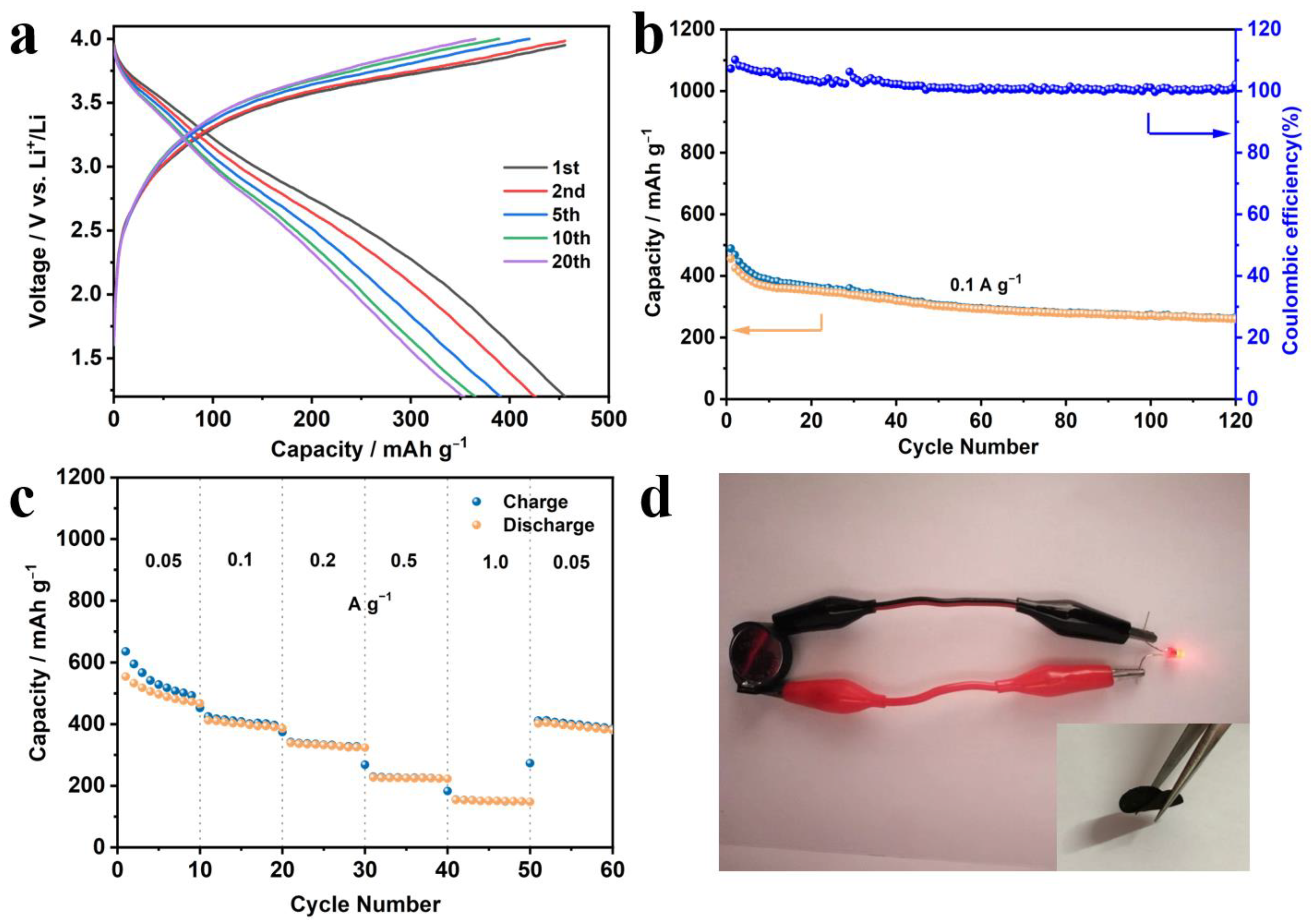
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tarascon, J.-M.; Armand, M. Issues and Challenges Facing Rechargeable Lithium Batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Goodenough, J.B.; Park, K.-S. The Li-Ion Rechargeable Battery: A Perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef]
- Lu, L.; Hu, Y.; Dai, K. The Advance of Fiber-Shaped Lithium Ion Batteries. Mater. Today Chem. 2017, 5, 24–33. [Google Scholar] [CrossRef]
- Xu, J.; Cai, X.; Cai, S.; Shao, Y.; Hu, C.; Lu, S.; Ding, S. High-Energy Lithium-Ion Batteries: Recent Progress and a Promising Future in Applications. Energy Environ. Mater. 2022, e12450. [Google Scholar] [CrossRef]
- Mukherjee, S.; Albertengo, A.; Djenizian, T. Beyond Flexible-Li-Ion Battery Systems for Soft Electronics. Energy Storage Mater. 2021, 42, 773–785. [Google Scholar] [CrossRef]
- Chen, K.; Yang, H.; Liang, F.; Xue, D. Microwave-Irradiation-Assisted Combustion toward Modified Graphite as Lithium Ion Battery Anode. ACS Appl. Mater. Interfaces 2018, 10, 909–914. [Google Scholar] [CrossRef]
- Du, J.; Li, Q.; Chai, J.; Jiang, L.; Zhang, Q.; Han, N.; Zhang, W.; Tang, B. Review of Metal Oxides as Anode Materials for Lithium-Ion Batteries. Dalton Trans. 2022, 51, 9584–9590. [Google Scholar] [CrossRef]
- Yuan, T.; Tang, R.; Xiao, F.; Zuo, S.; Wang, Y.; Liu, J. Modifying SiO as a Ternary Composite Anode Material((SiOx/G/SnO2)@C) for Lithium Battery with High Li-Ion Diffusion and Lower Volume Expansion. Electrochim. Acta 2023, 439, 141655. [Google Scholar] [CrossRef]
- Tran, Q.N.; Choi, H.W. Development of Cellulose Nanofiber-SnO2 Supported Nanocomposite as Substrate Materials for High-Performance Lithium-Ion Batteries. Nanomaterials 2023, 13, 1080. [Google Scholar] [CrossRef]
- Qin, J.; He, C.; Zhao, N.; Wang, Z.; Shi, C.; Liu, E.-Z.; Li, J. Graphene Networks Anchored with Sn@Graphene as Lithium Ion Battery Anode. ACS Nano 2014, 8, 1728–1738. [Google Scholar] [CrossRef]
- Li, B.; Cao, H.; Zhang, J.; Qu, M.; Lian, F.; Kong, X. SnO2-Carbon-RGO Heterogeneous Electrode Materials with Enhanced Anode Performances in Lithium Ion Batteries. J. Mater. Chem. 2012, 22, 2851–2854. [Google Scholar] [CrossRef]
- Yue, L.; Xue, C.; Huang, B.; Xu, N.; Guan, R.; Zhang, Q.; Zhang, W. High Performance Hollow Carbon@SnO2@graphene Composite Based on Internal-External Double Protection Strategy for Lithium Ion Battery. Electrochim. Acta 2016, 220, 222–230. [Google Scholar] [CrossRef]
- Gervillié, C.; Boisard, A.; Labbé, J.; Guérin, K.; Berthon-Fabry, S. Relationship between Tin Environment of SnO2 Nanoparticles and Their Electrochemical Behaviour in a Lithium Ion Battery. Mater. Chem. Phys. 2021, 257, 123461. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, Y.; Li, L.; Ding, Z.; Chen, Y.; Yuan, Q.; Sun, R.; Li, K.; Liu, C.; Wu, J. SnO2 Nanoparticles Embedded in 3D Hierarchical Honeycomb-like Carbonaceous Network for High-Performance Lithium Ion Battery. J. Alloys Compd. 2021, 858, 157716. [Google Scholar] [CrossRef]
- Zhang, L.; Deng, N.; Kang, J.; Wang, X.; Gao, H.; Liu, Y.; Wang, H.; Wang, G.; Cheng, B.; Kang, W. Enhanced Ionic Conductivity in a Novel Composite Electrolyte Based on Gd-Doped SnO2 Nanotubes for Ultra-Long-Life All-Solid-State Lithium Metal Batteries. J. Energy Chem. 2023, 77, 326–337. [Google Scholar] [CrossRef]
- Suzuki, T.; Cheng, J.; Qiao, L.; Xing, Y.; Zhang, M.F.; Nishijima, H.; Yano, T.; Pan, W. Preparation of SnO2 Nanotubes via a Template-Free Electrospinning Process. RSC Adv. 2020, 10, 22113–22119. [Google Scholar] [CrossRef]
- Yan, J.; Zhou, C.; Wang, L.; Zhuang, Y.; Zhong, M.; Zhang, J.; Lei, P.; Li, Y.; Shen, W.; Guo, S. Effects of Pulverization and Dead Sn Accumulation in SnO2 Nanorods Grown on Carbon Cloth on Their Electrochemical Performances as the Anode in Lithium Ion Batteries. ACS Appl. Energy Mater. 2022, 5, 3536–3544. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, Y.; Zhao, C.; Pan, E.; Jia, M. 1D Ultrafine SnO2 Nanorods Anchored on 3D Graphene Aerogels with Hierarchical Porous Structures for High-Performance Lithium/Sodium Storage. J. Colloid Interface Sci. 2018, 532, 352–362. [Google Scholar] [CrossRef]
- Long, J.; Han, T.; Ding, Y.; Hu, C.; Liu, J. Co-Coating ZnCo2O4 and Carbon on a Biomimetic Sea Anemone-Shaped SnO2 Mesostructure for High-Performance Lithium-Ion Batteries and Semi-Solid Lithium Slurry Batteries. Appl. Surf. Sci. 2022, 591, 153220. [Google Scholar] [CrossRef]
- Huang, B.; Li, X.; Pei, Y.; Li, S.; Cao, X.; Massé, R.C.; Cao, G. Novel Carbon-Encapsulated Porous SnO2 Anode for Lithium-Ion Batteries with Much Improved Cyclic Stability. Small 2016, 12, 1945–1955. [Google Scholar] [CrossRef]
- Huang, J.; Dai, Q.; Wu, Q.; Ren, H.; Lu, X.; Gu, C.; Zhang, Y.; Woo Joo, S. Preparation of Hollow SnO2@N-C Nanospheres for High Performance Lithium-Ion Battery. J. Electroanal. Chem. 2022, 922, 116741. [Google Scholar] [CrossRef]
- Choi, J.; Myung, Y.; Gu, M.G.; Kim, S.-K. Nanohybrid Electrodes of Porous Hollow SnO2 and Graphene Aerogel for Lithium Ion Battery Anodes. J. Ind. Eng. Chem. 2019, 71, 345–350. [Google Scholar] [CrossRef]
- Li, B.; Bi, R.; Yang, M.; Gao, W.; Wang, J. Coating Conductive Polypyrrole Layers on Multiple Shells of Hierarchical SnO2 Spheres and Their Enhanced Cycling Stability as Lithium-Ion Battery Anode. Appl. Surf. Sci. 2022, 586, 152836. [Google Scholar] [CrossRef]
- Tang, M.; Ma, X.; Niu, Y.-B.; Zhao, W. Engineering Design of Graphene Tightly Wrapped Hollow Structure for Boosted Lithium Storage in Conversion/Alloying Electrode: A Case of SnO2. Scr. Mater. 2022, 214, 114649. [Google Scholar] [CrossRef]
- Zhu, S.; Liu, J.; Sun, J. Growth of Ultrathin SnO2 on Carbon Nanotubes by Atomic Layer Deposition and Their Application in Lithium Ion Battery Anodes. Appl. Surf. Sci. 2019, 484, 600–609. [Google Scholar] [CrossRef]
- Liu, K.; Zhu, S.; Dong, X.; Huang, H.; Qi, M. Ionic Liquid-Assisted Anchoring SnO2 Nanoparticles on Carbon Nanotubes as Highly Cyclable Anode of Lithium Ion Batteries. Adv. Mater. Interfaces 2020, 7, 1901916. [Google Scholar] [CrossRef]
- Mao, H.; Shi, L.; Song, S.; Xiao, C.; Liang, J.; Dong, B.; Ding, S. N-Doped Hollow Carbon Nanosheet Supported SnO2 Nanoparticles. Inorg. Chem. Front. 2017, 4, 1742–1747. [Google Scholar] [CrossRef]
- Jiang, W.; Xiong, D.; Wu, S.; Gao, J.; Wu, K.; Li, W.; Feng, Y.; He, M.; Feng, Z. Carbon Nanosheets Wrapped in SnO2-TiO2 Nanoparticles as a High Performance Anode Material for Lithium Ion Batteries. Ceram. Int. 2022, 48, 27174–27181. [Google Scholar] [CrossRef]
- Gao, X.; Tang, Z.; Meng, M.; Yu, Q.; Zhu, Y.; Shen, S.; Yang, J. Synthesis of Crumpled SnO2/RGO Nanocomposites with 2D-in-3D Structure and High Performance. Mater. Chem. Phys. 2020, 253, 123298. [Google Scholar] [CrossRef]
- Zhang, D.; Bi, C.; Wu, Q.; Hou, G.; Zheng, G.; Wen, M.; Tang, Y. Co3Sn2/SnO2 Nanocomposite Loaded on Cu Foam as High-Performance Three-Dimensional Anode for Lithium-Ion Batteries. New J. Chem. 2019, 43, 1238–1246. [Google Scholar] [CrossRef]
- Liu, W.; Lu, B.; Liu, X.; Gan, Y.; Zhang, S.; Shi, S. In Situ Synthesis of the Peapod-Like Cu-SnO2@Copper Foam as Anode with Excellent Cycle Stability and High Area Specific Capacity. Adv. Funct. Mater. 2021, 31, 2101999. [Google Scholar] [CrossRef]
- Keshavarz, S.; Okoro, O.V.; Hamidi, M.; Derakhshankhah, H.; Azizi, M.; Mohammad Nabavi, S.; Gholizadeh, S.; Amini, S.M.; Shavandi, A.; Luque, R.; et al. Synthesis, Surface Modifications, and Biomedical Applications of Carbon Nanofibers: Electrospun vs Vapor-Grown Carbon Nanofibers. Coord. Chem. Rev. 2022, 472, 214770. [Google Scholar] [CrossRef]
- Lu, L.; Cao, X.; Shen, Z.; Li, L.; Huo, J.; Chen, W.; Liu, C.; Liu, H. Electrospun Nitrogen-Doped Carbon Nanofibers for Electrocatalysis. Sustain. Mater. Technol. 2020, 26, e00221. [Google Scholar] [CrossRef]
- Lee, K.H.; Lee, S.H.; Ruoff, R.S. Synthesis of Diamond-Like Carbon Nanofiber Films. ACS Nano 2020, 14, 13663–13672. [Google Scholar] [CrossRef]
- Guo, J.; Liu, J.; Dai, H.; Zhou, R.; Wang, T.; Zhang, C.; Ding, S.; Wang, H. Nitrogen Doped Carbon Nanofiber Derived from Polypyrrole Functionalized Polyacrylonitrile for Applications in Lithium-Ion Batteries and Oxygen Reduction Reaction. J. Colloid Interface Sci. 2017, 507, 154–161. [Google Scholar] [CrossRef]
- Xu, Y.; Zhu, Y.; Liu, Y.; Wang, C. Electrochemical Performance of Porous Carbon/Tin Composite Anodes for Sodium-Ion and Lithium-Ion Batteries. Adv. Energy Mater. 2013, 3, 128–133. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman Spectra of Disordered and Amorphous Carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef] [Green Version]
- Shao, Q.; Liu, J.; Wu, Q.; Li, Q.; Wang, H.; Li, Y.; Duan, Q. In Situ Coupling Strategy for Anchoring Monodisperse Co9S8 Nanoparticles on S and N Dual-Doped Graphene as a Bifunctional Electrocatalyst for Rechargeable Zn-Air Battery. Nano-Micro Lett. 2019, 11, 4. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Song, Z.; Sun, T.; Shen, Y.; Lv, X.; Xu, D.; Wang, H. Well-Dispersed Sb2O3 Nanoparticles Encapsulated in Multi-Channel-Carbon Nanofibers as High-Performance Anode Materials for Li/Dual-Ion Batteries. Int. J. Hydrogen Energy 2021, 46, 26308–26317. [Google Scholar] [CrossRef]
- Cheng, Y.; Huang, J.; Qi, H.; Cao, L.; Yang, J.; Xi, Q.; Luo, X.; Yanagisawa, K.; Li, J. Adjusting the Chemical Bonding of SnO2@CNT Composite for Enhanced Conversion Reaction Kinetics. Small 2017, 13, 1700656. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, Z.; Liang, Y.; Yang, Y.; An, N.; Li, Z.; Wu, H. Growth of 3D SnO2 Nanosheets on Carbon Cloth as a Binder-Free Electrode for Supercapacitors. J. Mater. Chem. A 2015, 3, 15057–15067. [Google Scholar] [CrossRef]
- Malitesta, C.; Losito, I.; Sabbatini, L.; Zambonin, P.G. New Findings on Polypyrrole Chemical Structure by XPS Coupled to Chemical Derivatization Labelling. J. Electron Spectrosc. Relat. Phenom. 1995, 76, 629–634. [Google Scholar] [CrossRef]
- Ma, C.; Shao, X.; Cao, D. Nitrogen-Doped Graphene Nanosheets as Anode Materials for Lithium Ion Batteries: A First-Principles Study. J. Mater. Chem. 2012, 22, 8911–8915. [Google Scholar] [CrossRef]
- Yu, X.-Y.; Hu, H.; Wang, Y.; Chen, H.; Lou, X.W. (David) Ultrathin MoS2 Nanosheets Supported on N-Doped Carbon Nanoboxes with Enhanced Lithium Storage and Electrocatalytic Properties. Angew. Chem. Int. Ed. 2015, 127, 7503–7506. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, S.; Liu, Q.; Gu, J.; Guo, Z.; Chen, Z.; Feng, C.; Zhang, D.; Moon, W.-J. Carbon-Coated SnO2@C with Hierarchically Porous Structures and Graphite Layers inside for a High-Performance Lithium-Ion Battery. J. Mater. Chem. 2012, 22, 2766–2773. [Google Scholar] [CrossRef]
- Xia, L.; Wang, S.; Liu, G.; Ding, L.; Li, D.; Wang, H.; Qiao, S. Flexible SnO2/N-Doped Carbon Nanofiber Films as Integrated Electrodes for Lithium-Ion Batteries with Superior Rate Capacity and Long Cycle Life. Small 2016, 12, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; He, Y.; Yue, G.; Li, H.; Li, S.; Liu, J.; Miao, B.; Bai, J.; Cui, Z.; Wang, N.; et al. Pea-like MoS2@NiS1.03-Carbon Heterostructured Hollow Nanofibers for High-Performance Sodium Storage. Carbon Energy 2023, 5, e319. [Google Scholar] [CrossRef]
- Jiang, Y.; Xie, M.; Wu, F.; Ye, Z.; Zhang, Y.; Wang, Z.; Zhou, Y.; Li, L.; Chen, R. Cobalt Selenide Hollow Polyhedron Encapsulated in Graphene for High-Performance Lithium/Sodium Storage. Small 2021, 17, 2102893. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, S.; Zhou, L.; Chang, L.; Liu, W.; Yin, D.; Yi, Z.; Wang, L. SnO2 Quantum Dots: Rational Design to Achieve Highly Reversible Conversion Reaction and Stable Capacities for Lithium and Sodium Storage. Small 2020, 16, 2000681. [Google Scholar] [CrossRef]
- Xie, F.; Zhao, S.; Bo, X.; Li, G.; Fei, J.; Ahmed, E.-A.M.A.; Zhang, Q.; Jin, H.; Wang, S.; Lin, Z. A Robust Solvothermal-Driven Solid-to-Solid Transition Route from Micron SnC2O4 to Tartaric Acid-Capped Nano-SnO2 Anchored on Graphene for Superior Lithium and Sodium Storage. J. Mater. Chem. 2023, 11, 53–67. [Google Scholar] [CrossRef]
- Xu, Q.; Li, Y.; Wu, C.; Sun, X.; Li, Q.; Zhang, H.; Yu, L.; Pan, Y.; Wang, Y.; Guo, S.; et al. Kinetically Accelerated and High-Mass Loaded Lithium Storage Enabled by Atomic Iron Embedded Carbon Nanofibers. Nano Res. 2022, 15, 6176–6183. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shao, Q.; Liu, J.; Yang, X.; Guan, R.; Yu, J.; Li, Y. Construction of Carbon Nanofiber-Wrapped SnO2 Hollow Nanospheres as Flexible Integrated Anode for Half/Full Li-Ion Batteries. Nanomaterials 2023, 13, 2226. https://doi.org/10.3390/nano13152226
Shao Q, Liu J, Yang X, Guan R, Yu J, Li Y. Construction of Carbon Nanofiber-Wrapped SnO2 Hollow Nanospheres as Flexible Integrated Anode for Half/Full Li-Ion Batteries. Nanomaterials. 2023; 13(15):2226. https://doi.org/10.3390/nano13152226
Chicago/Turabian StyleShao, Qi, Jiaqi Liu, Xiantao Yang, Rongqiang Guan, Jing Yu, and Yan Li. 2023. "Construction of Carbon Nanofiber-Wrapped SnO2 Hollow Nanospheres as Flexible Integrated Anode for Half/Full Li-Ion Batteries" Nanomaterials 13, no. 15: 2226. https://doi.org/10.3390/nano13152226



