Methane Catalytic Combustion under Lean Conditions over Pristine and Ir-Loaded La1−xSrxMnO3 Perovskites: Efficiency, Hysteresis, and Time-on-Stream and Thermal Aging Stabilities
Abstract
:1. Introduction
2. Materials and Methods
2.1. LSxM and Ir/LSxM Catalysts Synthesis
2.2. Catalyst Characterization Methods
2.3. Catalytic Activity and Stability Evaluation Experiments
3. Results and Discussion
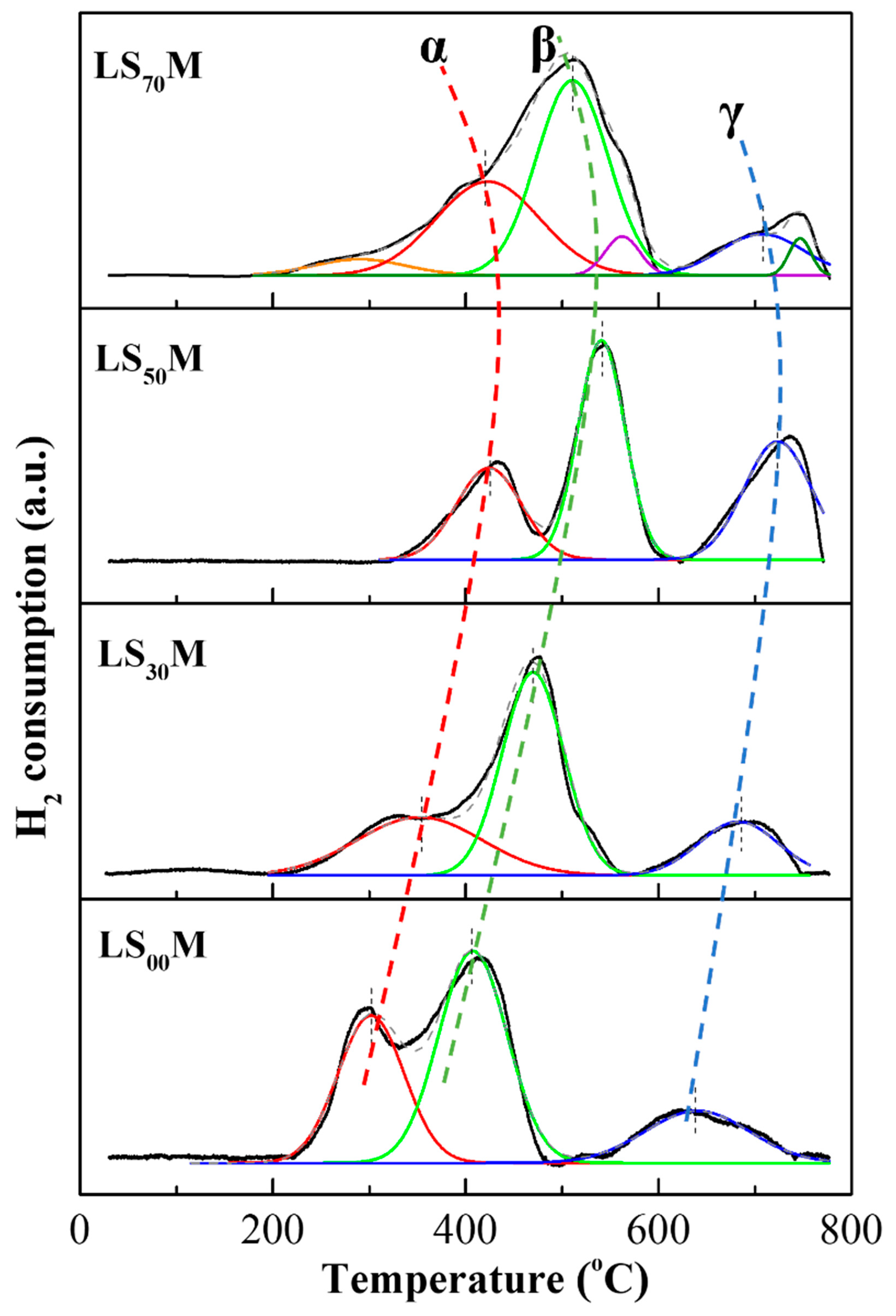
3.1. Catalyst Characteristics and Physicochemical Properties
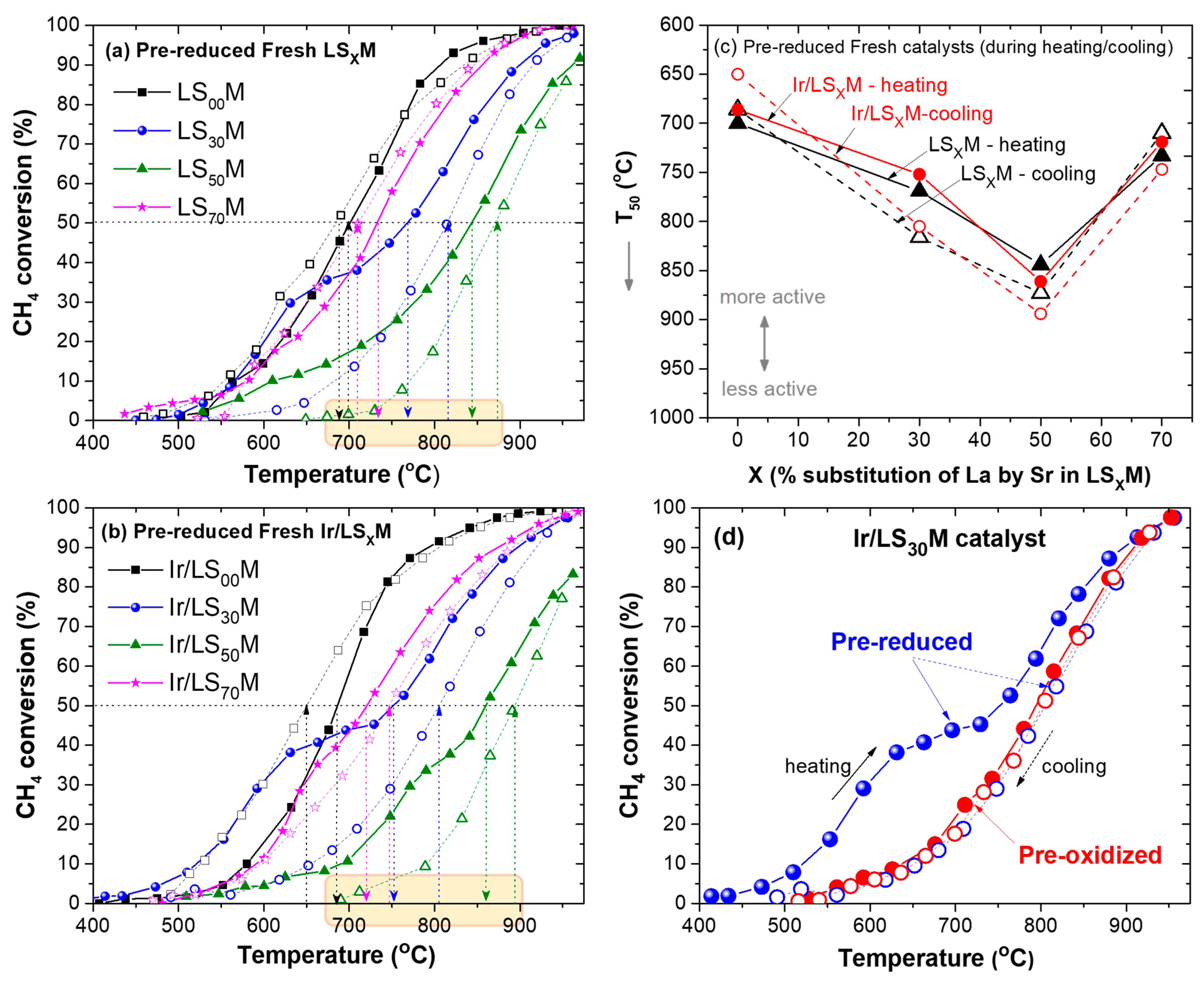
3.2. Light-Off/Light-Out Performance of LSXM and Ir/LSXM Catalysts
3.2.1. Pre-Oxidized Fresh LSXM and Ir/LSXM Catalysts
3.2.2. Pre-Reduced Fresh LSXM and Ir/LSXM Catalysts
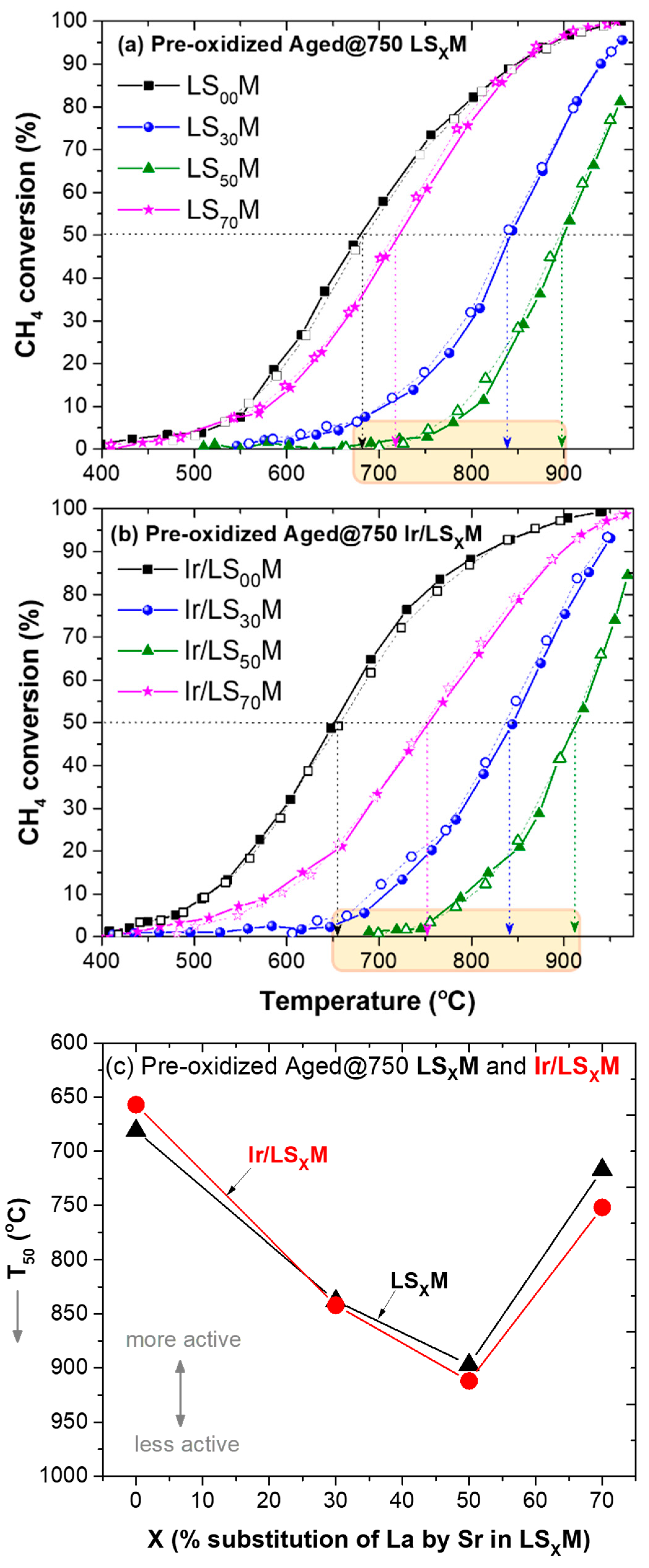
3.3. Light-Off/Light-Out Performance of LSXM and Ir/LSXM Catalysts Aged at 750 °C
3.3.1. Pre-Oxidized Catalysts Aged at 750 °C
3.3.2. Pre-Reduced Catalysts Aged at 750 °C
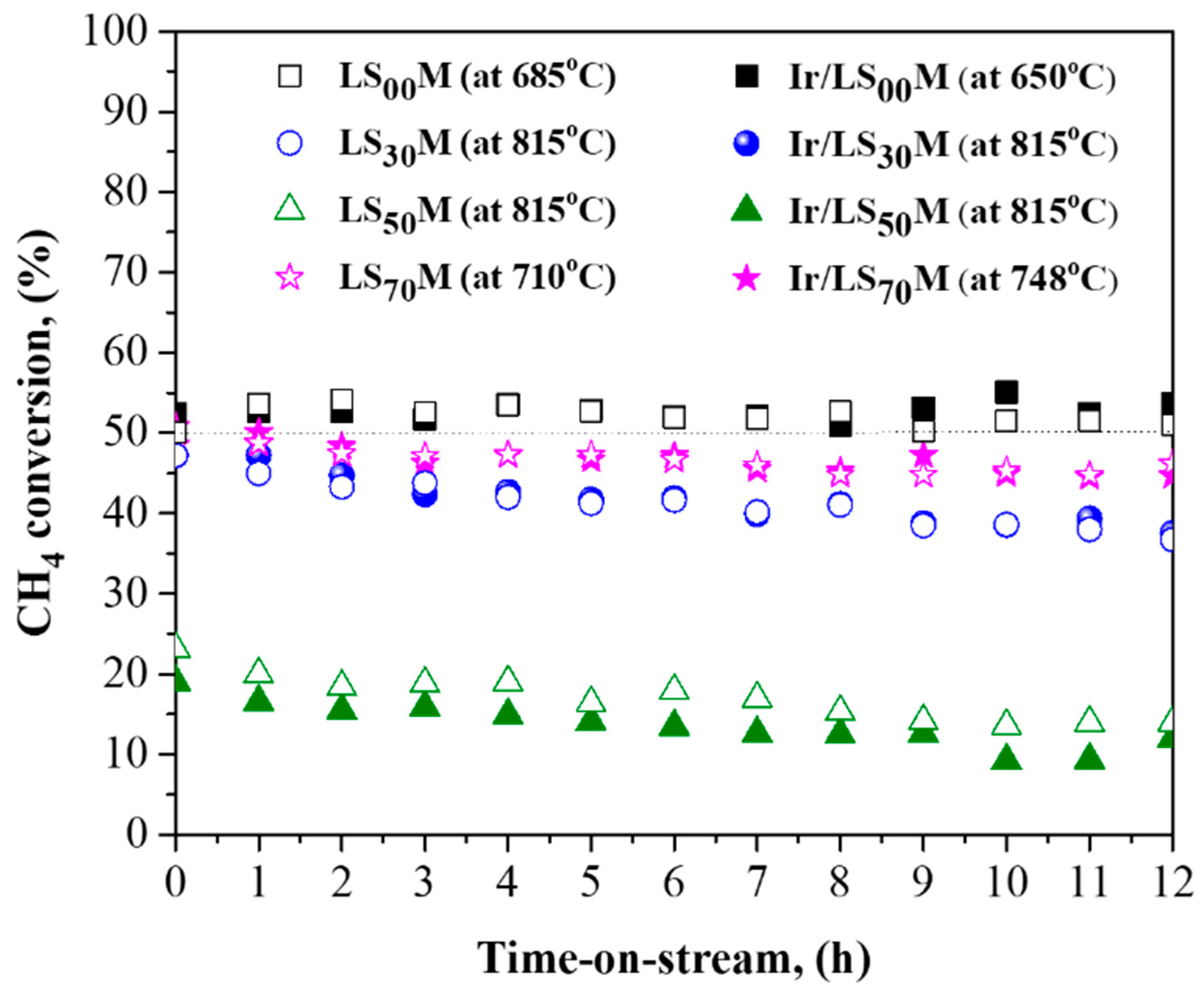
3.4. Thermal Aging and Time-on-Stream Stability of Catalysts
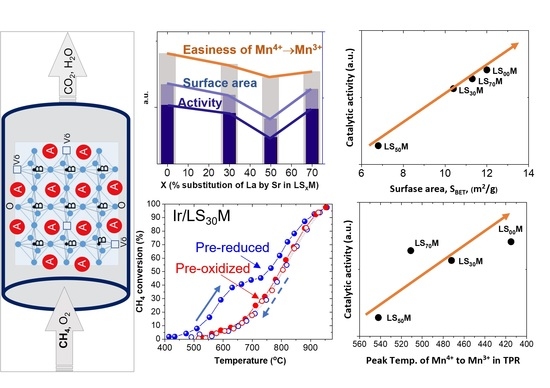
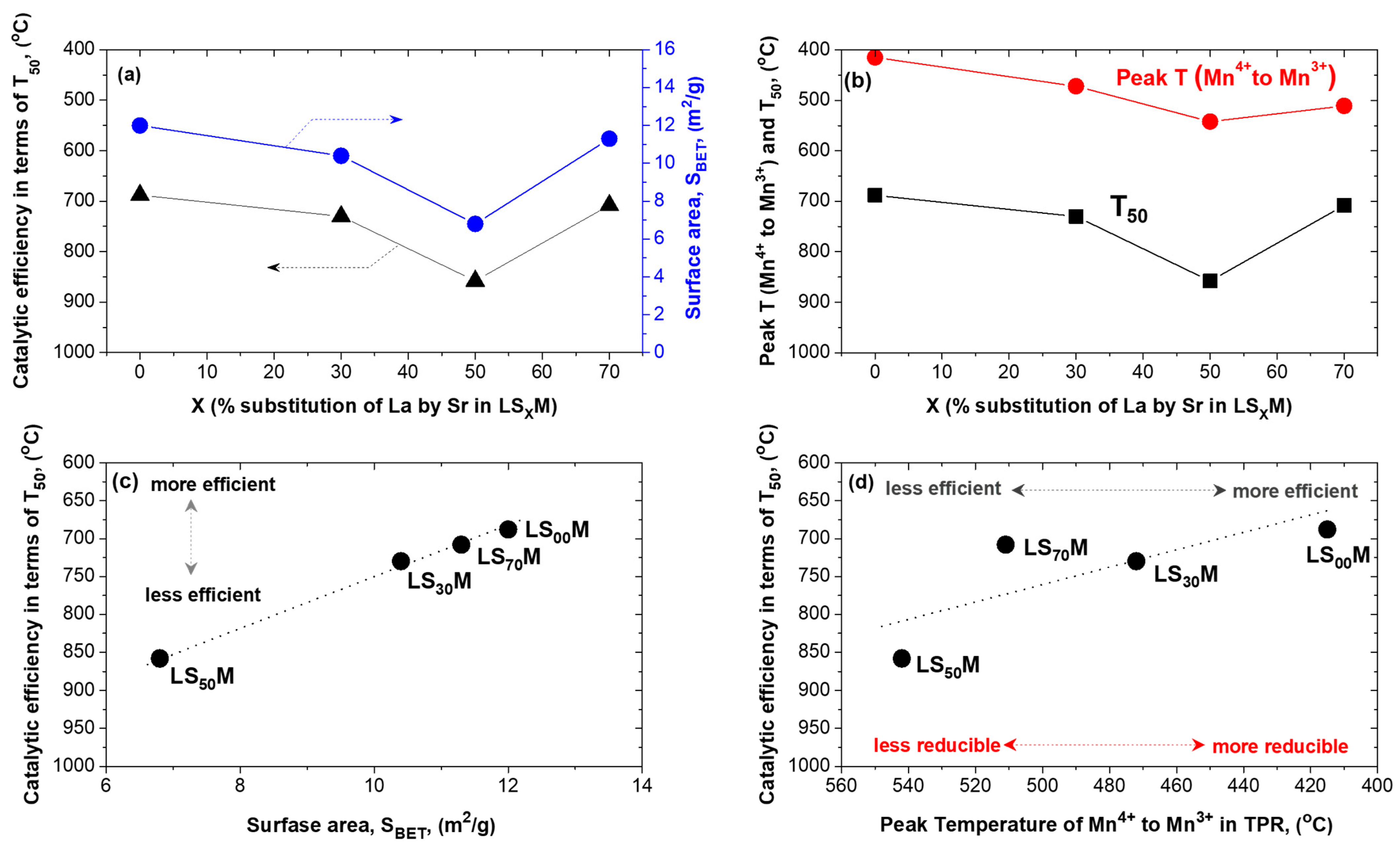
3.5. Main Observations and Material Properties—Catalytic Efficiency Correlations
- (i)
- The most important determining factor of the materials, for all cases (unloaded or Ir-loaded LSXM, pre-oxidized or pre-reduced, and fresh or aged materials), in terms of efficiency, was the composition of the LSXM perovskite, specifically, the amount of La that was substituted with Sr (X = 0, 30, 50, and 70%). This key factor typically causes shifts in T50 of up to ca. 300 °C, whereas other parameters appeared capable of shifts in T50 that were one order of magnitude lower (ca. 30 °C) (Figure 2, Figure 3, Figure 4 and Figure 5).
- (ii)
- X variation produced an inverted volcanic-type effect in terms of catalytic efficiency, with the most active in all cases the catalysts that did not contain Sr, i.e., X = 0% (LS00M = LaMnO3 and Ir/LS00M = Ir/LaMnO3), and less active the catalysts with X = 50% (i.e., LS50M and Ir/LS50M). Catalysts with larger X value (X = 70%; La0.3Sr0.7MnO3 and Ir/La0.3Sr0.7MnO3), tended to be similar to the behavior of the optimal catalysts (LaMnO3 and Ir/LaMnO3) (Figure 2c, Figure 3c, Figure 4c, Figure 5c and Figure 6).
- (iii)
- The addition of Ir nanoparticles onto the LSXM surface did not perform as expected. It had a negative effect on the efficiency of pre-oxidized catalysts (Ir/LSXM show T50 values that were ~30°C higher than those of their non-loaded LSXM counterparts) (Figure 2c and Figure 4c), whereas the pre-reduced catalysts exhibited a small positive effect at high temperatures, but it was more noticeable for lower temperatures (Figure 3c and Figure 5c).
- (iv)
- (v)
- (vi)
- The oxidative thermal aging of the materials caused marginal decreases in their efficiency (i.e., less than ~40 °C shifts of T50 towards higher temperatures). However, for the most active catalysts (with X = 0 and 70%), no activity deterioration was recorded (Figure 6).
- (vii)
- The time-on-stream stability of the materials was generally good, as the efficiency (methane conversion) only declined by 5–10%; this was observed after 12 h of operation. Notably, no degradation in the catalytic efficiency of the optimal LS00M (LaMnO3) and Ir/LS00M (Ir/LaMnO3) catalysts was recorded (Figure 7).
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, X.; Myhrvold, N.P.; Hausfather, Z.; Caldeira, K. Climate benefits of natural gas as a bridge fuel and potential delay of near-zero energy systems. Appl. Energy 2016, 167, 317–322. [Google Scholar] [CrossRef] [Green Version]
- Brauers, H. Natural gas as a barrier to sustainability transitions? A systematic mapping of the risks and challenges. Energy Res. Soc. Sci. 2022, 89, 102538. [Google Scholar] [CrossRef]
- Weissman, S. Natural Gas as a Bridge Fuel. Measuring the Bridge. Center for Sustainable Energy. Available online: https://energycenter.org/sites/default/files/docs/nav/policy/research-and-reports/Natural_Gas_Bridge_Fuel.pdf (accessed on 9 February 2023).
- Jiang, D.; Khivantsev, K.; Wang, Y. Low-Temperature Methane Oxidation for Efficient Emission Control in Natural Gas Vehicles: Pd and Beyond. ACS Catal. 2020, 10, 14304–14314. [Google Scholar] [CrossRef]
- Stoian, M.; Roge, V.; Lazar, L.; Maurer, T.; Vedrine, J.C.; Marcu, I.-C.; Fechete, I. Total Oxidation of Methane on Oxide and Mixed Oxide Ceria-Containing Catalysts. Catalysts 2021, 11, 427. [Google Scholar] [CrossRef]
- Yentekakis, I.V.; Goula, G. Biogas management: Advanced utilization for production of renewable energy and added-value chemicals. Front. Environ. Sci. 2017, 5, 7. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Yentekakis, I.V.; Vayenas, C.G. Methane to ethylene with 85 percent yield in a gas recycle electrocatalytic reactor-separator. Science 1994, 264, 1563–1566. [Google Scholar] [CrossRef]
- Siakavelas, G.I.; Charisiou, N.D.; AlKhoori, A.; Sebestian, V.; Hinder, S.J.; Baker, M.A.; Yentekakis, I.V.; Polychronopoulou, K.; Goula, M.A. Cerium oxide catalysts for oxidative coupling of methane reaction: Effect of lithium, samarium and lanthanum dopants. J. Environ. Chem. Eng. 2022, 10, 107259. [Google Scholar] [CrossRef]
- Yentekakis, I.V.; Dong, F. Grand Challenges for Catalytic Remediation in Environmental and Energy Applications Toward a Cleaner and Sustainable Future. Front. Environ. Chem. 2020, 1, 5. [Google Scholar] [CrossRef]
- Yentekakis, I.V.; Panagiotopoulou, P.; Artemakis, G. A review of recent efforts to promote dry reforming of methane (DRM) to syngas production via bimetallic catalyst formulations. Appl. Catal. B Environ. 2021, 296, 120210. [Google Scholar] [CrossRef]
- Osazuwa, O.U.; Abidin, S.Z.; Fan, X.; Amenaghawon, A.N.; Azizan, M.T. An insight into the effects of synthesis methods on catalysts properties for methane reforming. J. Environ. Chem. Eng. 2021, 9, 105052. [Google Scholar] [CrossRef]
- Karam, L.; El Hassan, N. Advantages of mesoporous silica based catalysts in methane reforming by CO2 from kinetic perspective. J. Environ. Chem. Eng. 2018, 6, 4289–4297. [Google Scholar] [CrossRef]
- Yentekakis, I.V.; Goula, G.; Hatzisymeon, M.; Betsi-Argyropoulou, I.; Botzolaki, G.; Kousi, K.; Kondarides, D.I.; Taylor, M.J.; Parlett, C.M.A.; Osatiashtiani, A.; et al. Effect of support oxygen storage capacity on the catalytic performance of Rh nanoparticles for CO2 reforming of methane. Appl. Catal. B Environ. 2019, 24, 490–501. [Google Scholar] [CrossRef] [Green Version]
- Yentekakis, I.V.; Goula, G.; Panagiotopoulou, P.; Katsoni, A.; Diamadopoulos, E.; Mantzavinos, D.; Delimitis, A. Dry reforming of methane: Catalytic performance and stability of Ir catalysts supported on γ-Al2O3, Zr0.92Y0.08O2−δ (YSZ) or Ce0.9Gd0.1O2−δ (GDC) supports. Top. Catal. 2015, 58, 1228–1241. [Google Scholar] [CrossRef]
- Nikolaraki, E.; Goula, G.; Panagiotopoulou, P.; Taylor, M.J.; Kousi, K.; Kyriakou, G.; Kondarides, D.I.; Lambert, R.M.; Yentekakis, I.V. Support Induced Effects on the Ir Nanoparticles Activity, Selectivity and Stability Performance under CO2 Reforming of Methane. Nanomaterials 2021, 11, 2880. [Google Scholar] [CrossRef]
- Barelli, L.; Ottaviano, A. Solid oxide fuel cell technology coupled with methane dry reforming: A viable option for high efficiency plant with reduced CO2 emissions. Energy 2014, 71, 118–129. [Google Scholar] [CrossRef]
- Gur, T.M. Comprehensive review of methane conversion in solid oxide fuel cells: Prospects for efficient electricity generation from natural gas. Progr. Energy Combust. Sci. 2016, 54, 1–64. [Google Scholar] [CrossRef] [Green Version]
- Sengodan, S.; Lan, R.; Humphreys, J.; Du, D.; Xu, W.; Wang, H.; Tao, S. Advances in reforming and partial oxidation of hydrocarbons for hydrogen production and fuel cell applications. Renew. Sustain. Energy Rev. 2018, 82, 761–780. [Google Scholar] [CrossRef]
- Yentekakis, I.V.; Papadam, T.; Goula, G. Electricity production from wastewater treatment via a novel biogas-SOFC aided process. Solid State Ion. 2008, 179, 15211525. [Google Scholar] [CrossRef]
- Yentekakis, I.V. Open- and closed-circuit study of an intermediate temperature SOFC directly fueled with simulated biogas mixtures. J. Power Sources 2006, 160, 422–425. [Google Scholar] [CrossRef]
- Miniajluk, N.; Trawczynski, J.; Zawadzki, M.; Tylus, W. LaMnO3 (La0.8Sr0.2MnO3) Perovskites for Lean Methane Combustion: Effect of Synthesis Method. Adv. Mater. Phys. Chem. 2018, 8, 193–215. Available online: http://creativecommons.org/licenses/by/4.0/ (accessed on 1 May 2023).
- Gelin, P.; Primet, M. Complete oxidation of methane at low temperature over noble metal based catalysts: A review. Appl. Catal. B Environ. 2002, 39, 1–37. [Google Scholar] [CrossRef]
- Li, H.; Fu, R.; Duan, W.; Jiang, Z. The preparation effect on activity and thermal stability of La0.8Ca0.2FeO3 perovskite honeycombs dispersed by MgAl2O4 spinel washcoat for catalytic combustion of dilute methane. J. Environ. Chem. Eng. 2006, 4, 2187–2195. [Google Scholar] [CrossRef]
- Chen, J.; Zhong, J.; Wu, Y.; Hu, W.; Qu, P.; Xiao, X.; Zhang, G.; Liu, X.; Jiao, Y.; Zhong, L.; et al. Particle Size Effects in Stoichiometric Methane Combustion: Structure–Activity Relationship of Pd Catalyst Supported on Gamma-Alumina. ACS Catal. 2020, 10, 10339–10349. [Google Scholar] [CrossRef]
- Cimino, S.; Lisi, L.; Pirone, R.; Russo, G.; Turco, M. Methane combustion on perovskites-based structured catalysts. Catal. Today 2000, 59, 19–31. [Google Scholar] [CrossRef]
- Gao, Y.; Jiang, M.; Yang, L.; Li, Z.; Tian, F.-X.; He, Y. Recent progress of catalytic methane combustion over transition metal oxide catalysts. Front. Chem. 2022, 10, 959442. [Google Scholar] [CrossRef]
- Zhang, X.; Long, E.; Li, Y.; Guo, J.; Zhang, L.; Gong, M.; Wang, M.; Chen, Y. CeO2-ZrO2-La2O3-Al2O3 composite oxide and its supported palladium catalyst for the treatment of exhaust of natural gas engined vehicles. J. Nat. Gas Chem. 2009, 18, 139–144. [Google Scholar] [CrossRef]
- Mortensen, R.L.; Noack, H.-D.; Pedersen, K.; Mossin, S.; Mielby, J. Recent Advances in Complete Methane Oxidation using Zeolite-Supported Metal Nanoparticle Catalysts. ChemCatChem 2022, 14, e202101924. [Google Scholar] [CrossRef]
- Santos, V.P.; Carabineiro, S.A.C.; Tavares, P.B.; Pereira, M.F.R.; Orfao, J.J.M.; Figueirdo, J.L. Oxidation of CO, ethanol and toluene over TiO2 supported noble metal catalysts. Appl. Catal. B Environ. 2010, 99, 198–205. [Google Scholar] [CrossRef]
- Drosou, C.; Nikolaraki, E.; Nikolaou, V.; Koilia, E.; Artemakis, G.; Stratakis, A.; Evdou, A.; Charisiou, N.D.; Goula, M.A.; Zaspalis, V.; et al. Activity and Thermal Aging Stability of La1−xSrxMnO3 (x = 0.0, 0.3, 0.5, 0.7) and Ir/La1−xSrxMnO3 Catalysts for CO Oxidation with Excess O2. Nanomaterials 2023, 13, 663. [Google Scholar] [CrossRef]
- Schick, L.; Sanchis, R.; Gonzalez-Alfaro, V.; Agouram, S.; Lopez, J.M.; Torrente-Murciano, L.; Garcia, T.; Solsona, B. Size-activity relationship of iridium particles supported on silica for the total oxidation of volatile organic compounds (VOCs). Chem. Eng. J. 2019, 366, 100–111. [Google Scholar] [CrossRef]
- Pachatouridou, E.; Papista, E.; Iliopoulou, E.F.; Delimitis, A.; Goula, G.; Yentekakis, I.V.; Marnellos, G.E.; Konsolakis, M. Nitrous oxide decomposition over Al2O3 supported noble metals (Pt, Pd, Ir): Effect of metal loading and feed composition. J. Environ. Chem. Eng. 2015, 3, 815–821. [Google Scholar] [CrossRef]
- Yentekakis, I.V.; Goula, G.; Kampouri, S.; Betsi-Argyropoulou, I.; Panagiotopoulou, P.; Taylor, M.J.; Kyriakou, G.; Lambert, R.M. Ir-catalyzed nitrous oxide (N2O) decomposition: Effect of the Ir particle size and metal-support interactions. Catal. Lett. 2018, 148, 341–347. [Google Scholar] [CrossRef] [Green Version]
- Goula, M.A.; Charisiou, N.D.; Papageridis, K.N.; Delimitis, A.; Papista, E.; Pachatouridou, E.; Iliopoulou, E.F.; Marnellos, G.; Konsolakis, M.; Yentekakis, I.V. A comparative study of the H2-assisted selective catalytic reduction of nitric oxide by propene over noble metal (Pt, Pd, Ir)/γ-Al2O3 catalysts. J. Environ. Chem. Eng. 2016, 4, 1629–1641. [Google Scholar] [CrossRef]
- Li, H.; Hao, C.; Tian, J.; Wang, S.; Zhao, C. Ultra-durable Ni-Ir/MgAl2O4 catalysts for dry reforming of methane enabled by dynamic balance between carbon deposition and elimination. Chem. Catal. 2022, 2, 1748–1763. [Google Scholar] [CrossRef]
- Nakagawa, K.; Ikenaga, N.; Suzuki, T.; Kobayashi, T.; Haruta, M. Partial oxidation of methane to synthesis gas over supported iridium catalysts. Appl. Catal. A 1998, 169, 281–290. [Google Scholar] [CrossRef]
- Fiedorow, R.M.J.; Chahar, B.S.; Wanke, S.E. The sintering of supported metal catalysts. II. Comparison of sintering rates of supported Pt, Ir, and Rh Catalysts in hydrogen and oxygen. J. Catal. 1978, 51, 193–202. [Google Scholar] [CrossRef]
- Argyle, M.D.; Bartholomew, C.H. Heterogeneous Catalyst Deactivation and Regeneration: A Review. Catalysts 2015, 5, 145–269. [Google Scholar] [CrossRef] [Green Version]
- Yentekakis, I.V.; Goula, G.; Panagiotopoulou, P.; Kampouri, S.; Taylor, M.J.; Kyriakou, G.; Lambert, R.M. Stabilization of catalyst particles against sintering on oxide supports with high oxygen ion lability exemplified by Ir-catalysed decomposition of N2O. Appl. Catal. B Environ. 2016, 192, 357–364. [Google Scholar] [CrossRef]
- Goula, G.; Botzolaki, G.; Osatiashtiani, A.; Parlett, C.M.A.; Kyriakou, G.; Lambert, R.M.; Yentekakis, I.V. Oxidative Thermal Sintering and Redispersion of Rh Nanoparticles on Supports with High Oxygen Ion Lability. Catalysts 2019, 9, 541. [Google Scholar] [CrossRef] [Green Version]
- Yentekakis, I.V. The effective-double-layer as an efficient tool for the design of sinter-resistant catalysts. In Recent Advances in Electrochemical Promotion of Catalysis; Vayenas, C.G., Vernoux, P., Eds.; Springer-Nature: Berlin/Heidelberg, Germany, 2023; pp. 117–149. Available online: https://link.springer.com/chapter/10.1007/978-3-031-13893-5_4 (accessed on 1 May 2023).
- Royer, S.; Duprez, D.; Can, F.; Courtois, X.; Batiot-Dupeyrat, C.; Laassiri, S.; Alamdari, H. Perovskites as substitutes of noble metals for heterogeneous catalysis: Dream or reality. Chem. Rev. 2014, 114, 10292–10368. [Google Scholar] [CrossRef]
- Zhu, J.; Li, H.; Zhong, L.; Xiao, P.; Xu, X.; Yang, X.; Zhao, Z.; Li, J. Perovskite oxides: Preparation, characterizations, and applications in heterogeneous catalysis. ACS Catal. 2014, 4, 2917–2940. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, P.; Dai, S. Recent Advances of Lanthanum-Based Perovskite Oxides for Catalysis. ACS Catal. 2015, 5, 6370–6385. [Google Scholar] [CrossRef]
- Peña, M.A.; Fierro, J.L.G. Chemical Structures and Performance of Perovskite Oxides. Chem. Rev. 2001, 101, 1981–2018. [Google Scholar] [CrossRef]
- Yentekakis, I.V.; Georgiadis, A.G.; Drosou, C.; Charisiou, N.D.; Goula, M.A. Selective Catalytic Reduction of NOx over Perovskite-Based Catalysts Using CxHy(Oz), H2 and CO as Reducing Agents—A Review of the Latest Developments. Nanomaterials 2022, 12, 1042. [Google Scholar] [CrossRef] [PubMed]
- Evdou, A.; Georgitsis, T.; Matsouka, C.; Pachatouridou, E.; Iliopoulou, E.; Zaspalis, V. Defect Chemistry and Chemical Looping Performance of La1−xMxMnO3 (M=Sr, Ca, (x = 0–0.5)) Perovskites. Nanomaterials 2022, 12, 3461. [Google Scholar] [CrossRef] [PubMed]
- Gálvez, M.E.; Jacot, R.; Scheffe, J.; Cooper, T.; Patzke, G.; Steinfeld, A. Physico-chemical changes in Ca, Sr and Al-doped La–Mn–O perovskites upon thermochemical splitting of CO2 via redox cycling. Phys. Chem. Chem. Phys. 2015, 17, 6629–6634. [Google Scholar] [CrossRef] [PubMed]
- Matsouka, C.; Zaspalis, V.; Nalbandian, L. Perovskites as oxygen carriers in chemical looping reforming process—Preparation of dense perovskite membranes and ionic conductivity measurement. Mater. Today Proc. 2018, 5, 27543–27552. [Google Scholar] [CrossRef]
- Nishihata, Y.; Mizuki, J.; Akao, T.; Tanaka, H.; Uenishi, M.; Kimura, M.; Okamoto, T.; Hamada, N. Self-regeneration of a Pd-perovskite catalyst for automotive emissions control. Nature 2002, 418, 164–167. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Uenishi, M.; Taniguchi, M.; Tan, I.; Narita, K.; Kimura, M.; Kaneko, K.; Nishihata, Y.; Mizuki, J. The intelligent catalyst having the self-regenerative function of Pd, Rh and Pt for automotive emissions control. Catal. Today 2006, 117, 321–328. [Google Scholar] [CrossRef]
- Kousi, K.; Tang, C.; Metcalfe, I.S.; Neagu, D. Emergence and Future of Exsolved Materials. Small 2021, 17, 2006479. [Google Scholar] [CrossRef]
- Cali, E.; Kerherve, G.; Naufal, F.; Kousi, K.; Neagu, D.; Papaioannou, E.I.; Thomas, M.P.; Guiton, B.S.; Metcalfe, I.S.; Irvine, J.T.; et al. Exsolution of Catalytically Active Iridium Nanoparticles from Strontium Titanate. ACS Appl. Mater. Interfaces 2020, 12, 37444–37453. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Jiao, Y.; Xu, X.; Pan, Y.; Su, C.; Duan, X.; Sun, H.; Liu, S.; Wang, S.; Shao, Z. Superstructures with Atomic-Level Arranged Perovskite and Oxide Layers for Advanced Oxidation with an Enhanced Non-Free Radical Pathway. ACS Sustain. Chem. Eng. 2022, 10, 1899–1909. [Google Scholar] [CrossRef]
- Xu, X.; Wang, W.; Zhou, W.; Shao, Z. Recent Advances in Novel Nanostructuring Methods of Perovskite Electrocatalysts for Energy-Related Applications. Small Methods 2018, 2, 1800071. [Google Scholar] [CrossRef]
- Yentekakis, I.V.; Konsolakis, M. Three-way Catalysis. In Perovskites and Related Mixed Oxides; Wiley-VCH, Vergal GmbH & Co. KGaA: Weinheim, Germany, 2016; pp. 559–586. [Google Scholar] [CrossRef]
- Shen, M.; Zhao, Z.; Chen, J.; Su, Y.; Wang, J.; Wang, X. Effects of calcium substitute in LaMnO3 perovskites for NO catalytic oxidation. J. Rare Earths 2013, 31, 119–123. [Google Scholar] [CrossRef]
- Tarjomannejad, A.; Niaei, A.; Gómez, M.J.I.; Farzi, A.; Salari, D.; Albaladejo-Fuentes, V. NO+CO reaction over LaCu0.7B0.3O3 (B = Mn, Fe, Co) and La0.8A0.2Cu0.7Mn0.3O3 (A = Rb, Sr, Cs, Ba) perovskite-type catalysts. J. Therm. Anal. Calorim. 2017, 129, 671–680. [Google Scholar] [CrossRef]
- Hwang, J.; Rao, R.R.; Giordano, L.; Katayama, Y.; Yu, Y.; Shao-Horn, Y. Perovskites in catalysis and electrocatalysis. Science 2017, 358, 751–756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, X.; Pan, Y.; Ge, L.; Chen, Y.; Mao, X.; Guan, D.; Li, M.; Zhong, Y.; Hu, Z.; Peterson, V.K.; et al. High-Performance Perovskite Composite Electrocatalysts Enabled by Controllable Interface Engineering. Small 2021, 17, 2101573. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, T.; Dong, X.; Li, M.; Wang, H. Effects of Ce substitution at the A-site of LaNi0.5Fe0.5O3 perovskite on the enhanced catalytic activity for dry reforming of methane. Appl. Catal. B Environ. 2018, 224, 214–221. [Google Scholar] [CrossRef]
- Sim, Y.; Kwon, D.; An, S.; Ha, J.M.; Oh, T.S.; Jung, J.C. Catalytic behavior of ABO3 perovskites in the oxidative coupling of methane. Mol. Catal. 2020, 489, 110925. [Google Scholar] [CrossRef]
- Bashan, V.; Ust, Y. Perovskite catalysts for methane combustion: Applications, design, effects for reactivity and partial oxidation. Int. J. Energy Res. 2019, 43, 7755–7789. [Google Scholar] [CrossRef]
- Bhattar, S.; Abedin, M.A.; Kanitkar, S.; Spivey, J.J. A review on dry reforming of methane over perovskite derived catalysts. Catal. Today 2021, 365, 2–23. [Google Scholar] [CrossRef]
- Zou, G.; Wang, Z.; Sun, M.; Luo, X.; Wang, X. A novel solid-gas process to synthesize LaMnO3 perovskite with high surface area and excellent activity for methane combustion. J. Nat. Gas Chem. 2021, 20, 294–298. [Google Scholar] [CrossRef]
- He, L.; Fan, Y.; Bellettre, J.; Yue, J.; Luo, L. A review on catalytic methane combustion at low temperatures: Catalysts, mechanisms, reaction conditions and reactor designs. Renew. Sustain. Energy Rev. 2020, 119, 109589. [Google Scholar] [CrossRef]
- Arai, H.; Yamada, T.; Eguchi, K.; Seiyama, T. Catalytic combustion of methane over various perovskite-type oxides. Appl. Catal. 1986, 26, 265–276. [Google Scholar] [CrossRef]
- Luo, L.; Wang, S.; Wu, Z.; Qin, Z.; Zhu, H.; Fan, W.; Wang, J. Structure and performance of supported iridium catalyst for the lean methane oxidation at low temperature. Appl. Catal. A 2022, 641, 118699. [Google Scholar] [CrossRef]
- Pliangos, A.; Yentekakis, I.V.; Papadakis, V.G.; Vayenas, C.G.; Verykios, X.E. Support-induced promotional effects on the activity of automotive exhaust catalysts: 1. The case of oxidation of light hydrocarbons (C2H4). Appl. Catal. B 1997, 14, 161–173. [Google Scholar] [CrossRef]
- Vayenas, C.G.; Brosda, S.; Pliangos, C. The double-layer approach to promotion, electrocatalysis, electrochemical promotion, and metal–support interactions. J. Catal. 2003, 216, 487–504. [Google Scholar] [CrossRef]
- Vernoux, P.; Lizarraga, L.; Tsampas, M.N.; Sapountzi, F.M.; DeLucas-Consuegra, A.; Valverde, J.L.; Souentie, S.; Vayenas, C.G.; Tsiplakides, D.; Balomenou, S.; et al. Ionically conducting ceramics as active catalyst supports. Chem. Rev. 2013, 113, 8192–8260. [Google Scholar] [CrossRef]
- Papadakis, V.G.; Pliangos, C.A.; Yentekakis, I.V.; Verykios, X.E.; Vayenas, C.G. Development of high performance, Pd-based, three way catalysts. Catal. Today 1996, 29, 71–75. [Google Scholar] [CrossRef]
- Konsolakis, M.; Drosou, C.; Yentekakis, I.V. Support mediated promotional effects of rare earth oxides (CeO2 and La2O3) on N2O decomposition and N2O reduction by CO or C3H6 over Pt/Al2O3 structured catalysts. Appl. Catal. B Environ. 2012, 123–124, 405–413. [Google Scholar] [CrossRef]
- Fornasiero, P.; Di Monte, R.; Ranga Rao, G.; Kaspar, J.; Meriani, S.; Trovarelli, A.; Graziani, M. Rh-loaded CeO2-ZrO2 solid-solutions as highly efficient oxygen exchangers: Dependence of the reduction behavior and the oxygen storage capacity on the structural properties. J. Catal. 1995, 151, 168–177. [Google Scholar] [CrossRef]
- Botzolaki, G.; Goula, G.; Rontogianni, A.; Nikolaraki, E.; Chalmpes, N.; Zygouri, P.; Karakassides, M.; Gournis, D.; Charisiou, N.D.; Goula, M.A.; et al. CO2 Methanation on Supported Rh Nanoparticles: The combined Effect of Support Oxygen Storage Capacity and Rh Particle Size. Catalysts 2020, 10, 944. [Google Scholar] [CrossRef]
- Dalla Betta, R.A.; Rostrup-Nielsen, T. Application of catalytic combustion to a 1.5 MW industrial gas turbine. Catal. Today 1999, 47, 369–375. [Google Scholar] [CrossRef]
- Haron, W.; Wisitsoraat, A.; Wongnawa, S. Comparison of monocrystalline LaMO3 (M = Co, Al) Perovskite Oxide Prepared by Co-Precipitation Method. Int. J. Chem. Eng. Appl. 2014, 5, 123–126. [Google Scholar] [CrossRef]
- Ponce, S.; Peña, M.A.; Fierro, J.L.G. Surface properties and catalytic performance in methane combustion of Sr-substituted lanthanum manganites. Appl. Catal. B Environ. 2000, 24, 193–205. [Google Scholar] [CrossRef]
- Saracco, G.; Geobaldo, F.; Baldi, G. Methane combustion on Mg-doped LaMnO3 perovskite catalysts. Appl. Catal. B Environ. 1999, 20, 277–288. [Google Scholar] [CrossRef]
- Kaliaquine, S.; Van Neste, A.; Szabo, V.; Gallot, J.E.; Bassir, M.; Muzychuk, R. Perovskite-type oxides synthesized by reactive grinding: Part I. Preparation and characterization. Appl. Catal. A 2001, 209, 345–358. [Google Scholar] [CrossRef]
- Patcas, F.; Buciuman, F.C.; Zsako, J. Oxygen non-stoichiometry and reducibility of B-site substituted lanthanum manganites. Thermoch. Acta 2000, 360, 71–76. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Y.; Chu, P.; Liu, J.; Wang, M.; Zhang, P.; Duan, E. Different active sites of LaCoO3 and LaMnO3 for CH4 oxidation by regulation of precursor’s ion concentration. Glob. Environ. Eng. 2020, 7, 28–39. [Google Scholar] [CrossRef]
- Nitadori, T.; Kurihara, S.; Misono, M. Catalytic properties of La1−xA′xMnO3 (A′ = Sr, Ce, Hf). J. Catal. 1986, 98, 221–228. [Google Scholar] [CrossRef]
- Kröger, F.A.; Vink, H.J.; Seitz, F.; Turnbull, D. (Eds.) Relations between the concentrations of imperfections in crystalline solids. In Solid State Physics; Academic Press: Cambridge, MA, USA, 1956; Volume 3, pp. 307–435. ISBN 9780126077032. [Google Scholar] [CrossRef]
- Nowotny, j.; Rekas, M. Defect chemistry of (La,Sr)MnO3. J. Am. Ceram. Soc. 1998, 81, 67–80. [Google Scholar] [CrossRef]
- Tang, W.; Wu, X.; Li, D.; Wang, Z.; Liu, G.; Liu, H.; Chen, Y. Oxalate route for promoting activity of manganese oxide catalysts in total VOCs’ oxidation: Effect of calcination temperature and preparation method. J. Mater. Chem. A 2014, 2, 2544–2554. [Google Scholar] [CrossRef]
- Hauptmann, W.; Votsmeier, M.; Gieshoff, J.; Drochner, A.; Vogel, H. Inverse hysteresis during the no oxidation on Pt under lean conditions. Appl. Catal. B Environ. 2009, 93, 22–29. [Google Scholar] [CrossRef]
- Grunwaldt, J.D.; van Vegten, N.; Baiker, A. Insight into the structure of supported palladium catalysts during the total oxidation of methane. Chem. Commun. 2007, 44, 4635–4637. [Google Scholar] [CrossRef] [PubMed]
- Al Soubaihi, R.M.; Saoud, K.M.; Dutta, J. Critical review of low-temperature CO oxidation and hysteresis phenomenon on heterogeneous catalysts. Catalysts 2018, 8, 660. [Google Scholar] [CrossRef] [Green Version]
- Lashina, E.A.; Slavinskaya, E.M.; Chumakova, N.A.; Stadnichenko, A.I.; Salanov, A.N.; Chumakov, G.A.; Boronin, A.I. Inverse temperature hysteresis and self-sustained oscillations in CO oxidation over Pd at elevated pressures of reaction mixture: Experiment and mathematical modeling. Chem. Eng. Sci. 2020, 212, 115312. [Google Scholar] [CrossRef]
- Chen, J.; Wang, X.; Zhang, L.; Rui, Z. Strong metal-support interaction assisted redispersion strategy for obtaining ultrafine and stable IrO2/Ir active sites with exceptional methane oxidation activity. Appl. Catal. B 2021, 297, 120410. [Google Scholar] [CrossRef]
- Wang, Y.; Arandiyan, H.; Tahini, H.A.; Scott, J.; Tan, X.; Dai, H.; Gale, J.D.; Rohl, A.L.; Smith, S.C.; Smith, R. The controlled disassembly of mesostructured perovskites as an avenue to fabricating high performance nanohybrid catalysts. Nat. Commun. 2017, 8, 15553. [Google Scholar] [CrossRef] [Green Version]
- Reinke, M.; Mantzaras, J.; Schaeren, R.; Bombach, R.; Inauen, A.; Schenker, S. High-pressure catalytic combustion of methane over platinum: In situ experiments and detailed numerical predictions. Combust. Flame 2004, 136, 217–240. [Google Scholar] [CrossRef]




| Catalysts Code | Chemical Formula | SBET (m2/g) | Average Pore Diameter (nm) | Total OSC (μmol O2/g) | Mean Ir Particle Size (nm) |
|---|---|---|---|---|---|
| LS00M | LaMnO3 | 12.0 | 10.9 | 671 | n.a |
| LS30M | La0.7Sr0.3MnO3 | 10.4 | 9.8 | 766 | n.a |
| LS50M | La0.5Sr0.5MnO3 | 6.8 | 8.9 | 886 | n.a |
| LS70M | La0.3Sr0.7MnO3 | 11.3 | 8.8 | 1219 | n.a |
| Ir/LS00M | 2 wt% Ir/LaMnO3 | 9.7 | 11.9 | 753 | 1.1 |
| Ir/LS30M | 2 wt% Ir/La0.7Sr0.3MnO3 | 10.5 | 10.0 | 981 | 1.1 |
| Ir/LS50M | 2 wt% Ir/La0.5Sr0.5MnO3 | 6.2 | 8.1 | 1203 | 1.0 |
| Ir/LS70M | 2 wt% Ir/La0.3Sr0.7MnO3 | 11.0 | 13.7 | 1348 | 1.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drosou, C.; Nikolaraki, E.; Georgakopoulou, T.; Fanourgiakis, S.; Zaspalis, V.T.; Yentekakis, I.V. Methane Catalytic Combustion under Lean Conditions over Pristine and Ir-Loaded La1−xSrxMnO3 Perovskites: Efficiency, Hysteresis, and Time-on-Stream and Thermal Aging Stabilities. Nanomaterials 2023, 13, 2271. https://doi.org/10.3390/nano13152271
Drosou C, Nikolaraki E, Georgakopoulou T, Fanourgiakis S, Zaspalis VT, Yentekakis IV. Methane Catalytic Combustion under Lean Conditions over Pristine and Ir-Loaded La1−xSrxMnO3 Perovskites: Efficiency, Hysteresis, and Time-on-Stream and Thermal Aging Stabilities. Nanomaterials. 2023; 13(15):2271. https://doi.org/10.3390/nano13152271
Chicago/Turabian StyleDrosou, Catherine, Ersi Nikolaraki, Theodora Georgakopoulou, Sotiris Fanourgiakis, Vassilios T. Zaspalis, and Ioannis V. Yentekakis. 2023. "Methane Catalytic Combustion under Lean Conditions over Pristine and Ir-Loaded La1−xSrxMnO3 Perovskites: Efficiency, Hysteresis, and Time-on-Stream and Thermal Aging Stabilities" Nanomaterials 13, no. 15: 2271. https://doi.org/10.3390/nano13152271
APA StyleDrosou, C., Nikolaraki, E., Georgakopoulou, T., Fanourgiakis, S., Zaspalis, V. T., & Yentekakis, I. V. (2023). Methane Catalytic Combustion under Lean Conditions over Pristine and Ir-Loaded La1−xSrxMnO3 Perovskites: Efficiency, Hysteresis, and Time-on-Stream and Thermal Aging Stabilities. Nanomaterials, 13(15), 2271. https://doi.org/10.3390/nano13152271