Flexible High-Performance and Screen-Printed Symmetric Supercapacitor Using Hierarchical Rodlike V3O7 Inks
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Preparation of V3O7 Nanorod
2.3. Formulation of V3O7 Ink
2.4. Screen Printing of Electrode and Symmetric Supercapacitor
2.5. Electrochemical Measurements
2.6. Materials Characterizations
3. Results
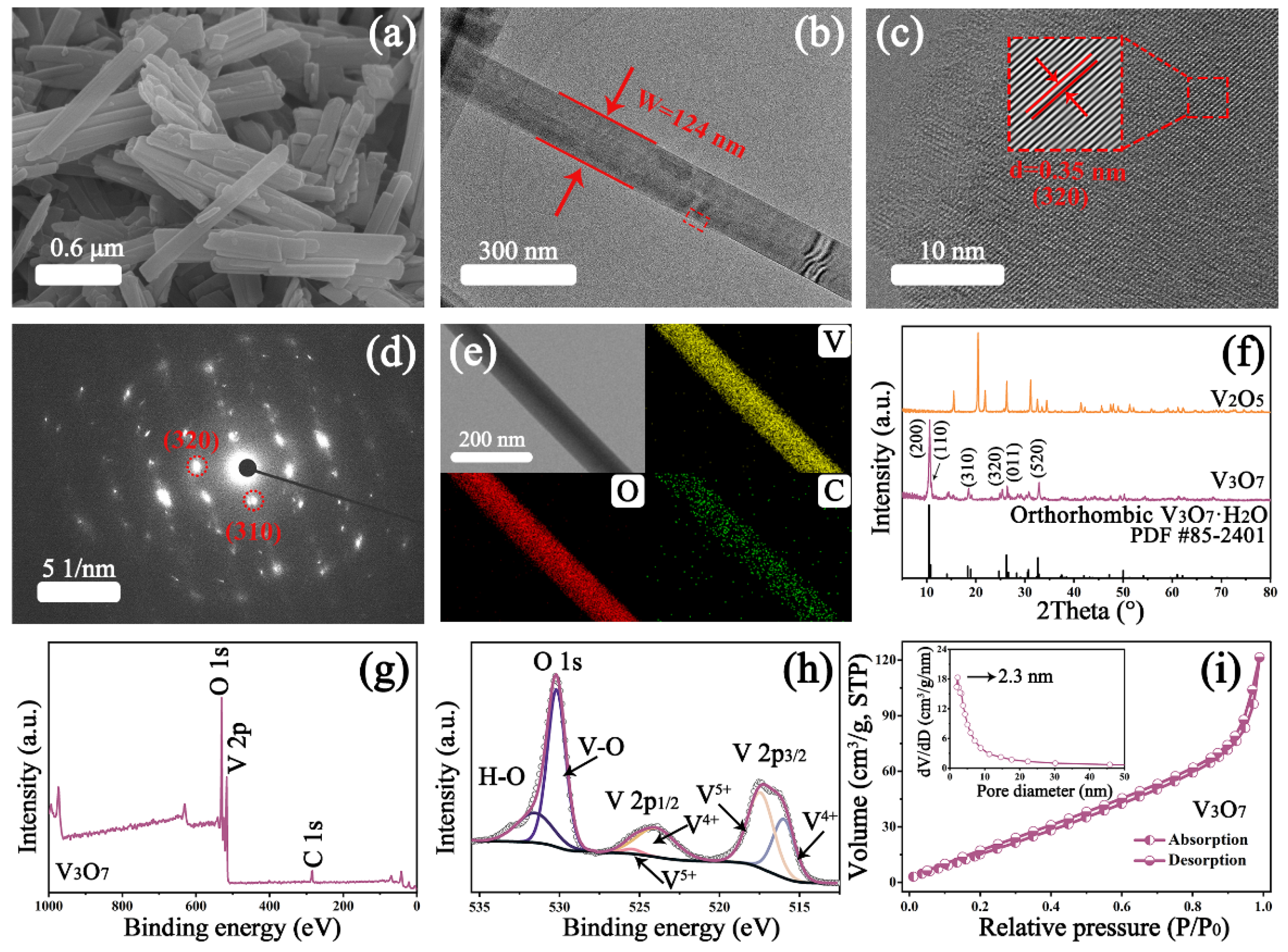
3.1. Microscopic and Structural Analysis
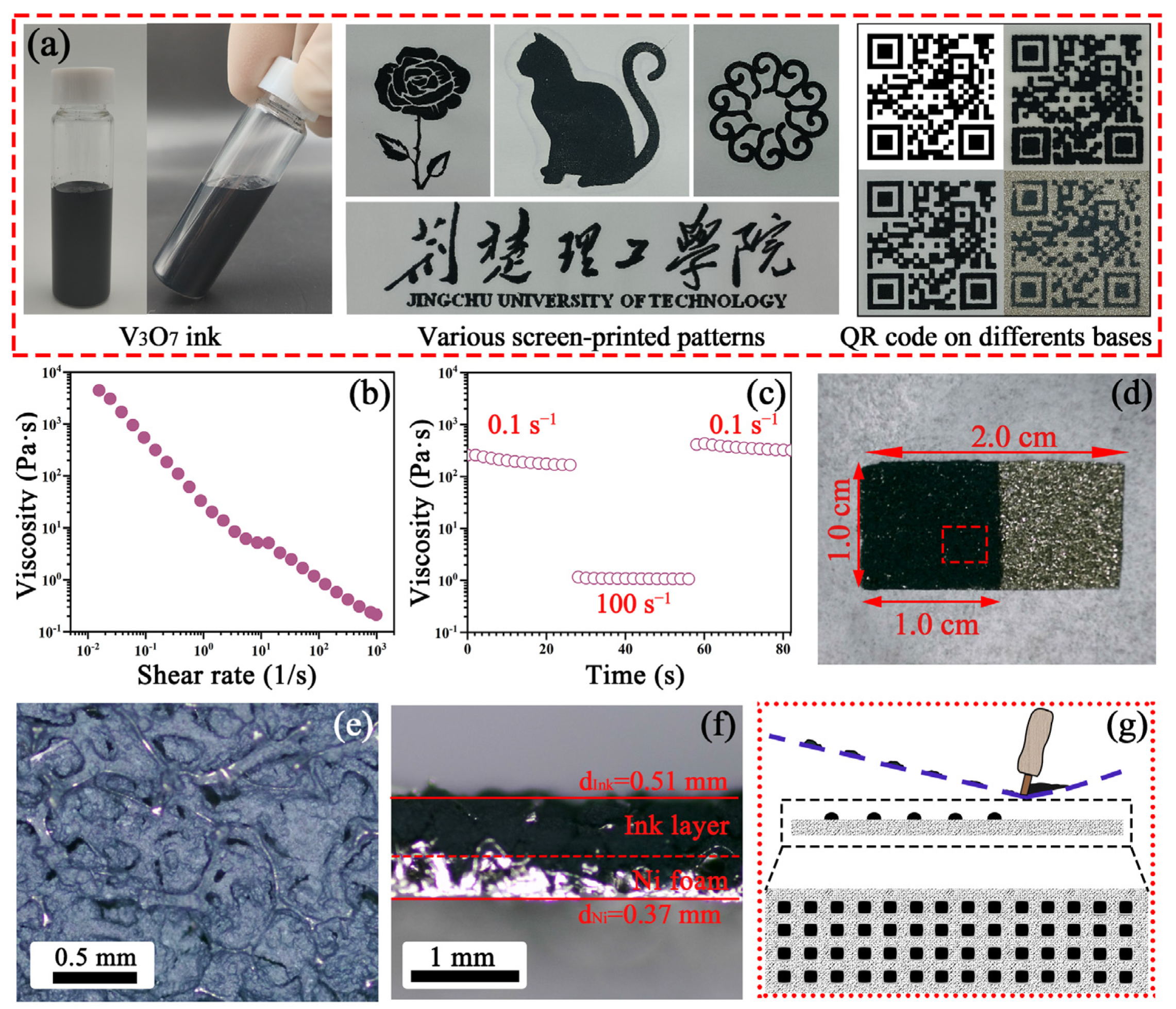
3.2. Ink Performances
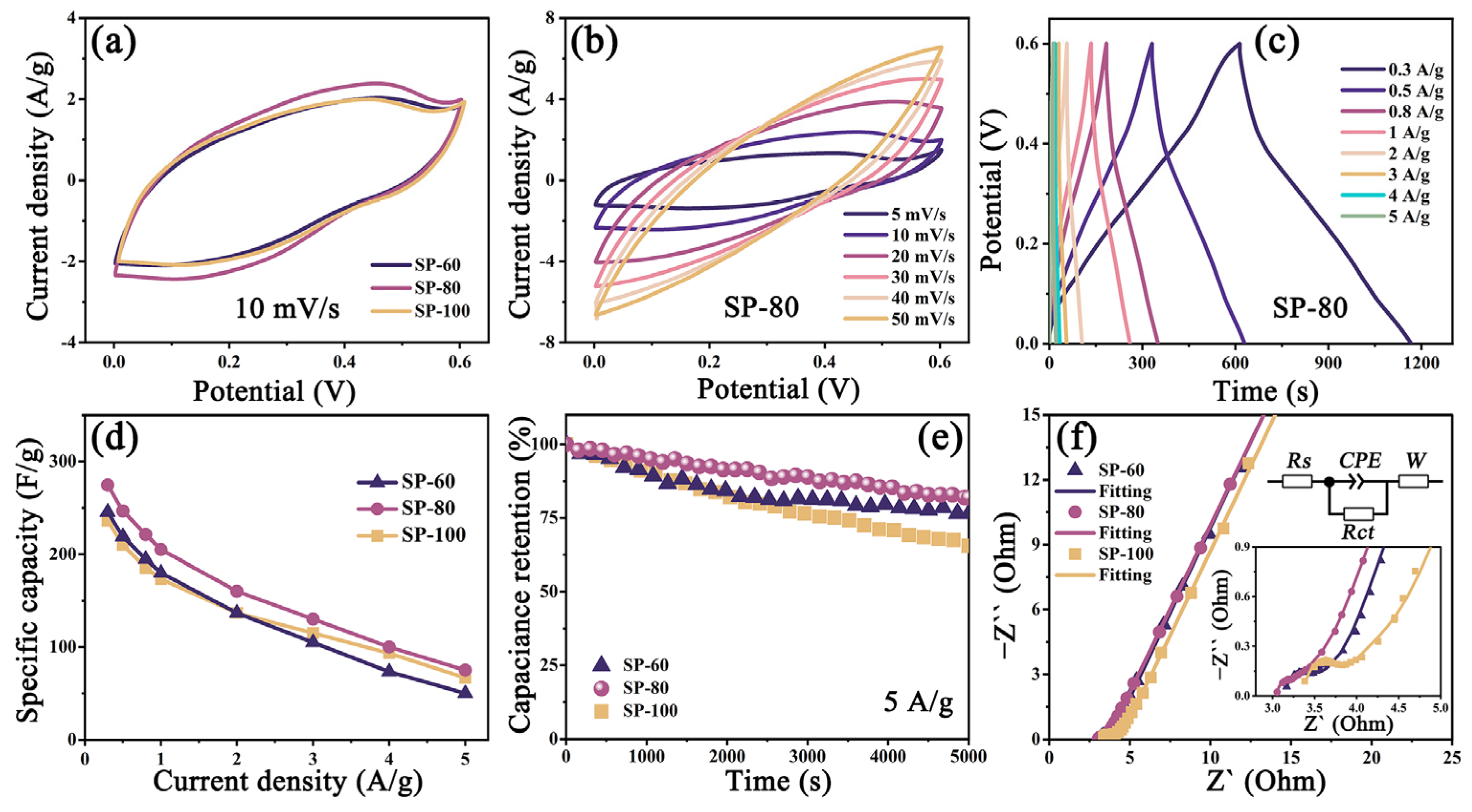
3.3. Electrochemical Study
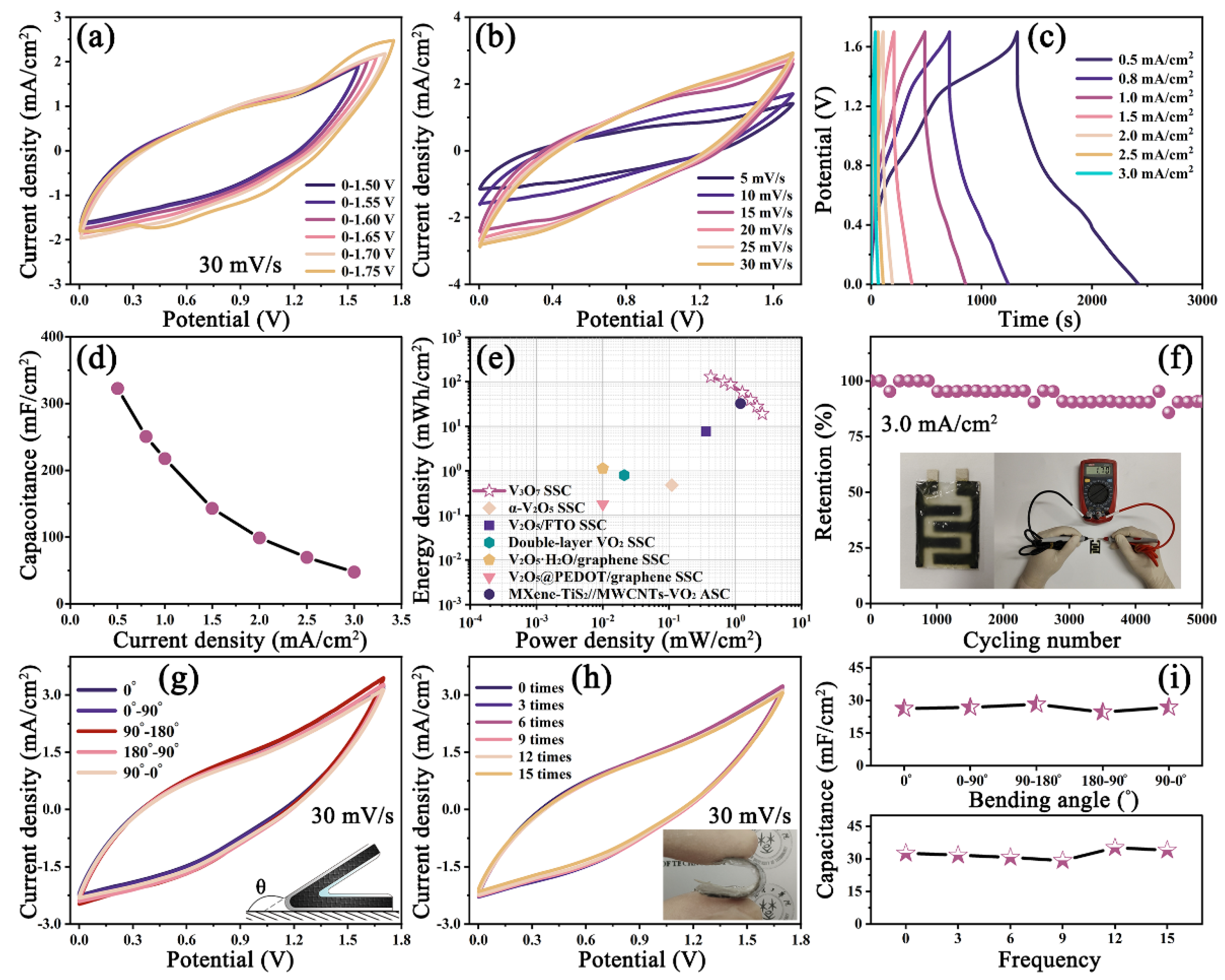
3.4. Characterization of the V3O7 SSC
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Na, J.; Zheng, D.; Kim, J.; Gao, M.; Azhar, A.; Lin, J.; Yamauchi, Y. Material nanoarchitectonics of functional polymers and inorganic nanomaterials for smart supercapacitors. Small 2022, 18, 2102397. [Google Scholar] [CrossRef]
- Benzigar, M.R.; Dasireddy, V.D.B.C.; Guan, X.; Wu, T.; Liu, G. Advances on emerging materials for flexible supercapacitors: Current trends and beyond. Adv. Funct. Mater. 2020, 30, 2002993. [Google Scholar] [CrossRef]
- Lv, Z.; Luo, Y.; Tang, Y.; Wei, J.; Zhu, Z.; Zhou, X.; Li, W.; Zeng, Y.; Zhang, W.; Zhang, Y.; et al. Editable supercapacitors with customizable stretchability based on mechanically strengthened ultralong MnO2 nanowire composite. Adv. Mater. 2018, 30, 1704531. [Google Scholar] [CrossRef]
- Pomerantseva, E.; Bonaccorso, F.; Feng, X.; Cui, Y.; Gogotsi, Y. Energy storage: The future enabled by nanomaterials. Science 2019, 366, eaan8285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simon, P.; Gogotsi, Y. Perspectives for electrochemical capacitors and related devices. Nat. Mater. 2020, 19, 1151–1163. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; McKeon, L.; Kremer, M.P.; Park, S.H.; Ronan, O.; Seral-Ascaso, A.; Barwich, S.; Coileáin, C.Ó.; McEvoy, N.; Nerl, H.C.; et al. Additive-free MXene inks and direct printing of micro-supercapacitors. Nat. Commun. 2019, 10, 1795. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Saeed, G.; Zhu, L.; Hui, K.N.; Kim, N.H.; Lee, J.H. 0D to 3D carbon-based networks combined with pseudocapacitive electrode material for high energy density supercapacitor: A review. Chem. Eng. J. 2021, 403, 126352. [Google Scholar] [CrossRef]
- Hu, C.; Li, M.; Qiu, J.; Sun, Y.P. Design and fabrication of carbon dots for energy conversion and storage. Chem. Soc. Rev. 2019, 48, 2315–2337. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, J.; Shifa, T.A.; Wang, D.; Wu, X.; Cui, Y. Hierarchical MnO2/activated carbon cloth electrode prepared by synchronized electrochemical activation and oxidation for flexible asymmetric supercapacitors. Chem. Eng. J. 2019, 372, 1047–1055. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, S.; Ma, J.; Das, P.; Zheng, S.; Wu, Z.S. Recent status and future perspectives of 2D MXene for micro-supercapacitors and micro-batteries. Energy Storage Mater. 2022, 51, 500–526. [Google Scholar] [CrossRef]
- Yan, J.; Dong, K.; Zhang, Y.; Wang, X.; Aboalhassan, A.A.; Yu, J.; Ding, B. Multifunctional flexible membranes from sponge-like porous carbon nanofibers with high conductivity. Nat. Commun. 2019, 10, 5584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsiao, Y.J.; Lin, L.Y. Efficient pore engineering in carbonized zeolitic imidazolate Framework-8 via chemical and physical methods as active materials for supercapacitors. J. Power Sources 2021, 486, 229370. [Google Scholar] [CrossRef]
- Dhandapani, P.; Balan, B.; Dinadayalane, T.; Angaiah, S. In-situ grown of FeCo2O4@2D-Carbyne coated nickel foam-A newer nanohybrid electrode for high performance asymmetric supercapacitors. J. Energy Storage 2022, 56, 105943. [Google Scholar] [CrossRef]
- Dhakal, G.; Kumar, D.R.; Sahoo, S.; Shim, J.J. Litchi seed biowaste-derived activated carbon supporting matrix for efficient symmetric and asymmetric supercapacitors. Carbon 2023, 208, 277–289. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, Y.; Zheng, S.; Zhang, L.; Shi, X.; He, J.; Chou, X.; Wu, Z.S. Ink formulation, scalable applications and challenging perspectives of screen printing for emerging printed microelectronics. J. Energy Chem. 2021, 63, 498–513. [Google Scholar] [CrossRef]
- Liang, J.; Tian, B.; Li, S.; Jiang, C.; Wu, W. All-printed MnHCF-MnOx-based high-performance flexible supercapacitors. Adv. Energy Mater. 2020, 10, 2000022. [Google Scholar] [CrossRef]
- Li, H.; Liang, J. Recent development of printed micro-supercapacitors: Printable materials, printing technologies, and perspectives. Adv. Mater. 2020, 32, 1805864. [Google Scholar] [CrossRef]
- Sajedi-Moghaddam, A.; Rahmanian, E.; Naseri, N. Inkjet-printing technology for supercapacitor application: Current state and perspectives. ACS Appl. Mater. Interfaces 2020, 12, 34487–34504. [Google Scholar] [CrossRef]
- Liu, B.T.; Shi, X.M.; Lang, X.Y.; Gu, L.; Wen, Z.; Zhao, M.; Jiang, Q. Extraordinary pseudocapacitive energy storage triggered by phase transformation in hierarchical vanadium oxides. Nat. Commun. 2018, 9, 1375. [Google Scholar] [CrossRef] [Green Version]
- Hu, T.; Liu, Y.; Zhang, Y.; Chen, M.; Zheng, J.; Tang, J.; Meng, C. 3D hierarchical porous V3O7·H2O nanobelts/CNT/reduced graphene oxide integrated composite with synergistic effect for supercapacitors with high capacitance and long cycling life. Journal of Colloid And Interface. Science 2018, 531, 382–393. [Google Scholar] [CrossRef]
- Tian, M.; Li, R.; Liu, C.; Long, D.; Cao, G. Aqueous Al-ion supercapacitor with V2O5 mesoporous carbon electrodes. ACS Appl. Mater. Interfaces 2019, 11, 15573–15580. [Google Scholar] [CrossRef] [PubMed]
- Oefner, N.; Shuck, C.E.; Schumacher, L.; Heck, F.; Hofmann, K.; Schmidpeter, J.; Li, W.; Bahri, M.; Mehdi, B.L.; Drochner, A.; et al. MXene Aerogel Derived Ultra-Active Vanadia Catalyst for Selective Conversion of Sustainable Alcohols to Base Chemicals. ACS Appl. Mater. Interfaces 2023, 15, 16714–16722. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Chen, Z.; Wei, M.; Wei, K.; Zhou, H. Single crystal nanobelts of V3O7·H2O: A lithium intercalation host with a large capacity. Electrochim. Acta 2009, 54, 1115–1118. [Google Scholar] [CrossRef]
- Devaraju, M.K.; Honma, I. Hydrothermal and solvothermal process towards development of LiMPO4 (M = Fe, Mn) nanomaterials for lithium-ion batteries. Adv. Energy Mater. 2012, 2, 284–297. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, P.; Wang, X.; Sun, J.; Sun, J.; Wen, Z. Fabrication of V3O7·H2O/graphene cathode for high performance zinc-Ion batteries. Mater. Lett. 2022, 317, 132124. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, J.; Xu, S.; Shao, M.; Zhang, Q.; Wei, F.; Jing, M.; Wei, M.; Evans, D.G.; Duan, X. Hierarchical NiMn layered double hydroxide/carbon nanotubes architecture with superb energy density for flexible supercapacitors. Adv. Funct. Mater. 2014, 24, 2938–2946. [Google Scholar] [CrossRef]
- Zhang, H.; Tahir, M.U.; Yan, X.; Liu, X.; Su, X.; Zhang, L. Ni-Al layered double hydroxide with regulated interlayer spacing as electrode for aqueous asymmetric supercapacitor. Chem. Eng. J. 2019, 368, 905–913. [Google Scholar] [CrossRef]
- Hu, P.; Hu, P.; Vu, T.D.; Li, M.; Wang, S.; Ke, Y.; Zeng, X.; Mai, L.; Long, Y. Vanadium Oxide: Phase Diagrams, Structures, Synthesis, and Applications. Chem. Rev. 2023, 123, 4353–4415. [Google Scholar] [CrossRef]
- Wang, P.; Shi, X.; Wu, Z.; Guo, S.; Zhou, J.; Liang, S. Layered hydrated vanadium oxide as highly reversible intercalation cathode for aqueous Zn-ion batteries. Carbon Energy 2020, 2, 294–301. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Xie, K.; Liu, Z.X. Determination of the position of V4+ as minor component in XPS spectra by difference spectra. Appl. Surf. Sci. 1998, 133, 221–224. [Google Scholar] [CrossRef]
- Cui, J.; Da, D.; Jiang, W. Structure characterization of vanadium oxide thin films prepared by magnetron sputtering methods. Appl. Surf. Sci. 1998, 133, 225–229. [Google Scholar] [CrossRef]
- Ureña-Begara, F.; Crunteanu, A.; Raskin, J.P. Raman and XPS characterization of vanadium oxide thin films with temperature. Appl. Surf. Sci. 2017, 403, 717–727. [Google Scholar] [CrossRef]
- Zhou, H.; Li, D.; Hibino, M.; Honma, I. A self-ordered, crystalline-glass, mesoporous nanocomposite for use as a lithium-based storage device with both high power and high energy densities. Angew. Chem. Int. Edit. 2005, 44, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.F.; Yuan, G.H.; Jiang, Z.H.; Yao, Z.P.; Yue, M. Preparation of Ni(OH)2-graphene sheet-carbon nanotube composite as electrode material for supercapacitors. J. Alloys Compd. 2015, 618, 37–43. [Google Scholar] [CrossRef]
- Saadi, M.A.S.R.; Maguire, A.; Pottackal, N.T.; Thakur, M.S.H.; Ikram, M.M.; Hart, A.J.; Ajayan, P.M.; Rahman, M.M. Direct ink writing: A 3D printing technology for diverse materials. Adv. Mater. 2022, 34, 2108855. [Google Scholar] [CrossRef]
- Yu, L.H.; Fan, Z.; Shao, Y.; Tian, Z.; Sun, J.; Liu, Z. Versatile N-Doped MXene Ink for Printed Electrochemical Energy Storage Application. Adv. Energy Mater. 2019, 9, 1901839. [Google Scholar] [CrossRef]
- Cong, G.; Zhou, Y.; Li, Z.; Lu, Y.C. A highly concentrated catholyte enabled by a low-melting-point ferrocene derivative. ACS Energy Lett. 2017, 2, 869–875. [Google Scholar] [CrossRef]
- Adewinbi, S.A.; Busari, R.A.; Animasahun, L.O.; Omotoso, E.; Taleatu, B.A. Effective pseudocapacitive performance of binder free transparent α-V2O5 thin film electrode: Electrochemical and some surface probing. Phys. B 2021, 621, 413260. [Google Scholar] [CrossRef]
- Azadian, F.; Rastogi, A.C. Energy storage performance of thin film nanocrystalline vanadium oxide with fluorinated tin oxide current carrier electrode for solid-state transparent supercapacitors based on ionic liquid gel electrolyte. Electrochim. Acta 2020, 330, 135339. [Google Scholar] [CrossRef]
- Alhebshi, N.A.; Vaseem, M.; Minyawi, B.A.; AlAmri, A.M.; Shamim, A. Single and double layer of monoclinic VO2 ink-based printed and interdigitated supercapacitors. Energy Technol. 2022, 10, 2200432. [Google Scholar] [CrossRef]
- Bao, J.; Zhang, X.; Bai, L.; Bai, W.; Zhou, M.; Xie, J.; Guan, M.; Zhou, J.; Xie, Y. All-solid-state flexible thin-film supercapacitors with high electrochemical performance based on a two-dimensional V2O5·H2O/graphene composite. J. Mater. Chem. A 2014, 2, 10876–10881. [Google Scholar] [CrossRef]
- Wang, L.; Shu, T.; Guo, S.; Lu, Y.; Li, M.; Nzabahimana, J.; Hu, X. Fabricating strongly coupled V2O5@PEDOT nanobelts/graphene hybrid films with high areal capacitance and facile transferability for transparent solid-state supercapacitors. Energy Storage Mater. 2020, 27, 150–158. [Google Scholar] [CrossRef]
- Zhao, B.; Wang, S.; Yu, Q.; Wang, Q.; Wang, M.; Ni, T.; Ruan, L.; Zeng, W. A flexible, heat-resistant and self-healable “rock-ing-chair” zinc ion microbattery based on MXene-TiS2 (de) intercalation anode. J. Power Sources 2021, 504, 230076. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, B.; Zheng, Y.; Wang, J.; Tu, Q.; Tang, W.; Chen, L. Flexible High-Performance and Screen-Printed Symmetric Supercapacitor Using Hierarchical Rodlike V3O7 Inks. Nanomaterials 2023, 13, 2282. https://doi.org/10.3390/nano13162282
Lin B, Zheng Y, Wang J, Tu Q, Tang W, Chen L. Flexible High-Performance and Screen-Printed Symmetric Supercapacitor Using Hierarchical Rodlike V3O7 Inks. Nanomaterials. 2023; 13(16):2282. https://doi.org/10.3390/nano13162282
Chicago/Turabian StyleLin, Baoying, Yinyin Zheng, Jinglu Wang, Qian Tu, Wentao Tang, and Liangzhe Chen. 2023. "Flexible High-Performance and Screen-Printed Symmetric Supercapacitor Using Hierarchical Rodlike V3O7 Inks" Nanomaterials 13, no. 16: 2282. https://doi.org/10.3390/nano13162282




