Solid-State Structural Transformation in Zn(II) Metal–Organic Frameworks in a Single-Crystal-to-Single-Crystal Fashion
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Procedure
2.2. Preparation of [Zn2(spy)2(tdc)2] (1)
2.3. Preparation of [Zn2(spy)4(tdc)2] (2)
2.4. Preparation of [Zn2(rctt-ppcb)(tdc)2] (3)
2.5. [Zn2(spy)2(rctt-ppcb)(tdc)2] (4)
2.6. X-ray Crystallographic Analysis
3. Results and Discussion
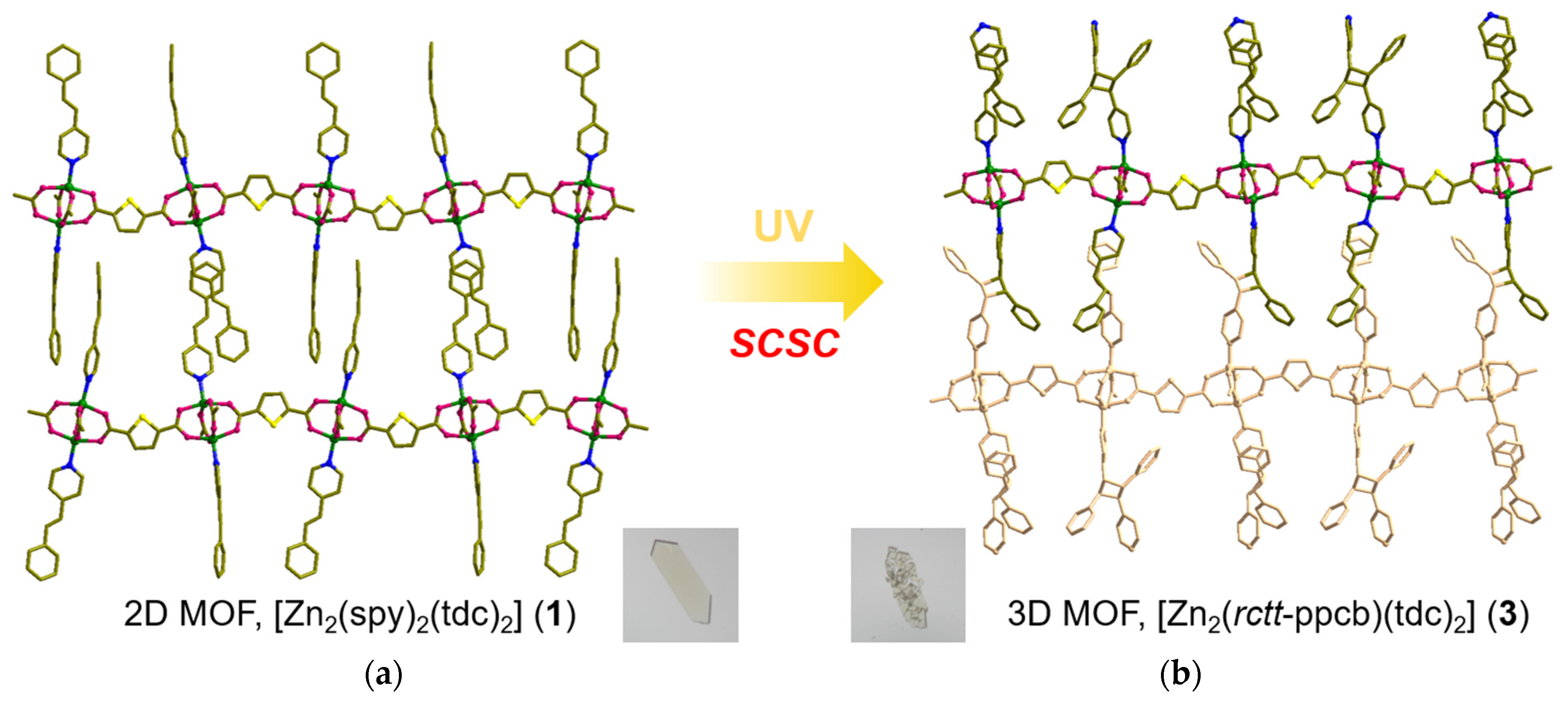
3.1. Syntheses of MOFs 1–4
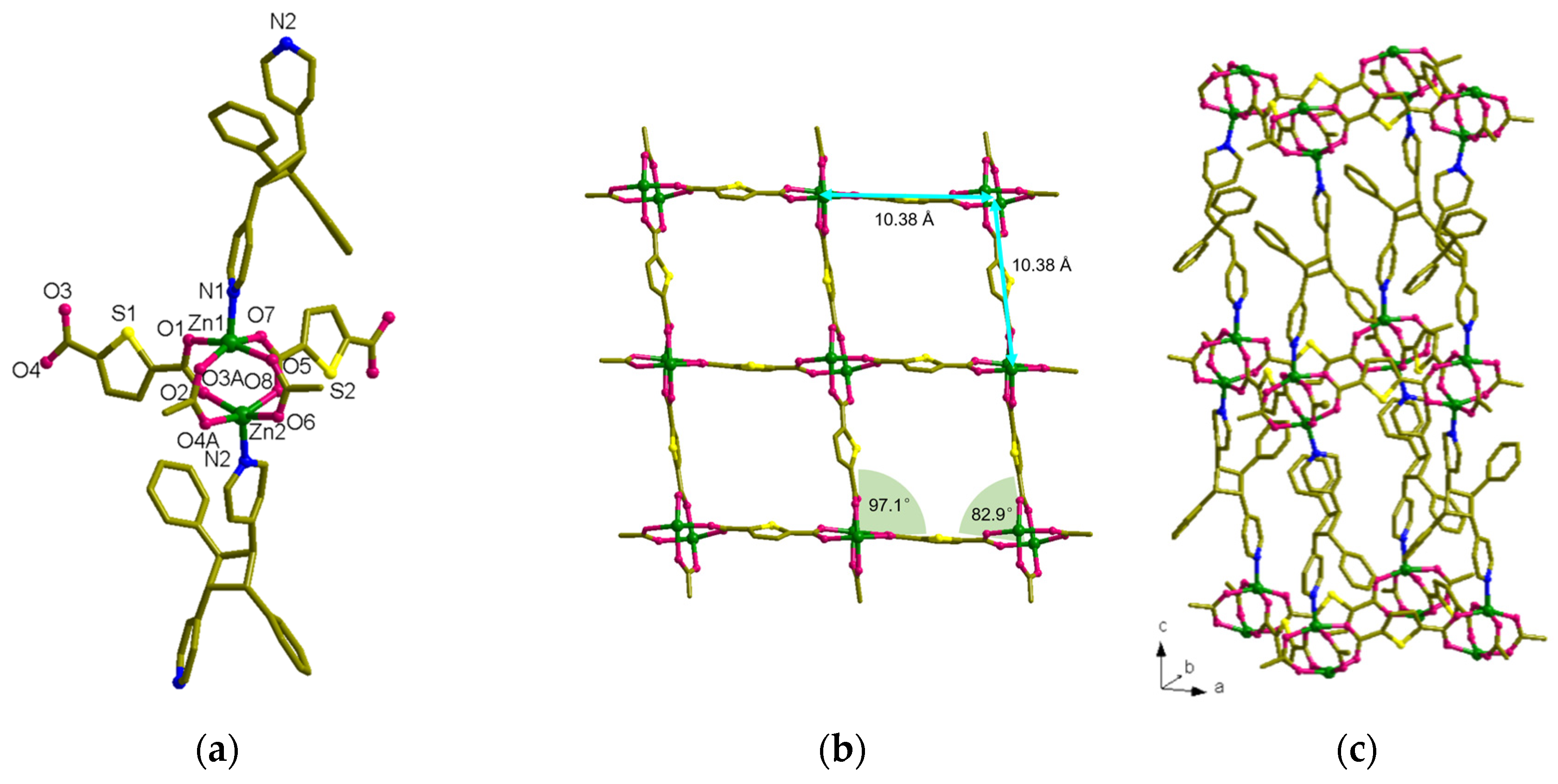
3.2. Structural Description of MOF 1
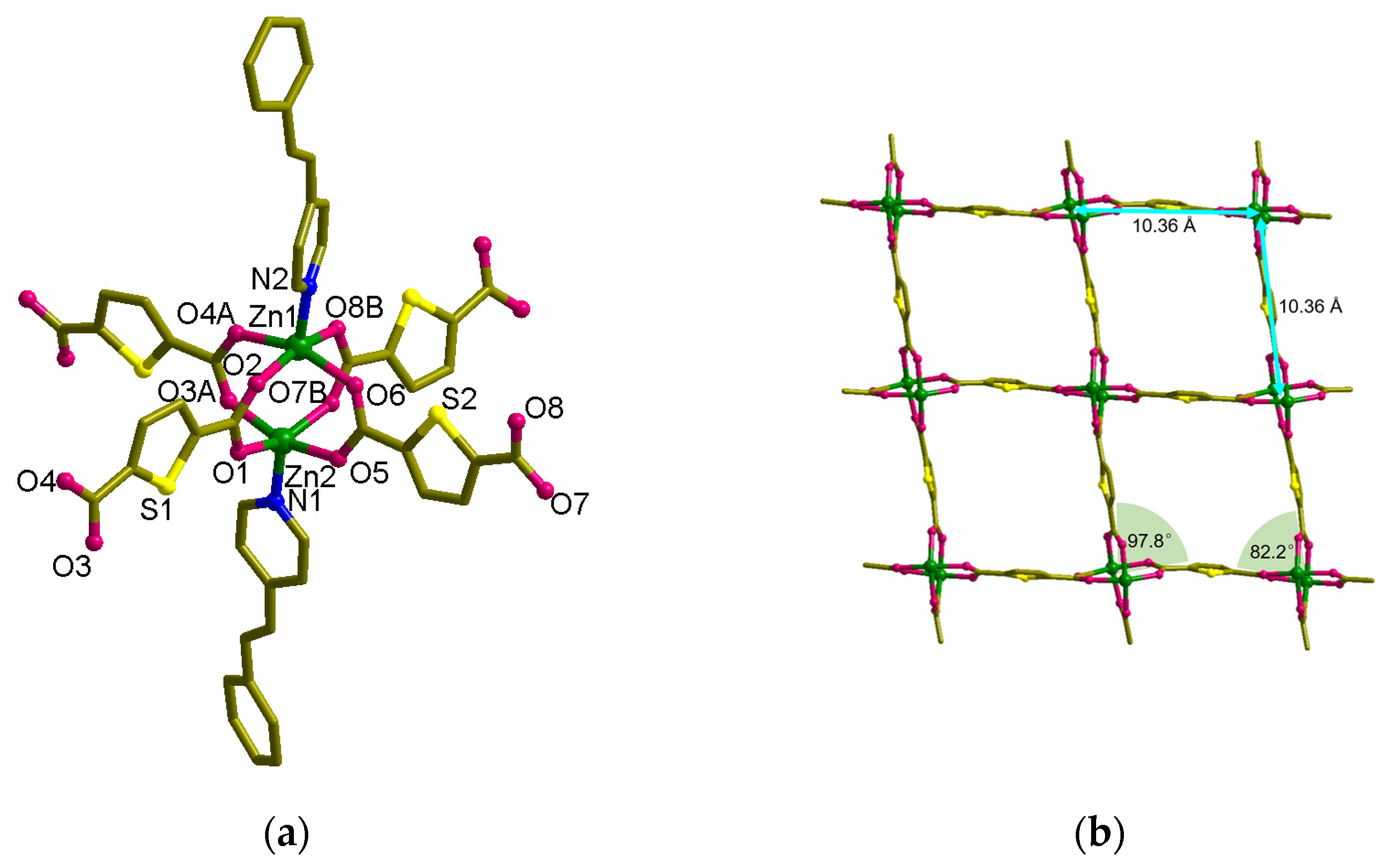
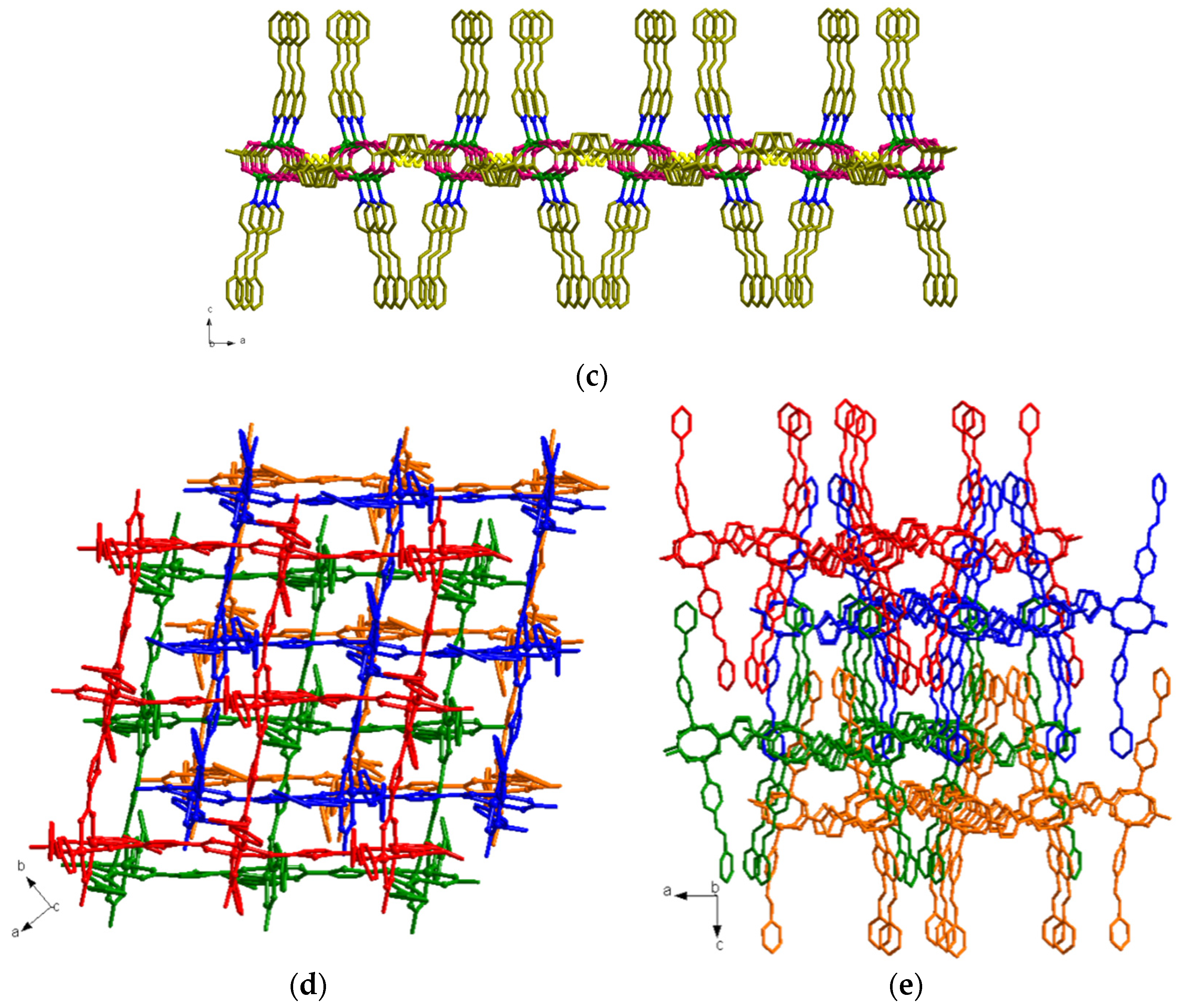
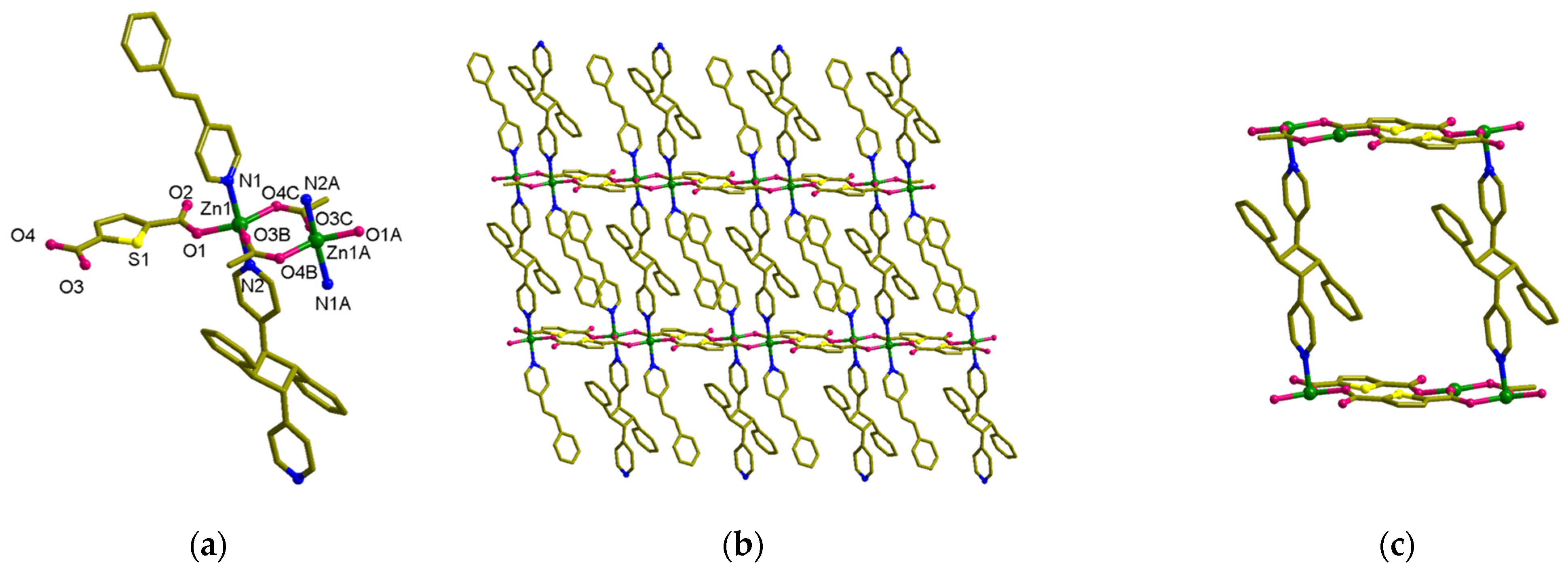
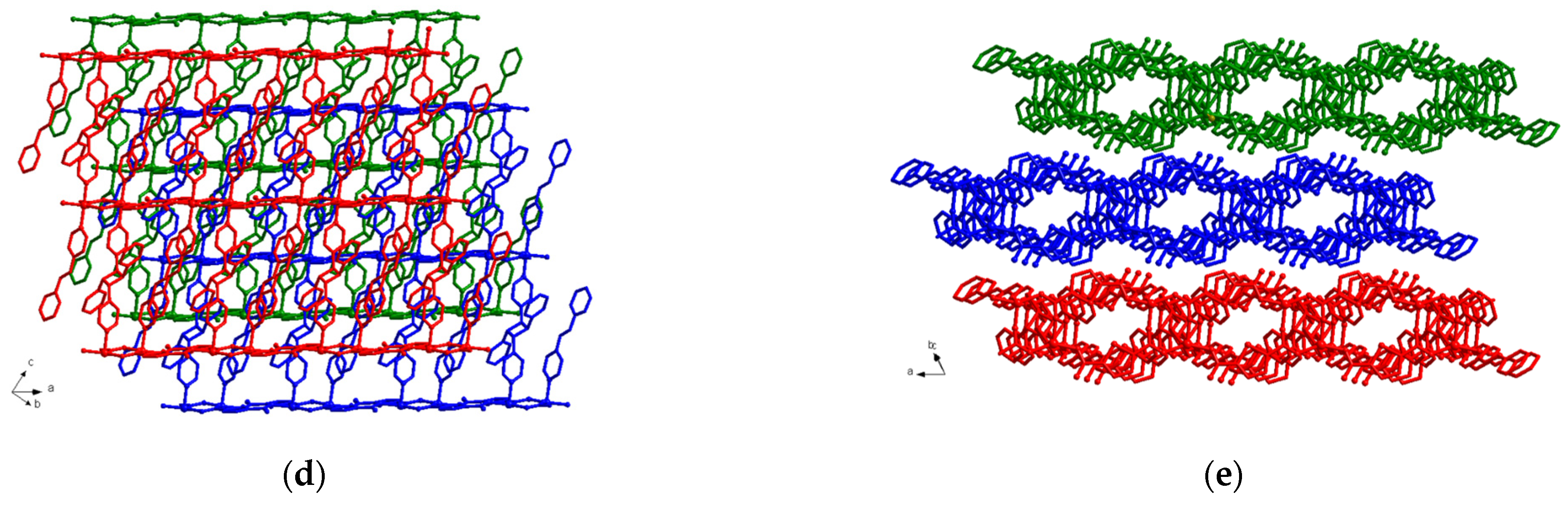
3.3. Structural Description of MOF 3
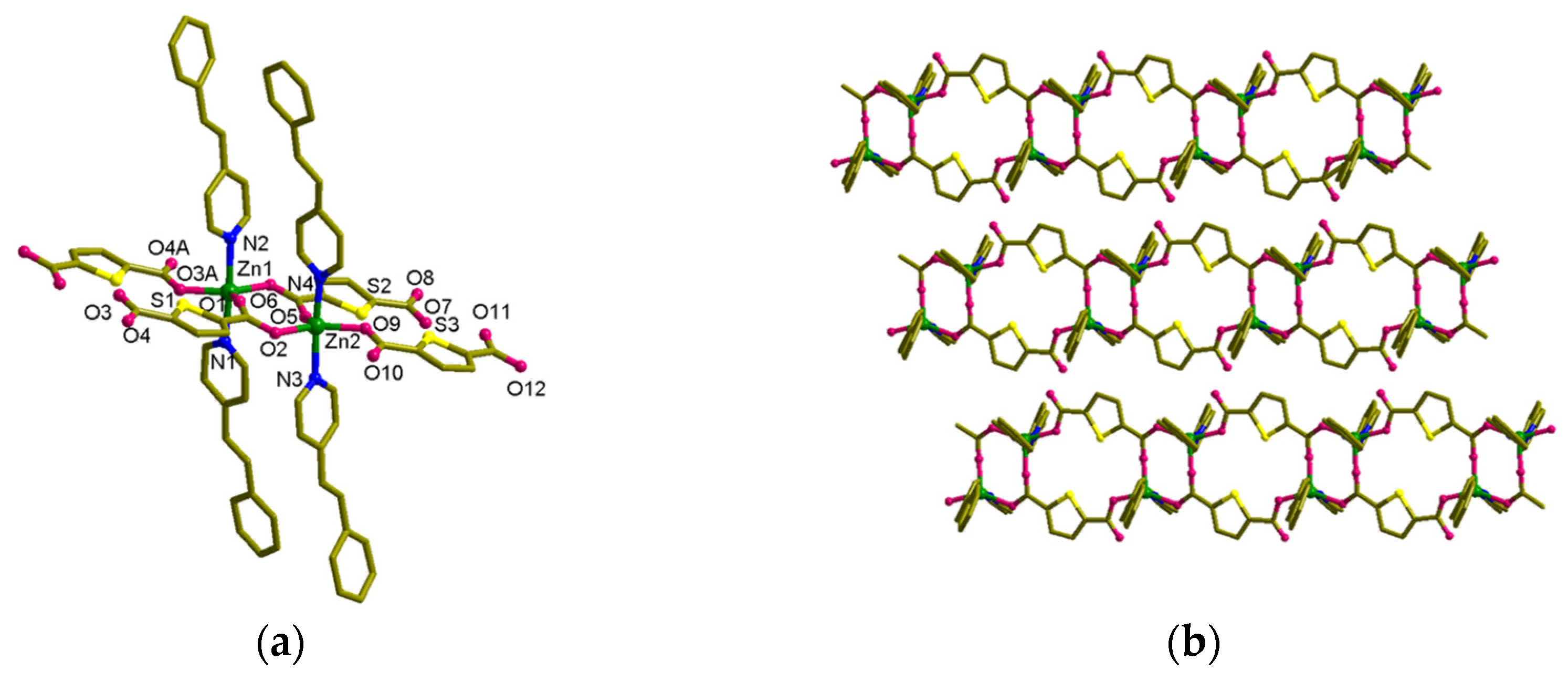
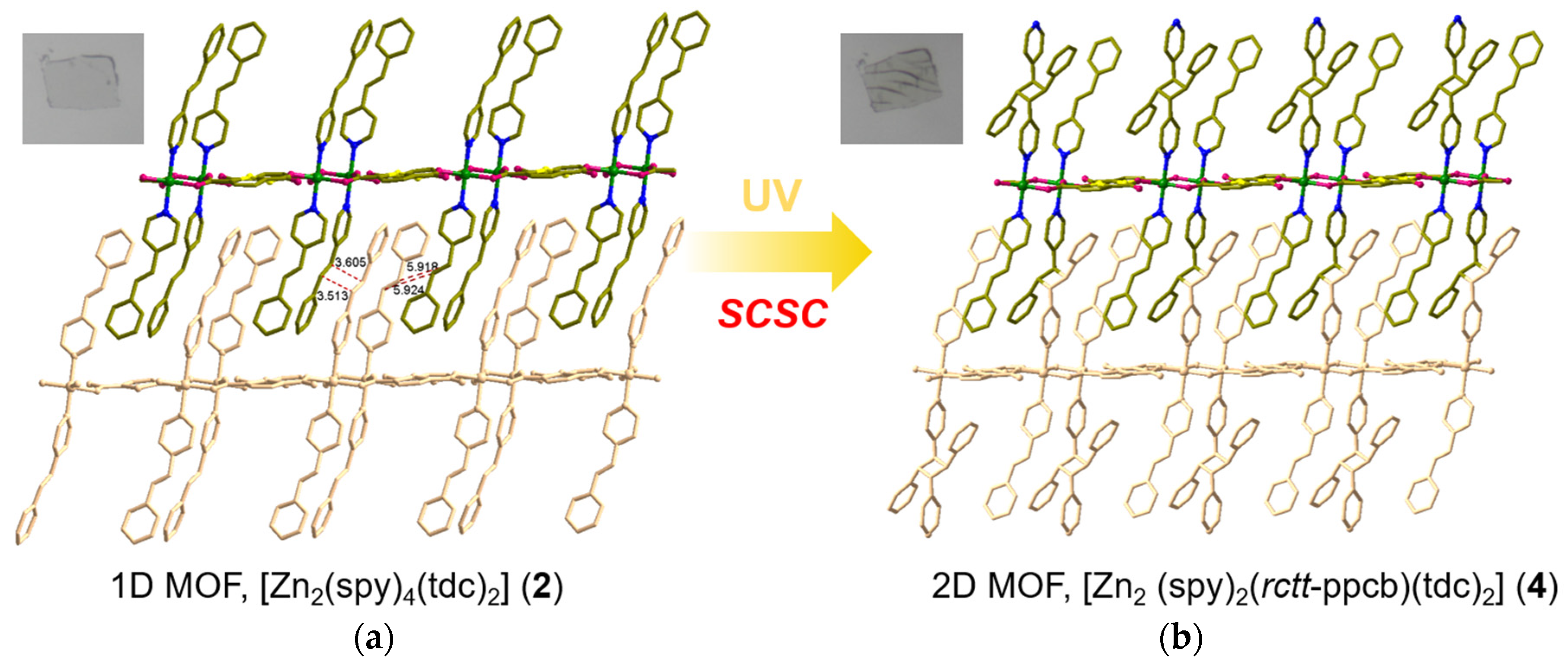
3.4. Structural Description of MOF 2
3.5. Structural Description of MOF 4
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chakraborty, G.; Park, I.-H.; Medishetty, R.; Vittal, J.J. Two-Dimensional Metal-Organic Framework Materials: Synthesis, Structures, Properties and Applications. Chem. Rev. 2021, 121, 3751–3891. [Google Scholar] [CrossRef] [PubMed]
- Leong, W.L.; Vittal, J.J. One-Dimensional Coordination Polymers: Complexity and Diversity in Structures, Properties, and Applications. Chem. Rev. 2011, 111, 688–764. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.-L.; Hor, T.S.A.; Jin, G.-X. Photodriven single-crystal-to-single-crystal transformation. Coord. Chem. Rev. 2017, 346, 112–122. [Google Scholar] [CrossRef]
- Kole, G.K.; Vittal, J.J. Solid-state reactivity and structural transformations involving coordination polymers. Chem. Soc. Rev. 2013, 42, 1755–1775. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Vittal, J.J. Control of interpenetration and structural transformations in the interpenetrated MOFs. Coord. Chem. Rev. 2021, 435, 213789. [Google Scholar] [CrossRef]
- Jin, K.; Lee, B.; Park, J. Metal-organic frameworks as a versatile platform for radionuclide management. Coord. Chem. Rev. 2021, 427, 213473. [Google Scholar] [CrossRef]
- Jeoung, S.; Kim, S.; Kim, M.; Moon, H.R. Pore engineering of metal-organic frameworks with coordinating functionalities. Coord. Chem. Rev. 2020, 420, 213377. [Google Scholar] [CrossRef]
- Kim, D.; Yoo, H.; Kim, K.; Kim, D.; Kim, K.T.; Kim, C.; Kim, J.Y.; Moon, H.R.; Kim, M. Post-synthetic ligand cyclization in metal–organic frameworks through functional group connection with regioisomerism. Chem. Commun. 2022, 58, 5948–5951. [Google Scholar] [CrossRef]
- Kim, J.; Na, C.; Son, Y.; Prabu, M.; Yoon, M. Stilbene ligand-based metal–organic frameworks for efficient dye adsorption and nitrobenzene detection. Bull. Korean Chem. Soc. 2023, 44, 507–515. [Google Scholar] [CrossRef]
- Bae, C.; Gu, M.; Jeon, Y.; Kim, D.; Kim, J. Metal–organic frameworks for NH3 adsorption by different NH3 operating pressures. Bull. Korean Chem. Soc. 2023, 44, 112–124. [Google Scholar] [CrossRef]
- Jeong, J.Y.; Joung, H.; Jang, G.J.; Han, S.Y. Probing emergence of biomolecular coronas around drug-loaded liposomal nanoparticles in the solution by using nanoparticle tracking analysis. Bull. Korean Chem. Soc. 2023, 44, 551–557. [Google Scholar] [CrossRef]
- Lee, D.; Lee, S.; Son, Y.; Kim, J.Y.; Cha, S.; Kwak, D.; Lee, J.; Kwak, J.; Yoon, M.; Kim, M. Uncoordinated tetrazole ligands in metal–organic frameworks for proton-conductivity studies. Bull. Korean Chem. Soc. 2022, 43, 912–917. [Google Scholar] [CrossRef]
- Medishetty, R.; Koh, L.L.; Kole, G.K.; Vittal, J.J. Solid-State Structural Transformations from 2D Interdigitated Layers to 3D Interpenetrated Structures. Angew. Chem. Int. Ed. 2011, 50, 10949–10952. [Google Scholar] [CrossRef] [PubMed]
- Park, I.-H.; Medishetty, R.; Lee, S.S.; Vittal, J.J. Solid-state polymerization in a polyrotaxane coordination polymer via a [2+2] cycloaddition reaction. Chem. Commun. 2014, 50, 6585–6588. [Google Scholar] [CrossRef] [PubMed]
- Dincă, M.; Long, J.R. Introduction: Porous Framework Chemistry. Chem. Rev. 2020, 120, 8037–8038. [Google Scholar] [CrossRef] [PubMed]
- Desiraju, G.R.; Vittal, J.J.; Ramanan, A. Crystal Engineering: A Textbook; World Scientific: Singapore, 2011. [Google Scholar]
- MacGillivray, L.R. Organic Synthesis in the Solid State via Hydrogen-Bond-Driven Self-Assembly. J. Org. Chem. 2008, 73, 3311–3317. [Google Scholar] [CrossRef] [PubMed]
- MacGillivray, L.R.; Papaefstathiou, G.S.; Friščić, T.; Hamilton, T.D.; Bučar, D.K.; Chu, Q.; Varshney, D.B.; Georgiev, I.G. Supramolecular Control of Reactivity in the Solid State: From Templates to Ladderanes to Metal−Organic Frameworks. Acc. Chem. Res. 2008, 41, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Kole, G.K.; Mir, M.H. Isolation of elusive cyclobutane ligands via a template-assisted photochemical [2 + 2] cycloaddition reaction and their utility in engineering crystalline solids. CrystEngComm 2022, 24, 3993–4007. [Google Scholar] [CrossRef]
- Khan, S.; Akhtaruzzaman; Medishetty, R.; Ekka, A.; Mir, M.H. Mechanical Motion in Crystals Triggered by Solid State Photochemical [2+2] Cycloaddition Reaction. Chem.–Asian J. 2021, 16, 2806–2816. [Google Scholar] [CrossRef]
- Hu, F.-L.; Mi, Y.; Zhu, C.; Abrahams, B.F.; Braunstein, P.; Lang, J.-P. Stereoselective Solid-State Synthesis of Substituted Cyclobutanes Assisted by Pseudorotaxane-like MOFs. Angew. Chem. Int. Ed. 2018, 57, 12696–12701. [Google Scholar] [CrossRef]
- Schmidt, G.M.J. Photodimerization in the solid state. Pure Appl. Chem. 1971, 27, 647–678. [Google Scholar] [CrossRef] [Green Version]
- Rath, B.B.; Vittal, J.J. Photoreactive Crystals Exhibiting [2 + 2] Photocycloaddition Reaction and Dynamic Effects. Acc. Chem. Res. 2022, 55, 1445–1455. [Google Scholar] [CrossRef] [PubMed]
- Yelgaonkar, S.P.; Campillo-Alvarado, G.; MacGillivray, L.R. Phototriggered Guest Release from a Nonporous Organic Crystal: Remarkable Single-Crystal-to-Single-Crystal Transformation of a Binary Cocrystal Solvate to a Ternary Cocrystal. J. Am. Chem. Soc. 2020, 142, 20772–20777. [Google Scholar] [CrossRef] [PubMed]
- Claassens, I.E.; Barbour, L.J.; Haynes, D.A. A Multistimulus Responsive Porous Coordination Polymer: Temperature-Mediated Control of Solid-State [2+2] Cycloaddition. J. Am. Chem. Soc. 2019, 141, 11425–11429. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, I.G.; MacGillivray, L.R. Metal-mediated reactivity in the organic solid state: From self-assembled complexes to metal-organic frameworks. Chem. Soc. Rev. 2007, 36, 1239–1248. [Google Scholar] [CrossRef] [PubMed]
- Biradha, K.; Santra, R. Crystal engineering of topochemical solid state reactions. Chem. Soc. Rev. 2013, 42, 950–967. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Dutta, B.; Mir, M.H. Impact of solid-state photochemical [2+2] cycloaddition on coordination polymers for diverse applications. Dalton Trans. 2020, 49, 9556–9563. [Google Scholar] [CrossRef] [PubMed]
- Vittal, J.J.; Quah, H.S. Photochemical reactions of metal complexes in the solid state. Dalton Trans. 2017, 46, 7120–7140. [Google Scholar] [CrossRef]
- Medishetty, R.; Park, I.-H.; Lee, S.S.; Vittal, J.J. Solid-state polymerisation via [2+2] cycloaddition reaction involving coordination polymers. Chem. Commun. 2016, 52, 3989–4001. [Google Scholar] [CrossRef]
- Yang, Z.-Y.; Sang, X.; Liu, D.; Li, Q.-Y.; Lang, F.; Abrahams, B.F.; Hou, H.; Braunstein, P.; Lang, J.-P. Photopolymerization-Driven Macroscopic Mechanical Motions of a Composite Film Containing a Vinyl Coordination Polymer. Angew. Chem. Int. Ed. 2023, 62, e202302429. [Google Scholar] [CrossRef]
- Sato, H.; Matsuda, R.; Mir, M.H.; Kitagawa, S. Photochemical cycloaddition on the pore surface of a porous coordination polymer impacts the sorption behavior. Chem. Commun. 2012, 48, 7919–7921. [Google Scholar] [CrossRef] [PubMed]
- Kusaka, S.; Kiyose, A.; Sato, H.; Hijikata, Y.; Hori, A.; Ma, Y.; Matsuda, R. Dynamic Topochemical Reaction Tuned by Guest Molecules in the Nanospace of a Metal–Organic Framework. J. Am. Chem. Soc. 2019, 141, 15742–15746. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, M.; Kusaka, S.; Kiyose, A.; Nakajo, T.; Iguchi, H.; Mizuno, M.; Matsuda, R. Beyond the Conventional Limitation of Photocycloaddition Reaction in the Roomy Nanospace of a Metal–Organic Framework. J. Am. Chem. Soc. 2023, 145, 12059–12065. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.-X.; Zhang, W.-H.; Abrahams, B.F.; Braunstein, P.; Lang, J.-P. Fabrication of Photoactuators: Macroscopic Photomechanical Responses of Metal–Organic Frameworks to Irradiation by UV Light. Angew. Chem. Int. Ed. 2019, 58, 9453–9458. [Google Scholar] [CrossRef] [PubMed]
- Karothu, D.P.; Halabi, J.M.; Ahmed, E.; Ferreira, R.; Spackman, P.R.; Spackman, M.A.; Naumov, P. Global Analysis of the Mechanical Properties of Organic Crystals. Angew. Chem. Int. Ed. 2022, 61, e202113988. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Tong, F.; Al-Kaysi, R.O.; Bardeen, C.J. Photomechanical Effects in Photochromic Crystals. In Photomechanical Materials, Composites, and Systems; White, T.J., Ed.; Wiley: Hoboken, NJ, USA, 2017; pp. 233–274. [Google Scholar]
- Horwitz, L. Notes–Studies in cis- and trans-Stilbazoles. J. Org. Chem. 1956, 21, 1039–1041. [Google Scholar] [CrossRef]
- Williams, J.L.R.; Adel, R.E.; Carlson, J.M.; Reynolds, G.A.; Borden, D.G.; Ford, J.A., Jr. A Comparison of Methods for the Preparation of 2- and 4-Styrylpyridines1. J. Org. Chem. 1963, 28, 387–390. [Google Scholar] [CrossRef]
- Efange, S.M.N.; Michelson, R.H.; Remmel, R.P.; Boudreau, R.J.; Dutta, A.K.; Freshler, A. Flexible N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine analog: Synthesis and monoamine oxidase catalyzed bioactivation. J. Med. Chem. 1990, 33, 3133–3138. [Google Scholar] [CrossRef]
- Bruker, A.I.; Madison, W. USA, SMART, SAINT and SADABS; Bruker AXS Inc.: Billerica, MA, USA, 2016. [Google Scholar]
- Sheldrick, G.M. SADABS Version 2.03; University of Göttingen: Göttingen, Germany, 2002; pp. 1600–5368. [Google Scholar]
- Bruker, A.I.; Madison, W. USA, Inc. SHELXTL v 6.10; Bruker AXS: Billerica, MA, USA, 2000. [Google Scholar]
- Shin, J.W.; Eom, K.; Moon, D. BL2D-SMC, the supramolecular crystallography beamline at the Pohang Light Source II, Korea. J. Synchrotron Radiat. 2016, 23, 369–373. [Google Scholar] [CrossRef]
- Otwinowski, Z.; Minor, W. [20] Processing of X-ray diffraction data collected in oscillation mode. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1997; Volume 276, pp. 307–326. [Google Scholar]
- Sheldrick, G.M. SADABS: A Program for Empirical Absorption Correction of Area Detector Data; University of Göttingen: Göttingen, Germany, 1996. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Spek, A.L. Single-crystal structure validation with the program PLATON. J. Appl. Crystallogr. 2003, 36, 7–13. [Google Scholar] [CrossRef] [Green Version]
- Chanthapally, A.; Oh, W.T.; Vittal, J.J. Photoreactivity of polymorphs of a ladder polymer with criss-cross and parallel orientations of C=C bonds. Chem. Commun. 2014, 50, 451–453. [Google Scholar] [CrossRef]



| 1 | 2 | 3 | 4 | |
|---|---|---|---|---|
| Formula | C304H208N16O64S16Zn16 | C224H168N14O28S7Zn7 | C304H208N16O64S16Zn16 | C32H24N2O4SZn |
| Formula weight | 6667.73 | 4185.72 | 6667.73 | 597.96 |
| Temperature (K) | 223(2) | 223(2) | 223(2) | 223(2) |
| Crystal system | Orthorhombic | Triclinic | Orthorhombic | |
| Space group | Pbca | P-1 | Pbca | P-1 |
| Z | 1 | 2 | 1 | 2 |
| a (Å) | 15.619(10) | 12.327(6) | 15.565(3) | 10.511(2) |
| b (Å) | 13.628(9) | 19.995(4) | 13.750(3) | 12.313(3) |
| c (Å) | 34.631(17) | 38.859(8) | 33.213(7) | 12.463(3) |
| α (°) | 90 | 91.48(2) | 90 | 69.62(3) |
| β (°) | 90 | 93.97(4) | 90 | 72.22(3) |
| γ (°) | 90 | 95.486(19) | 90 | 65.06(3) |
| V (Å3) | 7372(8) | 9506(5) | 7108(3) | 1346.9(6) |
| Dcalc (g/cm3) | 1.502 | 1.462 | 1.558 | 1.474 |
| 2θmax (°) | 52.00 | 52.00 | 52.00 | 52.00 |
| R1, wR2 [I > 2σ(I)] | 0.0618, 0.1721 | 0.0625, 0.1314 | 0.0415, 0.1124 | 0.0346, 0.0966 |
| R1, wR2 [all data] | 0.0968, 0.2238 | 0.3039, 0.1549 | 0.0425, 0.1130 | 0.0413, 0.0991 |
| Goodness-of-fit on F2 | 1.012 | 1.050 | 1.081 | 1.054 |
| No. of reflection used [>2σ(I)] | 7230 [Rint = 0.1216] | 35774 [Rint = 0.0743] | 9950 [Rint = 0.0647] | 7151 [Rint = 0.0204] |
| Refinement | 83,844 | 67,872 | 72,761 | 14,241 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, J.; Oh, J.; Kurakula, U.; Lee, D.H.; Choudhury, A.; Lee, E.; Medishetty, R.; Park, I.-H. Solid-State Structural Transformation in Zn(II) Metal–Organic Frameworks in a Single-Crystal-to-Single-Crystal Fashion. Nanomaterials 2023, 13, 2319. https://doi.org/10.3390/nano13162319
An J, Oh J, Kurakula U, Lee DH, Choudhury A, Lee E, Medishetty R, Park I-H. Solid-State Structural Transformation in Zn(II) Metal–Organic Frameworks in a Single-Crystal-to-Single-Crystal Fashion. Nanomaterials. 2023; 13(16):2319. https://doi.org/10.3390/nano13162319
Chicago/Turabian StyleAn, Jaewook, Jihye Oh, Uma Kurakula, Dong Hee Lee, Aditya Choudhury, Eunji Lee, Raghavender Medishetty, and In-Hyeok Park. 2023. "Solid-State Structural Transformation in Zn(II) Metal–Organic Frameworks in a Single-Crystal-to-Single-Crystal Fashion" Nanomaterials 13, no. 16: 2319. https://doi.org/10.3390/nano13162319
APA StyleAn, J., Oh, J., Kurakula, U., Lee, D. H., Choudhury, A., Lee, E., Medishetty, R., & Park, I.-H. (2023). Solid-State Structural Transformation in Zn(II) Metal–Organic Frameworks in a Single-Crystal-to-Single-Crystal Fashion. Nanomaterials, 13(16), 2319. https://doi.org/10.3390/nano13162319







