A Study of the Microstructure and Tribological Properties of Copper-Based Cr@graphite Alloy Modified by Nano Cr3C2 and CrC
Abstract
:1. Introduction
2. Experiments
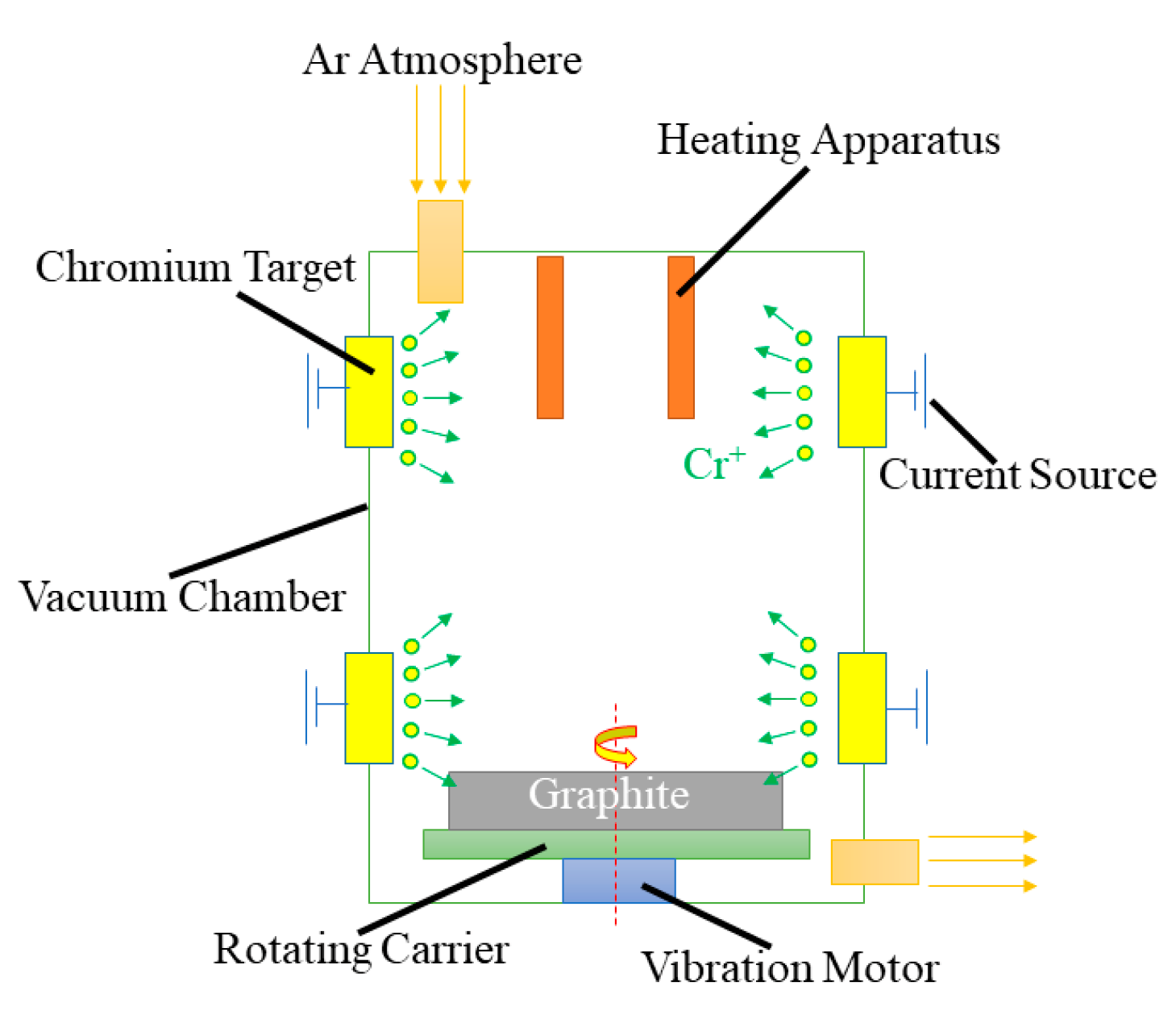
2.1. Preparation of Cr@graphite
2.2. Copper-Based Cr@graphite Alloy
2.3. Microstructure Investigation
2.4. Mechanical Properties Test
2.5. Tribological Properties Test
3. Results
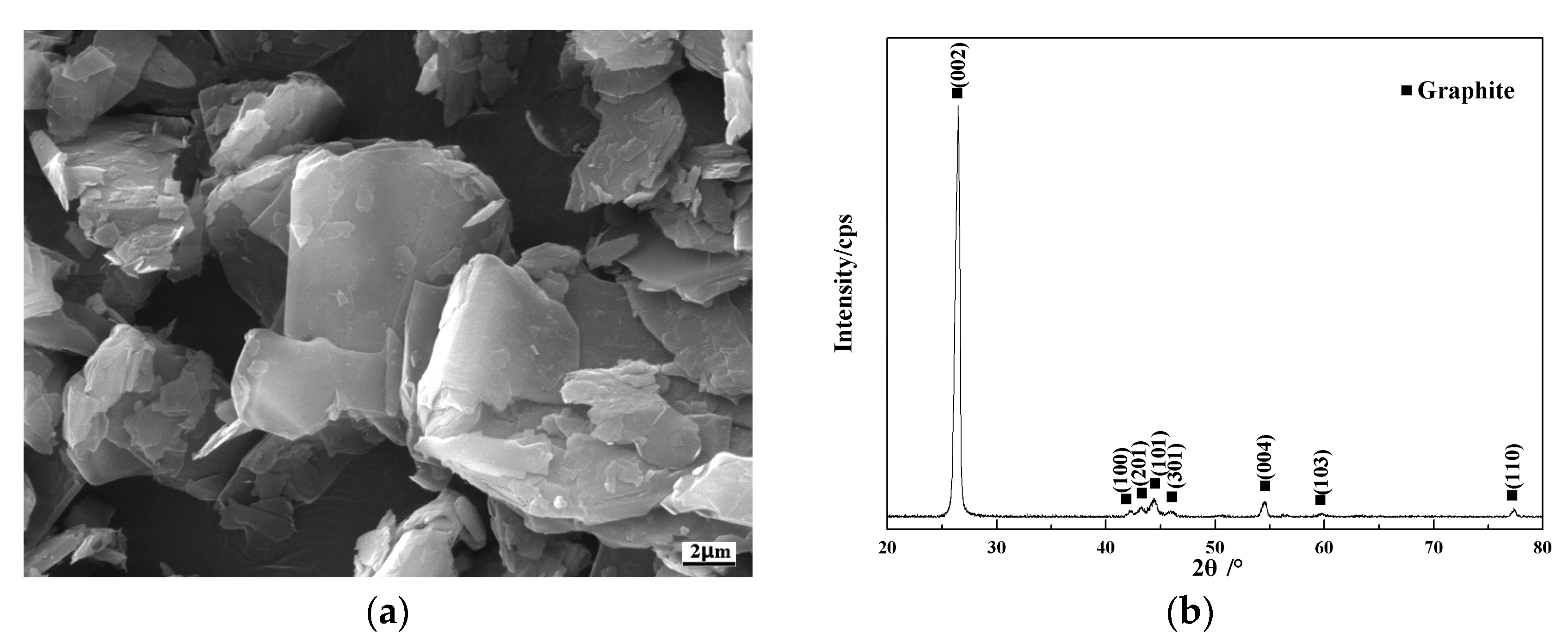
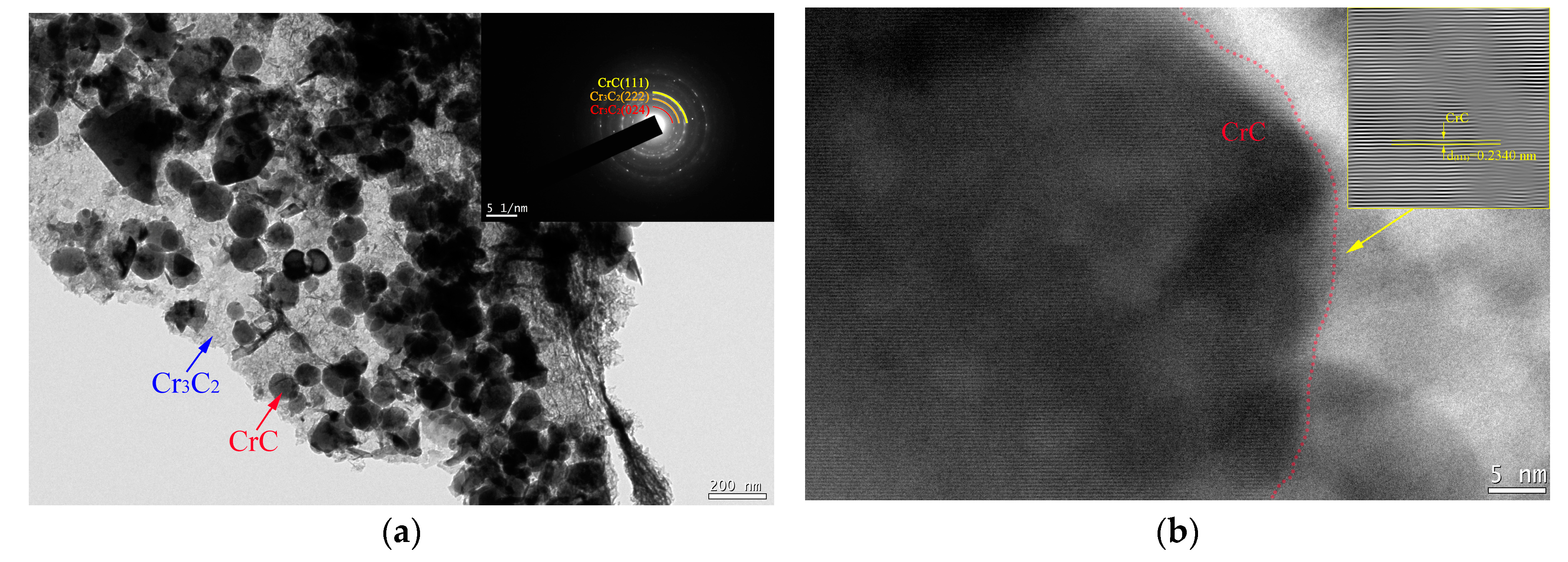
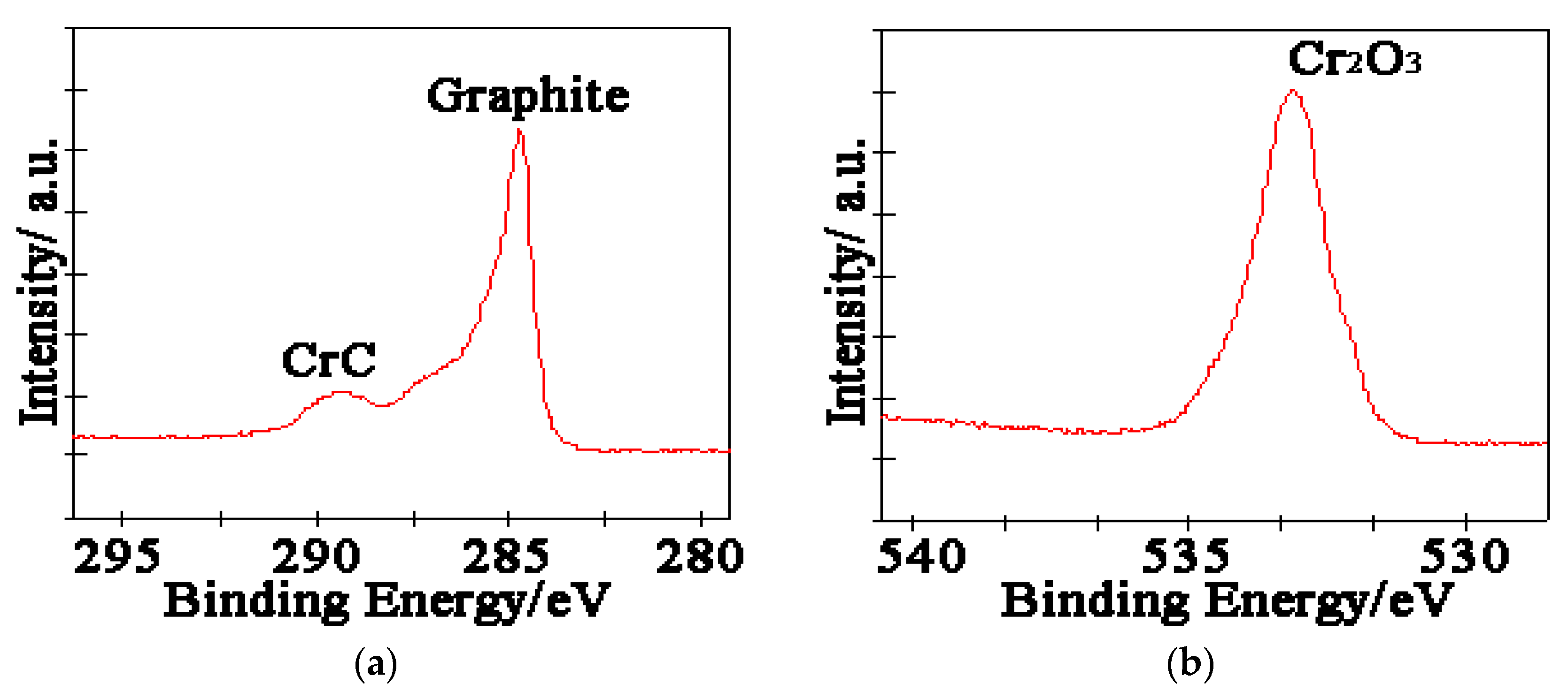
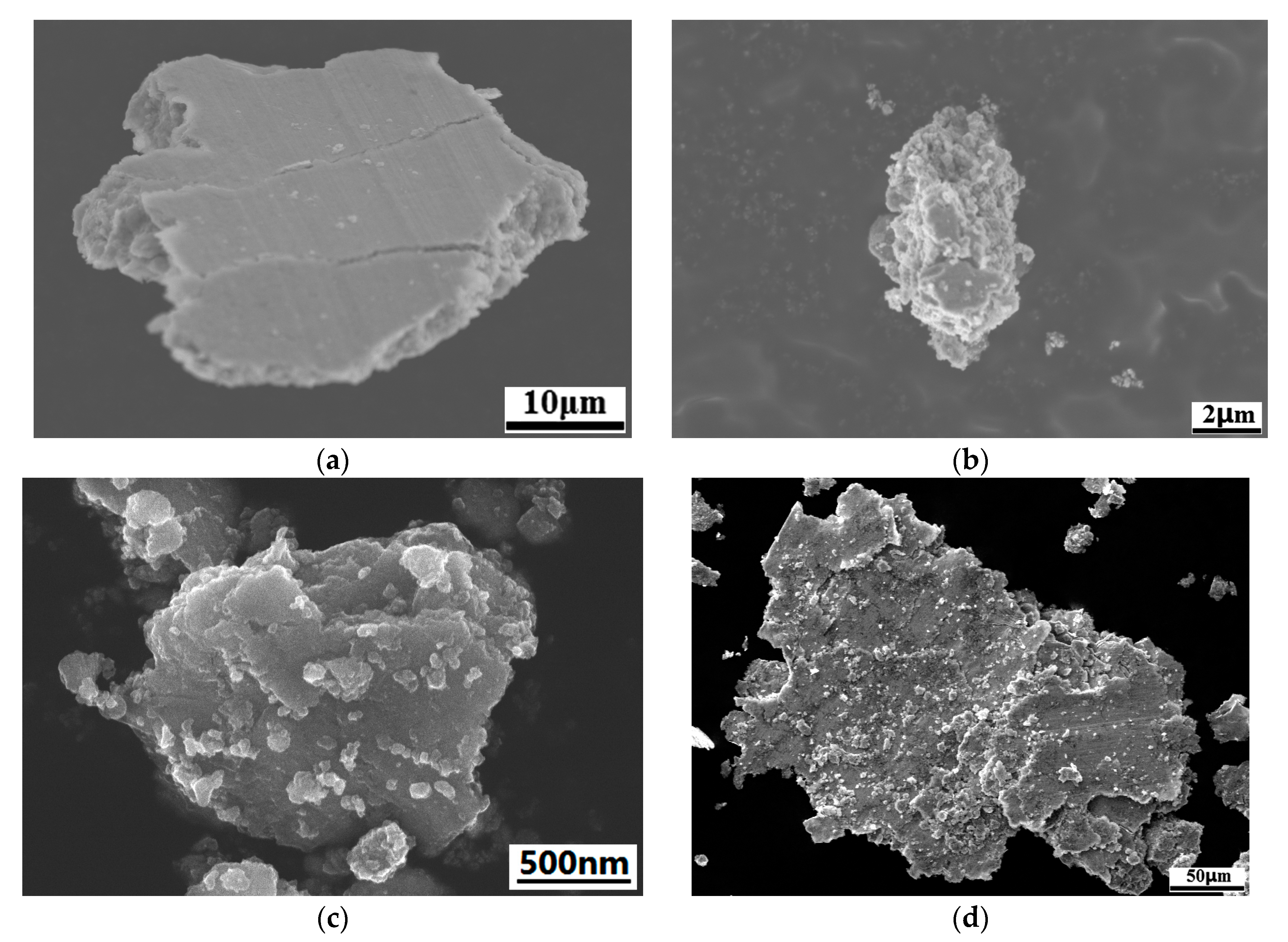
3.1. Microstructure of Cr@graphite
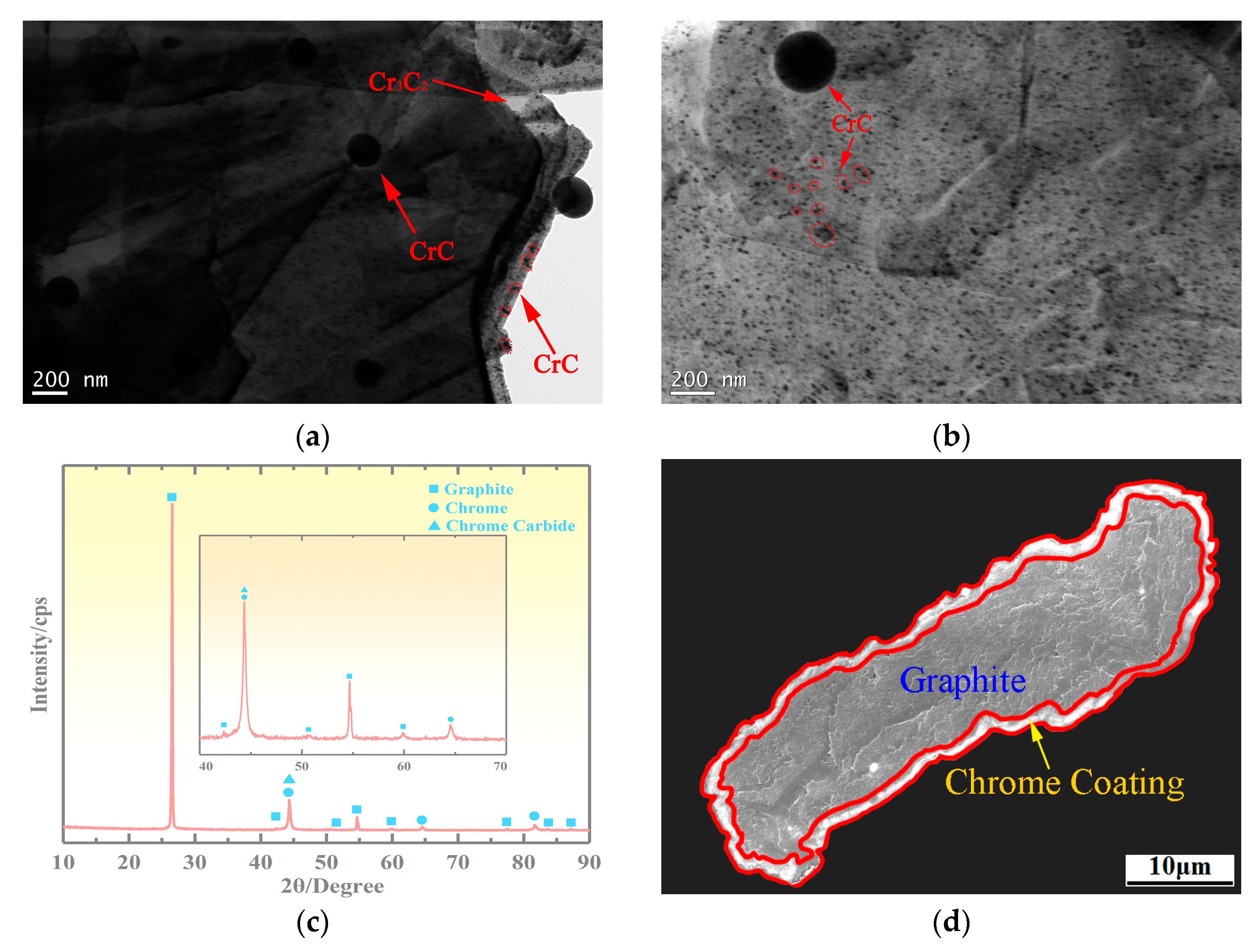
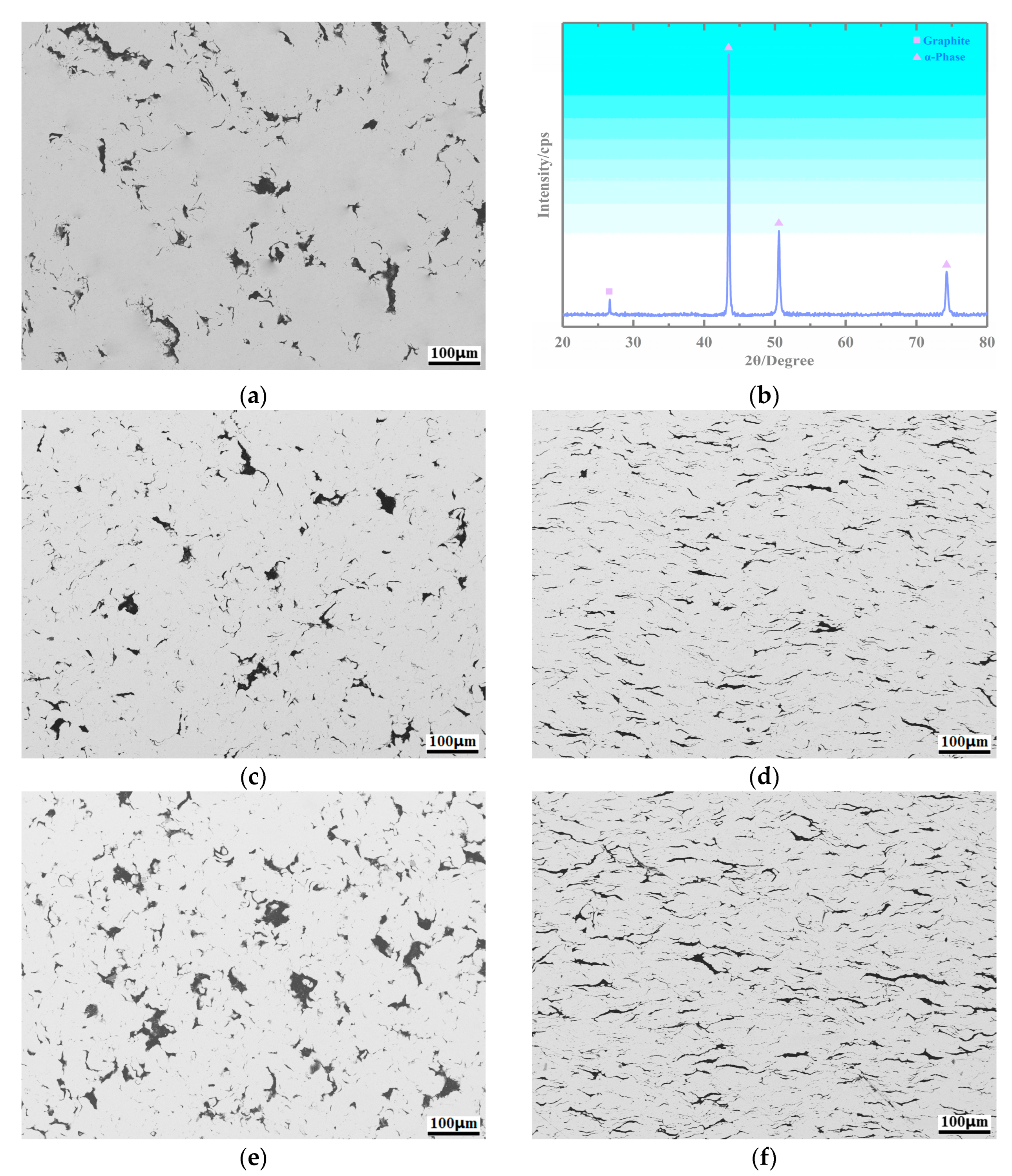
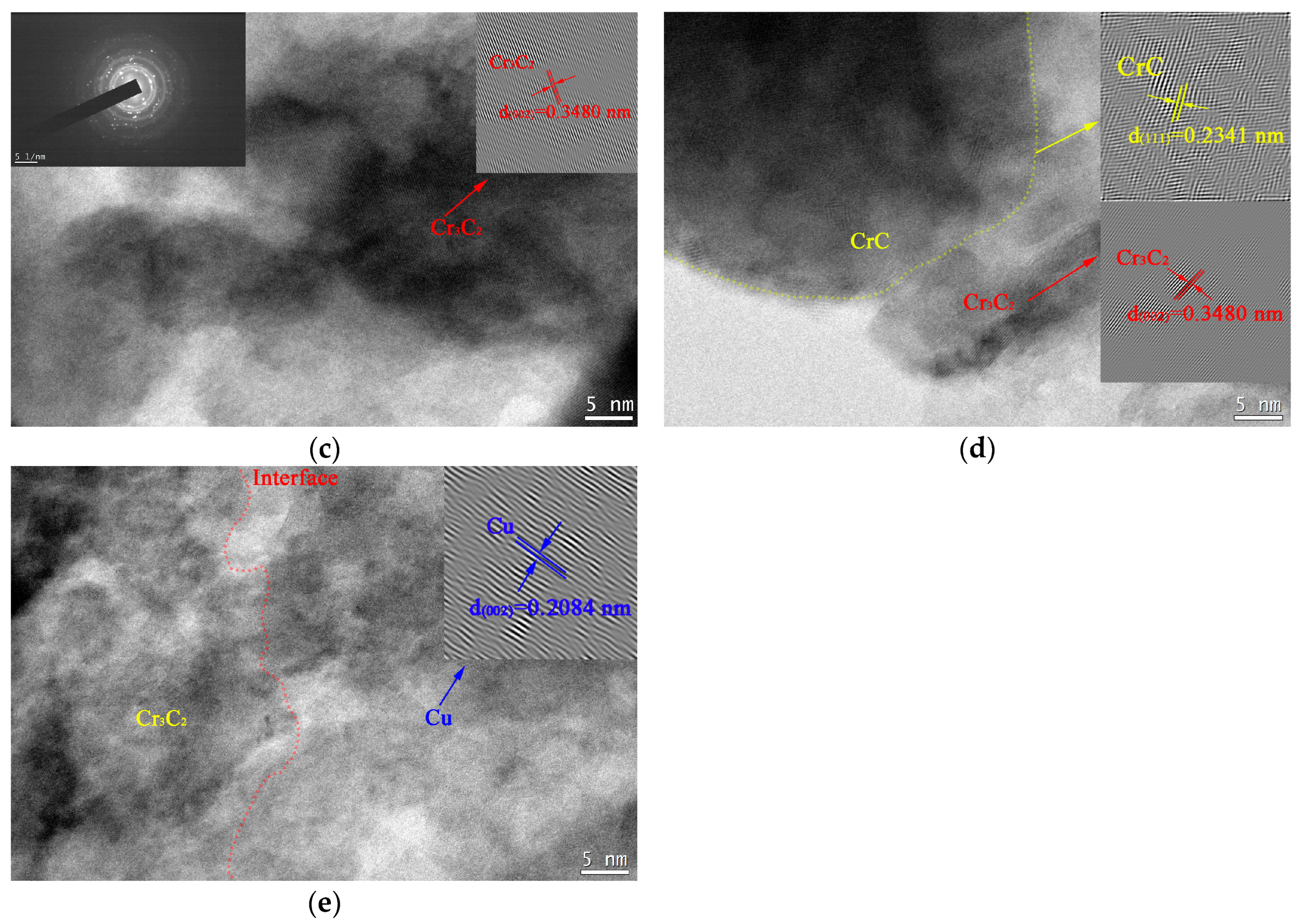
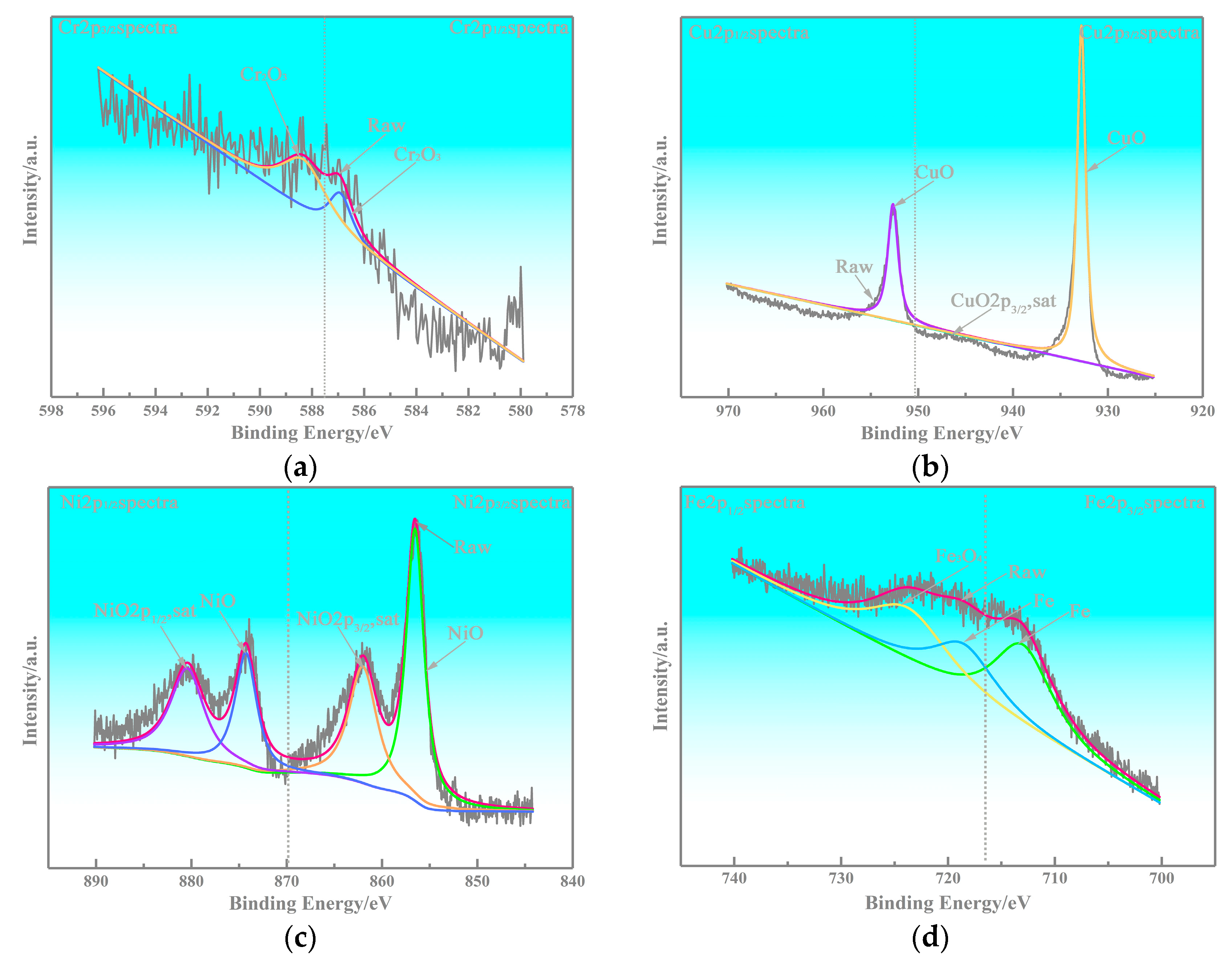
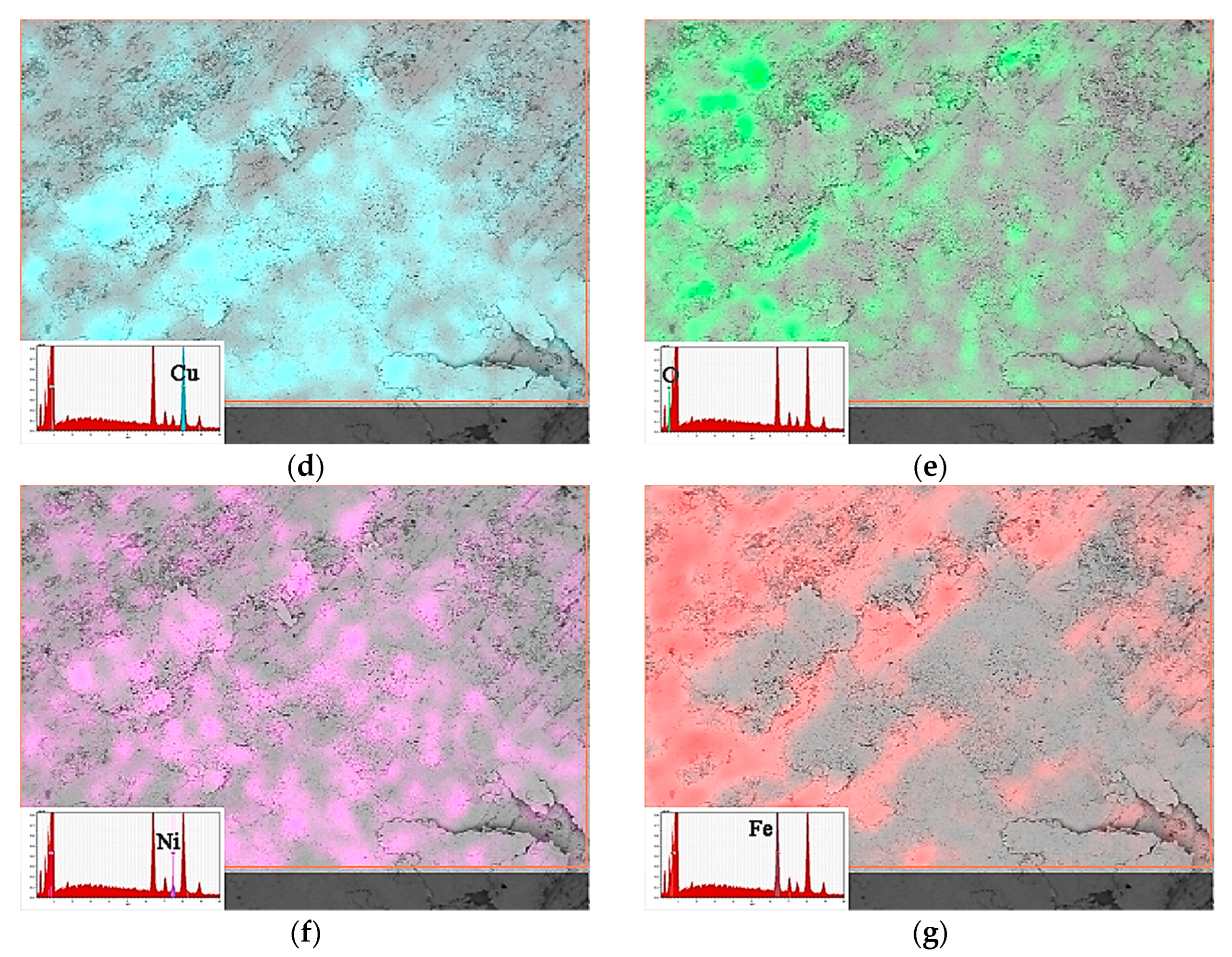
3.2. Microstructure of the Copper-Based Cr@graphite Alloy
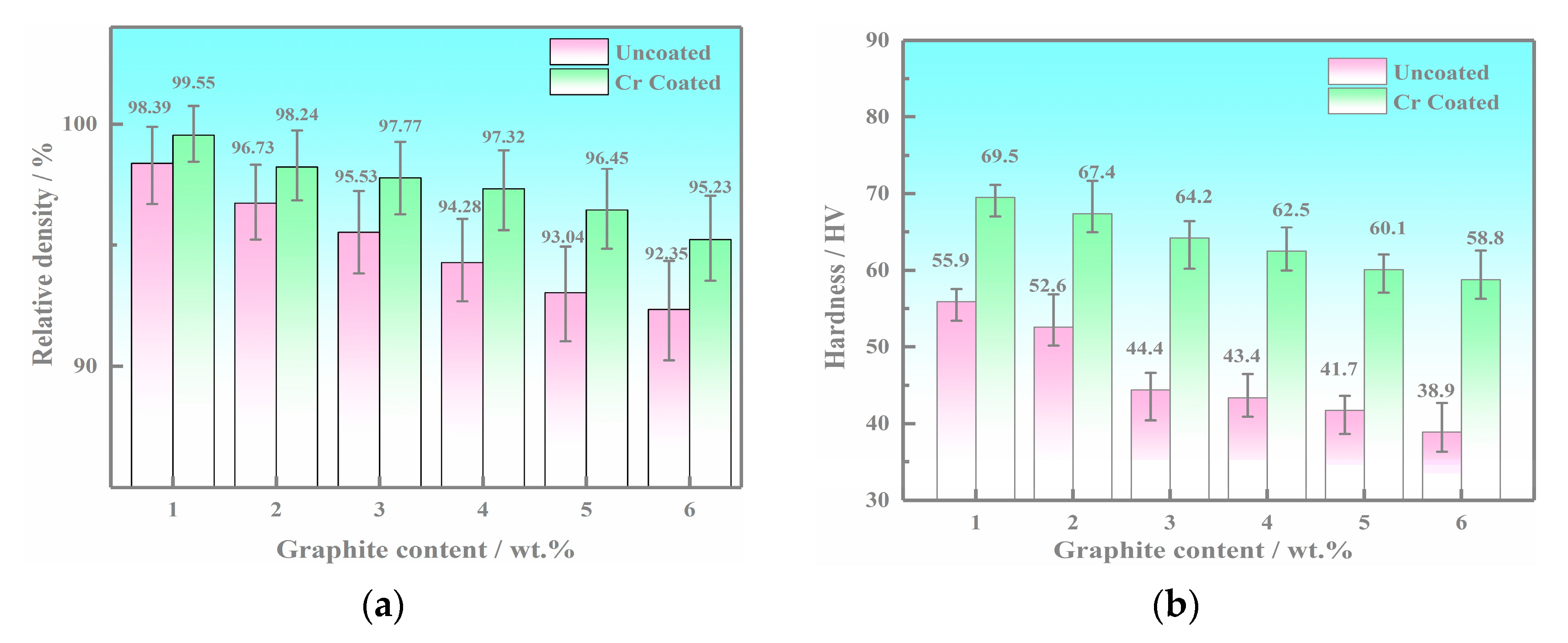
3.3. Mechanical Properties Results
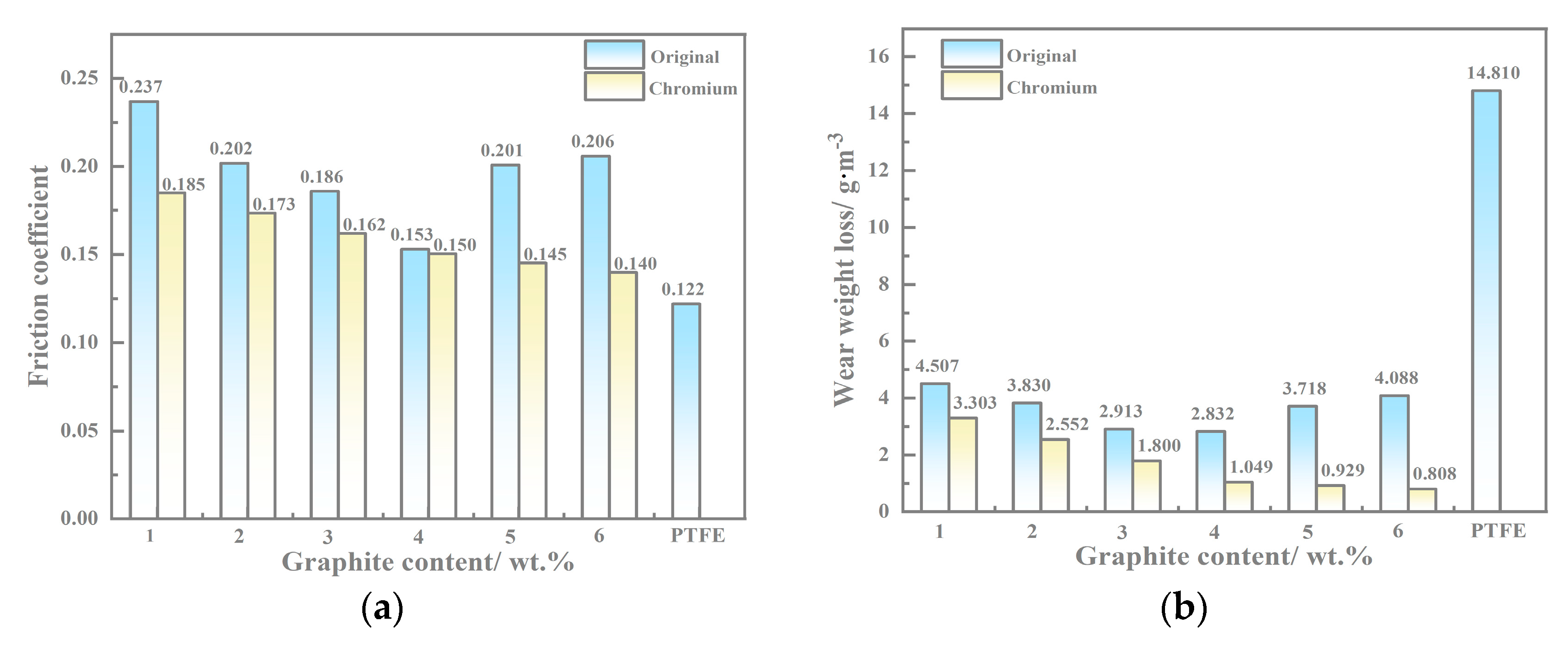
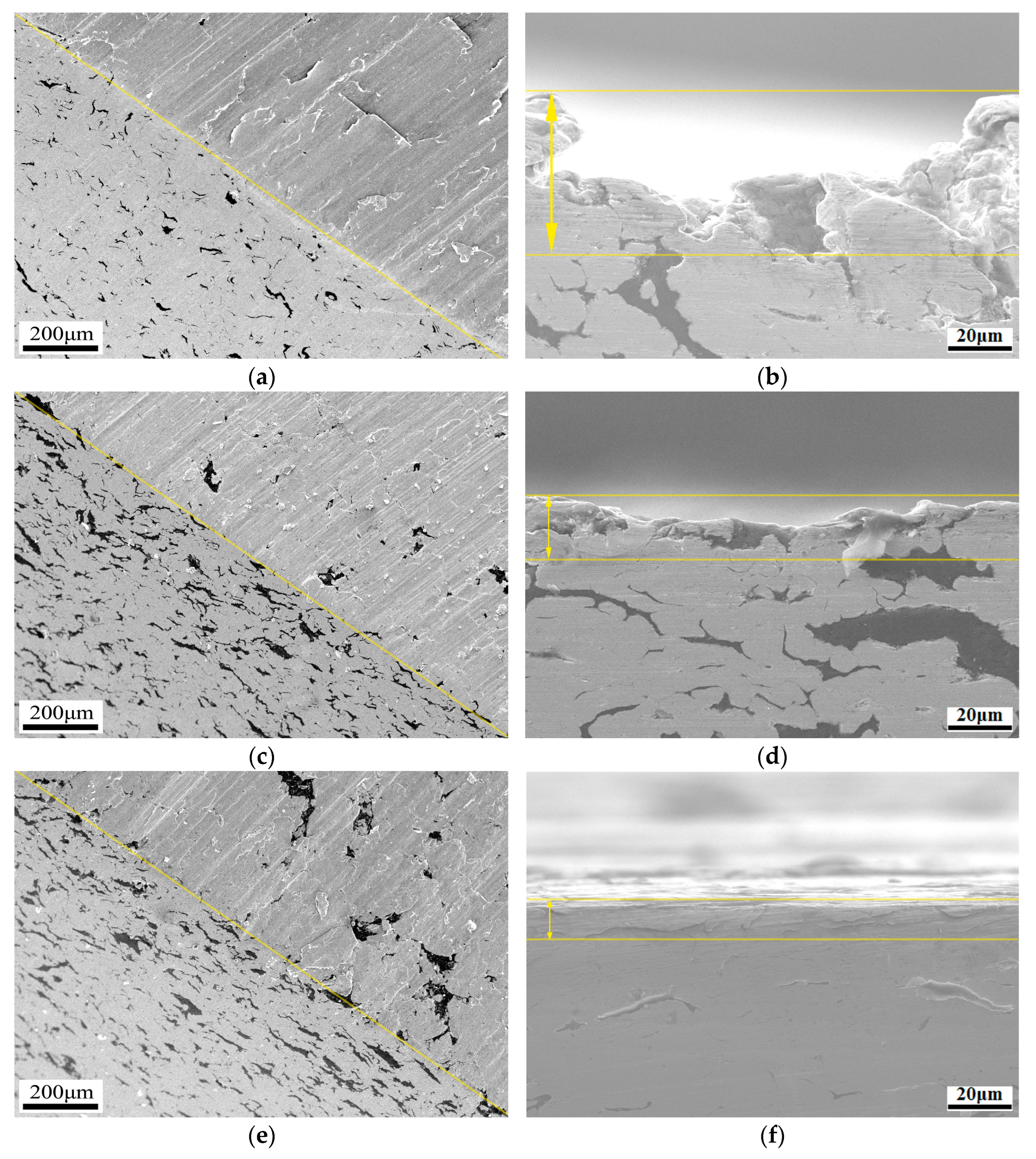
3.4. Wear Test Results
4. Discussion
4.1. Interfacial Improvement of Copper-Based Cr@graphite Alloy
4.2. Anti-Friction Performance of Cr2O3
4.3. Wear Mechanism
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luo, Y. Research on the switch slide baseplates under the conditions of dry friction. J. Shanghai Tiedao Univ. 1998, 19, 37–42. [Google Scholar]
- Bishop, D.M.; Chambers, J. Plastic dry bearings in switch slide baseplates. J. Rep. Proc. Perm. Way Inst. 1990, 108, 155–173. [Google Scholar]
- Liu, Q.; Enxue, Z.; Ding, S. Study on Wear-Resistant-Lubricating Paste for the Bed Plate of HZt13-3. Rail Switch. Tribol. 1983, 3, 216–220. [Google Scholar]
- Li, X.; Gao, Y.; Xing, J.; Wang, Y.; Fang, L. Wear reduction mechanism of graphite and MoS2 in epoxy composites. Wear 2004, 257, 279–283. [Google Scholar] [CrossRef]
- Shen, W.; Cui, D.; Shi, Y. Research and manufacture of self-lubricating switch glide. China Railw. Sci. 1998, 19, 103–110. [Google Scholar]
- Kato, H.; Takama, M.; Iwai, Y.; Washida, K.; Sasaki, Y. Wear and mechanical properties of sintered copper-tin composites containing graphite or molybdenum disulfide. Wear 2003, 255, 573–578. [Google Scholar]
- Manory, R. A novel electrical contact material with improved self-lubrication for railway current collectors. Wear 2001, 249, 626–636. [Google Scholar]
- Kováčik, J.; Emmer, Š.; Bielek, J.; Keleši, L.U. Effect of composition on friction coefficient of Cu–graphite composites. Wear 2008, 265, 417–421. [Google Scholar] [CrossRef]
- Francis, J.C. (Ed.) Solid Lubricants and Self-Lubricating Solids; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Wang, Y.; Gao, Y.; Takahashi, J.; Wan, Y.; Zhang, Y.; Xiao, B.; He, X.; Li, J. Effect of graphite content on Cu–Ni–graphite composite for use as switch slide baseplate materials sliding against U75V steel. J. Tribo. 2019, 141, 121603. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, Y.; Li, Y.; Zhang, C.; Sun, L.; Zhai, W. Research on nickel modified graphite/Cu composites interface. Surf. Coat. Technol. 2017, 328, 70–79. [Google Scholar] [CrossRef]
- Moustafa, S.F.; El-Badry, S.A.; Sanad, A.M.; Kieback, B. Friction and wear of copper–graphite composites made with Cu-coated and uncoated graphite powders. Wear 2002, 253, 699–710. [Google Scholar]
- Wang, Y.; Gao, Y.; Takahashi, J.; Wan, Y.; Xiao, B.; Zhang, Y.; He, X.; Li, J. Titanium-modified graphite reinforced Cu-Ni composite by multi-arc ion plating technology. Vacuum 2019, 168, 108829. [Google Scholar]
- Wang, Y.; Gao, Y.; Li, Y.; Zhao, S.; Li, M. Effect of Sn modification on microstructure and mechanical properties of graphite/Cu composites. Acta Mater. Compos. Sin. 2021, 38, 2–10. [Google Scholar]
- Wang, Y.; Gao, Y.; Li, Y.; Li, M.; Sun, L.; Zhai, W.; Li, K. Research on synergistic lubrication effect of silver modified Cu–Ni-graphite composite. Wear 2020, 444–445, 203140. [Google Scholar]
- Zhai, W.; Zhang, K.; Gao, Y.; Sun, L.; Xu, L.; Wang, Y.; Dong, H.; Wang, S.; Gao, Q. Preparation and oxidation kinetics behavior of bulk Cr3C2-20 wt % Ni cermets. Ceramics Inter. 2021, 47, 6573–6583. [Google Scholar]
- Bai, X.; Li, J.; Zhu, L.; Wang, L. Effect of Cu content on microstructure, mechanical and anti-fouling properties of TiSiN-Cu coating deposited by multi-arc ion plating. Appl. Surf. Sci. 2018, 427, 444–451. [Google Scholar]
- Cao, Z.; Ding, X.; Bagheri, R.; Wattoo, A.G.; Xu, C.; Yang, L.; Song, L.; Wen, Y.; Song, Z. The deposition, microstructure and properties of Al protective coatings for NdFeB magnets by multi-arc ion plating. Vacuum 2017, 142, 37–44. [Google Scholar]
- Zhan, Y.; Zhang, G.; Wu, Y. Effect of surface metallization of graphite on the tribological properties of copper hybrid composites. Scand. J. Metall. 2004, 33, 80–84. [Google Scholar]
- Li, F.; Yan, F.Y.; Yu, L.G.; Liu, W.M. The tribological behaviors of copper-coated graphite filled PTFE composites. Wear 2000, 237, 33–38. [Google Scholar]
- Dong, R.; Cui, Z.; Zhu, S.; Xu, X.; Yang, X. Preparation, Characterization and Mechanical Properties of Cu-Sn Alloy/Graphite Composites. Metall. Mater. Trans. A 2014, 45, 5194–5200. [Google Scholar] [CrossRef]





| Properties | Density/g·m3 | Purity /% | Melt Point /K | Specific Heat Capacity/J·(kg·K)−1 | Conductivity /m·Ω | Thermal Conductivity/W·(m·K)−1 |
|---|---|---|---|---|---|---|
| Parameters | 2.25 | 99.5 | 4123 K | 710 | 0.061 × 10−6 | 129 |
| Element | Cr | C | Si | Fe | P | S |
|---|---|---|---|---|---|---|
| Weight/% | 99.9 | 0.008 | 0.09 | 0.17 | 0.005 | 0.003 |
| Contents | Cr@graphite/wt.% | Cu/wt.% | Ni/wt.% | |
|---|---|---|---|---|
| Samples | ||||
| Cr1 | 1 | 91 | 8 | |
| Cr2 | 2 | 90 | ||
| Cr3 | 3 | 89 | ||
| Cr4 | 4 | 88 | ||
| Cr5 | 5 | 87 | ||
| Cr6 | 6 | 86 | ||
| Sequence of Steps | Processing | Parameters |
|---|---|---|
| 1 | Ball-milling | 200 rpm × 15 h |
| 2 | Cold Compacting | 600 MPa × 3 min |
| 3 | Sintering | 1123 k × 1.5 h at vacuum |
| 4 | Re-Compacting | 600 MPa × 3 min |
| 5 | Re-Sintering | 1073 k × 0.5 h at vacuum |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Gao, Y.; Li, Y. A Study of the Microstructure and Tribological Properties of Copper-Based Cr@graphite Alloy Modified by Nano Cr3C2 and CrC. Nanomaterials 2023, 13, 2347. https://doi.org/10.3390/nano13162347
Wang Y, Gao Y, Li Y. A Study of the Microstructure and Tribological Properties of Copper-Based Cr@graphite Alloy Modified by Nano Cr3C2 and CrC. Nanomaterials. 2023; 13(16):2347. https://doi.org/10.3390/nano13162347
Chicago/Turabian StyleWang, Yiran, Yimin Gao, and Yefei Li. 2023. "A Study of the Microstructure and Tribological Properties of Copper-Based Cr@graphite Alloy Modified by Nano Cr3C2 and CrC" Nanomaterials 13, no. 16: 2347. https://doi.org/10.3390/nano13162347
APA StyleWang, Y., Gao, Y., & Li, Y. (2023). A Study of the Microstructure and Tribological Properties of Copper-Based Cr@graphite Alloy Modified by Nano Cr3C2 and CrC. Nanomaterials, 13(16), 2347. https://doi.org/10.3390/nano13162347








