A Meta-Analysis of Influencing Factors on the Activity of BiVO4-Based Photocatalysts
Abstract
:1. Introduction
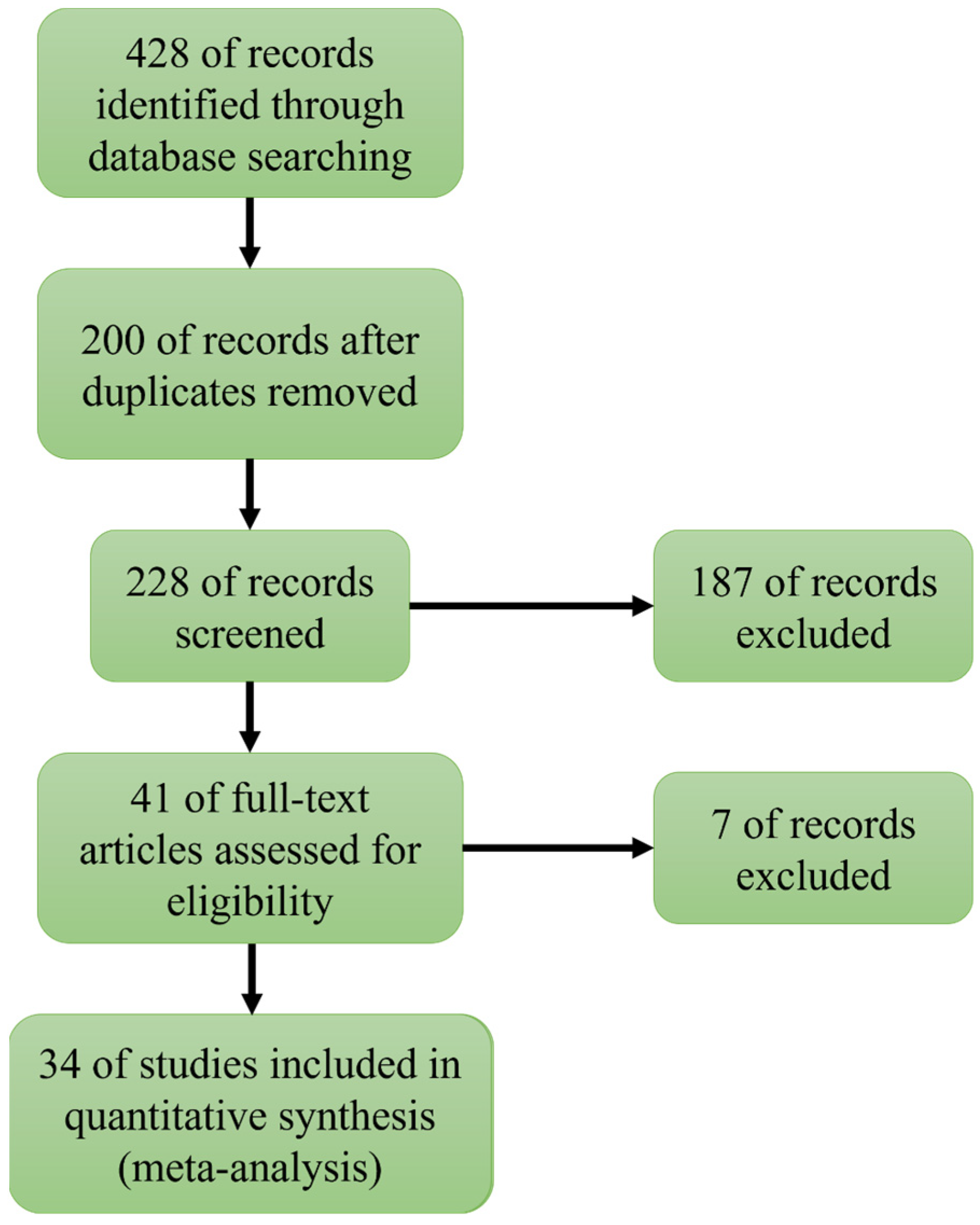
2. Literature Extraction and Data Screening
2.1. Literature Search
2.2. Criteria for Data Inclusion
2.3. Data Extraction and Grouping
3. Meta Analysis
3.1. Selection of Models
3.2. Calculation of the Effect Size
3.3. Subgroup Analysis
3.4. Data Analysis
4. Results and Discussion
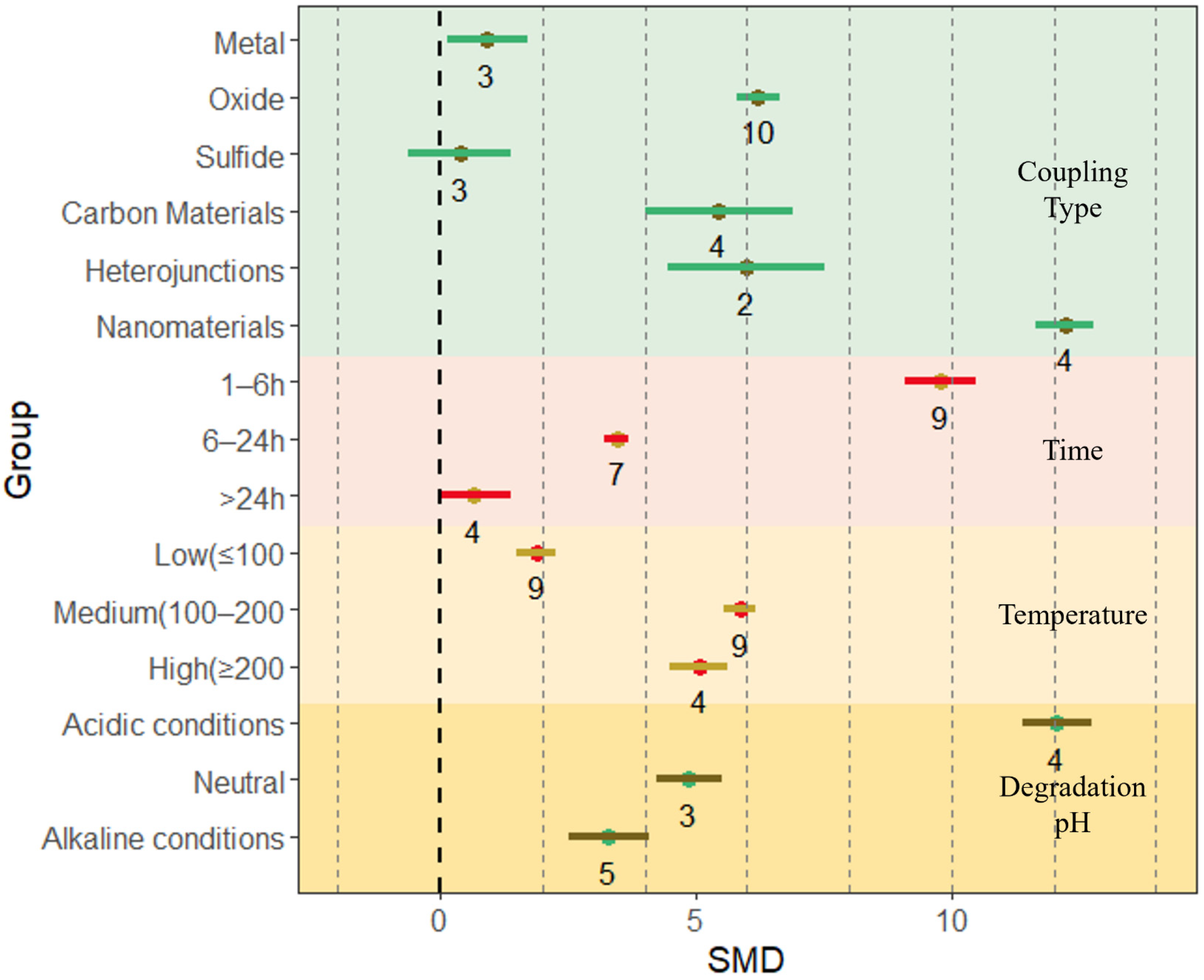
4.1. Effects of Different Preparation Conditions on BET of BiVO4-Based Composites
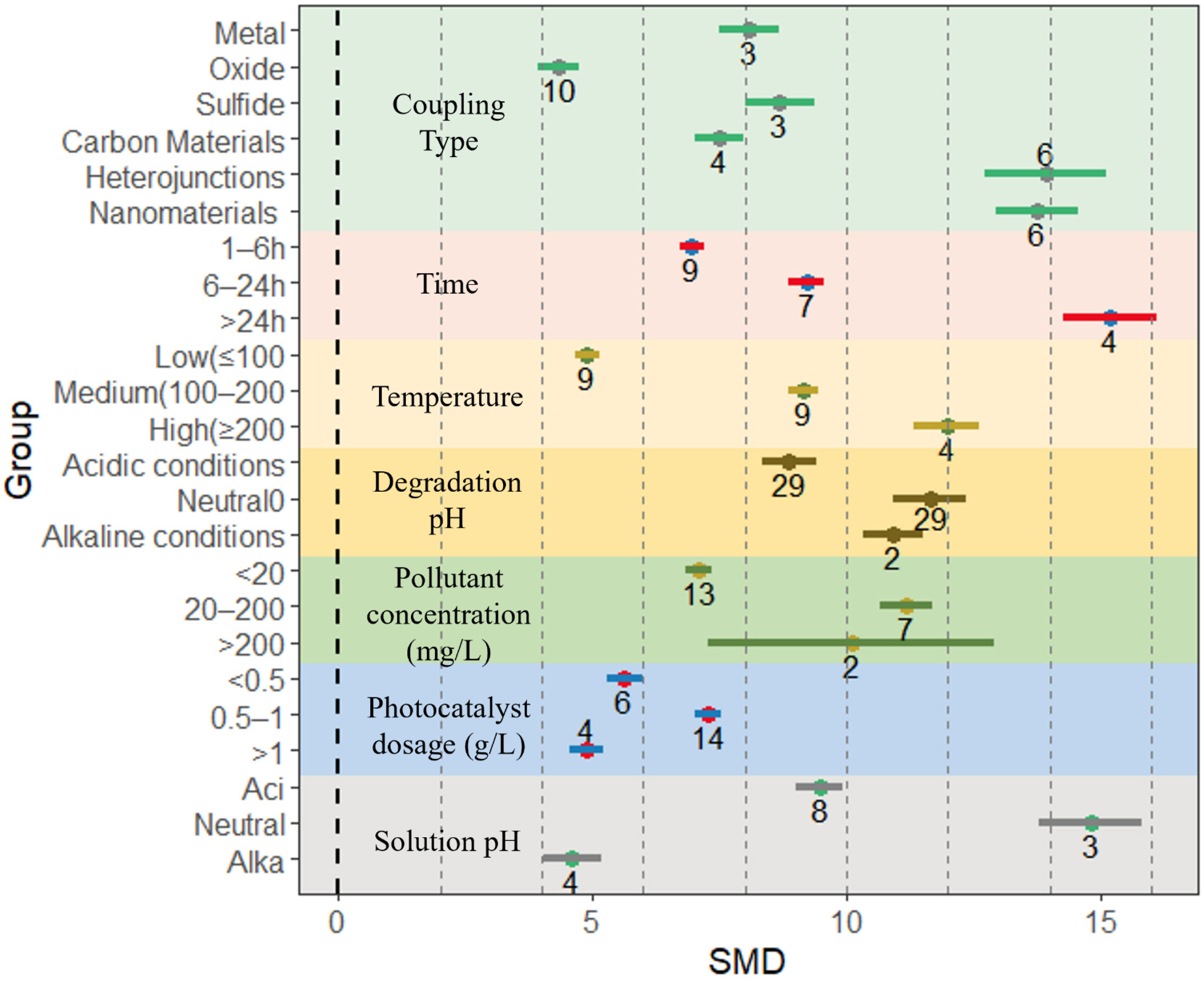
4.2. Effects of Different Preparation Conditions on Kinetic Constant of BiVO4-Based Composites
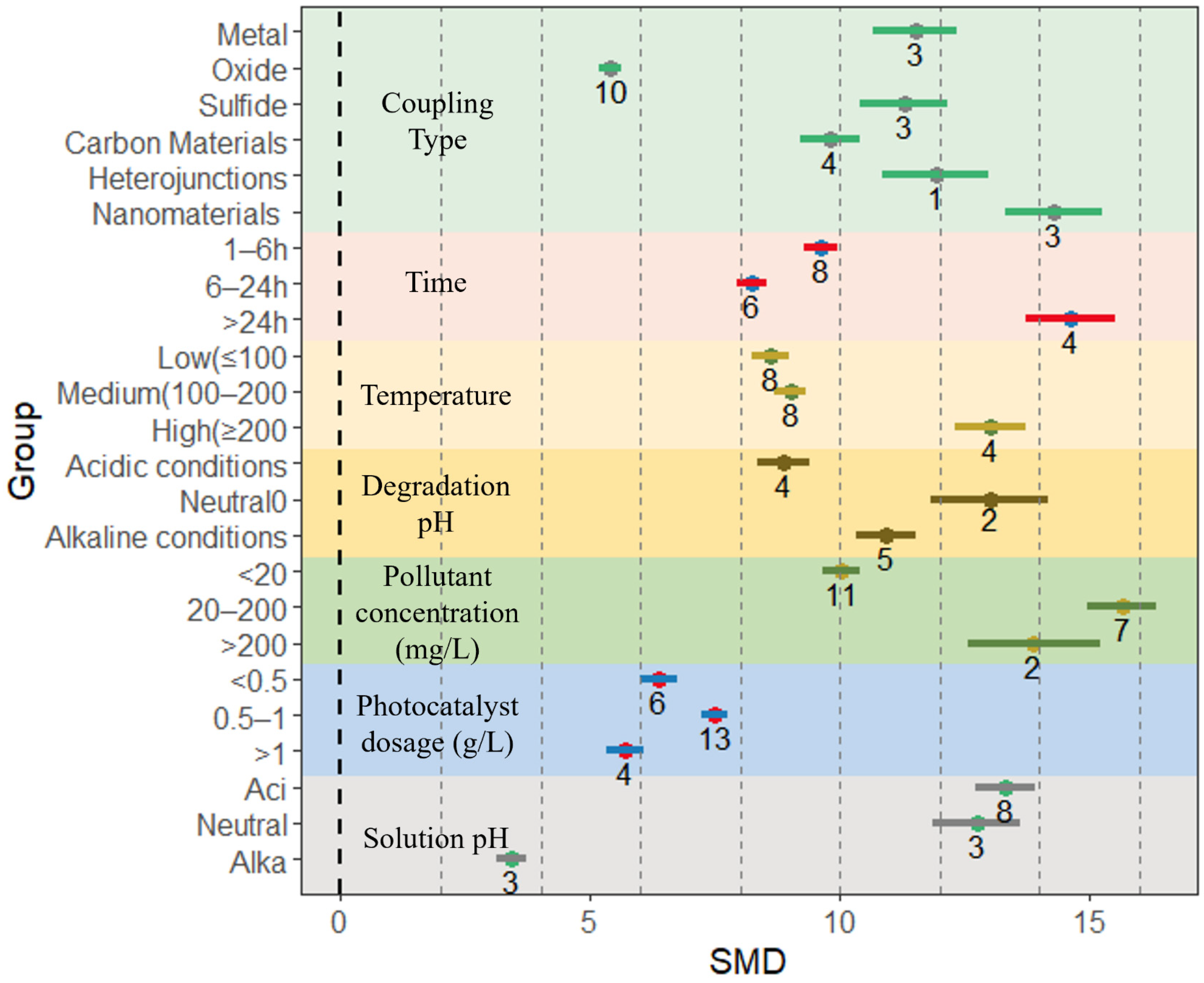
4.3. Study on the Degradation Efficiency of BiVO4-Based Composites for Environmental Pollutants
4.3.1. Efficiency of Degradation of Organic Pollutants
4.3.2. Efficiency of Degradation of Inorganic Pollutants
4.4. Multiple Factors Jointly Determine the Photocatalytic Activity of BiVO4-Based Photocatalysts
4.4.1. Effect of Coupling Type on Photocatalytic Activity of Composites
4.4.2. Effect of Preparation Time and Preparation Temperature on Photocatalytic Activity of Composites
4.4.3. Effect of Degradation pH on Photocatalytic Activity of Composites
4.4.4. Other Factors Affecting Photocatalytic Efficiency
5. Conclusions and Outlook
5.1. Conclusions
5.2. Outlook and Future Perspective
- (1)
- According to the results of our meta-analysis, in order to make the photocatalytic performance of BiVO4-based composites as high as possible, the method should be designed to optimize the various preparation and reaction conditions of the materials to construct nanoscale heterojunctions with large specific surface area. And the preparation pH should be controlled to be around neutral, while allowing each preparation material be fully mixed at a higher temperature.
- (2)
- BiVO4-based photocatalysts have demonstrated a promising capacity for effectively degrading organic pollutants present in wastewater, including substances like rhodamine B and methylene blue. Additionally, these photocatalysts exhibit a notable proficiency in reducing inorganic heavy metals, such as Cr(VI), within aqueous environments. Although current research primarily encompasses laboratory-based simulations and verifications, there exists a substantial opportunity for future endeavors to transition toward large-scale applications in real-world wastewater treatment processes.
- (3)
- BiVO4-based photocatalysts, responsive to visible light, present a cost-effective alternative to conventional photocatalytic devices, offering greater environmental compatibility. In the material synthesis process, prioritizing the utilization of economically viable and environmentally benign raw materials for coupling with BiVO4 helps prevent potential secondary pollution. This strategic approach not only ensures the mitigation of material preparation-related environmental impacts, but also paves the way for feasible large-scale industrial applications.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Singla, S.; Devi, P.; Basu, S. Highly Effectual Photocatalytic Remediation of Tetracycline under the Broad Spectrum of Sunlight by Novel BiVO4/Sb2S3 Nanocomposite. Catalysts 2023, 13, 731. [Google Scholar] [CrossRef]
- Jing, Q.; Liu, J.; Chen, A.; Chen, C.; Liu, J. The Spatial–Temporal Chemical Footprint of Pesticides in China from 1999 to 2018. Environ. Sci. Pollut. Res. 2022, 29, 75539–75549. [Google Scholar] [CrossRef] [PubMed]
- Paul, B.; Patnaik, U.; Sasidharan, S.; Murari, K.K.; Bahinipati, C.S. Fertilizer Use, Value, and Knowledge Capital: A Case of Indian Farming. Sustainability 2022, 14, 12491. [Google Scholar] [CrossRef]
- Sarkar, B.; Daware, A.V.; Gupta, P.; Krishnani, K.K.; Baruah, S.; Bhattacharjee, S. Nanoscale Wide-Band Semiconductors for Photocatalytic Remediation of Aquatic Pollution. Environ. Sci. Pollut. Res. 2017, 24, 25775–25797. [Google Scholar] [CrossRef]
- Ahemed, J.; Pasha, J.; Rao, D.V.; Kore, R.; Gade, R.; Bhongiri, Y.; Chetti, P.; Pola, S. Synthesis of New Zn (II) Complexes for Photo Decomposition of Organic Dye Pollutants, Industrial Wastewater and Photo-Oxidation of Methyl Arenes under Visible-Light. J. Photochem. Photobiol. A Chem. 2021, 419, 113455. [Google Scholar] [CrossRef]
- Gupta, R.; Pandit, C.; Pandit, S.; Gupta, P.K.; Lahiri, D.; Agarwal, D.; Pandey, S. Potential and Future Prospects of Biochar-Based Materials and Their Applications in Removal of Organic Contaminants from Industrial Wastewater. J. Mater. Cycles Waste Manag. 2022, 24, 852–876. [Google Scholar] [CrossRef]
- Maru, K.; Kalla, S.; Jangir, R. Dye Contaminated Wastewater Treatment through Metal–Organic Framework (MOF) Based Materials. New J. Chem. 2022, 46, 3054–3072. [Google Scholar] [CrossRef]
- Mariappan, A.; Dharman, R.K.; Oh, T.H. Efficient Visible Light Photocatalytic Degradation of Heavy Metal Pollutant Using Carbon Doped WS2 Nanostructure. Opt. Mater. 2023, 135, 113366. [Google Scholar] [CrossRef]
- Cai, Y.; Ran, Z.; Cang, Y.; Chen, X.; Shaaban, M.; Peng, Q.-A. Efficient Removal of Cr(VI) and As(V) from an Aquatic System Using Iron Oxide Supported Typha Biochar. Environ. Res. 2023, 225, 115588. [Google Scholar] [CrossRef] [PubMed]
- Dou, K.; Peng, C.; Wang, R.; Cao, H.; Yao, C.; Qiu, J.; Liu, J.; Tsidaeva, N.; Wang, W. S-Scheme Tubular g-C3N4/BiOI Heterojunctions for Boosting Photodegradation of Tetracycline and Cr(VI): Mechanism Insight, Degradation Pathway and DFT Calculation. Chem. Eng. J. 2023, 455, 140813. [Google Scholar] [CrossRef]
- Zuo, W.; Yu, Y.; Huang, H. Making Waves: Microbe-Photocatalyst Hybrids May Provide New Opportunities for Treating Heavy Metal Polluted Wastewater. Water Res. 2021, 195, 116984. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, Y.; Ma, J.; Peng, Y.; Wang, A. A Review on Bidirectional Analogies between the Photocatalysis and Antibacterial Properties of ZnO. J. Alloys Compd. 2019, 783, 898–918. [Google Scholar] [CrossRef]
- Wang, C.-C.; Du, X.-D.; Li, J.; Guo, X.-X.; Wang, P.; Zhang, J. Photocatalytic Cr(VI) Reduction in Metal-Organic Frameworks: A Mini-Review. Appl. Catal. B Environ. 2016, 193, 198–216. [Google Scholar] [CrossRef]
- Bard, A.J.; Fox, M.A. Artificial Photosynthesis: Solar Splitting of Water to Hydrogen and Oxygen. Acc. Chem. Res. 1995, 28, 141–145. [Google Scholar] [CrossRef]
- Cheng, C.; He, B.; Fan, J.; Cheng, B.; Cao, S.; Yu, J. An Inorganic/Organic S-Scheme Heterojunction H2-Production Photocatalyst and Its Charge Transfer Mechanism. Adv. Mater. 2021, 33, 2100317. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, H.; Chen, Z.; Wong, P.K.; Liu, J. Bi2WO6 Micro/Nano-Structures: Synthesis, Modifications and Visible-Light-Driven Photocatalytic Applications. Appl. Catal. B Environ. 2011, 106, 1–13. [Google Scholar] [CrossRef]
- Malathi, A.; Madhavan, J.; Ashokkumar, M.; Arunachalam, P. A Review on BiVO4 Photocatalyst: Activity Enhancement Methods for Solar Photocatalytic Applications. Appl. Catal. A Gen. 2018, 555, 47–74. [Google Scholar] [CrossRef]
- Wen, J.; Xie, J.; Chen, X.; Li, X. A Review on G-C3N4-Based Photocatalysts. Appl. Surf. Sci. 2017, 391, 72–123. [Google Scholar] [CrossRef]
- Xu, F.; Tan, H.; Fan, J.; Cheng, B.; Yu, J.; Xu, J. Electrospun TiO2-Based Photocatalysts. Sol. RRL 2021, 5, 2000571. [Google Scholar] [CrossRef]
- He, R.; Xu, D.; Cheng, B.; Yu, J.; Ho, W. Review on Nanoscale Bi-Based Photocatalysts. Nanoscale Horiz. 2018, 3, 464–504. [Google Scholar] [CrossRef]
- Sun, Y.; Ahmadi, Y.; Kim, K.-H.; Lee, J. The Use of Bismuth-Based Photocatalysts for the Production of Ammonia through Photocatalytic Nitrogen Fixation. Renew. Sustain. Energy Rev. 2022, 170, 112967. [Google Scholar] [CrossRef]
- Su, K.; Liu, H.; Gao, Z.; Fornasiero, P.; Wang, F. Nb2O5-Based Photocatalysts. Adv. Sci. 2021, 8, 2003156. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, K.; Aadil, M.; Zulfiqar, S.; Ullah, S.; Haider, S.; Agboola, P.O.; Warsi, M.F.; Shakir, I. Graphene Oxide and Reduced Graphene Oxide Supported ZnO Nanochips for Removal of Basic Dyes from the Industrial Effluents. Fuller. Nanotub. Carbon Nanostruct. 2021, 29, 915–928. [Google Scholar] [CrossRef]
- Xu, D.; Liu, B.; Zou, W.; Wang, H.; Zhang, C. In Situ Synthesis of TiO2 Nanosheets@CdSe Nanocomposites and the Improved Photocatalytic Performance on Removal of Methylene Blue. Appl. Surf. Sci. 2019, 487, 91–100. [Google Scholar] [CrossRef]
- Bashir, S.; Jamil, A.; Khan, M.S.; Alazmi, A.; Abuilaiwi, F.A.; Shahid, M. Gd-Doped BiVO4 Microstructure and Its Composite with a Flat Carbonaceous Matrix to Boost Photocatalytic Performance. J. Alloys Compd. 2022, 913, 165214. [Google Scholar] [CrossRef]
- Lotfi, S.; Ouardi, M.E.; Ahsaine, H.A.; Assani, A. Recent Progress on the Synthesis, Morphology and Photocatalytic Dye Degradation of BiVO4 Photocatalysts: A Review. Catal. Rev. 2022, 1–45. [Google Scholar] [CrossRef]
- Peng, Y.; Cai, J.; Shi, Y.; Jiang, H.; Li, G. Thin P-Type NiO Nanosheet Modified Peanut-Shaped Monoclinic BiVO4 for Enhanced Charge Separation and Photocatalytic Activities. Catal. Sci. Technol. 2022, 12, 5162–5170. [Google Scholar] [CrossRef]
- Phanichphant, S.; Nakaruk, A.; Chansaenpak, K.; Channei, D. Evaluating the Photocatalytic Efficiency of the BiVO4/RGO Photocatalyst. Sci. Rep. 2019, 9, 16091. [Google Scholar] [CrossRef]
- Ke, G.; Liu, B.; Duan, F.; Liu, X.; Wen, J.; Jia, B.; Liu, X.; He, H.; Zhou, Y. Resorcinol-Formaldehyde Resin Nanoparticles as Surface Charge Transfer and Separation Sites for the Improvement of BiVO4 Film Photoanodes’ Performance in Solar Water Oxidation. Appl. Surf. Sci. 2022, 601, 154236. [Google Scholar] [CrossRef]
- Yang, L.; Wang, R.; Chu, D.; Chen, Z.; Zhong, F.; Xu, X.; Deng, C.; Yu, H.; Lv, J. BiVO4 Photoelectrodes for Unbiased Solar Water Splitting Devices Enabled by Electrodepositing of Cu2O Simultaneously as Photoanode and Photocathode. J. Alloys Compd. 2023, 945, 169336. [Google Scholar] [CrossRef]
- Liang, J.; Deng, J.; Li, M.; Tong, M. Bactericidal Activity and Mechanism of AgI/AgBr/BiOBr0.75I0.25 under Visible Light Irradiation. Colloids Surf. B Biointerfaces 2016, 138, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, X.; Gu, X.; Guo, R.; Zhong, W. Cu2−xSe Modification onto Monoclinic BiVO4 for Enhanced Photocatalytic Activity Under Visible Light. Nanoscale Res. Lett. 2019, 14, 95. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wu, Y.; Wang, Z.; Dai, D.; Liu, X.; Zhang, Q.; Wang, Z.; Liu, Y.; Zheng, Z.; Cheng, H.; et al. Multi-Strategy Preparation of BiVO4 Photoanode with Abundant Oxygen Vacancies for Efficient Water Oxidation. Appl. Surf. Sci. 2023, 614, 156164. [Google Scholar] [CrossRef]
- Kang, Z.; Sun, Z.; Zang, Y.; Wan, S.; Zheng, Y.-Z.; Tao, X. Dual Functions of Heterometallic FeCo Oxyhydroxides in Borate-Treated BiVO4 Photoanodes toward Boosted Activity and Photostability in Photoelectrochemical Water Oxidation. Chem. Eng. J. 2022, 431, 133379. [Google Scholar] [CrossRef]
- Wen, P.; Lei, R.; Cao, X.; Ma, Q.; Zhang, G.; Guo, C.; Wang, X.; Qiu, Y. Anchored Ni Nanocrystals Boosting BiVO4 Photoanode for Highly Efficient Water Oxidation via In-Situ Generation of Ni@NiOOH Co-Catalyst. Chem. Eng. J. 2023, 454, 139983. [Google Scholar] [CrossRef]
- Choi, W.; Termin, A.; Hoffmann, M.R. The Role of Metal Ion Dopants in Quantum-Sized TiO2: Correlation between Photoreactivity and Charge Carrier Recombination Dynamics. J. Phys. Chem. 1994, 98, 13669–13679. [Google Scholar] [CrossRef]
- Yu, C.; Yu, J.C.; Fan, C.; Wen, H.; Hu, S. Synthesis and Characterization of Pt/BiOI Nanoplate Catalyst with Enhanced Activity under Visible Light Irradiation. Mater. Sci. Eng. B 2010, 166, 213–219. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, Y.; Zhou, Z.; Zhao, Y.; Liu, L. Enhanced Photocatalytic Properties in BiOBr Nanosheets with Dominantly Exposed (102) Facets. J. Phys. Chem. C 2014, 18, 14662–14669. [Google Scholar] [CrossRef]
- Wang, H.; Thangamuthu, M.; Wu, Z.; Yang, J.; Yuan, H.; Bayazit, M.K.; Tang, J. Self-Assembled Sulphur Doped Carbon Nitride for Photocatalytic Water Reforming of Methanol. Chem. Eng. J. 2022, 445, 136790. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Zhao, S.; Huang, Z.; Chen, W.; Zhou, Y.; Lv, X.; Yuan, S. Bio-Template Synthesis of Mo-Doped Polymer Carbon Nitride for Photocatalytic Hydrogen Evolution. Appl. Catal. B Environ. 2019, 248, 44–53. [Google Scholar] [CrossRef]
- Zhang, J.; Tse, K.; Wong, M.; Zhang, Y.; Zhu, J. A Brief Review of Co-Doping. Front. Phys. 2016, 11, 117405. [Google Scholar] [CrossRef]
- Selvarajan, S.; Suganthi, A.; Rajarajan, M.; Arunprasath, K. Highly Efficient BiVO4/WO3 Nanocomposite towards Superior Photocatalytic Performance. Powder Technol. 2017, 307, 203–212. [Google Scholar] [CrossRef]
- Wang, G.; Cheng, H. Boosting Catalytic Efficiency via BiVO4 Surface Heterojunction-Induced Interfacial Z-Scheme NiFe2O4/{010}BiVO4 Composite. Sep. Purif. Technol. 2023, 318, 123949. [Google Scholar] [CrossRef]
- Reli, M.; Troppová, I.; Šihor, M.; Pavlovský, J.; Praus, P.; Kočí, K. Photocatalytic Decomposition of N2O over G-C3N4/BiVO4 Composite. Appl. Surf. Sci. 2019, 469, 181–191. [Google Scholar] [CrossRef]
- Su, J.; Liu, C.; Liu, D.; Li, M.; Zhou, J. Enhanced Photoelectrochemical Performance of the BiVO4/Zn:BiVO4 Homojunction for Water Oxidation. ChemCatChem 2016, 8, 3279–3286. [Google Scholar] [CrossRef]
- Zou, L.; Wang, H.; Wu, C.; Li, L.; Yuan, G.; Wang, X. Construction of All-Solid-State Z-Scheme 2D BiVO4/Ag/CdS Composites with Robust Photoactivity and Stability. Appl. Surf. Sci. 2019, 498, 143900. [Google Scholar] [CrossRef]
- Lebedev, A.; Anariba, F.; Li, X.; Wu, P. Rational Design of Visible-Light-Sensitive Ag-BiVO4 Oxides by Matching Redox Potentials of Catalyst, Dyes, and Reactive Oxygen Species towards More Efficient Photocatalytic Degradation. J. Environ. Chem. Eng. 2020, 8, 103748. [Google Scholar] [CrossRef]
- Yuan, Q.; Chen, L.; Xiong, M.; He, J.; Luo, S.-L.; Au, C.-T.; Yin, S.-F. Cu2O/BiVO4 Heterostructures: Synthesis and Application in Simultaneous Photocatalytic Oxidation of Organic Dyes and Reduction of Cr(VI) under Visible Light. Chem. Eng. J. 2014, 255, 394–402. [Google Scholar] [CrossRef]
- Mohamed, N.A.; Safaei, J.; Ismail, A.F.; Khalid, M.N.; Mohd Jailani, M.F.A.; Noh, M.F.M.; Arzaee, N.A.; Zhou, D.; Sagu, J.S.; Teridi, M.A.M. Boosting Photocatalytic Activities of BiVO4 by Creation of G-C3N4/ZnO@BiVO4 Heterojunction. Mater. Res. Bull. 2020, 125, 110779. [Google Scholar] [CrossRef]
- Liaqat, M.; Khalid, N.R.; Tahir, M.B.; Znaidia, S.; Alrobei, H.; Alzaid, M. Visible Light Induced Photocatalytic Activity of MnO2/BiVO4 for the Degradation of Organic Dye and Tetracycline. Ceram. Int. 2023, 49, 10455–10461. [Google Scholar] [CrossRef]
- Wang, R.; Bai, J.; Li, Y.; Zeng, Q.; Li, J.; Zhou, B. BiVO4/TiO2(N2) Nanotubes Heterojunction Photoanode for Highly Efficient Photoelectrocatalytic Applications. Nano-Micro Lett. 2016, 9, 14. [Google Scholar] [CrossRef]
- Yuan, D.; Sun, M.; Tang, S.; Zhang, Y.; Wang, Z.; Qi, J.; Rao, Y.; Zhang, Q. All-Solid-State BiVO4/ZnIn2S4 Z-Scheme Composite with Efficient Charge Separations for Improved Visible Light Photocatalytic Organics Degradation. Chin. Chem. Lett. 2020, 31, 547–550. [Google Scholar] [CrossRef]
- Lai, C.; Zhang, M.; Li, B.; Huang, D.; Zeng, G.; Qin, L.; Liu, X.; Yi, H.; Cheng, M.; Li, L.; et al. Fabrication of CuS/BiVO4 (0 4 0) Binary Heterojunction Photocatalysts with Enhanced Photocatalytic Activity for Ciprofloxacin Degradation and Mechanism Insight. Chem. Eng. J. 2019, 358, 891–902. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Wang, X.-T.; Jian, L.-J.; Abdallah, N.I.M.; Dong, X.-F.; Wang, C.-W. P-n Heterostructured Design of Decahedral NiS/BiVO4 with Efficient Charge Separation for Enhanced Photodegradation of Organic Dyes. Colloids Surf. A Physicochem. Eng. Asp. 2021, 608, 125565. [Google Scholar] [CrossRef]
- Liang, Q.; Ploychompoo, S.; Chen, J.; Zhou, T.; Luo, H. Simultaneous Cr(VI) Reduction and Bisphenol A Degradation by a 3D Z-Scheme Bi2S3-BiVO4 Graphene Aerogel under Visible Light. Chem. Eng. J. 2020, 384, 123256. [Google Scholar] [CrossRef]
- Naing, H.H.; Wang, K.; Li, Y.; Mishra, A.K.; Zhang, G. Sepiolite Supported BiVO4 Nanocomposites for Efficient Photocatalytic Degradation of Organic Pollutants: Insight into the Interface Effect towards Separation of Photogenerated Charges. Sci. Total Environ. 2020, 722, 137825. [Google Scholar] [CrossRef]
- Oladipo, A.A.; Mustafa, F.S. Bismuth-Based Nanostructured Photocatalysts for the Remediation of Antibiotics and Organic Dyes. Beilstein J. Nanotechnol. 2023, 14, 291–321. [Google Scholar] [CrossRef]
- Sajid, M.M.; Amin, N.; Shad, N.A.; Khan, S.B.; Javed, Y.; Zhang, Z. Hydrothermal Fabrication of Monoclinic Bismuth Vanadate (m-BiVO4) Nanoparticles for Photocatalytic Degradation of Toxic Organic Dyes. Mater. Sci. Eng. B 2019, 242, 83–89. [Google Scholar] [CrossRef]
- Bano, K.; Mittal, S.K.; Singh, P.P.; Kaushal, S. Sunlight Driven Photocatalytic Degradation of Organic Pollutants Using a MnV2O6/BiVO4 Heterojunction: Mechanistic Perception and Degradation Pathways. Nanoscale Adv. 2021, 3, 6446–6458. [Google Scholar] [CrossRef]
- Chawla, H.; Garg, S.; Rohilla, J.; Szamosvölgyi, Á.; Efremova, A.; Szenti, I.; Ingole, P.P.; Sápi, A.; Kónya, Z.; Chandra, A. Visible LED-Light Driven Photocatalytic Degradation of Organochlorine Pesticides (2,4-D & 2,4-DP) by Curcuma Longa Mediated Bismuth Vanadate. J. Clean. Prod. 2022, 367, 132923. [Google Scholar] [CrossRef]
- Ahmed, T.; Zhang, H.; Gao, Y.-Y.; Xu, H.; Zhang, Y. Surfactant-Free Synthesis of m-BiVO4 Nanoribbons and Enhanced Visible-Light Photocatalytic Properties. Mater. Res. Bull. 2018, 99, 298–305. [Google Scholar] [CrossRef]
- Mosleh, S.; Rahimi, M.R.; Ghaedi, M.; Dashtian, K. HKUST-1-MOF–BiVO4 Hybrid as a New Sonophotocatalyst for Simultaneous Degradation of Disulfine Blue and Rose Bengal Dyes: Optimization and Statistical Modelling. RSC Adv. 2016, 6, 61516–61527. [Google Scholar] [CrossRef]
- Shang, M.; Wang, W.; Zhou, L.; Sun, S.; Yin, W. Nanosized BiVO4 with High Visible-Light-Induced Photocatalytic Activity: Ultrasonic-Assisted Synthesis and Protective Effect of Surfactant. J. Hazard. Mater. 2009, 172, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Yan, J.; Jia, H.; Zhong, S.; Zhang, X.; Xu, L. BiVO4/g-C3N4 Composite Visible-Light Photocatalyst for Effective Elimination of Aqueous Organic Pollutants. J. Mol. Catal. A Chem. 2016, 424, 162–170. [Google Scholar] [CrossRef]
- Huang, L.; Liu, H.; Wang, Y.; Zhang, T.C.; Yuan, S. Construction of Ternary Bi2O3/Biochar/g-C3N4 Heterojunction to Accelerate Photoinduced Carrier Separation for Enhanced Tetracycline Photodegradation. Appl. Surf. Sci. 2023, 616, 156509. [Google Scholar] [CrossRef]
- Gu, J.; Ban, C.; Meng, J.; Li, Q.; Long, X.; Zhou, X.; Liu, N.; Li, Z. Construction of Dual Z-Scheme UNiMOF/BiVO4/S-C3N4 Photocatalyst for Visible-Light Photocatalytic Tetracycline Degradation and Cr(VI) Reduction. Appl. Surf. Sci. 2023, 611, 155575. [Google Scholar] [CrossRef]
- Fatima, U.; Tahir, M.B.; Gouadria, S.; Khalid, N.R.; Nawaz, T.; Sagir, M.; Siddeeg, S.M.; Alrobei, H.; Alzaid, M. Synthesis of Ternary Photocatalysts BiVO4/Ag/Black Phosphorene for the Degradation of Dyes and Pharmaceuticals. Appl. Nanosci. 2023, 13, 5501–5507. [Google Scholar] [CrossRef]
- Zhu, P.; Zhang, S.; Liu, R.; Luo, D.; Yao, H.; Zhu, T.; Bai, X. Investigation of an Enhanced Z-Scheme Magnetic Recyclable BiVO4/GO/CoFe2O4 Photocatalyst with Visible-Light-Driven for Highly Efficient Degradation of Antibiotics. J. Solid State Chem. 2022, 314, 123379. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, Y.; Xi, L.; Zhu, G.; Li, X.; Shi, D.; Fan, J. Highly Efficient Photocatalytic Removal of Multiple Refractory Organic Pollutants by BiVO4/CH3COO(BiO) Heterostructured Nanocomposite. Sci. Total Environ. 2019, 647, 245–254. [Google Scholar] [CrossRef]
- Xu, G.; Du, M.; Zhang, J.; Li, T.; Guan, Y.; Guo, C. Facile Fabrication of Magnetically Recyclable Fe3O4/BiVO4/CuS Heterojunction Photocatalyst for Boosting Simultaneous Cr(VI) Reduction and Methylene Blue Degradation under Visible Light. J. Alloys Compd. 2022, 895, 162631. [Google Scholar] [CrossRef]
- Soltani, T.; Tayyebi, A.; Lee, B.-K. Photolysis and Photocatalysis of Tetracycline by Sonochemically Heterojunctioned BiVO4/Reduced Graphene Oxide under Visible-Light Irradiation. J. Environ. Manag. 2019, 232, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Li, Y.; Peng, H.; Huang, Z.; Wang, H.; Wen, J. Efficient Solar-Light Photodegradation of Tetracycline Hydrochloride Using BiVO4/MoO3 Composites. Colloids Surf. A Physicochem. Eng. Asp. 2021, 621, 126599. [Google Scholar] [CrossRef]
- Zhang, M.; Shi, Q.; Cheng, X.; Yang, J.; Liu, Z.; Chen, T.; Qu, Y.; Wang, J.; Xie, M.; Han, W. Accelerated Generation of Hydroxyl Radical through Surface Polarization on BiVO4 Microtubes for Efficient Chlortetracycline Degradation. Chem. Eng. J. 2020, 400, 125871. [Google Scholar] [CrossRef]
- Yan, X.; Wang, B.; Zhao, J.; Liu, G.; Ji, M.; Zhang, X.; Chu, P.K.; Li, H.; Xia, J. Hierarchical Columnar ZnIn2S4/BiVO4 Z-Scheme Heterojunctions with Carrier Highway Boost Photocatalytic Mineralization of Antibiotics. Chem. Eng. J. 2023, 452, 139271. [Google Scholar] [CrossRef]
- Zheng, Y.; Cao, L.; Xing, G.; Bai, Z.; Huang, J.; Zhang, Z. Microscale Flower-like Magnesium Oxide for Highly Efficient Photocatalytic Degradation of Organic Dyes in Aqueous Solution. RSC Adv. 2019, 9, 7338–7348. [Google Scholar] [CrossRef]
- Wei, L.; Shi, D.; Qi, Y.; Zhang, Y. Synthetic Mechanism of UiO-66-NH2/BiVO4/BiOBr Spherical and Lamellar Dual Z-scheme Heterojunction and Efficient Photocatalytic Degradation of Tetracycline under Visible Light. ChemistrySelect 2022, 7, e202103742. [Google Scholar] [CrossRef]
- Tahir, M.B.; Iqbal, T.; Kiran, H.; Hasan, A. Insighting Role of Reduced Graphene Oxide in BiVO4 Nanoparticles for Improved Photocatalytic Hydrogen Evolution and Dyes Degradation. Int. J. Energy Res. 2019, 43, 2410–2417. [Google Scholar] [CrossRef]
- Cai, Z.; Fan, X. A Comparison of Fixed-Effects and Random-Effects Models for Multivariate Meta-Analysis Using an SEM Approach. Multivar. Behav. Res. 2020, 55, 839–854. [Google Scholar] [CrossRef] [PubMed]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.T.; Rothstein, H.R. A Basic Introduction to Fixed-Effect and Random-Effects Models for Meta-Analysis. Res. Synth. Methods 2010, 1, 97–111. [Google Scholar] [CrossRef]
- Hedges, L.V.; Gurevitch, J.; Curtis, P.S. The Meta-Analysis of Response Ratios in Experimental Ecology. Ecology 1999, 80, 1150–1156. [Google Scholar] [CrossRef]
- Osenberg, C.W.; Sarnelle, O.; Cooper, S.D.; Holt, R.D. Resolving Ecological Questions through Meta-Analysis: Goals, Metrics, and Models. Ecology 1999, 80, 1105–1117. [Google Scholar] [CrossRef]
- Harindintwali, J.D.; He, C.; Xiang, L.; Dou, Q.; Liu, Y.; Wang, M.; Wen, X.; Fu, Y.; Islam, M.U.; Chang, S.X.; et al. Effects of Ball Milling on Biochar Adsorption of Contaminants in Water: A Meta-Analysis. Sci. Total Environ. 2023, 882, 163643. [Google Scholar] [CrossRef]
- Islam, M.U.; Jiang, F.; Guo, Z.; Peng, X. Does Biochar Application Improve Soil Aggregation? A Meta-Analysis. Soil Tillage Res. 2021, 209, 104926. [Google Scholar] [CrossRef]
- Andrade, C. Mean Difference, Standardized Mean Difference (SMD), and Their Use in Meta-Analysis: As Simple as It Gets. J. Clin. Psychiatry 2020, 81, 11349. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Sun, X.; Yu, Y.; Wang, H.; Wang, Y. Photocatalytic Degradation of Flumequine with B/N Codoped TiO2 Catalyst: Kinetics, Main Active Species, Intermediates and Pathways. Chem. Eng. J. 2019, 378, 122226. [Google Scholar] [CrossRef]
- Lakhera, S.K.; Venkataramana, R.; Mathew, G.; Hafeez, H.Y.; Neppolian, B. Fabrication of High Surface Area AgI Incorporated Porous BiVO4 Heterojunction Photocatalysts. Mater. Sci. Semicond. Process. 2020, 106, 104756. [Google Scholar] [CrossRef]
- Fan, Z.; Wei, T.; Shi, H.; Tang, B.; Zhao, G. Adsorption Driven Preferential Degradation of Alkyl Phenols on Hydrophobic Perfluoroalkyl Modified {0 0 1}-TiO2. Chem. Eng. J. 2019, 357, 689–697. [Google Scholar] [CrossRef]
- Wang, S.; Li, Y.; Liu, Q.; Wang, J.; Zhao, Y.; Cai, Y.; Li, H.; Chen, Z. Photo-/Electro-/Piezo-Catalytic Elimination of Environmental Pollutants. J. Photochem. Photobiol. A Chem. 2023, 437, 114435. [Google Scholar] [CrossRef]
- Deng, Y.; Tang, L.; Zeng, G.; Wang, J.; Zhou, Y.; Wang, J.; Tang, J.; Wang, L.; Feng, C. Facile Fabrication of Mediator-Free Z-Scheme Photocatalyst of Phosphorous-Doped Ultrathin Graphitic Carbon Nitride Nanosheets and Bismuth Vanadate Composites with Enhanced Tetracycline Degradation under Visible Light. J. Colloid Interface Sci. 2018, 509, 219–234. [Google Scholar] [CrossRef] [PubMed]
- Kamble, G.S.; Natarajan, T.S.; Patil, S.S.; Thomas, M.; Chougale, R.K.; Sanadi, P.D.; Siddharth, U.S.; Ling, Y.-C. BiVO4 as a Sustainable and Emerging Photocatalyst: Synthesis Methodologies, Engineering Properties, and Its Volatile Organic Compounds Degradation Efficiency. Nanomaterials 2023, 13, 1528. [Google Scholar] [CrossRef]
- Silvestri, S.; Fajardo, A.R.; Iglesias, B.A. Supported Porphyrins for the Photocatalytic Degradation of Organic Contaminants in Water: A Review. Environ. Chem. Lett. 2022, 20, 731–771. [Google Scholar] [CrossRef]
- Chengli, Z.; Ronghua, M.; Qi, W.; Mingrui, Y.; Rui, C.; Xiaonan, Z. Photocatalytic Degradation of Organic Pollutants in Wastewater by Heteropolyacids: A Review. J. Coord. Chem. 2021, 74, 1751–1764. [Google Scholar] [CrossRef]
- Murakami, N.; Takebe, N.; Tsubota, T.; Ohno, T. Improvement of Visible Light Photocatalytic Acetaldehyde Decomposition of Bismuth Vanadate/Silica Nanocomposites by Cocatalyst Loading. J. Hazard. Mater. 2012, 211–212, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, X.; Chen, Y.; Zhuang, Z.; Chen, F.-F.; Zhu, Y.-J.; Yu, Y. Recycling Heavy Metals from Wastewater for Photocatalytic CO2 Reduction. Chem. Eng. J. 2020, 402, 125922. [Google Scholar] [CrossRef]
- Talaiekhozani, A.; Rezania, S.; Kim, K.H.; Sanaye, R.; Amani, A.M. Recent Advances in Photocatalytic Removal of Organic and Inorganic Pollutants in Air. J. Clean. Prod. 2021, 278, 123895. [Google Scholar] [CrossRef]
- Min, S.; Wang, F.; Jin, Z.; Xu, J. Cu2O Nanoparticles Decorated BiVO4 as an Effective Visible-Light-Driven p-n Heterojunction Photocatalyst for Methylene Blue Degradation. Superlattices Microstruct. 2014, 74, 294–307. [Google Scholar] [CrossRef]
- Zheng, Z.; Zu, X.; Zhang, Y.; Zhou, W. Rational Design of Type-II Nano-Heterojunctions for Nanoscale Optoelectronics. Mater. Today Phys. 2020, 15, 100262. [Google Scholar] [CrossRef]
- Yang, Q.; Tan, G.; Zhang, B.; Feng, S.; Bi, Y.; Wang, Z.; Xia, A.; Ren, H.; Liu, W. Cs0.33WO3/(t-m)-BiVO4 Double Z-Type Heterojunction Photothermal Synergistic Enhanced Full-Spectrum Degradation of Antibiotics. Chem. Eng. J. 2023, 458, 141378. [Google Scholar] [CrossRef]
- Lin, Z.; Ye, S.; Xu, Y.; Lin, X.; Qin, Z.; Bao, J.; Peng, H. Construction of a Novel Efficient Z-Scheme BiVO4/EAQ Heterojunction for the Photocatalytic Inactivation of Antibiotic-Resistant Pathogens: Performance and Mechanism. Chem. Eng. J. 2023, 453, 139747. [Google Scholar] [CrossRef]
- Li, H.; Gong, H.; Jin, Z. Phosphorus Modified Ni-MOF–74/BiVO4 S-Scheme Heterojunction for Enhanced Photocatalytic Hydrogen Evolution. Appl. Catal. B Environ. 2022, 307, 121166. [Google Scholar] [CrossRef]
- Huang, J.; Luo, W.; Ma, W.; Yuan, X. Fabrication of BiVO4 Photoanode Catalyzed with Bimetallic Sulfide Nanoparticles for Enhanced Photoelectrochemical Water Oxidation. Int. J. Hydrogen Energy 2022, 47, 17600–17610. [Google Scholar] [CrossRef]
- Linic, S.; Christopher, P.; Ingram, D.B. Plasmonic-Metal Nanostructures for Efficient Conversion of Solar to Chemical Energy. Nat. Mater. 2011, 10, 911–921. [Google Scholar] [CrossRef]
- Zaleska-Medynska, A.; Marchelek, M.; Diak, M.; Grabowska, E. Noble Metal-Based Bimetallic Nanoparticles: The Effect of the Structure on the Optical, Catalytic and Photocatalytic Properties. Adv. Colloid Interface Sci. 2016, 229, 80–107. [Google Scholar] [CrossRef] [PubMed]
- Grigioni, I.; Di Liberto, G.; Dozzi, M.V.; Tosoni, S.; Pacchioni, G.; Selli, E. WO3/BiVO4 Photoanodes: Facets Matching at the Heterojunction and BiVO4 Layer Thickness Effects. ACS Appl. Energy Mater. 2021, 4, 8421–8431. [Google Scholar] [CrossRef] [PubMed]
- Dhas, C.R.; Arivukarasan, D.; Venkatesh, R.; Josephine, A.J.; Gnana Malar, K.C.M.; Santhoshi Monica, S.E.; Subramanian, B. Influence of Precursor Aging Time Period on Physical and Photocatalytic Properties of Nebulizer Spray Coated BiVO4 Thin Films. Solid State Sci. 2019, 92, 36–45. [Google Scholar] [CrossRef]
- He, W.; Zhang, X.; Dong, X.; Zhang, X.; Ma, C.; Ma, H. Preparation of Mesoporous BiVO4 for Efficient Photocatalytic Degradation of RhB Under Illuminated Visible Light. J. Adv. Oxid. Technol. 2014, 17, 33–38. [Google Scholar] [CrossRef]
- Fan, G.; Cai, C.; Yang, S.; Du, B.; Luo, J.; Chen, Y.; Lin, X.; Li, X.; Wang, Y. Sonophotocatalytic Degradation of Ciprofloxacin by Bi2MoO6/FeVO4 Heterojunction: Insights into Performance, Mechanism and Pathway. Sep. Purif. Technol. 2022, 303, 122251. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, Y.; Zhu, Y.; Wang, J.; Qiao, L.; Zhao, Y.; Tao, Y.; Xiao, Y.; Tang, L. Insights into Adsorption and High Photocatalytic Oxidation of Ciprofloxacin under Visible Light by Intra-Molecular Donor-Acceptor like p-n Isotype Heterojunction: Performance and Mechanism. Chem. Eng. J. 2023, 464, 142533. [Google Scholar] [CrossRef]
- Martín-Sómer, M.; Pablos, C.; van Grieken, R.; Marugán, J. Influence of Light Distribution on the Performance of Photocatalytic Reactors: LED vs Mercury Lamps. Appl. Catal. B Environ. 2017, 215, 1–7. [Google Scholar] [CrossRef]
- Li, D.; Wang, Y.; Song, X.; Jiang, M.; Zhao, X.; Cao, X. The Inhibitory Effects of Simulated Light Sources on the Activity of Algae Cannot Be Ignored in Photocatalytic Inhibition. Chemosphere 2022, 309, 136611. [Google Scholar] [CrossRef]
- Melchor-Lagar, V.; Ramos-Ramírez, E.; Morales-Pérez, A.-A.; Rangel-Vázquez, I.; Del Angel, G. Photocatalytic Removal of 4-Chlorophenol Present in Water Using ZrO2/LDH under UV Light Source. J. Photochem. Photobiol. A Chem. 2020, 389, 112251. [Google Scholar] [CrossRef]
- Lakshmana Reddy, N.; Rao, V.N.; Kumari, M.M.; Ravi, P.; Sathish, M.; Shankar, M.V. Effective Shuttling of Photoexcitons on CdS/NiO Core/Shell Photocatalysts for Enhanced Photocatalytic Hydrogen Production. Mater. Res. Bull. 2018, 101, 223–231. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, J.; Tan, G.; Huang, J.; Zhang, L. Controllable Synthesis and Photocatalytic Properties of C-Doped BiVO4 with Self-Heterostructure Under Different Light Sources. NANO 2015, 10, 1550008. [Google Scholar] [CrossRef]
- Zhang, L.; Meng, Y.; Shen, H.; Li, J.; Yang, C.; Xie, B.; Xia, S. Photocatalytic Degradation of Rhodamine B by Bi2O3@LDHs S–Scheme Heterojunction: Performance, Kinetics and Mechanism. Appl. Surf. Sci. 2021, 567, 150760. [Google Scholar] [CrossRef]
- Srinivasan, N.; Anbuchezhiyan, M.; Harish, S.; Ponnusamy, S. Efficient Catalytic Activity of BiVO4 Nanostructures by Crystal Facet Regulation for Environmental Remediation. Chemosphere 2022, 289, 133097. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Wu, H.; Fang, Y.; Li, R.; Huang, Y. Hydrothermal Synthesis of M-BiVO4/t-BiVO4 Heterostructure for Organic Pollutants Degradation: Insight into the Photocatalytic Mechanism of Exposed Facets from Crystalline Phase Controlling. J. Hazard. Mater. 2020, 399, 123159. [Google Scholar] [CrossRef]
- Sonkusare, V.N.; Chaudhary, R.G.; Bhusari, G.S.; Rai, A.R.; Juneja, H.D. Microwave-Mediated Synthesis, Photocatalytic Degradation and Antibacterial Activity of α-Bi2O3 Microflowers/Novel γ-Bi2O3 Microspindles. Nano-Struct. Nano-Objects 2018, 13, 121–131. [Google Scholar] [CrossRef]
- Gao, G.; Niu, X.; Xu, B.; Wang, X.L.; Yao, Y.-F. Shape and Size Effects on Photocatalytic Hydrogen Production via Pd/C3N4 Photocatalysts under Visible Light. Catal. Sci. Technol. 2020, 10, 5438–5442. [Google Scholar] [CrossRef]
- Lu, M.; Li, Q.; Zhang, C.; Fan, X.; Li, L.; Dong, Y.; Chen, G.; Shi, H. Remarkable Photocatalytic Activity Enhancement of CO2 Conversion over 2D/2D g-C3N4/BiVO4 Z-Scheme Heterojunction Promoted by Efficient Interfacial Charge Transfer. Carbon 2020, 160, 342–352. [Google Scholar] [CrossRef]
- Khezli, S.; Abedini, A. Effect of Amino Acids as a Capping Agent on the Size and Morphology of Pure AgO Nanoparticles and Its Photocatalyst Application. J. Mater. Sci. Mater. Electron. 2017, 28, 10535–10540. [Google Scholar] [CrossRef]
- Le, S.; Li, W.; Wang, Y.; Jiang, X.; Yang, X.; Wang, X. Carbon Dots Sensitized 2D-2D Heterojunction of BiVO4/Bi3TaO7 for Visible Light Photocatalytic Removal towards the Broad-Spectrum Antibiotics. J. Hazard. Mater. 2019, 376, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, K.; Tahir, M.B.; Sagir, M.; Kabli, M.R. Role of CuCo2S4 in Z-Scheme MoSe2/BiVO4 Composite for Efficient Photocatalytic Reduction of Heavy Metals. Ceram. Int. 2019, 45, 23225–23232. [Google Scholar] [CrossRef]


| Appearance | Preparation Method | Degradation Efficiency of Single BiVO4 (%) | Degradation Efficiency after Loading (%) | Cycle Performance (%)/Cycle Count | Advantages | References |
|---|---|---|---|---|---|---|
| Microsphere | Photo-deposition method | 12.1 | 90.5 | - | High efficiency; potential applications in solar energy, water decomposition, and medicine | [47] |
| N-decahedron | Hydrothermal method | 31.7 | 99.0 | 99.0/5 times | Good photocatalytic stability; significant improvement of photocatalytic performance | [54] |
| 3D z-shape | Hydrothermal method | 45 | 100 | ≥85/5 times | Novel and efficient in removing both heavy metals and organic pollutants from water | [55] |
| Nanorod | Hydrothermal and co-precipitation methods | 34 | 87 | 77.5/5 times | Good structure and compound performance; can effectively degrade organic pollutants such as RhB and TC; good stability | [50] |
| Peanut-like nanorods with monoclinic structure | Hydrothermal method | 45 | 96 | 92/5 times | Low cost and high photocatalytic activity; can be loaded on clay to degrade organic pollutants | [56] |
| Nanosheet | Hydrothermal and photoreduction methods | 58.6 | 96.2 | - | Simultaneous removal of Cr(VI) and CIP pollutants; high photocatalytic activity and stability for degradation | [57] |
| Nanoparticle aggregation | Hydrothermal method | 56 | 96.23 | - | Excellent size and structural homogeneity; good photocatalytic activity for visible-light-driven photocatalysts for water remediation | [58] |
| Band | Hydrothermal method | 56 | 98 | 87/4 times | - | [59] |
| Nano-floral | Hydrolysis method | 45.94 | 90.20 | 88.58/4 times | Large specific surface area and high photocatalytic efficiency | [60] |
| Ribbon | Simple solvent heat path | - | - | - | Higher surface area and crystallinity; high activity; recyclable | [61] |
| Sheet | Ultrasound-assisted solvent heat method | 67 | 99.98 | 94/5 times | - | [62] |
| Rod-shaped pellet | Ultrasonic-assisted method | 28 | 100 | 97/5 times | Large surface area; suitable band gap and small crystal size; excellent visible light photocatalytic activity | [63] |
| Sheet | Hydrothermal method | 43 | 85 | - | - | [48] |
| Sheet | Ultrasonication | 49 | 92 | 95/5 times | Good contact surface structure; high photocatalytic activity; strong optical absorption ability; good adsorption for organic pollutants; low level of electron–hole pair compounding | [64] |
| Nanosheet aggregation | Hydrothermal method | 36 | 86.7 | - | High reusability and photocatalytic performance | [65] |
| Lump | Ultrasonic-assisted method | 47 | 93.6 | 78.7/6 times | Large specific surface area; high number of increased active sites; good visible light absorption range and construction of heterojunctions; high stability | [66] |
| Nanowires or nanorods | Hydrothermal method | 48 | 92 | - | High oxidation and reduction capacity; economical and effective | [67] |
| Spherical nanoparticles | Liquid precipitation mechanical mixing method | 14.57 | 90.14 | 78/3 times | Large specific surface area; high photocatalytic activity | [68] |
| Olivine nanoparticles | One-step solvent heat method | 34 | 99 | - | Synthesis strategy is simple, easy, environmentally friendly, and scalable | [69] |
| Regular octahedron | In situ simple precipitation method | 47.50 | 93.67 | 89.29/5 times | Simultaneous removal of Cr(VI) and MB-mixed contamination, with good reusability and stability | [70] |
| Dumbbell-shaped | Visible-light-assisted photocatalysis | 85 | 99 | - | BVO/rGO has small particle size, strong adsorption capacity and high photocatalytic activity | [71] |
| Hexagonal nanorods | Chemical precipitation method | - | - | 91.35/5 times | Strong adsorption capacity; good photocatalytic activity | [72] |
| Porous tubular | - | 58.60 | 97.66 | 80/5 times | High separation rate of photogenerated charges and high efficiency of catalytic degradation of organic pollutants | [73] |
| Flowery sphere | In situ technology | 26 | 60 | 32/9 times | Photogenerated carriers can be effectively separated with high oxidation and reduction capacities | [74] |
| Sheet | Easy precipitation method | 38 | 94 | - | Excellent performance in degrading organic dyes and good reusability | [75] |
| Spherical cluster | Two-step solvent heat method | 77 | 89 | 85.3/5 times | Excellent adsorption performance and abundant active sites | [76] |
| Irregular spherical/oval clusters | Hydrothermal method | 71 | 97 | - | Low compounding rate of electron–hole pairs; small energy band gap; strong electron capture ability; high photocatalytic activity | [77] |
| Variable | Group | Sample Size (n) | 95% CI (SMD) | Effect Size (%) | ||
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Preparation conditions | Coupling type | Metal | 3 | 7.49 | 8.69 | 134.79 |
| Oxide | 10 | 3.94 | 4.73 | 62.75 | ||
| Sulfide | 3 | 8 | 9.36 | 152.19 | ||
| Carbon materials | 4 | 7.02 | 7.96 | 79.31 | ||
| Heterojunctions | 2 | 12.73 | 15.13 | 136.40 | ||
| Nanomaterials | 4 | 12.96 | 14.56 | 90.10 | ||
| Time | 1–6 h | 9 | 6.72 | 7.2 | 91.90 | |
| 6–24 h | 7 | 8.87 | 9.56 | 59.59 | ||
| >24 h | 4 | 14.26 | 16.12 | 145.34 | ||
| Temperature | Low (≤100) | 9 | 4.65 | 5.12 | 83.37 | |
| Medium (100~200) | 9 | 8.84 | 9.46 | 77.69 | ||
| High (≥200) | 4 | 11.31 | 12.62 | 121.33 | ||
| Degradation pH | Acidic conditions | 4 | 8.33 | 9.41 | 88.08 | |
| Neutral | 4 | 10.92 | 12.36 | 125.47 | ||
| Alkaline conditions | 5 | 10.33 | 11.52 | 72.56 | ||
| Degradation condition | Pollution concentration (mg/L) | <20 | 13 | 6.84 | 7.33 | 93.48 |
| 20–200 | 7 | 10.66 | 11.69 | 105.83 | ||
| >200 | 2 | 7.26 | 12.92 | 285.03 | ||
| Photocatalyst dosage (g/L) | <0.5 | 6 | 5.28 | 5.97 | 95.45 | |
| 0.5–1 | 14 | 7.00 | 7.52 | 112.02 | ||
| >1 | 4 | 4.56 | 5.21 | 65.32 | ||
| Solution pH | Acidic | 8 | 9.02 | 9.91 | 119.93 | |
| Neutral | 3 | 13.79 | 15.82 | 115.74 | ||
| Alkaline | 4 | 4.02 | 5.19 | 52.82 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Che, R.; Zhu, Y.; Tu, B.; Miao, J.; Dong, Z.; Liu, M.; Wang, Y.; Li, J.; Chen, S.; Wang, F. A Meta-Analysis of Influencing Factors on the Activity of BiVO4-Based Photocatalysts. Nanomaterials 2023, 13, 2352. https://doi.org/10.3390/nano13162352
Che R, Zhu Y, Tu B, Miao J, Dong Z, Liu M, Wang Y, Li J, Chen S, Wang F. A Meta-Analysis of Influencing Factors on the Activity of BiVO4-Based Photocatalysts. Nanomaterials. 2023; 13(16):2352. https://doi.org/10.3390/nano13162352
Chicago/Turabian StyleChe, Ruijie, Yining Zhu, Biyang Tu, Jiahe Miao, Zhongtian Dong, Mengdi Liu, Yupeng Wang, Jining Li, Shuoping Chen, and Fenghe Wang. 2023. "A Meta-Analysis of Influencing Factors on the Activity of BiVO4-Based Photocatalysts" Nanomaterials 13, no. 16: 2352. https://doi.org/10.3390/nano13162352





