Fountain Pen-Inspired 3D Colloidal Assembly, Consisting of Metallic Nanoparticles on a Femtoliter Scale
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabrication of Nano-Fountain Pen
2.2. Preparation of Nanoparticle-Dispersed Ink
2.3. Motorized Printing Process
2.4. Structure and Optical Spectra of 3D Colloidal Assembly
3. Results and Discussion
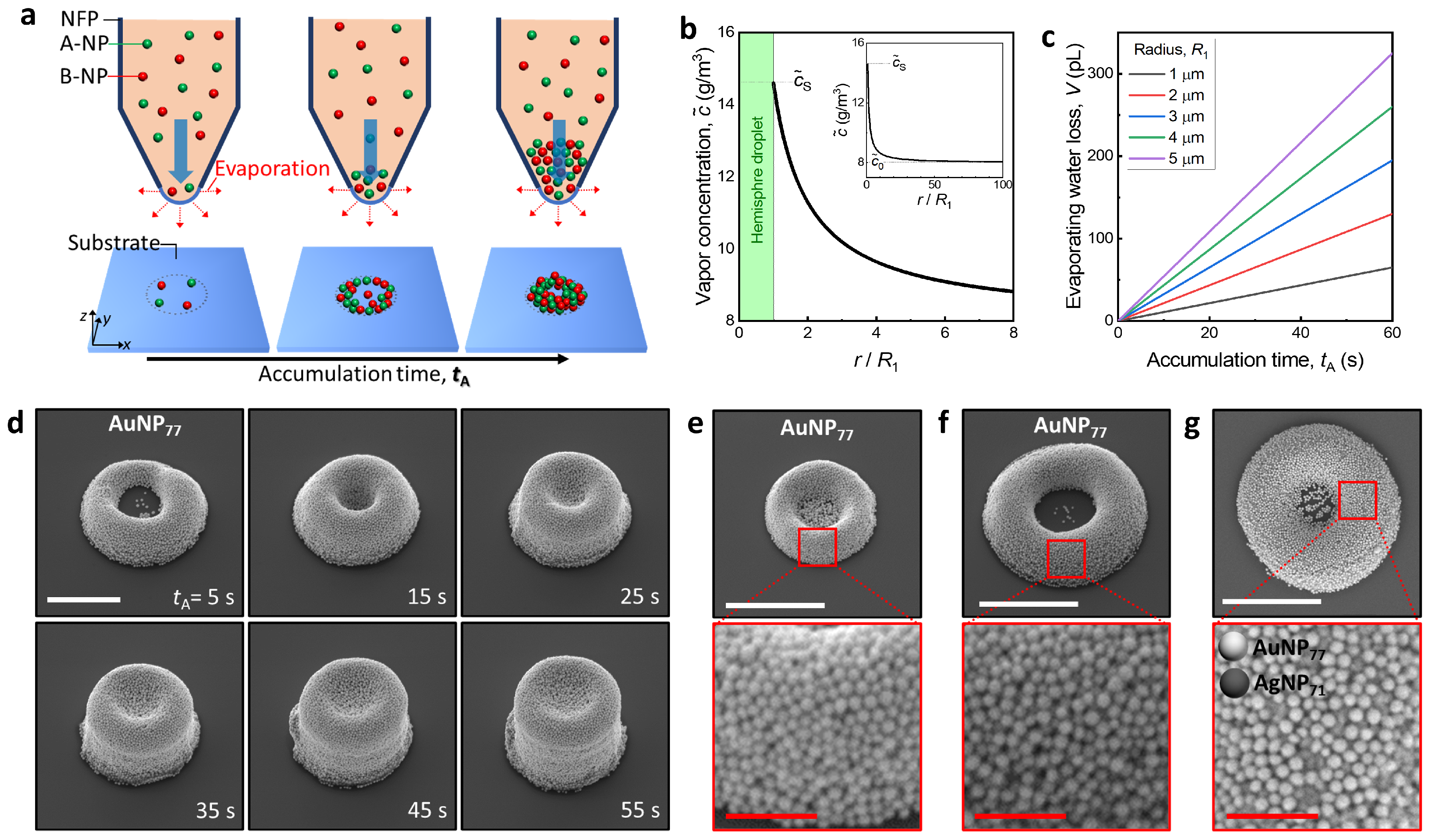
3.1. 3D Colloidal Assembly Using a Nano-Fountain Pen
3.2. Fabrication Mechanism of a Femtoliter Level of 3D Colloidal Assembly
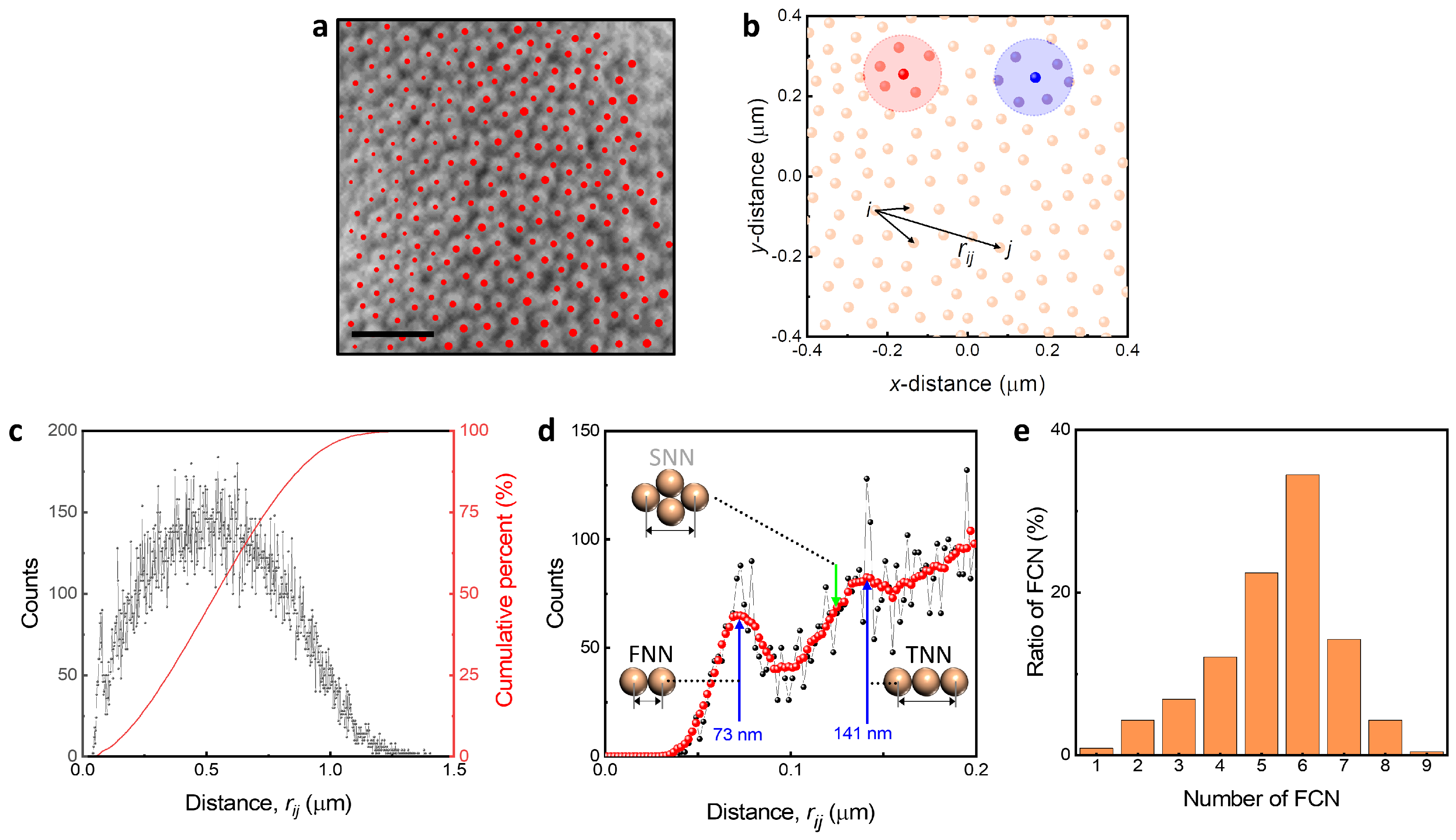
3.3. Packing Structures of 3D Colloidal Assembly
3.4. Optical Properties Regarding the Structural Changes of 3D Colloidal Assembly
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| NP | Nanoparticle |
| NFP | Nano-Fountain Pen |
| SEM | Scanning Electron Microscope |
| FNN | First Nearest Neighbor |
| SNN | Second Nearest Neighbor |
| TNN | Third Nearest Neighbor |
| LSPR | Localized Surface Plasmon Resonance |
References
- Hutter, E.; Fendler, J.H. Exploitation of localized surface plasmon resonance. Adv. Mater. 2004, 16, 1685–1706. [Google Scholar] [CrossRef]
- Jensen, T.R.; Malinsky, M.D.; Haynes, C.L.; Van Duyne, R.P. Nanosphere lithography: Tunable localized surface plasmon resonance spectra of silver nanoparticles. J. Phys. Chem. 2000, 104, 10549–10556. [Google Scholar] [CrossRef]
- Ringe, E.; McMahon, J.M.; Sohn, K.; Cobley, C.; Xia, Y.; Huang, J.; Schatz, G.C.; Marks, L.D.; Van Duyne, R.P. Unraveling the effects of size, composition, and substrate on the localized surface plasmon resonance frequencies of gold and silver nanocubes: A systematic single-particle approach. J. Phys. Chem. C 2010, 114, 12511–12516. [Google Scholar] [CrossRef]
- Mayer, K.M.; Hafner, J.H. Localized surface plasmon resonance sensors. Chem. Rev. 2011, 111, 3828–3857. [Google Scholar] [CrossRef] [PubMed]
- Petryayeva, E.; Krull, U.J. Localized surface plasmon resonance: Nanostructures, bioassays and biosensing—A review. Anal. Chim. Acta 2011, 706, 8–24. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Sun, T.; Grattan, K.T. Gold nanorod-based localized surface plasmon resonance biosensors: A review. Sens. Actuators B Chem. 2014, 195, 332–351. [Google Scholar] [CrossRef]
- Wu, J.L.; Chen, F.C.; Hsiao, Y.S.; Chien, F.C.; Chen, P.; Kuo, C.H.; Huang, M.H.; Hsu, C.S. Surface plasmonic effects of metallic nanoparticles on the performance of polymer bulk heterojunction solar cells. ACS Nano 2011, 5, 959–967. [Google Scholar] [CrossRef] [PubMed]
- Du, P.; Jing, P.; Li, D.; Cao, Y.; Liu, Z.; Sun, Z. Plasmonic Ag@ oxide nanoprisms for enhanced performance of organic solar cells. Small 2015, 11, 2454–2462. [Google Scholar] [CrossRef]
- Lee, H.B.; Kim, W.G.; Lee, M.; Lee, J.M.; He, S.; Kumar, N.; Devaraj, V.; Choi, E.J.; Jeon, I.; Song, M.; et al. Gap Plasmon of Virus-Templated Biohybrid Nanostructures Uplifting the Performance of Organic Optoelectronic Devices. Adv. Opt. Mater. 2020, 8, 1902080. [Google Scholar] [CrossRef]
- Xiao, Q.; Jaatinen, E.; Zhu, H. Direct Photocatalysis for Organic Synthesis by Using Plasmonic-Metal Nanoparticles Irradiated with Visible Light. Chem. Asian J. 2014, 9, 3046–3064. [Google Scholar] [CrossRef]
- Kolemen, S.; Ozdemir, T.; Lee, D.; Kim, G.M.; Karatas, T.; Yoon, J.; Akkaya, E.U. Remote-controlled release of singlet oxygen by the plasmonic heating of endoperoxide-modified gold nanorods: Towards a paradigm change in photodynamic therapy. Angew. Chem. 2016, 128, 3670–3674. [Google Scholar] [CrossRef]
- Hung, W.H.; Aykol, M.; Valley, D.; Hou, W.; Cronin, S.B. Plasmon resonant enhancement of carbon monoxide catalysis. Nano Lett. Mater. 2010, 10, 1314–1318. [Google Scholar] [CrossRef] [PubMed]
- Mao, P.; Liu, C.; Favraud, G.; Chen, Q.; Han, M.; Fratalocchi, A.; Zhang, S. Broadband single molecule SERS detection designed by warped optical spaces. Nat. Commun. 2018, 9, 5428. [Google Scholar] [CrossRef] [PubMed]
- Kneipp, J.; Kneipp, H.; Kneipp, K. SERS—A single-molecule and nanoscale tool for bioanalytics. Chem. Soc. Rev. 2008, 37, 1052–1060. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Puebla, R.A.; Liz-Marzán, L.M. SERS detection of small inorganic molecules and ions. Angew. Chem. Int. Ed. 2012, 51, 11214–11223. [Google Scholar] [CrossRef] [PubMed]
- Sonnefraud, Y.; Verellen, N.; Sobhani, H.; Vandenbosch, G.A.; Moshchalkov, V.V.; Van Dorpe, P.; Nordlander, P.; Maier, S.A. Experimental realization of subradiant, superradiant, and Fano resonances in ring/disk plasmonic nanocavities. ACS Nano 2010, 4, 1664–1670. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.A.; Bao, K.; Wu, C.; Bao, J.; Bardhan, R.; Halas, N.J.; Manoharan, V.N.; Shvets, G.; Nordlander, P.; Capasso, F. Fano-like interference in self-assembled plasmonic quadrumer clusters. Nano Lett. 2010, 10, 4680–4685. [Google Scholar] [CrossRef] [PubMed]
- Shao, T.; Wang, X.; Dong, H.; Liu, S.; Duan, D.; Li, Y.; Song, P.; Jiang, H.; Hou, Z.; Gao, C.; et al. A Stacked Plasmonic Metamaterial with Strong Localized Electric Field Enables Highly Efficient Broadband Light-Driven CO2 Hydrogenation. Adv. Mater. 2022, 34, 2202367. [Google Scholar] [CrossRef]
- Ji, T.; Peng, L.; Zhu, Y.; Yang, F.; Cui, Y.; Wu, X.; Liu, L.; He, S.; Zhu, F.; Hao, Y. Plasmonic broadband absorber by stacking multiple metallic nanoparticle layers. Appl. Phys. Lett. 2015, 106, 161107. [Google Scholar] [CrossRef]
- Saeed, A.; Panaro, S.; Zaccaria, R.P.; Raja, W.; Liberale, C.; Dipalo, M.; Messina, G.C.; Wang, H.; De Angelis, F.; Toma, A. Stacked optical antennas for plasmon propagation in a 5 nm-confined cavity. Sci. Rep. 2015, 5, 11237. [Google Scholar] [CrossRef]
- Kim, W.G.; Lee, J.M.; Yang, Y.; Kim, H.; Devaraj, V.; Kim, M.; Jeong, H.; Choi, E.J.; Yang, J.; Jang, Y.; et al. Three-dimensional plasmonic nanocluster-driven light–matter interaction for photoluminescence enhancement and picomolar-level biosensing. Nano Lett. 2022, 22, 4702–4711. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhen, Y.R.; Neumann, O.; Day, J.K.; Nordlander, P.; Halas, N.J. Coherent anti-Stokes Raman scattering with single-molecule sensitivity using a plasmonic Fano resonance. Nat. Commun. 2014, 5, 4424. [Google Scholar] [CrossRef] [PubMed]
- Chu, M.W.; Myroshnychenko, V.; Chen, C.H.; Deng, J.P.; Mou, C.Y.; de Abajo, F.J.G. Probing bright and dark surface-plasmon modes in individual and coupled noble metal nanoparticles using an electron beam. Nano Lett. 2009, 9, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; You, E.A.; Hwang, D.W.; Kang, S.; Wi, J.S. Active accumulation of spherical analytes on plasmonic hot spots of double-bent Au strip arrays by multiple dip-coating. Nanomaterials 2019, 9, 660. [Google Scholar] [CrossRef] [PubMed]
- Tahghighi, M.; Mannelli, I.; Janner, D.; Ignés-Mullol, J. Tailoring plasmonic response by Langmuir-Blodgett gold nanoparticle templating for the fabrication of SERS substrates. Appl. Surf. Sci. 2018, 447, 416–422. [Google Scholar] [CrossRef]
- Hanske, C.; Tebbe, M.; Kuttner, C.; Bieber, V.; Tsukruk, V.V.; Chanana, M.; König, T.A.; Fery, A. Strongly coupled plasmonic modes on macroscopic areas via template-assisted colloidal self-assembly. Nano Lett. 2014, 14, 6863–6871. [Google Scholar] [CrossRef] [PubMed]
- Rana, A.S.; Lockerby, D.A.; Sprittles, J.E. Lifetime of a nanodroplet: Kinetic effects and regime transitions. Phys. Rev. Lett. 2019, 123, 154501. [Google Scholar] [CrossRef]
- CVR. Lange’s Handbook of Chemistry; Revised 10th Edition; McGraw-Hill: New York, NY, USA, 1968; p. 449. [Google Scholar]
- Cui, J.; Ju, Y.; Liang, K.; Ejima, H.; Lörcher, S.; Gause, K.T.; Richardson, J.J.; Caruso, F. Nanoscale engineering of low-fouling surfaces through polydopamine immobilisation of zwitterionic peptides. Soft Matter 2014, 10, 2656–2663. [Google Scholar] [CrossRef]
- Shuturminska, K.; Tarakina, N.V.; Azevedo, H.S.; Bushby, A.J.; Mata, A.; Anderson, P.; Al-Jawad, M. Elastin-like protein, with statherin derived peptide, controls fluorapatite formation and morphology. Front. Physiol. 2017, 8, 368. [Google Scholar] [CrossRef]
- Cussler, E.L. Diffusion: Mass Transfer in Fluid Systems; Cambridge University Press: Cambridge, UK, 2009. [Google Scholar]
- Berryman, J.G. Random close packing of hard spheres and disks. Phys. Rev. A 1983, 27, 1053. [Google Scholar] [CrossRef]
- Qin, Z.; Li, Z.; Sharma, S.; Peng, Y.; Jin, R.; Li, G. Self-assembly of silver clusters into one-and two-dimensional structures and highly selective methanol sensing. Research 2022, 2022, 0018. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Li, Z.; Qin, Z.; Sharma, S.; Li, G. Atomically precise copper dopants in metal clusters boost up stability, fluorescence, and photocatalytic activity. Commun. Chem. 2023, 6, 24. [Google Scholar] [CrossRef]
- Shi, Q.; Qin, Z.; Sharma, S.; Li, G. Recent progress in heterogeneous catalysis by atomically and structurally precise metal nanoclusters. Chem. Rec. 2021, 21, 879–892. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-J.; Lee, I.-H.; Kim, W.-G.; Hwang, Y.-H.; Oh, J.-W. Fountain Pen-Inspired 3D Colloidal Assembly, Consisting of Metallic Nanoparticles on a Femtoliter Scale. Nanomaterials 2023, 13, 2403. https://doi.org/10.3390/nano13172403
Kim S-J, Lee I-H, Kim W-G, Hwang Y-H, Oh J-W. Fountain Pen-Inspired 3D Colloidal Assembly, Consisting of Metallic Nanoparticles on a Femtoliter Scale. Nanomaterials. 2023; 13(17):2403. https://doi.org/10.3390/nano13172403
Chicago/Turabian StyleKim, Sung-Jo, Il-Hyun Lee, Won-Geun Kim, Yoon-Hwae Hwang, and Jin-Woo Oh. 2023. "Fountain Pen-Inspired 3D Colloidal Assembly, Consisting of Metallic Nanoparticles on a Femtoliter Scale" Nanomaterials 13, no. 17: 2403. https://doi.org/10.3390/nano13172403
APA StyleKim, S.-J., Lee, I.-H., Kim, W.-G., Hwang, Y.-H., & Oh, J.-W. (2023). Fountain Pen-Inspired 3D Colloidal Assembly, Consisting of Metallic Nanoparticles on a Femtoliter Scale. Nanomaterials, 13(17), 2403. https://doi.org/10.3390/nano13172403





