Spectral Behavior of a Conjugated Polymer MDMO-PPV Doped with ZnO Nanoparticles: Thin Films
Abstract
1. Introduction
2. Experimental Procedures
2.1. Materials Preparation
2.2. Materials Characterization
3. Results and Discussion
3.1. Optical Properties of ZnO NPs Doped MDMO-PPV
3.1.1. Absorption Spectra
3.1.2. Energy Band Gap () and Refractive Index (n)
3.1.3. Photoluminescence Spectra
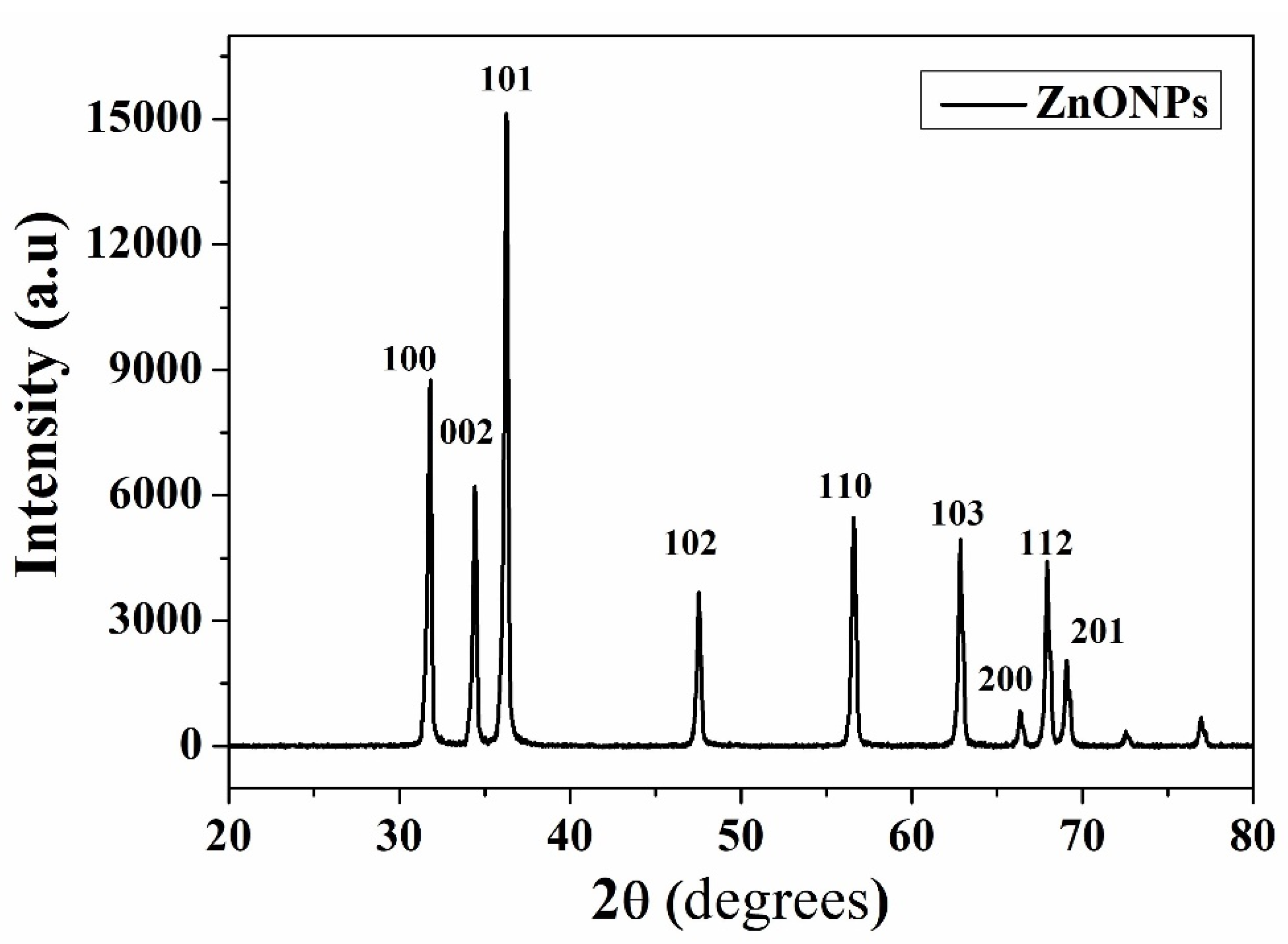
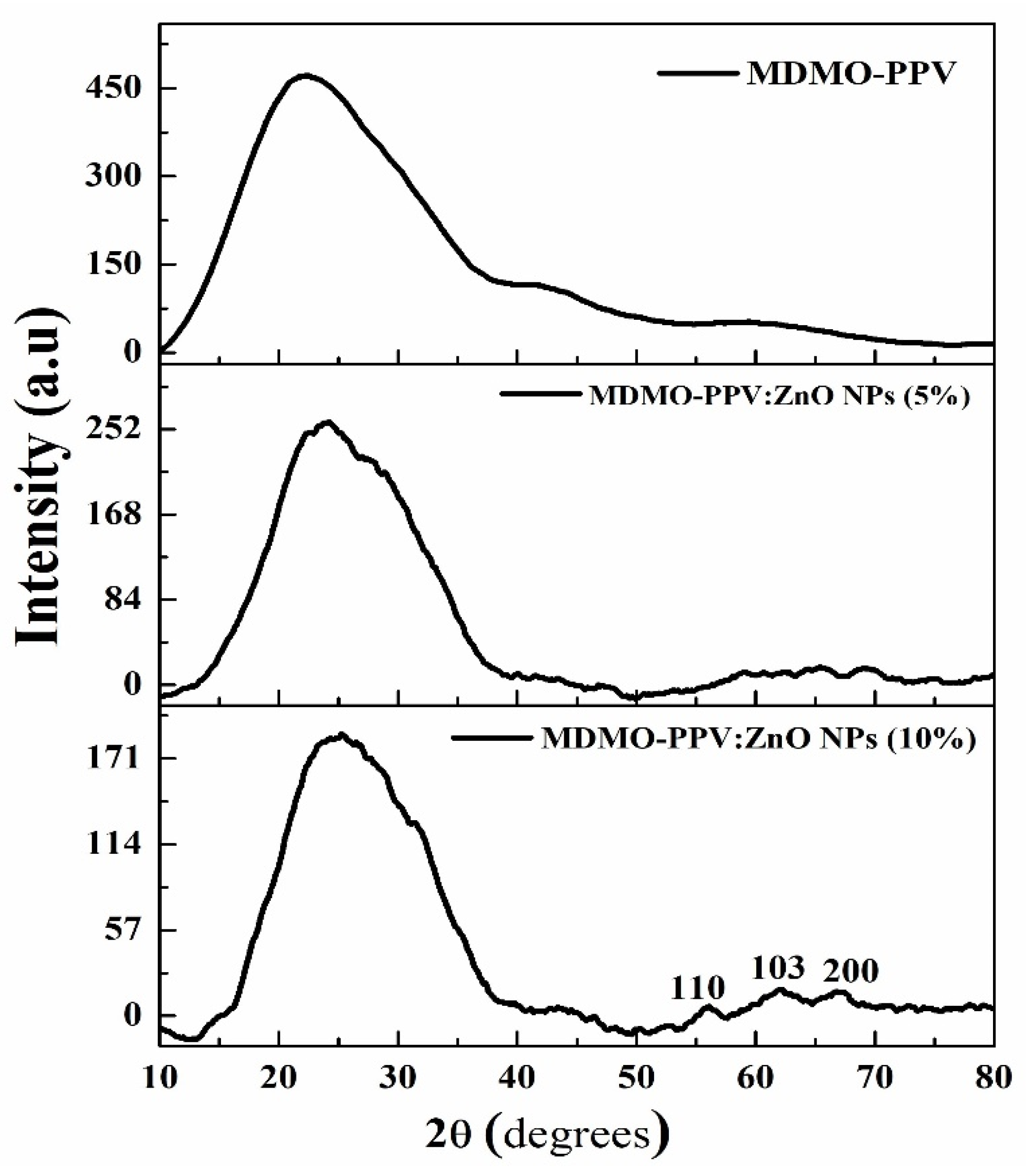
3.2. X-ray Diffraction (XRD) Measurements
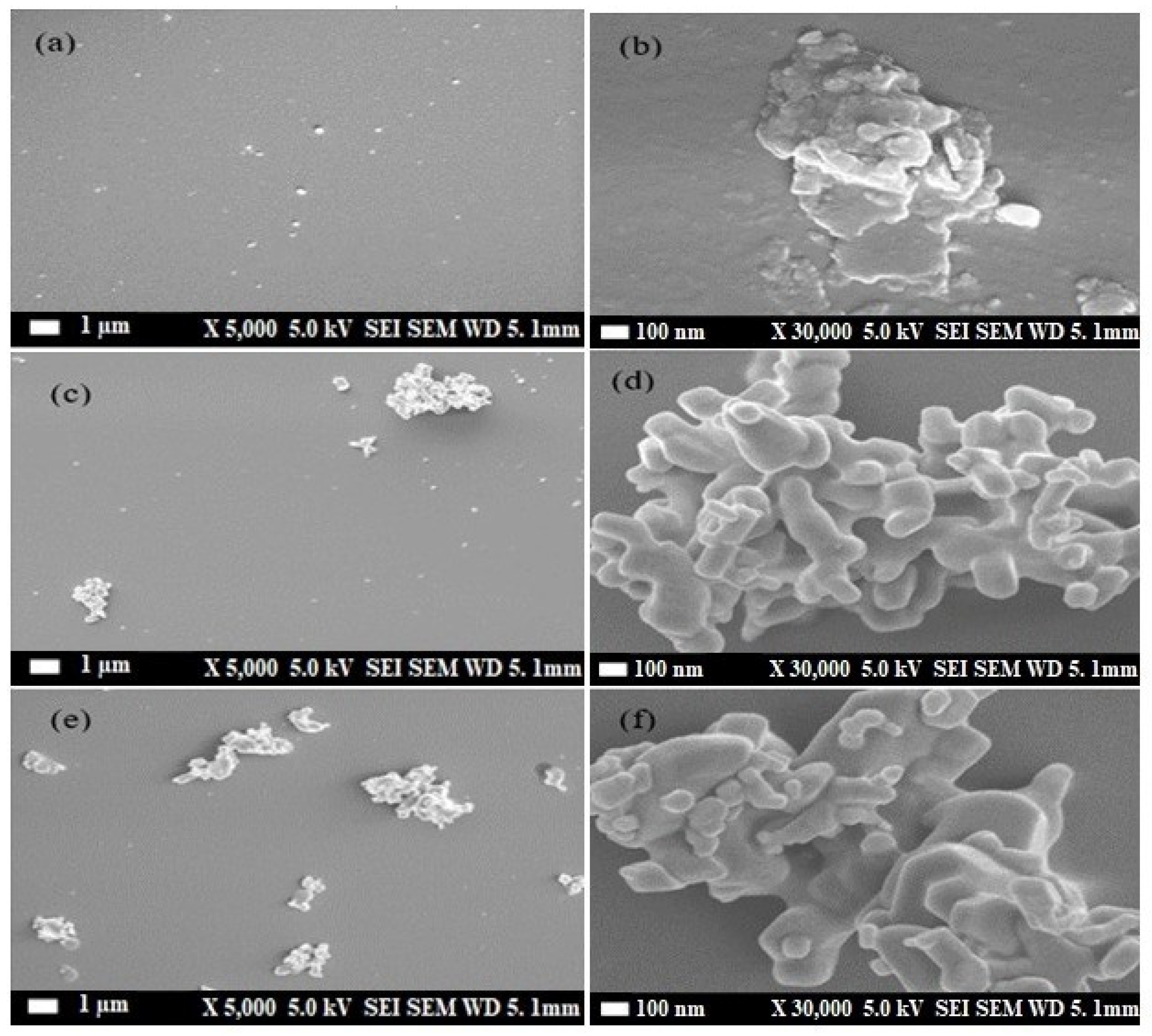
3.3. Scanning Electron Microscope (SEM)
3.4. Theoretical Analysis of the PL Spectra
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- An, Z.; Huang, Y.; Zhang, R. High-temperature multispectral stealth metastructure from the microwave-infrared compatible design. Compos. Part B Eng. 2023, 259, 110737. [Google Scholar] [CrossRef]
- An, Z.; Li, Y.; Luo, X.; Huang, Y.; Zhang, R.; Fang, D. Multilaminate metastructure for high-temperature radar-infrared bi-stealth: Topological optimization and near-room-temperature synthesis. Matter 2022, 5, 1937–1952. [Google Scholar] [CrossRef]
- Komlev, A.S.; Gimaev, R.R.; Zverev, V.I. Smart magnetocaloric coatings for implants: Controlled drug release for targeted delivery. Phys. Open 2021, 7, 100063. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, X.; Chen, X.; He, Y.; Cheng, L.; Huo, M.; Yin, J.; Hao, F.; Chen, S.; Wang, P.; et al. Additive manufacturing of structural materials. Mater. Sci. Eng. R Rep. 2021, 145, 100596. [Google Scholar] [CrossRef]
- Yang, Y.; Song, X.; Li, X.; Chen, Z.; Zhou, C.; Zhou, Q.; Chen, Y. Recent Progress in Biomimetic Additive Manufacturing Technology: From Materials to Functional Structures. Adv. Mater. 2018, 30, 1706539. [Google Scholar] [CrossRef]
- Friend, R.H.; Gymer, R.; Holmes, A.; Burroughes, J.; Marks, R.; Taliani, C.; Bradley, D.; Santos, D.D.; Bredas, J.-L.; Lögdlund, M. Electroluminescence in conjugated polymers. Nature 1999, 397, 121–128. [Google Scholar] [CrossRef]
- Jagadesan, P.; Yu, Z.; Barboza-Ramos, I.; Lara, H.H.; Vazquez-Munoz, R.; López-Ribot, J.L.; Schanze, K.S. Light-activated antifungal properties of imidazolium-functionalized cationic conjugated polymers. Chem. Mater. 2020, 32, 6186–6196. [Google Scholar] [CrossRef]
- Hestand, N.J.; Spano, F.C. The effect of chain bending on the photophysical properties of conjugated polymers. J. Phys. Chem. B 2014, 118, 8352–8363. [Google Scholar]
- Li, X.-J.; Sun, G.-P.; Gong, Y.-F.; Li, Y.-F. Recent research progress of n-type conjugated polymer acceptors and all-polymer solar cells. Chin. J. Polym. Sci. 2023, 41, 640–651. [Google Scholar] [CrossRef]
- Ture, S.A.; Pattathil, S.D.; Zing, B.Z.; Abbaraju, V. Fluorescence Sensing of Some Important Nitroaromatic Compounds by Using Polyaniline Ag Composite. Micro 2023, 3, 16. [Google Scholar] [CrossRef]
- Desu, M.; Sharma, S.; Cheng, K.-H.; Wang, Y.-H.; Nagamatsu, S.; Chen, J.-C.; Pandey, S.S. Controlling the molecular orientation of a novel diketopyrrolopyrrole-based organic conjugated polymer for enhancing the performance of organic field-effect transistors. Org. Electron. 2023, 113, 106691. [Google Scholar] [CrossRef]
- Alfadhli, S.; Darwish, A.; Soliman, S.; El-Zaidia, E.; Yahia, I.; Laariedh, F.; Alatawi, A.; Bahamran, A.; Alatawi, N.M.; Hamdalla, T.A. Structural characterizations and photoelectric performance of non-crystalline boron subphthalocyanine chloride films/FTO for photodiode applications. J. Non-Cryst. Solids 2023, 601, 122044. [Google Scholar]
- Ibnaouf, K. Excimer state of a conjugated polymer (MEH-PPV) in thin films. Opt. Laser Technol. 2013, 48, 401–404. [Google Scholar] [CrossRef]
- Alsalhi, M.; Ibnaouf, K.; Masilamani, V.; Yassin, O. Excimer state of a conjugate polymer (MEH-PPV) in liquid solutions. Laser Phys. 2007, 17, 1361–1366. [Google Scholar] [CrossRef]
- Ibnaouf, K.; Prasad, S.; Masilamani, V.; AlSalhi, M. Evidence for amplified spontaneous emission from double excimer of conjugated polymer (PDHF) in a liquid solution. Polymer 2013, 54, 2401–2405. [Google Scholar] [CrossRef]
- Prasad, S.; Ibnaouf, K.; AlSalhi, M.; Masilamani, V. Laser from the dimer state of a conjugated polymer (PFO) in solution. Polymer 2014, 55, 727–732. [Google Scholar] [CrossRef]
- Rörich, I.; Schönbein, A.-K.; Mangalore, D.K.; Ribeiro, A.H.; Kasparek, C.; Bauer, C.; Crăciun, N.I.; Blom, P.W.; Ramanan, C. Temperature dependence of the photo-and electroluminescence of poly (p-phenylene vinylene) based polymers. J. Mater. Chem. C 2018, 6, 10569–10579. [Google Scholar]
- Bourbon, S.; Gao, M.; Kirstein, S. Electroluminescence of self-assembled films of poly (p-phenylene vinylene) and J-aggregates. Synth. Met. 1999, 101, 152–153. [Google Scholar] [CrossRef]
- Colaneri, N.; Bradley, D.; Friend, R.; Burn, P.; Holmes, A.; Spangler, C. Photoexcited states in poly (p-phenylene vinylene): Comparison with trans, trans-distyrylbenzene, a model oligomer. Phys. Rev. B 1990, 42, 11670. [Google Scholar]
- Wu, C.; Chun, J.; Burrows, P.; Sturm, J.; Thompson, M.; Forrest, S.; Register, R. Poly (p-phenylene vinylene)/tris (8-hydroxy) quinoline aluminum heterostructure light emitting diode. Appl. Phys. Lett. 1995, 66, 653–655. [Google Scholar] [CrossRef][Green Version]
- Masse, M.A.; Martin, D.C.; Thomas, E.L.; Karasz, F.E.; Petermann, J.H. Crystal morphology in pristine and doped films of poly (p-phenylene vinylene). J. Mater. Sci. 1990, 25, 311–320. [Google Scholar] [CrossRef]
- Marks, R.; Halls, J.; Bradley, D.; Friend, R.; Holmes, A. The photovoltaic response in poly (p-phenylene vinylene) thin-film devices. J. Phys. Condens. Matter 1994, 6, 1379. [Google Scholar] [CrossRef]
- Chasteen, S.V.; Sholin, V.; Carter, S.A.; Rumbles, G. Towards optimization of device performance in conjugated polymer photovoltaics: Charge generation, transfer and transport in poly (p-phenylene-vinylene) polymer heterojunctions. Sol. Energy Mater. Sol. Cells 2008, 92, 651–659. [Google Scholar] [CrossRef]
- Oo, T.; Mathews, N.; Tam, T.; Xing, G.; Sum, T.; Sellinger, A.; Wong, L.; Mhaisalkar, S. Investigation of photophysical, morphological and photovoltaic behavior of poly (p-phenylene vinylene) based polymer/oligomer blends. Thin Solid Film. 2010, 518, 5292–5299. [Google Scholar] [CrossRef]
- Blayney, A.J.; Perepichka, I.F.; Wudl, F.; Perepichka, D.F. Advances and Challenges in the Synthesis of Poly (p-phenylene vinylene)-Based Polymers. Isr. J. Chem. 2014, 54, 674–688. [Google Scholar] [CrossRef]
- Al-Asbahi, B.A.; Alanezi, A.A.; AlSalhi, M.S. Photophysical characteristics of multicolor emitting MDMO-PPV–DMP/ZnO hybrid nanocomposites. Molecules 2022, 27, 843. [Google Scholar] [CrossRef]
- Shin, M.J.; Gwon, D.-O.; Lee, G.S.; Ahn, H.S.; Yi, S.N.; Ha, D.H. Fabrication of n-GaN/MDMO-PPV hybrid structures for optoelectronic devices. J. Lumin. 2014, 147, 1–4. [Google Scholar] [CrossRef]
- Modwi, A.; Ali, M.; Taha, K.K.; Ibrahem, M.; El-Khair, H.; Eisa, M.; Elamin, M.; Aldaghri, O.; Alhathlool, R.; Ibnaouf, K. Structural and optical characteristic of chalcone doped ZnO nanoparticles. J. Mater. Sci. Mater. Electron. 2018, 29, 2791–2796. [Google Scholar] [CrossRef]
- Liao, S.-H.; Jhuo, H.-J.; Yeh, P.-N.; Cheng, Y.-S.; Li, Y.-L.; Lee, Y.-H.; Sharma, S.; Chen, S.-A. Single junction inverted polymer solar cell reaching power conversion efficiency 10.31% by employing dual-doped zinc oxide nano-film as cathode interlayer. Sci. Rep. 2014, 4, 6813. [Google Scholar] [CrossRef]
- Dimitrov, S.D.; Schroeder, B.C.; Nielsen, C.B.; Bronstein, H.; Fei, Z.; McCulloch, I.; Heeney, M.; Durrant, J.R. Singlet exciton lifetimes in conjugated polymer films for organic solar cells. Polymers 2016, 8, 14. [Google Scholar] [CrossRef]
- Ibnaouf, K. Photodynamic properties of poly [2-methoxy-5-(3′, 7′-dimethyloctyloxy)-1, 4-phenylenevinylene] under pulsed laser excitation. Opt. Laser Technol. 2020, 130, 106369. [Google Scholar] [CrossRef]
- Quist, P.A.; Sweelssen, J.; Koetse, M.M.; Savenije, T.J.; Siebbeles, L.D. Formation and decay of charge carriers in bulk heterojunctions of MDMO-PPV or P3HT with new n-type conjugated polymers. J. Phys. Chem. C 2007, 111, 4452–4457. [Google Scholar] [CrossRef]
- Rao, M.M.; Su, Y.K.; Huang, T.-S.; Tu, M.-L.; Wu, S.-S.; Huang, C.-Y. Enhanced performance of polymer light emitting devices using zinc oxide nanoparticle with poly (vinylcarbazole). J. Electrochem. Soc. 2010, 157, H832. [Google Scholar]
- Musa, I.; Massuyeau, F.; Faulques, E.; Nguyen, T.-P. Investigations of optical properties of MEH-PPV/ZnO nanocomposites by photoluminescence spectroscopy. Synth. Met. 2012, 162, 1756–1761. [Google Scholar] [CrossRef]
- Lee, C.-W.; Renaud, C.; Hsu, C.-S.; Nguyen, T.-P. Traps and performance of MEH-PPV/CdSe (ZnS) nanocomposite-based organic light-emitting diodes. Nanotechnology 2008, 19, 455202. [Google Scholar] [CrossRef] [PubMed]
- Zhan, X.; Zhu, D. Conjugated polymers for high-efficiency organic photovoltaics. Polym. Chem. 2010, 1, 409–419. [Google Scholar] [CrossRef]
- Beek, W.J.; Wienk, M.M.; Janssen, R.A. Efficient hybrid solar cells from zinc oxide nanoparticles and a conjugated polymer. Adv. Mater. 2004, 16, 1009–1013. [Google Scholar] [CrossRef]
- Dhole, S.G.; Dake, S.A.; Prajapati, T.A.; Helambe, S.N. Effect of ZnO filler on structural and optical properties of polyaniline-ZnO nanocomposites. Procedia Manuf. 2018, 20, 127–134. [Google Scholar] [CrossRef]
- Cho, M.S.; Park, S.Y.; Hwang, J.Y.; Choi, H.J. Synthesis and electrical properties of polymer composites with polyaniline nanoparticles. Mater. Sci. Eng. C 2004, 24, 15–18. [Google Scholar] [CrossRef]
- Aldaghri, O.A.; El-Badry, B.A.; Ali, M.K.M.; Ibnaouf, K.H. Effect of Gamma Irradiation on the Optical Properties of the Conjugated Copolymer B-co-MP. Appl. Sci. 2022, 12, 1606. [Google Scholar] [CrossRef]
- Li, J.; Xie, B.; Xia, K.; Zhao, C.; Li, Y.; Hu, S. Enhanced PL and EL properties of Alq3/nano-TiO2 with the modification of 8-vinyl POSS. Opt. Mater. 2018, 78, 279–284. [Google Scholar] [CrossRef]
- Uthirakumar, P.; Lee, Y.-S.; Suh, E.-K.; Hong, C.-H. Hybrid fluorescent polymer–zinc oxide nanoparticles: Improved efficiency for luminescence conversion LED. J. Lumin. 2008, 128, 287–296. [Google Scholar] [CrossRef]
- Jarka, P.; Tański, T.; Matysiak, W.; Krzemiński, Ł.; Hajduk, B.; Bilewicz, M. Manufacturing and investigation of surface morphology and optical properties of composite thin films reinforced by TiO2, Bi2O3 and SiO2 nanoparticles. Appl. Surf. Sci. 2017, 424, 206–212. [Google Scholar] [CrossRef]
- Shanshool, H.M.; Yahaya, M.; Yunus, W.M.M.; Abdullah, I.Y. Investigation of energy band gap in polymer/ZnO nanocomposites. J. Mater. Sci. Mater. Electron. 2016, 27, 9804–9811. [Google Scholar] [CrossRef]
- El-Zahed, H.; El-Korashy, A.; Rahem, M.A. Effect of heat treatment on some of the optical parameters of Cu9Ge11Te80 films. Vacuum 2002, 68, 19–27. [Google Scholar] [CrossRef]
- Urbach, F. The long-wavelength edge of photographic sensitivity and of the electronic absorption of solids. Phys. Rev. 1953, 92, 1324. [Google Scholar] [CrossRef]
- Elliot, S. The Physics and Chemistry of Solids, 1st, Corrected ed.; John Wiley & Sons Inc.: Chichester, UK, 2005. [Google Scholar]
- Tommalieh, M.; Zihlif, A. Optical properties of polyimide/silica nanocomposite. Phys. B Condens. Matter 2010, 405, 4750–4754. [Google Scholar] [CrossRef]
- Mohammed, M.; Khafagy, R.; Hussien, M.S.; Sakr, G.; Ibrahim, M.A.; Yahia, I.; Zahran, H. Enhancing the structural, optical, electrical, properties and photocatalytic applications of ZnO/PMMA nanocomposite membranes: Towards multifunctional membranes. J. Mater. Sci. Mater. Electron. 2021, 33, 1977–2002. [Google Scholar] [CrossRef]
- Yahia, I.; Farag, A.; Cavas, M.; Yakuphanoglu, F. Effects of stabilizer ratio on the optical constants and optical dispersion parameters of ZnO nano-fiber thin films. Superlattices Microstruct. 2013, 53, 63–75. [Google Scholar] [CrossRef]
- Zyoud, S.H.; Almoadi, A.; AlAbdulaal, T.H.; Alqahtani, M.S.; Harraz, F.A.; Al-Assiri, M.S.; Yahia, I.S.; Zahran, H.Y.; Mohammed, M.I.; Abdel-wahab, M.S. Structural, Optical, and Electrical Investigations of Nd2O3-Doped PVA/PVP Polymeric Composites for Electronic and Optoelectronic Applications. Polymers 2023, 15, 1351. [Google Scholar] [CrossRef]
- Mohan, S.R.; Joshi, M.; Dhami, T.; Awasthi, V.; Shalu, C.; Singh, B.; Singh, V. Charge transport in thin films of MDMO PPV dispersed with lead sulfide nanoparticles. Synth. Met. 2017, 224, 80–85. [Google Scholar] [CrossRef]
- Raj Mohan, S.; Joshi, M.; Shalu, C.; Ghosh, C.; Mukharjee, C.; Kukreja, L. Charge transport properties of MDMO PPV thin films cast in different solvents. J. Polym. Sci. Part B Polym. Phys. 2015, 53, 1431–1439. [Google Scholar] [CrossRef]
- Ortiz-Morales, A.; Ortiz-Lopez, J.; Cruz-Zaragoza, E.; Gómez-Aguilar, R. Thermoluminescence and photoluminescence analyses of MEH-PPV, MDMO-PPV and RU (bpy) 3 gamma-irradiated polymer thin films. Appl. Radiat. Isot. 2015, 102, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Samuel, I.; Rumbles, G.; Collison, C.; Friend, R.; Moratti, S.; Holmes, A. Picosecond time-resolved photoluminescence of PPV derivatives. Synth. Met. 1997, 84, 497–500. [Google Scholar] [CrossRef]
- Cuba, M.; Rathinavalli, U.; Thangaraju, K.; Muralidharan, G. Synthesis and optical properties of ZnO incorporated Tris-(8-hydroxyquinoline) aluminum. J. Lumin. 2014, 153, 188–193. [Google Scholar] [CrossRef]
- Uthirakumar, P.; Suh, E.-K.; Hong, C.-H. Effect of zinc oxide incorporation on the morphology of tris (8-hydroxyquinoline) aluminum/zinc oxide hybrid nanomaterials. Thin Solid Film. 2008, 516, 7299–7305. [Google Scholar] [CrossRef]
- Crupi, I.; Boscarino, S.; Strano, V.; Mirabella, S.; Simone, F.; Terrasi, A. Optimization of ZnO: Al/Ag/ZnO: Al structures for ultra-thin high-performance transparent conductive electrodes. Thin Solid Film. 2012, 520, 4432–4435. [Google Scholar] [CrossRef]
- He, G.; Li, Y.; Liu, J.; Yang, Y. Enhanced electroluminescence using polystyrene as a matrix. Appl. Phys. Lett. 2002, 80, 4247–4249. [Google Scholar] [CrossRef]
- Periyayya, U.; Kang, J.H.; Ryu, J.H.; Hong, C.-H. Synthesis and improved luminescence properties of OLED/ZnO hybrid materials. Vacuum 2011, 86, 254–260. [Google Scholar] [CrossRef]
- Atta, A.; Abdel Reheem, A.; Abdeltwab, E. Ion beam irradiation effects on surface morphology and optical properties of ZnO/PVA composites. Surf. Rev. Lett. 2020, 27, 1950214. [Google Scholar] [CrossRef]
- Quan, S.; Teng, F.; Xu, Z.; Zhang, T.; Qian, L.; Liu, D.; Hou, Y.; Wang, Y. Temperature effects on photoluminescence of poly [2-methoxy-5-(20-ethyl-hexyloxy)-1,4-phenylene vinylene]. Mater. Lett. 2006, 60, 1134–1136. [Google Scholar] [CrossRef]
- Scharsich, C.; Fischer, F.S.; Wilma, K.; Hildner, R.; Ludwigs, S.; Köhler, A. Revealing structure formation in PCPDTBT by optical spectroscopy. J. Polym. Sci. Part B Polym. Phys. 2015, 53, 1416–1430. [Google Scholar] [CrossRef]
- Clark, J.; Silva, C.; Friend, R.H.; Spano, F.C. Role of intermolecular coupling in the photophysics of disordered organic semiconductors: Aggregate emission in regioregular polythiophene. Phys. Rev. Lett. 2007, 98, 206406. [Google Scholar] [CrossRef] [PubMed]
- Saidani, M.; Benfredj, A.; Romdhane, S.; Kouki, F.; Bouchriha, H. Role of intermolecular coupling and electron-nuclear coupling in the photophysics of oligothiophenes. Phys. Rev. B 2012, 86, 165315. [Google Scholar] [CrossRef]
- Kanemoto, K.; Sudo, T.; Akai, I.; Hashimoto, H.; Karasawa, T.; Aso, Y.; Otsubo, T. Intrachain photoluminescence properties of conjugated polymers as revealed by long oligothiophenes and polythiophenes diluted in an inactive solid matrix. Phys. Rev. B 2006, 73, 235203. [Google Scholar] [CrossRef]
- Wise, A.J.; Precit, M.R.; Papp, A.M.; Grey, J.K. Effect of Fullerene Intercalation on the Conformation and Packing of Poly-(2-methoxy-5-(3′-7′-dimethyloctyloxy)-1,4-phenylenevinylene). ACS Appl. Mater. Interfaces 2011, 3, 3011–3019. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Le Rendu, P.; Musa, I.; Yang, S.-H.; Dan, Y.; That, C.T.; Nguyen, T. Investigation of the optical properties of polyfluorene/ZnO nanocomposites. Thin Solid Film. 2011, 519, 3997–4003. [Google Scholar] [CrossRef]
- Chang, R.; Hsu, J.; Fann, W.; Liang, K.; Chang, C.; Hayashi, M.; Yu, J.; Lin, S.; Chang, E.; Chuang, K. Experimental and theoretical investigations of absorption and emission spectra of the light-emitting polymer MEH-PPV in solution. Chem. Phys. Lett. 2000, 317, 142–152. [Google Scholar] [CrossRef]
- Lutich, A.A.; Jiang, G.; Susha, A.S.; Rogach, A.L.; Stefani, F.D.; Feldmann, J. Energy transfer versus charge separation in type-ii hybrid organic–inorganic nanocomposites. Nano Lett. 2009, 9, 2636–2640. [Google Scholar] [CrossRef]
- Quan, S.; Teng, F.; Xu, Z.; Qian, L.; Zhang, T.; Liu, D.; Hou, Y.; Wang, Y.; Xu, X. Temperature dependence of photoluminescence in MEH-PPV blend films. J. Lumin. 2007, 124, 81–84. [Google Scholar] [CrossRef]






| Active Layer | Zinc Oxide (mg) | Polymer (mg) | Toluene (mL) | Thickness (nm) |
|---|---|---|---|---|
| MDMO-PPV: ZnO NPs (0%) | 0 | 40 | 10 | 45 |
| MDMO-PPV: ZnO NPs (5%) | 2 | 38 | 10 | 55 |
| MDMO-PPV: ZnO NPs (10%) | 5 | 36 | 10 | 90 |
| Samples | ||||
|---|---|---|---|---|
| MDMO-PPV | 2.15 | 3.63 | 0.114 | 2.34 |
| MDMO-PPV: ZnO NPs (5%) | 2.06 | 3.82 | 0.091 | 2.31 |
| MDMO-PPV: ZnO NPs (10%) | 2.05 | 3.85 | 0.093 | 2.30 |
| Samples | × 1016 (s−1) | × 10−26 (Kg) | (eV.m. N−1) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MDMO-PPV | 2.163 | 2.050 | 0.055 | 0.548 | 0.016 | 0.11 | 1.9141 (S0) 1.9140 (S1) | 0.0049 (S0) 0.0156 (S1) | 11.974 (S0) 16.940 (S1) | 0.057 | 66.33 |
| MDMO-PPV: ZnO NPs (5%) | 2.165 | 2.060 | 0.070 | 0.570 | 0.016 | 0.11 | 1.9141(S0) 1.9140 (S1) | 0.0056 (S0) 0.0159 (S1) | 12.038 (S0) 16.870 (S1) | 0.060 | 70.40 |
| MDMO-PPV: ZnO NPs (10%) | 2.167 | 2.060 | 0.070 | 0.600 | 0.016 | 0.11 | 1.9141 (S0) 1.9140 (S1) | 0.0056 (S0) 0.0164 (S1) | 12.041 (S0) 16.750 (S1) | 0.061 | 73.70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelaziz, B.B.; Mustapha, N.; Bedja, I.M.; Aldaghri, O.; Idriss, H.; Ibrahem, M.; Ibnaouf, K.H. Spectral Behavior of a Conjugated Polymer MDMO-PPV Doped with ZnO Nanoparticles: Thin Films. Nanomaterials 2023, 13, 2405. https://doi.org/10.3390/nano13172405
Abdelaziz BB, Mustapha N, Bedja IM, Aldaghri O, Idriss H, Ibrahem M, Ibnaouf KH. Spectral Behavior of a Conjugated Polymer MDMO-PPV Doped with ZnO Nanoparticles: Thin Films. Nanomaterials. 2023; 13(17):2405. https://doi.org/10.3390/nano13172405
Chicago/Turabian StyleAbdelaziz, Boutheina Ben, Nazir Mustapha, Idriss M. Bedja, Osamah Aldaghri, Hajo Idriss, Moez Ibrahem, and Khalid H. Ibnaouf. 2023. "Spectral Behavior of a Conjugated Polymer MDMO-PPV Doped with ZnO Nanoparticles: Thin Films" Nanomaterials 13, no. 17: 2405. https://doi.org/10.3390/nano13172405
APA StyleAbdelaziz, B. B., Mustapha, N., Bedja, I. M., Aldaghri, O., Idriss, H., Ibrahem, M., & Ibnaouf, K. H. (2023). Spectral Behavior of a Conjugated Polymer MDMO-PPV Doped with ZnO Nanoparticles: Thin Films. Nanomaterials, 13(17), 2405. https://doi.org/10.3390/nano13172405








