Recent Advances on the Design and Applications of Antimicrobial Nanomaterials
Abstract
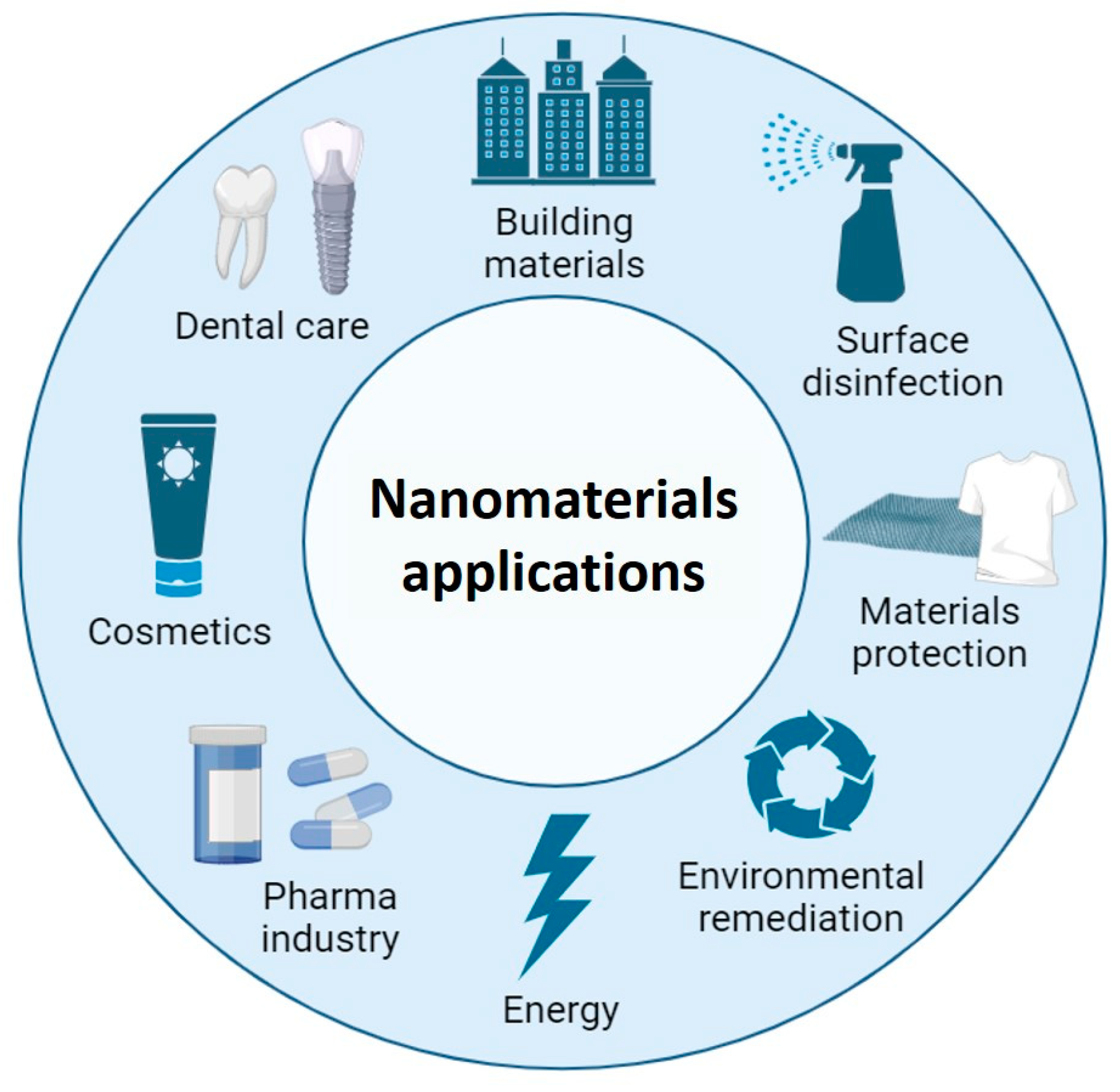
:1. Introduction
2. Design and Synthesis of Different Nanomaterials
2.1. Synthesis of Metal Nanoparticles (Ag, Cu, Zn, Ce)
2.2. Synthesis of Carbon-Based Metal Hybrid Nanomaterials
2.3. Multi-Metallic Hybrid Nanostructured Materials
2.4. Superhydrophobic Coating Materials
3. Antimicrobial Performance of Different Metal Combinations and Nanostructures
3.1. Metal Nanoparticles

3.2. Antimicrobial Mechanism of Carbon-Based Nanomaterials
4. Applications
4.1. Medical Application: Nanomaterials against Multi-Drug-Resistant Bacteria
4.2. Material Protection Application: Nanomaterials as Coating Agents
4.3. Building Materials Application: Nanomaterials as Additives
5. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goutam, S.P.; Saxena, G.; Roy, D.; Yadav, A.K.; Bharagava, R.N. Green Synthesis of Nanoparticles and Their Applications in Water and Wastewater Treatment. In Bioremediation of Industrial Waste for Environmental Safety; Saxena, G., Bharagava, R.N., Eds.; Springer: Singapore, 2020; pp. 349–379. [Google Scholar]
- Merugu, R.; Gothalwal, R.; Velamakanni, R.P.; Velamakanni, R.S.; Chitturi, K.L.; Naz, F. Green Synthesis of Nanoparticles by Plants and Their Renewable Energy Applications. In Green Nano Solution for Bioenergy Production Enhancement; Srivastava, M., Malik, M.A., Mishra, P.K., Eds.; Springer Nature: Singapore, 2022; pp. 225–244. [Google Scholar]
- Nam, N.H.; Luong, N.H. Nanoparticles: Synthesis and Applications. In Materials for Biomedical Engineering; Elsevier: Amsterdam, The Netherlands, 2019; pp. 211–240. [Google Scholar]
- Qing, Y.; Cheng, L.; Li, R.; Liu, G.; Zhang, Y.; Tang, X.; Wang, J.; Liu, H.; Qin, Y. Potential Antibacterial Mechanism of Silver Nanoparticles and the Optimization of Orthopedic Implants by Advanced Modification Technologies. Int. J. Nanomed. 2018, 13, 3311–3327. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Dong, S.; Ashour, A.; Han, B. Antimicrobial Concrete for Smart and Durable Infrastructures: A Review. Constr. Build. Mater. 2020, 260, 120456. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Wang, S.; Wang, Y.; Song, C.; Yao, Q.; Yuan, X.; Xie, J. Gold Nanocluster with AIE: A Novel Photodynamic Antibacterial and Deodorant Molecule. Biomaterials 2022, 288, 121695. [Google Scholar] [CrossRef] [PubMed]
- Abdulsada, F.W.; Hammood, A.S. Characterization of Corrosion and Antibacterial Resistance of Hydroxyapatite/Silver Nano Particles Powder on 2507 Duplex Stainless Steel. Mater. Today Proc. 2021, 42, 2301–2307. [Google Scholar] [CrossRef]
- Makabenta, J.M.V.; Nabawy, A.; Li, C.-H.; Schmidt-Malan, S.; Patel, R.; Rotello, V.M. Nanomaterial-Based Therapeutics for Antibiotic-Resistant Bacterial Infections. Nat. Rev. Microbiol. 2021, 19, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Cai, X.; Ge, J. Enzyme–Metal Nanocomposites for Antibacterial Applications. Particuology 2022, 64, 134–139. [Google Scholar] [CrossRef]
- Weldick, P.J.; Wang, A.; Halbus, A.F.; Paunov, V.N. Emerging Nanotechnologies for Targeting Antimicrobial Resistance. Nanoscale 2022, 14, 4018–4041. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.C.; Wang, A.Z. Nanoparticles and Their Applications in Cell and Molecular Biology. Integr. Biol. 2013, 6, 9–26. [Google Scholar] [CrossRef]
- Sorbiun, M.; Shayegan Mehr, E.; Ramazani, A.; Mashhadi Malekzadeh, A. Biosynthesis of Metallic Nanoparticles Using Plant Extracts and Evaluation of Their Antibacterial Properties. Nanochem. Res. 2018, 3, 1–16. [Google Scholar] [CrossRef]
- Gold, K.; Slay, B.; Knackstedt, M.; Gaharwar, A.K. Antimicrobial Activity of Metal and Metal-Oxide Based Nanoparticles. Adv. Ther. 2018, 1, 1700033. [Google Scholar] [CrossRef]
- Mathews, S.; Hans, M.; Mücklich, F.; Solioz, M. Contact Killing of Bacteria on Copper Is Suppressed If Bacterial-Metal Contact Is Prevented and Is Induced on Iron by Copper Ions. Appl. Environ. Microbiol. 2013, 79, 2605–2611. [Google Scholar] [CrossRef]
- Losada-Garcia, N.; Vazquez-Calvo, A.; Alcami, A.; Palomo, J.M. Preparation of Highly Stable and Cost-Efficient Antiviral Materials for Reducing Infections and Avoiding the Transmission of Viruses Such as SARS-CoV-2. ACS Appl. Mater. Interfaces 2023, 15, 22580–22589. [Google Scholar] [CrossRef] [PubMed]
- Meister, T.L.; Fortmann, J.; Breisch, M.; Sengstock, C.; Steinmann, E.; Köller, M.; Pfaender, S.; Ludwig, A. Nanoscale Copper and Silver Thin Film Systems Display Differences in Antiviral and Antibacterial Properties. Sci. Rep. 2022, 12, 7193. [Google Scholar] [CrossRef] [PubMed]
- Baig, N.; Kammakakam, I.; Wail Falath, W. Nanomaterials: A review of synthesis methods, properties, recent progress, and challenges. Mater. Adv. 2021, 2, 1821–1871. [Google Scholar] [CrossRef]
- Liu, S.; Zheng, J.; Hao, L.; Yegin, Y.; Bae, M.; Ulu-gun, B.; Taylor, T.M.; Scholar, E.A.; Cisneros-Zevallos, L.; Kyun Oh, J.K.; et al. Dual-Functional, Superhydrophobic Coatings with Bacterial Anticontact and Antimicrobial Characteristics. ACS Appl. Mat. Interfaces 2020, 12, 21311–21321. [Google Scholar] [CrossRef]
- Sun, Z.; Ma, L.; Sun, X.; Sloan, A.J.; O’Brien-Simpson, N.M.; Li, W. The overview of anti-microbial peptide-coated implants against oral bacterial infections. Aggregate 2023, 4, e309. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Umar, K.; Ibrahim, M.N.M. Silver Nanoparticles: Various Methods of Synthesis, Size Affecting Factors and Their Potential Applications–a Review. Appl. Nanosci. 2020, 10, 1369–1378. [Google Scholar] [CrossRef]
- Silva, L.P.; Silveira, A.P.; Bonatto, C.C.; Reis, I.G.; Milreu, P.V. Silver Nanoparticles as Antimicrobial Agents. In Nanostructures for Antimicrobial Therapy; Ficai, A., Grumezescu, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 577–596. [Google Scholar]
- Nie, P.; Zhao, Y.; Xu, H. Synthesis, Applications, Toxicity and Toxicity Mechanisms of Silver Nanoparticles: A Review. Ecotoxicol. Environ. Saf. 2023, 253, 114636. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Liu, Z.-G.; Shen, W.; Gurunathan, S. Silver Nanoparticles: Synthesis, Characterization, Properties, Applications, and Therapeutic Approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef]
- Krutyakov, Y.A.; Kudrinskiy, A.A.; Olenin, A.Y.; Lisichkin, G.V. Synthesis and Properties of Silver Nanoparticles: Advances and Prospects. Russ. Chem. Rev. 2008, 77, 233–257. [Google Scholar] [CrossRef]
- Ltaief, S.; Jabli, M.; Ben Abdessalem, S. Immobilization of Copper Oxide Nanoparticles onto Chitosan Biopolymer: Application to the Oxidative Degradation of Naphthol Blue Black. Carbohydr. Polym. 2021, 261, 117908. [Google Scholar] [CrossRef] [PubMed]
- Palomo, J.M. Nanobiohybrids: A New Concept for Metal Nanoparticles Synthesis. Chem. Commun. 2019, 55, 9583–9589. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Tejedor, D.; Benavente, R.; Palomo, J.M. Iron Nanostructured Catalysts: Design and Applications. Catal. Sci. Technol. 2018, 8, 1754–1776. [Google Scholar] [CrossRef]
- Filice, M.; Losada-Garcia, N.; Perez-Rizquez, C.; Marciello, M.; Morales, M.d.P.; Palomo, J.M. Palladium-Nanoparticles Biohybrids in Applied Chemistry. Appl. Nano 2020, 2, 1–13. [Google Scholar] [CrossRef]
- Zhang, L.; Han, F. Protein Coated Gold Nanoparticles as Template for the Directed Synthesis of Highly Fluorescent Gold Nanoclusters. Nanotechnology 2018, 29, 165702. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Lodeiro, A.; Djafari, J.; Fernández-Lodeiro, J.; Duarte, M.P.; Muchagato Mauricio, E.; Capelo-Martínez, J.L.; Lodeiro, C. Synthesis of Mesoporous Silica Coated Gold Nanorods Loaded with Methylene Blue and Its Potentials in Antibacterial Applications. Nanomaterials 2021, 11, 1338. [Google Scholar] [CrossRef]
- Das, S.K.; Khan, M.M.R.; Guha, A.K.; Naskar, N. Bio-Inspired Fabrication of Silver Nanoparticles on Nanostructured Silica: Characterization and Application as a Highly Efficient Hydrogenation Catalyst. Green Chem. 2013, 15, 2548. [Google Scholar] [CrossRef]
- Losada-Garcia, N.; Carranza, J.; Palomo, J.M. Graphene-TLL-Cu2ONPs Hybrid as Highly Efficient Catalyst for Degradation of Organic Compounds. Nanomaterials 2023, 13, 449. [Google Scholar] [CrossRef]
- Chen, Y.; Egan, G.C.; Wan, J.; Zhu, S.; Jacob, R.J.; Zhou, W.; Dai, J.; Wang, Y.; Danner, V.A.; Yao, Y.; et al. Ultra-Fast Self-Assembly and Stabilization of Reactive Nanoparticles in Reduced Graphene Oxide Films. Nat. Commun. 2016, 7, 12332. [Google Scholar] [CrossRef]
- Zhu, L.; Xu, Q.-Q.; Guo, J.; Niu, S.-L.; Lu, B.-N.; Yin, J.-Z. In Situ Growth of Ag Nanoparticles on Pristine Graphene and Their Applications in Conductive Ink. J. Nanopart. Res. 2023, 25, 98. [Google Scholar] [CrossRef]
- Moosavy, M.-H.; de la Guardia, M.; Mokhtarzadeh, A.; Khatibi, S.A.; Hosseinzadeh, N.; Hajipour, N. Green Synthesis, Characterization, and Biological Evaluation of Gold and Silver Nanoparticles Using Mentha Spicata Essential Oil. Sci. Rep. 2023, 13, 7230. [Google Scholar] [CrossRef] [PubMed]
- Pavithran, S.; Pappuswamy, M.; Annadurai, Y.; Armugam, V.A.; Periyaswamy, T. Green Synthesis of Copper Nanoparticles, Characterization and Their Applications. J. Appl. Life Sci. Int. 2020, 23, 10–24. [Google Scholar] [CrossRef]
- Ahmed, A.; Usman, M.; Ji, Z.; Rafiq, M.; Yu, B.; Shen, Y.; Cong, H. Nature-Inspired Biogenic Synthesis of Silver Nanoparticles for Antibacterial Applications. Mater. Today Chem. 2023, 27, 101339. [Google Scholar] [CrossRef]
- Beyene, H.D.; Werkneh, A.A.; Bezabh, H.K.; Ambaye, T.G. Synthesis Paradigm and Applications of Silver Nanoparticles (AgNPs), a Review. Sustain. Mater. Technol. 2017, 13, 18–23. [Google Scholar] [CrossRef]
- Elsupikhe, R.F.; Shameli, K.; Ahmad, M.B.; Ibrahim, N.A.; Zainudin, N. Green Sonochemical Synthesis of Silver Nanoparticles at Varying Concentrations of κ-Carrageenan. Nanoscale Res. Lett. 2015, 10, 302. [Google Scholar] [CrossRef]
- Sánchez-López, E.; Gomes, D.; Esteruelas, G.; Bonilla, L.; Lopez-Machado, A.L.; Galindo, R.; Cano, A.; Espina, M.; Ettcheto, M.; Camins, A.; et al. Metal-Based Nanoparticles as Antimicrobial Agents: An Overview. Nanomaterials 2020, 10, 292. [Google Scholar] [CrossRef]
- Gracia-Pinilla, M.; Martínez, E.; Vidaurri, G.S.; Pérez-Tijerina, E. Deposition of Size-Selected Cu Nanoparticles by Inert Gas Condensation. Nanoscale Res. Lett. 2010, 5, 180. [Google Scholar] [CrossRef]
- Mishra, G.; Verma, S.K.; Singh, D.; Yadawa, P.K.; Yadav, R.R. Synthesis and Ultrasonic Characterization of Cu/PVP Nanoparticles-Polymer Suspensions. Open J. Acoust. 2011, 01, 9–14. [Google Scholar] [CrossRef]
- Pham, L.Q.; Sohn, J.H.; Kim, C.W.; Park, J.H.; Kang, H.S.; Lee, B.C.; Kang, Y.S. Copper Nanoparticles Incorporated with Conducting Polymer: Effects of Copper Concentration and Surfactants on the Stability and Conductivity. J. Colloid Interface Sci. 2012, 365, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Parveen, F.; Sannakki, B.; Mandke, M.V.; Pathan, H.M. Copper Nanoparticles: Synthesis Methods and Its Light Harvesting Performance. Sol. Energy Mater. Sol. Cells 2016, 144, 371–382. [Google Scholar] [CrossRef]
- Bhagat, M.; Anand, R.; Sharma, P.; Rajput, P.; Sharma, N.; Singh, K. Review—Multifunctional Copper Nanoparticles: Synthesis and Applications. ECS J. Solid State Sci. Technol. 2021, 10, 063011. [Google Scholar] [CrossRef]
- Benassai, E.; Del Bubba, M.; Ancillotti, C.; Colzi, I.; Gonnelli, C.; Calisi, N.; Salvatici, M.C.; Casalone, E.; Ristori, S. Green and Cost-Effective Synthesis of Copper Nanoparticles by Extracts of Non-Edible and Waste Plant Materials from Vaccinium Species: Characterization and Antimicrobial Activity. Mater. Sci. Eng. C 2021, 119, 111453. [Google Scholar] [CrossRef] [PubMed]
- Losada-García, N.; Rodríguez-Otero, A.; Palomo, J.M. Tailorable Synthesis of Heterogeneous Enzyme–Copper Nanobiohybrids and Their Application in the Selective Oxidation of Benzene to Phenol. Catal. Sci. Technol. 2020, 10, 196–206. [Google Scholar] [CrossRef]
- Ortega-Nieto, C.; Losada-Garcia, N.; Pessela, B.C.; Domingo-Calap, P.; Palomo, J.M. Design and Synthesis of Copper Nanobiomaterials with Antimicrobial Properties. ACS Bio Med Chem Au 2023, 3, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Selvamani, V.; Zareei, A.; Elkashif, A.; Maruthamuthu, M.K.; Chittiboyina, S.; Delisi, D.; Li, Z.; Cai, L.; Pol, V.G.; Seleem, M.N.; et al. Hierarchical Micro/Mesoporous Copper Structure with Enhanced Antimicrobial Property via Laser Surface Texturing. Adv. Mater. Interfaces 2020, 7, 1901890. [Google Scholar] [CrossRef]
- Fan, C.; Ming, S.; Chen, Z.; Pang, L.; Guo, W.; Dong, C.; Liu, P.; Li, T. Cold Start Wetting Effect on the Catalytic Property and Hydrothermal Stability of a Cu-SSZ-13 Catalyst for NH3-SCR. Ind. Eng. Chem. Res. 2020, 59, 12304–12312. [Google Scholar] [CrossRef]
- Hlinka, J.; Fogarassy, Z.; Cziráki, Á.; Weltsch, Z. Wetting Properties, Recrystallization Phenomena and Interfacial Reactions between Laser Treated Cu Substrate and SAC305 Solder. Appl. Surf. Sci. 2020, 501, 144127. [Google Scholar] [CrossRef]
- Shakibaie, M.; Khalaf, A.K.; Rashidipour, M.; Mahmoudvand, H. Effects of Green Synthesized Zinc Nanoparticles Alone and along with Albendazole against Hydatid Cyst Protoscoleces. Ann. Med. Surg. 2022, 78, 103746. [Google Scholar] [CrossRef]
- Rajakumar, G.; Thiruvengadam, M.; Mydhili, G.; Gomathi, T.; Chung, I.-M. Green Approach for Synthesis of Zinc Oxide Nanoparticles from Andrographis Paniculata Leaf Extract and Evaluation of Their Antioxidant, Anti-Diabetic, and Anti-Inflammatory Activities. Bioprocess Biosyst. Eng. 2018, 41, 21–30. [Google Scholar] [CrossRef]
- Pop, O.L.; Mesaros, A.; Vodnar, D.C.; Suharoschi, R.; Tăbăran, F.; Magerușan, L.; Tódor, I.S.; Diaconeasa, Z.; Balint, A.; Ciontea, L.; et al. Cerium Oxide Nanoparticles and Their Efficient Antibacterial Application In Vitro against Gram-Positive and Gram-Negative Pathogens. Nanomaterials 2020, 10, 1614. [Google Scholar] [CrossRef]
- Algethami, J.S.; Hassan, M.S.; Amna, T.; Sheikh, F.A.; Alhamami, M.A.M.; Seliem, A.F.; Faisal, M.; Kim, H.Y. Nanotextured CeO2−SnO2 Composite: Efficient Photocatalytic, Antibacterial, and Energy Storage Fibers. Nanomaterials 2023, 13, 1001. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Choe, Y.-E.; Shin, S.-J.; Park, J.-H.; Dashnyam, K.; Kim, H.S.; Jun, S.-K.; Knowles, J.C.; Kim, H.-W.; Lee, J.-H.; et al. Photocatalytic Effect-Assisted Antimicrobial Activities of Acrylic Resin Incorporating Zinc Oxide Nanoflakes. Biomat. Adv. 2022, 139, 213025. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.; Li, T.; Wang, D.; Yi, S.; Li, J.; Li, X. Recent Advances in Carbon-Based Nanomaterials for Combating Bacterial Biofilm-Associated Infections. J. Hazard. Mater. 2022, 431, 128597. [Google Scholar] [CrossRef]
- Speranza, G. Carbon Nanomaterials: Synthesis, Functionalization and Sensing Applications. Nanomaterials 2021, 11, 967. [Google Scholar] [CrossRef]
- Lertcumfu, N.; Jaita, P.; Thammarong, S.; Lamkhao, S.; Tandorn, S.; Randorn, C.; Tunkasiri, T.; Rujijanagul, G. Influence of Graphene Oxide Additive on Physical, Microstructure, Adsorption, and Photocatalytic Properties of Calcined Kaolinite-Based Geopolymer Ceramic Composites. Colloids Surf. A Physicochem. Eng. Asp. 2020, 602, 125080. [Google Scholar] [CrossRef]
- Prodan, D.; Moldovan, M.; Furtos, G.; Saroși, C.; Filip, M.; Perhaița, I.; Carpa, R.; Popa, M.; Cuc, S.; Varvara, S.; et al. Synthesis and Characterization of Some Graphene Oxide Powders Used as Additives in Hydraulic Mortars. Appl. Sci. 2021, 11, 11330. [Google Scholar] [CrossRef]
- Lee, C.Y.; Bae, J.H.; Kim, T.Y.; Chang, S.H.; Kim, S.Y. Using Silane-Functionalized Graphene Oxides for Enhancing the Interfacial Bonding Strength of Carbon/Epoxy Composites. Compos. Part. A Appl. Sci. Manuf. 2015, 75, 11–17. [Google Scholar] [CrossRef]
- Vuppaladadium, S.S.R.; Agarwal, T.; Kulanthaivel, S.; Mohanty, B.; Barik, C.S.; Maiti, T.K.; Pal, S.; Pal, K.; Banerjee, I. Silanization Improves Biocompatibility of Graphene Oxide. Mat. Sci. Eng. C 2020, 110, 110647. [Google Scholar] [CrossRef]
- Han, F.; Lv, S.; Li, Z.; Jin, L.; Fan, B.; Zhang, J.; Zhang, R.; Zhang, X.; Han, L.; Li, J. Triple-Synergistic 2D Material-Based Dual-Delivery Antibiotic Platform. NPG Asia Mater. 2020, 12, 15. [Google Scholar] [CrossRef]
- Seo, Y.; Hwang, J.; Lee, E.; Kim, Y.J.; Lee, K.; Park, C.; Choi, Y.; Jeon, H.; Choi, J. Engineering Copper Nanoparticles Synthesized on the Surface of Carbon Nanotubes for Anti-Microbial and Anti-Biofilm Applications. Nanoscale 2018, 10, 15529–15544. [Google Scholar] [CrossRef]
- Li, H.; Zou, Y.; Jiang, J. Synthesis of Ag@CuO Nanohybrids and Their Photo-Enhanced Bactericidal Effect through Concerted Ag Ion Release and Reactive Oxygen Species Generation. Dalton Trans. 2020, 49, 9274–9281. [Google Scholar] [CrossRef]
- Cheng, J.; Tu, W.; Ang, E.H.; Aizudin, M.; Yang, F.; Zhou, X.; Yu, D.; Li, F.; Guo, Z.; Song, Y. Achieving Reinforced Broad-Spectrum and Sustained Antimicrobial Efficacy by Nickel-Doping AlOOH Nanoflower Accommodated with Uniform Silver Nanospecies. Colloids Surf. A Physicochem. Eng. Asp. 2022, 641, 128488. [Google Scholar] [CrossRef]
- Maruthapandi, M.; Saravanan, A.; Luong, J.H.T.; Gedanken, A. Antimicrobial Properties of Polyaniline and Polypyrrole Decorated with Zinc-Doped Copper Oxide Microparticles. Polymers 2020, 12, 1286. [Google Scholar] [CrossRef] [PubMed]
- Pormohammad, A.; Turner, R.J. Silver Antibacterial Synergism Activities with Eight Other Metal(Loid)-Based Antimicrobials against Escherichia Coli, Pseudomonas Aeruginosa, and Staphylococcus Aureus. Antibiotics 2020, 9, 853. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, L.; Zheng, H.; Meng, F.; Wang, F. Superhydrophobic, corrosion resistance, and antibacterial coating with delayed release of Ag ions. Compos. Commun. 2022, 31, 101134. [Google Scholar] [CrossRef]
- Wang, L.; Hu, C.; Shao, L. The Antimicrobial Activity of Nanoparticles: Present Situation and Prospects for the Future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef]
- Makvandi, P.; Gu, J.T.; Zare, E.N.; Ashtari, B.; Moeini, A.; Tay, F.R.; Niu, L. Polymeric and Inorganic Nanoscopical Antimicrobial Fillers in Dentistry. Acta Biomater. 2020, 101, 69–101. [Google Scholar] [CrossRef]
- Godoy-Gallardo, M.; Eckhard, U.; Delgado, L.M.; de Roo Puente, Y.J.D.; Hoyos-Nogués, M.; Gil, F.J.; Perez, R.A. Antibacterial Approaches in Tissue Engineering Using Metal Ions and Nanoparticles: From Mechanisms to Applications. Bioact. Mater. 2021, 6, 4470–4490. [Google Scholar] [CrossRef]
- Xin, Q.; Shah, H.; Nawaz, A.; Xie, W.; Akram, M.Z.; Batool, A.; Tian, L.; Jan, S.U.; Boddula, R.; Guo, B.; et al. Antibacterial Carbon-Based Nanomaterials. Adv. Mat. 2019, 31, 1804838. [Google Scholar] [CrossRef]
- Giraud, L.; Tourrette, A.; Flahaut, E. Carbon Nanomaterials-Based Polymer-Matrix Nanocomposites for Antimicrobial Applications: A Review. Carbon 2021, 182, 463–483. [Google Scholar] [CrossRef]
- Mohammed, H.; Kumar, A.; Bekyarova, E.; Al-Hadeethi, Y.; Zhang, X.; Chen, M.; Ansari, M.S.; Cochis, A.; Rimondini, L. Antimicrobial Mechanisms and Effectiveness of Graphene and Graphene-Functionalized Biomaterials. A Scope Review. Front. Bioeng. Biotechnol. 2020, 8, 498689. [Google Scholar] [CrossRef] [PubMed]
- Mangadlao, J.D.; Santos, C.M.; Felipe, M.J.L.; de Leon, A.C.C.; Rodrigues, D.F.; Advincula, R.C. On the Antibacterial Mechanism of Graphene Oxide (GO) Langmuir–Blodgett Films. Chem. Commun. 2015, 51, 2886–2889. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.; Babu, R.P.; Zhao, W.; Johnson, C.M.; Hedström, P.; Odnevall, I.; Leygraf, C. High-Resolution Microscopical Studies of Contact Killing Mechanisms on Copper-Based Surfaces. ACS Appl. Mater. Interfaces 2021, 13, 49402–49413. [Google Scholar] [CrossRef]
- Kadiyala, U.; Kotov, N.A.; VanEpps, J.S. Antibacterial Metal Oxide Nanoparticles: Challenges in Interpreting the Literature. Curr. Pharm. Des. 2018, 24, 896–903. [Google Scholar] [CrossRef]
- Vorobyev, S.A.; Novikova, G.V.; Demina, A.V.; Shidlovskiy, I.P.; Volochaev, M.N. Synthesis and Synergistic Effect of Antibacterial Composites Based on Concentrated Hydrosols of Silver Nanoparticles Combined with Cephalosporins Antibiotics. Inorg. Chem. Comm. 2022, 144, 109862. [Google Scholar] [CrossRef]
- Hu, Y.; Feng, Q.; Zeng, H.; Banat, I.M.; Si, Y.; Huang, P.; Li, X.; Sun, S.; Dong, H.; She, Y.; et al. Corrosion Inhibition of Sulphate-Reducing Bacterial by Ag/Cu Bimetallic Nanoparticles Synthesised from Ginger Extract. J. Clean. Prod. 2022, 377, 134204. [Google Scholar] [CrossRef]
- Rasheed, P.A.; Jabbar, K.A.; Mackey, H.R.; Mahmoud, K.A. Recent Advancements of Nanomaterials as Coatings and Biocides for the Inhibition of Sulfate Reducing Bacteria Induced Corrosion. Curr. Opin. Chem. Eng. 2019, 25, 35–42. [Google Scholar] [CrossRef]
- Zhang, S.; Fang, K.; Liu, X.; Cheng, M.; Liu, D.; Qiao, X.; Wang, J. Polymethylhydrosiloxane and ZIF-8/Color Nanoparticles Enhanced the UV-Resistance, Antibacterial and Hydrophobicity Performance of Cotton Fabrics. Prog. Org. Coat. 2023, 182, 107702. [Google Scholar] [CrossRef]
- Jamshidinia, N.; Mohammadipanah, F. Nanomaterial-Augmented Formulation of Disinfectants and Antiseptics in Controlling SARS-CoV-2. Food Environ. Virol. 2022, 14, 105–119. [Google Scholar] [CrossRef]
- Garcia, I.M.; Balhaddad, A.A.; Ibrahim, M.S.; Weir, M.D.; Xu, H.H.K.; Collares, F.M.; Melo, M.A.S. Antibacterial Response of Oral Microcosm Biofilm to Nano-Zinc Oxide in Adhesive Resin. Dent. Mater. 2021, 37, e182–e193. [Google Scholar] [CrossRef]
- Li, X.; Li, B.; Liu, R.; Dong, Y.; Zhao, Y.; Wu, Y. Development of PH-Responsive Nanocomposites with Remarkably Synergistic Antibiofilm Activities Based on Ultrasmall Silver Nanoparticles in Combination with Aminoglycoside Antibiotics. Colloids Surf. B 2021, 208, 112112. [Google Scholar] [CrossRef] [PubMed]
- Sacher, E. A Pragmatic Perspective of the Initial Stages of the Contact Killing of Bacteria on Copper-Containing Surfaces. Appl. Microbiol. 2022, 2, 449–452. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, X.X. Small Colony Variants Are More Susceptible to Copper-Mediated Contact Killing for Pseudomonas Aeruginosa and Staphylococcus Aureus. J. Med. Microbiol. 2016, 65, 1143–1151. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Lee, J.Y.; Yu, M.Y. Influence of Iron Oxide Pigments on the Properties of Concrete Interlocking Blocks. Cem. Concr. Res. 2003, 33, 1889–1896. [Google Scholar] [CrossRef]
- Papadaki, D.; Kiriakidis, G.; Tsoutsos, T. Applications of Nanotechnology in Construction Industry. In Fundamentals of Nanoparticles; Barhoum, A., Hamdy Makhlouf, A.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 343–370. [Google Scholar]
- Jindal, B.B.; Sharma, R. The Effect of Nanomaterials on Properties of Geopolymers Derived from Industrial By-Products: A State-of-the-Art Review. Constr. Build. Mater. 2020, 252, 119028. [Google Scholar] [CrossRef]
- Zidi, Z.; Ltifi, M.; Ayadi, Z.B.; Mir, L.E.L.; Nóvoa, X.R. Effect of Nano-ZnO on Mechanical and Thermal Properties of Geopolymer. J. Asian Ceram. Soc. 2020, 8, 1–9. [Google Scholar] [CrossRef]
- Nawaz, M.; Heitor, A.; Sivakumar, M. Geopolymers in Construction—Recent Developments. Constr. Build. Mater. 2020, 260, 120472. [Google Scholar] [CrossRef]
- Zhong, L.; Liu, H.; Samal, M.; Yun, K. Synthesis of ZnO Nanoparticles-Decorated Spindle-Shaped Graphene Oxide for Application in Synergistic Antibacterial Activity. J. Photochem. Photobiol. B 2018, 183, 293–301. [Google Scholar] [CrossRef]
- Gutiérrez, R.M.-d.; Villaquirán-Caicedo, M.; Ramírez-Benavides, S.; Astudillo, M.; Mejía, D. Evaluation of the Antibacterial Activity of a Geopolymer Mortar Based on Metakaolin Supplemented with TiO2 and CuO Particles Using Glass Waste as Fine Aggregate. Coatings 2020, 10, 157. [Google Scholar] [CrossRef]
- Singh, N.B.; Saxena, S.K.; Kumar, M. Effect of Nanomaterials on the Properties of Geopolymer Mortars and Concrete. Mater. Today Proc. 2018, 5, 9035–9040. [Google Scholar] [CrossRef]
- Giacobello, F.; Ielo, I.; Belhamdi, H.; Plutino, M.R. Geopolymers and Functionalization Strategies for the Development of Sustainable Materials in Construction Industry and Cultural Heritage Applications: A Review. Materials 2022, 15, 1725. [Google Scholar] [CrossRef] [PubMed]
- Haile, T.; Nakhla, G. The Inhibitory Effect of Antimicrobial Zeolite on the Biofilm of Acidithiobacillus Thiooxidans. Biodegradation 2010, 21, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Haile, T.; Nakhla, G. Inhibition of Microbial Concrete Corrosion by Acidithiobacillus Thiooxidans with Functionalised Zeolite-A Coating. Biofouling 2009, 25, 1–12. [Google Scholar] [CrossRef]
- Haile, T.; Nakhla, G.; Allouche, E. Evaluation of the Resistance of Mortars Coated with Silver Bearing Zeolite to Bacterial-Induced Corrosion. Corros. Sci. 2008, 50, 713–720. [Google Scholar] [CrossRef]
- Singh, V.P.; Sandeep, K.; Kushwaha, H.S.; Powar, S.; Vaish, R. Photocatalytic, Hydrophobic and Antimicrobial Characteristics of ZnO Nano Needle Embedded Cement Composites. Constr. Build. Mater. 2018, 158, 285–294. [Google Scholar] [CrossRef]
- Klapiszewska, I.; Ławniczak, Ł.; Balicki, S.; Gapiński, B.; Wieczorowski, M.; Wilk, K.A.; Jesionowski, T.; Klapiszewski, Ł.; Ślosarczyk, A. Influence of Zinc Oxide Particles Dispersion on the Functional and Antimicrobial Properties of Cementitious Composites. J. Mater. Res. Technol. 2023, 24, 2239–2264. [Google Scholar] [CrossRef]
















Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortega-Nieto, C.; Losada-Garcia, N.; Prodan, D.; Furtos, G.; Palomo, J.M. Recent Advances on the Design and Applications of Antimicrobial Nanomaterials. Nanomaterials 2023, 13, 2406. https://doi.org/10.3390/nano13172406
Ortega-Nieto C, Losada-Garcia N, Prodan D, Furtos G, Palomo JM. Recent Advances on the Design and Applications of Antimicrobial Nanomaterials. Nanomaterials. 2023; 13(17):2406. https://doi.org/10.3390/nano13172406
Chicago/Turabian StyleOrtega-Nieto, Clara, Noelia Losada-Garcia, Doina Prodan, Gabriel Furtos, and Jose M. Palomo. 2023. "Recent Advances on the Design and Applications of Antimicrobial Nanomaterials" Nanomaterials 13, no. 17: 2406. https://doi.org/10.3390/nano13172406






