All-in-One Photoactivated Inhibition of Butyrylcholinesterase Combined with Luminescence as an Activation and Localization Indicator: Carbon Quantum Dots@Phosphonate Hybrids
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Transmission Electron Microscopy
2.2.2. X-ray Photoelectron Spectroscopy
2.2.3. Dynamic Light Scattering (DLS)
2.2.4. NMR
2.2.5. Absorption Spectroscopy
2.2.6. Photoluminescence Spectroscopy
2.2.7. FTIR Absorption Spectroscopy
2.2.8. BChE Activity Measurements
2.2.9. Laser Irradiation of the Samples
2.2.10. Testing of CQD@PhAM Hybrids Functionality on Biological Models
3. Results
3.1. Phosphonates Synthesis
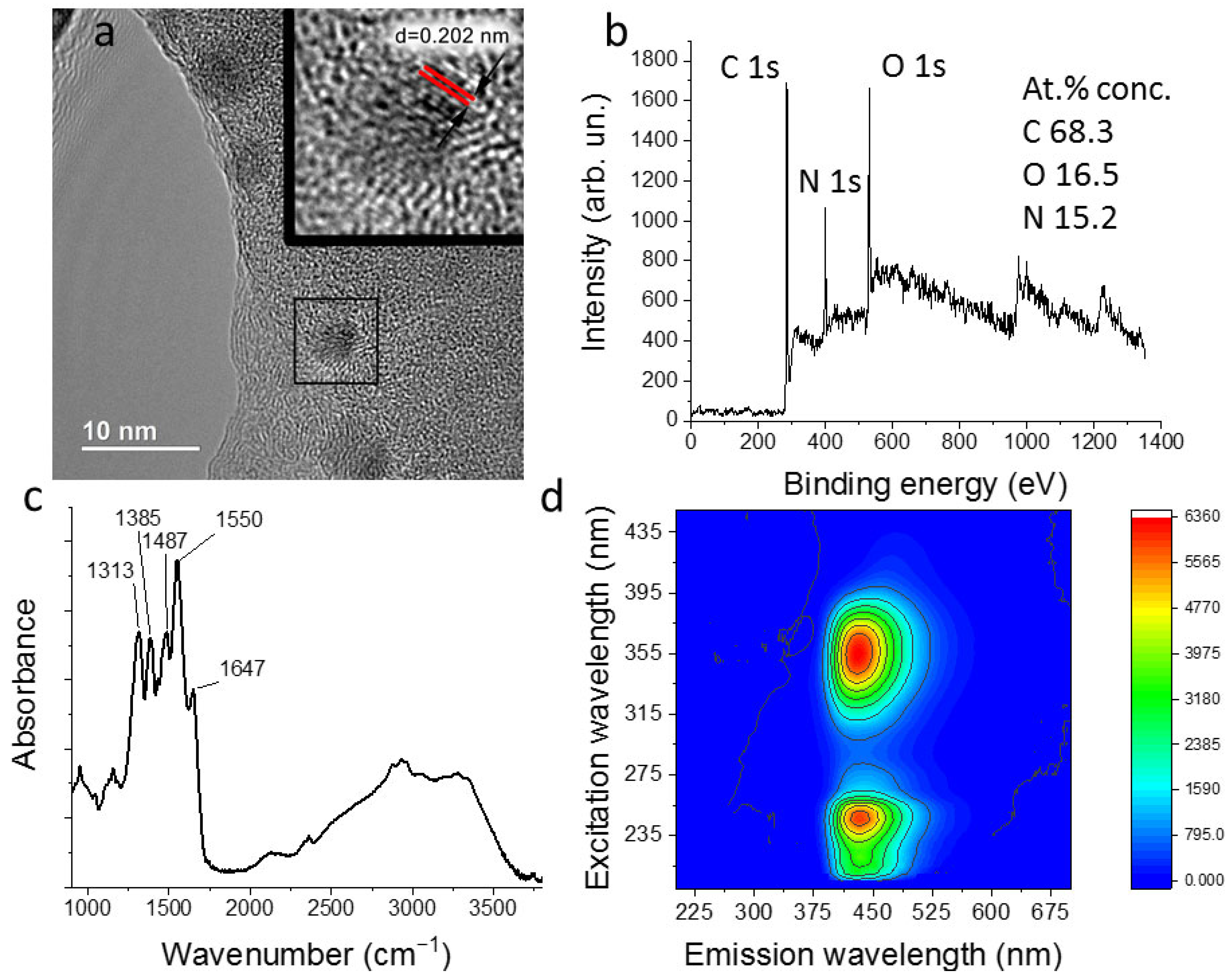
3.2. Carbon Quantum Dots Synthesis and Characterization
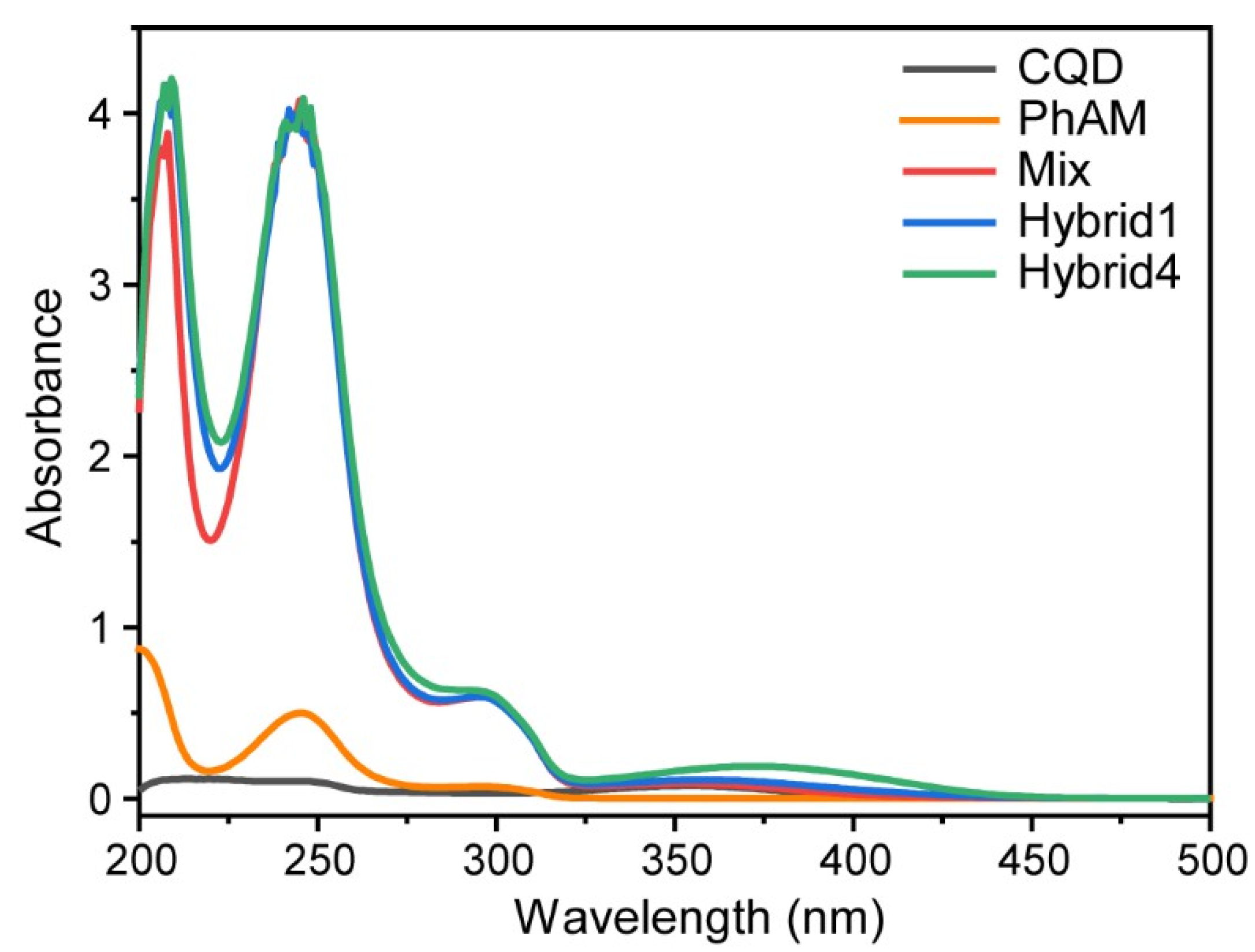
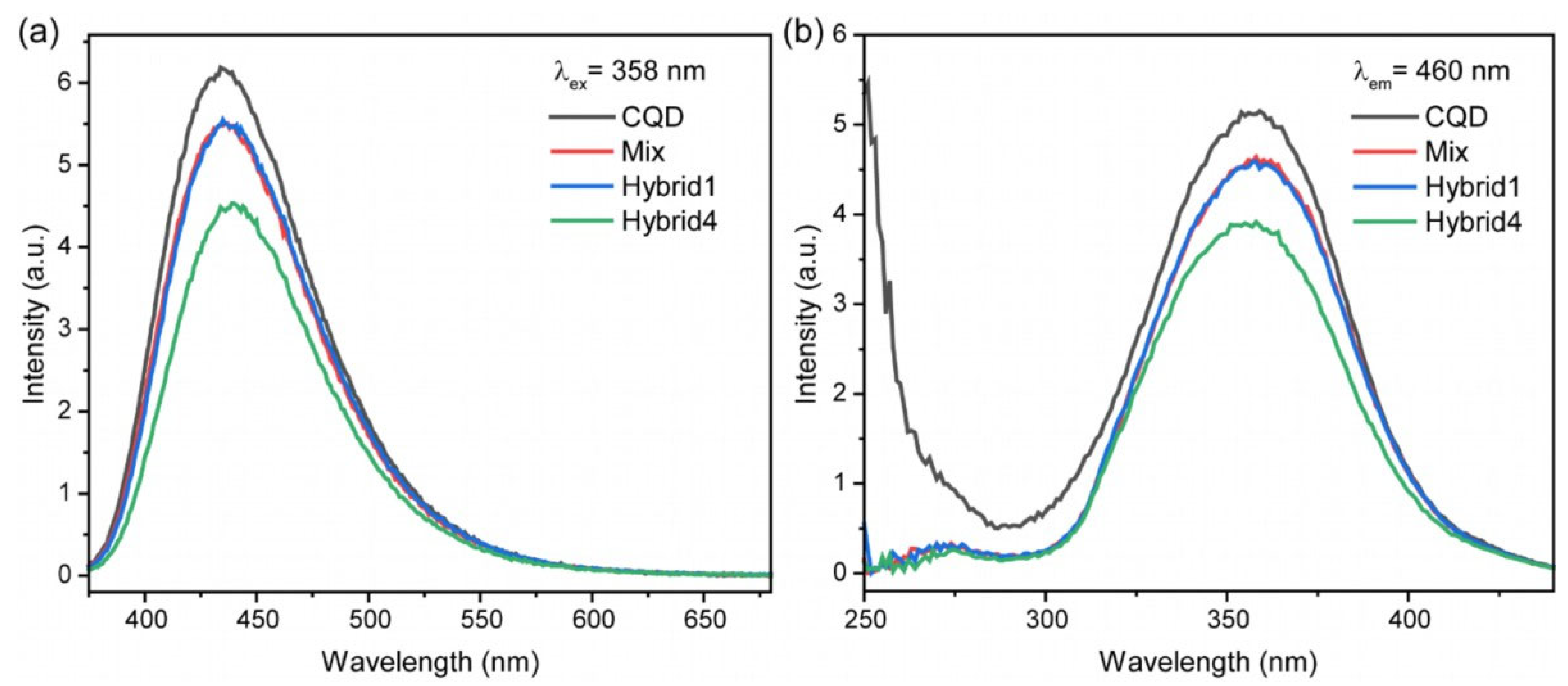
3.3. Phosphonate and Carbon Quantum Dots Hybrids
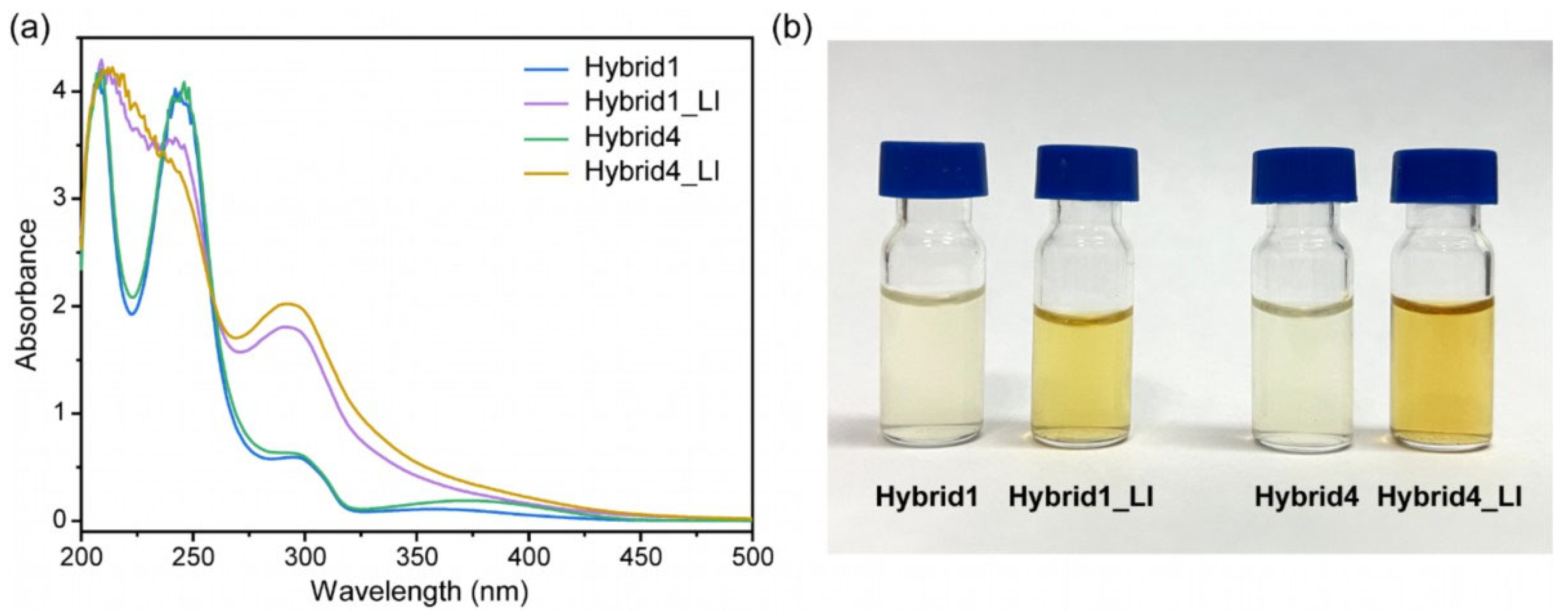
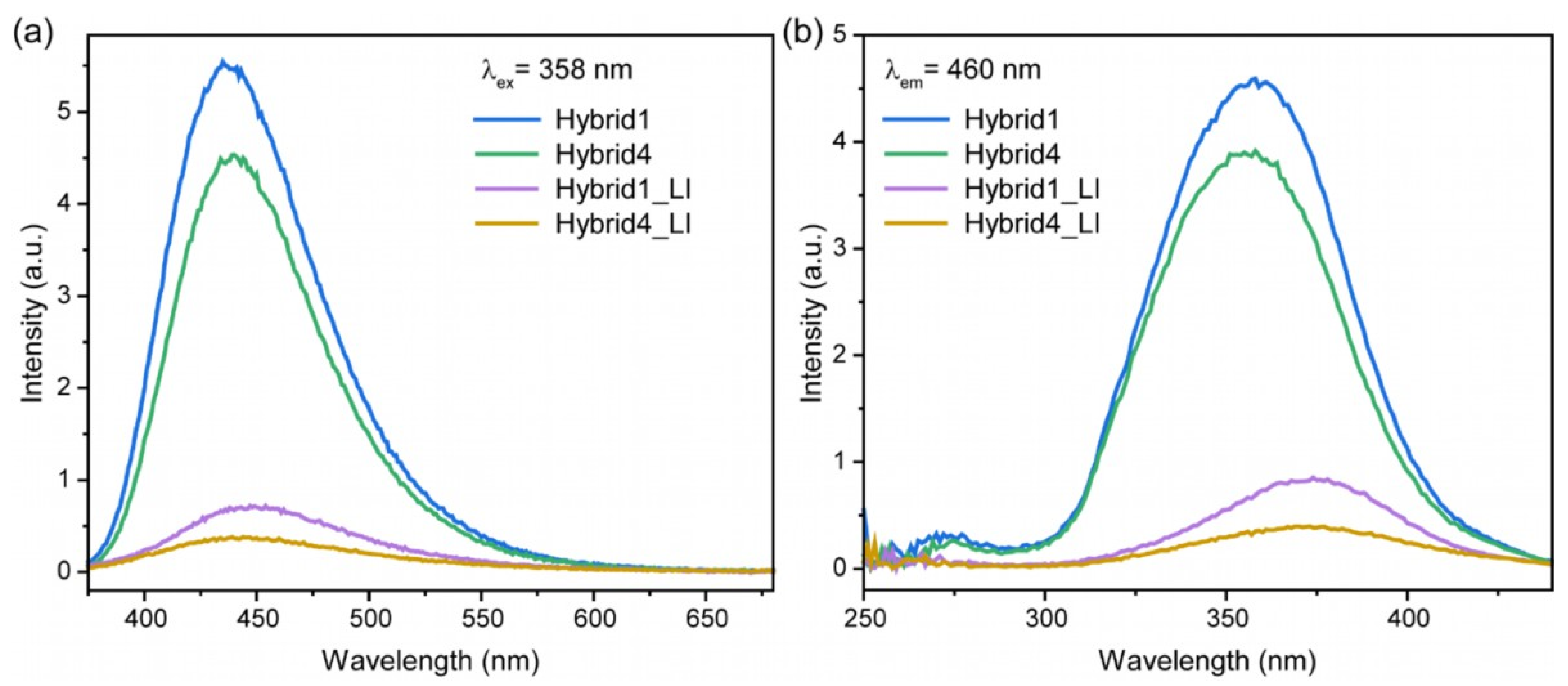
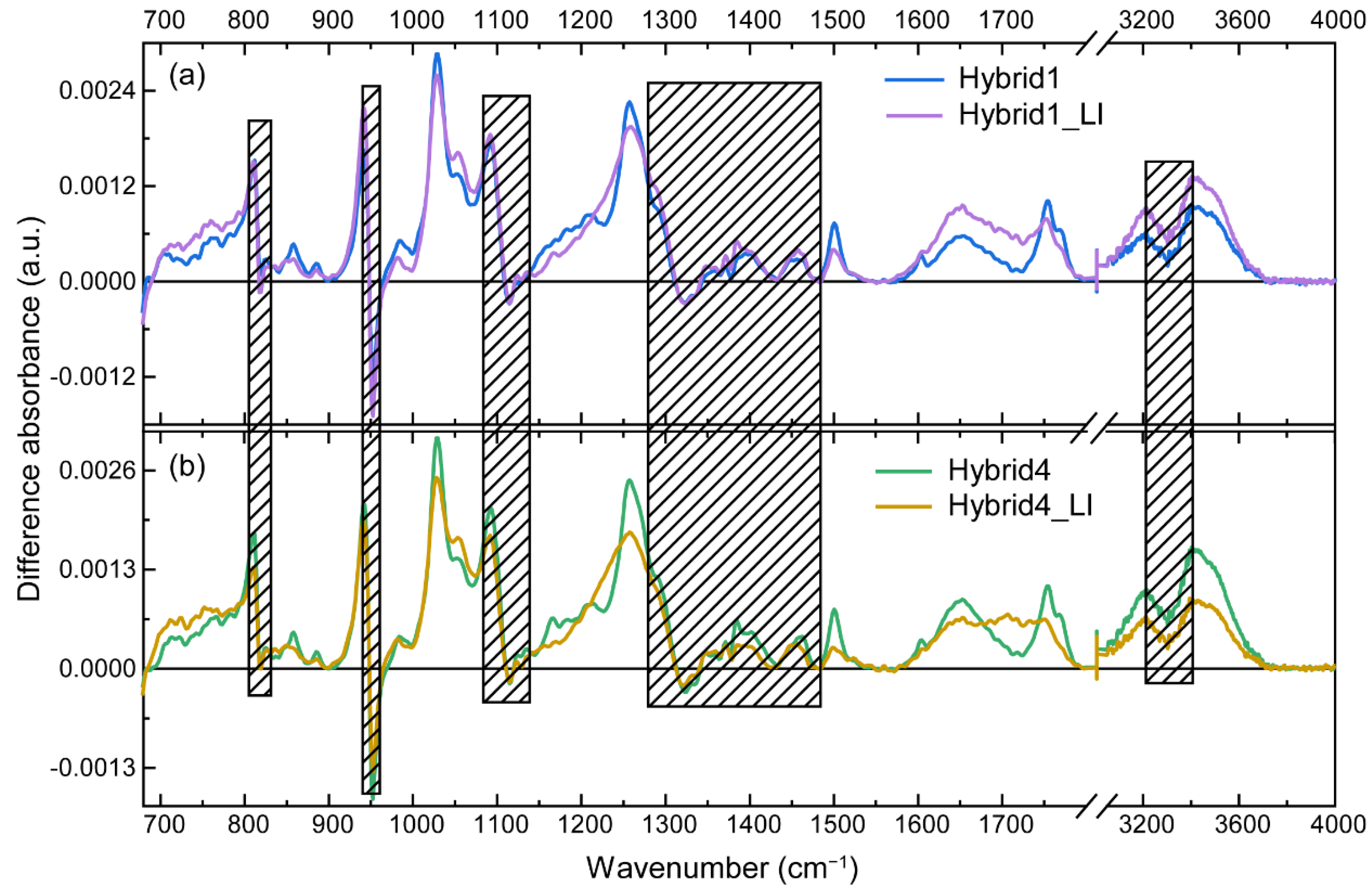
3.4. Laser Irradiation and Characterization of CQD@PhAM Hybrids
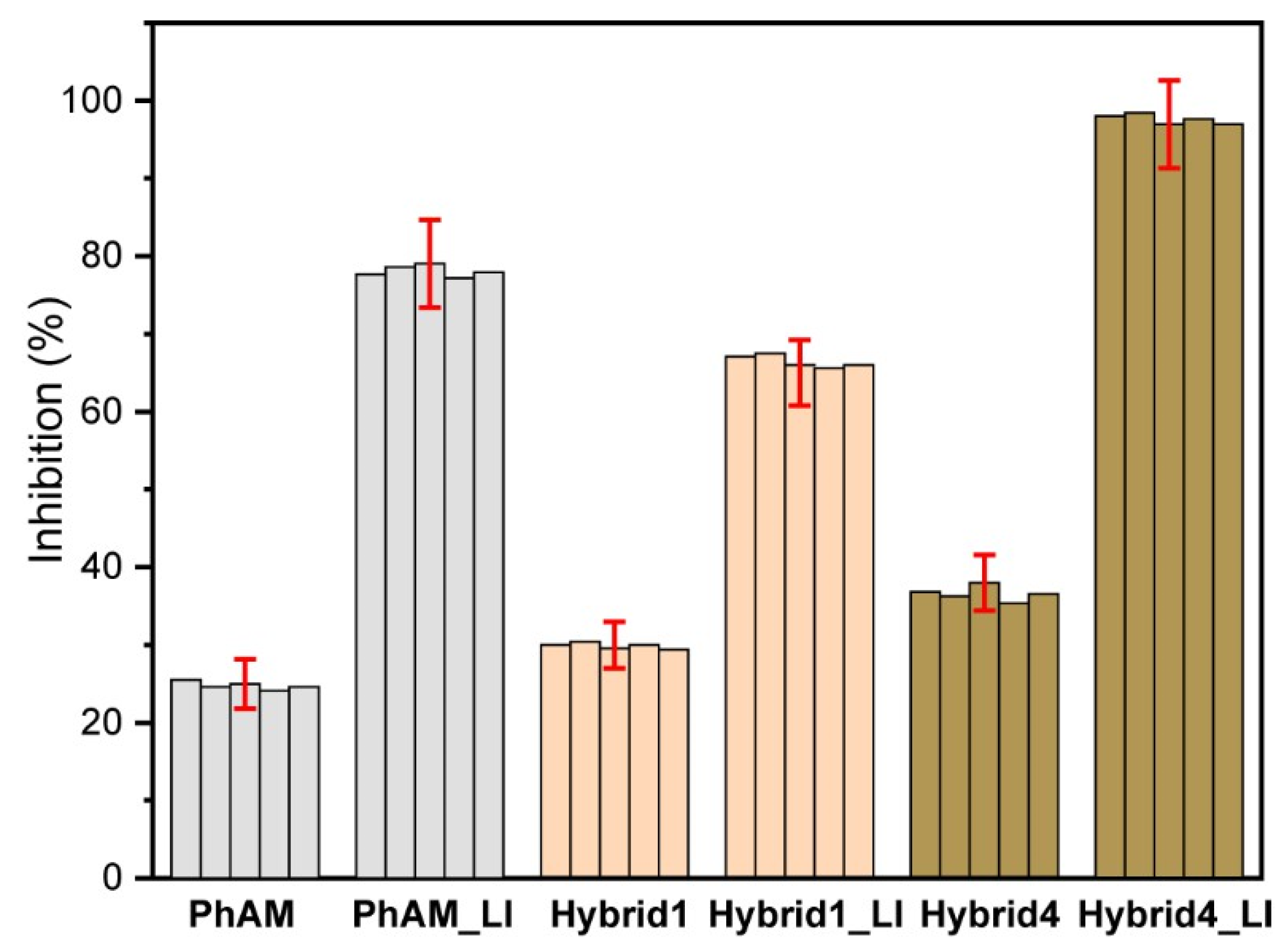
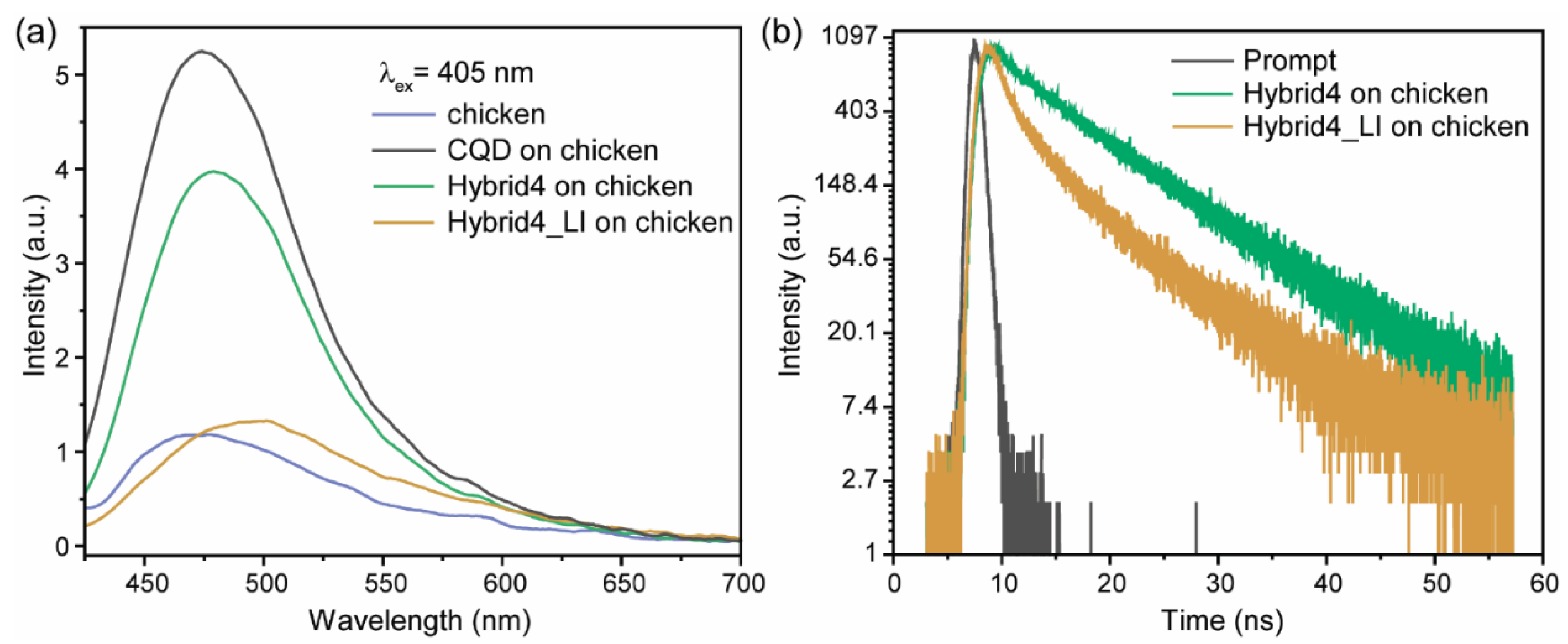
3.5. Testing of CQD@PhAM Hybrids Functionality on Biological Models
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berry, M.H.; Holt, A.; Broichhagen, J.; Donthamsetti, P.; Flannery, J.G.; Isacoff, E.Y. Photopharmacology for Vision Restoration. Curr. Opin. Pharmacol. 2022, 65, 102259. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Friedman, S.H. The Issue of Tissue: Approaches and Challenges to the Light Control of Drug Activity. ChemPhotoChem 2021, 5, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Velema, W.A.; Szymanski, W.; Feringa, B.L. Photopharmacology: Beyond Proof of Principle. J. Am. Chem. Soc. 2014, 136, 2178–2191. [Google Scholar] [CrossRef] [PubMed]
- Calbo, J.; Weston, C.E.; White, A.J.P.; Rzepa, H.S.; Contreras-García, J.; Fuchter, M.J. Tuning Azoheteroarene Photoswitch Performance through Heteroaryl Design. J. Am. Chem. Soc. 2017, 139, 1261–1274. [Google Scholar] [CrossRef] [PubMed]
- Weston, C.E.; Richardson, R.D.; Fuchter, M.J. Photoswitchable Basicity through the Use of Azoheteroarenes. Chem. Commun. 2016, 52, 4521–4524. [Google Scholar] [CrossRef]
- Shchelik, I.S.; Tomio, A.; Gademann, K. Design, Synthesis, and Biological Evaluation of Light-Activated Antibiotics. ACS Infect. Dis. 2021, 7, 681–692. [Google Scholar] [CrossRef]
- Scheiner, M.; Sink, A.; Spatz, P.; Endres, E.; Decker, M. Photopharmacology on Acetylcholinesterase: Novel Photoswitchable Inhibitors with Improved Pharmacological Profiles. ChemPhotoChem 2021, 5, 149–159. [Google Scholar] [CrossRef]
- Howerton, B.S.; Heidary, D.K.; Glazer, E.C. Strained Ruthenium Complexes Are Potent Light-Activated Anticancer Agents. J. Am. Chem. Soc. 2012, 134, 8324–8327. [Google Scholar] [CrossRef]
- Pankin, D.; Khokhlova, A.; Kolesnikov, I.; Vasileva, A.; Pilip, A.; Egorova, A.; Erkhitueva, E.; Zigel, V.; Gureev, M.; Manshina, A. Laser-Induced Twisting of Phosphorus Functionalized Thiazolotriazole as a Way of Cholinesterase Activity Change. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 246, 118979. [Google Scholar] [CrossRef]
- Kolesnikov, I.; Khokhlova, A.; Pankin, D.; Pilip, A.; Egorova, A.; Zigel, V.; Gureev, M.; Leuchs, G.; Manshina, A. Laser-Induced Switching of the Biological Activity of Phosphonate Molecules. N. J. Chem. 2021, 45, 15195–15199. [Google Scholar] [CrossRef]
- Noetzli, M.; Eap, C.B. Pharmacodynamic, Pharmacokinetic and Pharmacogenetic Aspects of Drugs Used in the Treatment of Alzheimer’s Disease. Clin. Pharmacokinet. 2013, 52, 225–241. [Google Scholar] [CrossRef] [PubMed]
- Valasani, K.R.; Hu, G.; Chaney, M.O.; Yan, S.S. Structure-Based Design and Synthesis of Benzothiazole Phosphonate Analogues with Inhibitors of Human ABAD-Aβ for Treatment of Alzheimer’s Disease. Chem. Biol. Drug Des. 2013, 81, 238–249. [Google Scholar] [CrossRef] [PubMed]
- Grando, S.A.; Horton, R.M.; Pereira, E.F.R.; Diethelm-Okita, B.M.; George, P.M.; Albuquerque, E.X.; Conti-Fine, B.M. A Nicotinic Acetylcholine Receptor Regulating Cell Adhesion and Motility Is Expressed in Human Keratinocytes. J. Investig. Dermatol. 1995, 105, 774–781. [Google Scholar] [CrossRef]
- GB2419093A; Acetylcholinesterase Inhibitors for the Treatment of Skin. American College of Healthcare Executives: Chicago, IL, USA, 2004.
- Kolesnikov, I.; Mamonova, D.; Pankin, D.; Bikbaeva, G.; Khokhlova, A.; Pilip, A.; Egorova, A.; Zigel, V.; Manshina, A. Photoswitchable Phosphonate–Fullerene Hybrids with Cholinesterase Activity. Photochem. Photobiol. 2022, 99, 929–935. [Google Scholar] [CrossRef]
- Jung, H.; You, S.; Lee, C.; You, S.; Kim, Y. One-Pot Synthesis of Monodispersed Silica Nanoparticles for Diarylethene-Based Reversible Fluorescence Photoswitching in Living Cells. Chem. Commun. 2013, 49, 7528. [Google Scholar] [CrossRef] [PubMed]
- Villa, M.; Angeloni, S.; Bianco, A.; Gradone, A.; Morandi, V.; Ceroni, P. Luminescent Silicon Nanocrystals Appended with Photoswitchable Azobenzene Units. Nanoscale 2021, 13, 12460–12465. [Google Scholar] [CrossRef]
- Arkhipova, V.; Fu, H.; Hoorens, M.W.H.; Trinco, G.; Lameijer, L.N.; Marin, E.; Feringa, B.L.; Poelarends, G.J.; Szymanski, W.; Slotboom, D.J.; et al. Structural Aspects of Photopharmacology: Insight into the Binding of Photoswitchable and Photocaged Inhibitors to the Glutamate Transporter Homologue. J. Am. Chem. Soc. 2021, 143, 1513–1520. [Google Scholar] [CrossRef]
- Wang, B.; Cai, H.; Waterhouse, G.I.N.; Qu, X.; Yang, B.; Lu, S. Carbon Dots in Bioimaging, Biosensing and Therapeutics: A Comprehensive Review. Small Sci. 2022, 2, 2200012. [Google Scholar] [CrossRef]
- Song, H.; Wu, M.; Tang, Z.; Tse, J.S.; Yang, B.; Lu, S. Single Atom Ruthenium-Doped CoP/CDs Nanosheets via Splicing of Carbon-Dots for Robust Hydrogen Production. Angew. Chem. Int. Ed. 2021, 60, 7234–7244. [Google Scholar] [CrossRef]
- Wu, J.; Chen, G.; Jia, Y.; Ji, C.; Wang, Y.; Zhou, Y.; Leblanc, R.M.; Peng, Z. Carbon Dot Composites for Bioapplications: A Review. J. Mater. Chem. B 2022, 10, 843–869. [Google Scholar] [CrossRef]
- Hou, L.; Chen, D.; Wang, R.; Wang, R.; Zhang, H.; Zhang, Z.; Nie, Z.; Lu, S. Transformable Honeycomb-Like Nanoassemblies of Carbon Dots for Regulated Multisite Delivery and Enhanced Antitumor Chemoimmunotherapy. Angew. Chem. Int. Ed. 2021, 60, 6581–6592. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Sapelkin, A.V.; Titirici, M.M.; Sukhorukov, G.B. In Situ Synthesis of Fluorescent Carbon Dots/Polyelectrolyte Nanocomposite Microcapsules with Reduced Permeability and Ultrasound Sensitivity. ACS Nano 2016, 10, 9608–9615. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.-H.; Li, C.-Q.; Hou, X.-L.; Wu, G.-Y.; Xie, X.-T.; Zhu, D.; Jin, F.; Zhao, Y.-D.; Liu, B. A Metal Ion-Drug-Induced Self-Assembly Nanosystems for Augmented Chemodynamic and Chemotherapy Synergetic Anticancer Therapy. Carbon. N. Y 2022, 188, 104–113. [Google Scholar] [CrossRef]
- Ardekani, S.M.; Dehghani, A.; Ye, P.; Nguyen, K.-A.; Gomes, V.G. Conjugated Carbon Quantum Dots: Potent Nano-Antibiotic for Intracellular Pathogens. J. Colloid. Interface Sci. 2019, 552, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Murali, G.; Kwon, B.; Kang, H.; Modigunta, J.K.R.; Park, S.; Lee, S.; Lee, H.; Park, Y.H.; Kim, J.; Park, S.Y.; et al. Hematoporphyrin Photosensitizer-Linked Carbon Quantum Dots for Photodynamic Therapy of Cancer Cells. ACS Appl. Nano Mater. 2022, 5, 4376–4385. [Google Scholar] [CrossRef]
- Wu, F.; Yue, L.; Su, H.; Wang, K.; Yang, L.; Zhu, X. Carbon Dots @ Platinum Porphyrin Composite as Theranostic Nanoagent for Efficient Photodynamic Cancer Therapy. Nanoscale Res. Lett. 2018, 13, 357. [Google Scholar] [CrossRef]
- Prabhakar, N.; Näreoja, T.; von Haartman, E.; Şen Karaman, D.; Burikov, S.A.; Dolenko, T.A.; Deguchi, T.; Mamaeva, V.; Hänninen, P.E.; Vlasov, I.I.; et al. Functionalization of Graphene Oxide Nanostructures Improves Photoluminescence and Facilitates Their Use as Optical Probes in Preclinical Imaging. Nanoscale 2015, 7, 10410–10420. [Google Scholar] [CrossRef]
- Nasrin, A.; Hassan, M.; Gomes, V.G. Two-Photon Active Nucleus-Targeting Carbon Dots: Enhanced ROS Generation and Photodynamic Therapy for Oral Cancer. Nanoscale 2020, 12, 20598–20603. [Google Scholar] [CrossRef]
- Laptinskiy, K.; Khmeleva, M.; Vervald, A.; Burikov, S.; Dolenko, T. Carbon Dots with Up-Conversion Luminescence as PH Nanosensor. Appl. Sci. 2022, 12, 12006. [Google Scholar] [CrossRef]
- Sarmanova, O.E.; Laptinskiy, K.A.; Khmeleva, M.Y.; Burikov, S.A.; Dolenko, S.A.; Tomskaya, A.E.; Dolenko, T.A. Development of the Fluorescent Carbon Nanosensor for PH and Temperature of Liquid Media with Artificial Neural Networks. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 258, 119861. [Google Scholar] [CrossRef]
- Sarmanova, O.E.; Laptinskiy, K.A.; Burikov, S.A.; Chugreeva, G.N.; Dolenko, T.A. Implementing Neural Network Approach to Create Carbon-Based Optical Nanosensor of Heavy Metal Ions in Liquid Media. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2023, 286, 122003. [Google Scholar] [CrossRef]
- Ugarriza, I.; Uria, U.; Carrillo, L.; Vicario, J.L.; Reyes, E. Base-Promoted C→N Acyl Rearrangement: An Unconventional Approach to A-Amino Acid Derivatives. Chem. A Eur. J. 2014, 20, 11650–11654. [Google Scholar] [CrossRef] [PubMed]
- Petrov, A.A.; Dogadina, A.V.; Ionin, B.I.; Garibina, V.A.; Leonov, A.A. Arbuzov Rearrangement with Halogen Acetylenes-New Way of Synthesis of Acetylene Phosphonates and Other Organo-Phosphorus Compounds. Usp. Khim 1983, 52, 1793–1802. [Google Scholar] [CrossRef]
- Eremenko, A.V.; Dontsova, E.A.; Nazarov, A.P.; Evtushenko, E.G.; Amitonov, S.V.; Savilov, S.V.; Martynova, L.F.; Lunin, V.V.; Kurochkin, I.N. Manganese Dioxide Nanostructures as a Novel Electrochemical Mediator for Thiol Sensors. Electroanalysis 2012, 24, 573–580. [Google Scholar] [CrossRef]
- Eremenko, A.; Prokopkina, T.; Kasatkin, V.; Zigel, V.; Pilip, A.; Russkikh, I.; Zhakovskaya, Z.; Kurochkin, I. Biosensing of Neurotoxicity to Prevent Bioterrorist Threats and Harmful Algal Blooms. In Biosensors for Security and Bioterrorism Applications. Advanced Sciences and Technologies for Security Applications; Nikolelis, D., Nikoleli, G.P., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 333–348. [Google Scholar]
- Egorova, A.V.; Viktorov, N.B.; Lyamenkova, D.V.; Svintsitskaya, N.I.; Garabadziu, A.V.; Dogadina, A.V. Phosphorylation of Aminomalonates. Russ. J. Gen. Chem. 2016, 86, 2446–2453. [Google Scholar] [CrossRef]
- Ţucureanu, V.; Matei, A.; Avram, A.M. FTIR Spectroscopy for Carbon Family Study. Crit. Rev. Anal. Chem. 2016, 46, 502–520. [Google Scholar] [CrossRef]
- Mohammad-Jafarieh, P.; Akbarzadeh, A.; Salamat-Ahangari, R.; Pourhassan-Moghaddam, M.; Jamshidi-Ghaleh, K. Solvent Effect on the Absorption and Emission Spectra of Carbon Dots: Evaluation of Ground and Excited State Dipole Moment. BMC Chem. 2021, 15, 53. [Google Scholar] [CrossRef]
- Yang, L.; Jiang, W.; Qiu, L.; Jiang, X.; Zuo, D.; Wang, D.; Yang, L. One Pot Synthesis of Highly Luminescent Polyethylene Glycol Anchored Carbon Dots Functionalized with a Nuclear Localization Signal Peptide for Cell Nucleus Imaging. Nanoscale 2015, 7, 6104–6113. [Google Scholar] [CrossRef]
- Snyder, L.R.; Kirkland, J.J.; Dolan, J.W. Appendix I: Properties of HPLC Solvents. In Introduction to Modern Liquid Chromatography; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; pp. 879–886. [Google Scholar]
- Mayo, D.W.; Miller, F.A.; Hannah, R.W. (Eds.) Course Notes on the Interpretation of Infrared and Raman Spectra; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2004; ISBN 978-0-471-24823-1. [Google Scholar]
- Fujii, T.; Kodaira, K.; Kawauchi, O.; Tanaka, N.; Yamashita, H.; Anpo, M. Photochromic Behavior in the Fluorescence Spectra of 9-Anthrol Encapsulated in Si−Al Glasses Prepared by the Sol−Gel Method. J. Phys. Chem. B 1997, 101, 10631–10637. [Google Scholar] [CrossRef]
- Pankin, D.; Martynova, N.; Smirnov, M.; Manshina, A. Spectral Properties of Triphenyltin Chloride Toxin and Its Detectivity by SERS: Theory and Experiment. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 245, 118933. [Google Scholar] [CrossRef]
- Stepanidenko, E.A.; Arefina, I.A.; Khavlyuk, P.D.; Dubavik, A.; Bogdanov, K.V.; Bondarenko, D.P.; Cherevkov, S.A.; Kundelev, E.V.; Fedorov, A.V.; Baranov, A.V.; et al. Influence of the Solvent Environment on Luminescent Centers within Carbon Dots. Nanoscale 2020, 12, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Ullal, N.; Muthamma, K.; Sunil, D. Carbon Dots from Eco-Friendly Precursors for Optical Sensing Application: An up-to-Date Review. Chem. Pap. 2022, 76, 6097–6127. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bikbaeva, G.; Pilip, A.; Egorova, A.; Kolesnikov, I.; Pankin, D.; Laptinskiy, K.; Vervald, A.; Dolenko, T.; Leuchs, G.; Manshina, A. All-in-One Photoactivated Inhibition of Butyrylcholinesterase Combined with Luminescence as an Activation and Localization Indicator: Carbon Quantum Dots@Phosphonate Hybrids. Nanomaterials 2023, 13, 2409. https://doi.org/10.3390/nano13172409
Bikbaeva G, Pilip A, Egorova A, Kolesnikov I, Pankin D, Laptinskiy K, Vervald A, Dolenko T, Leuchs G, Manshina A. All-in-One Photoactivated Inhibition of Butyrylcholinesterase Combined with Luminescence as an Activation and Localization Indicator: Carbon Quantum Dots@Phosphonate Hybrids. Nanomaterials. 2023; 13(17):2409. https://doi.org/10.3390/nano13172409
Chicago/Turabian StyleBikbaeva, Gulia, Anna Pilip, Anastasia Egorova, Ilya Kolesnikov, Dmitrii Pankin, Kirill Laptinskiy, Alexey Vervald, Tatiana Dolenko, Gerd Leuchs, and Alina Manshina. 2023. "All-in-One Photoactivated Inhibition of Butyrylcholinesterase Combined with Luminescence as an Activation and Localization Indicator: Carbon Quantum Dots@Phosphonate Hybrids" Nanomaterials 13, no. 17: 2409. https://doi.org/10.3390/nano13172409